ABSTRACT
Biofilm formation by Staphylococcus aureus involves the formation of an extracellular matrix, but the composition of this matrix has been uncertain. Here we report that the matrix is largely composed of cytoplasmic proteins that reversibly associate with the cell surface in a manner that depends on pH. We propose a model for biofilm formation in which cytoplasmic proteins are released from cells in stationary phase. These proteins associate with the cell surface in response to decreasing pH during biofilm formation. Rather than utilizing a dedicated matrix protein, S. aureus appears to recycle cytoplasmic proteins that moonlight as components of the extracellular matrix.
IMPORTANCE
Staphylococcus aureus is a leading cause of multiantibiotic-resistant nosocomial infections and is often found growing as a biofilm in catheters and chronic wounds. Biofilm formation is an important pathogenicity strategy that enhances resistance to antimicrobials, thereby limiting treatment options and ultimately contributing to increased morbidity and mortality. Cells in a biofilm are held together by an extracellular matrix that consists in whole or in part of protein, but the nature of the proteins in the S. aureus matrix is not well understood. Here we postulate that S. aureus recycles proteins from the cytoplasm to form the extracellular matrix. This strategy, of cytoplasmic proteins moonlighting as matrix proteins, could allow enhanced flexibility and adaptability for S. aureus in forming biofilms under infection conditions and could promote the formation of mixed-species biofilms in chronic wounds.
INTRODUCTION
Biofilms are surface-associated, multicellular communities in which cells are held together by means of a self-produced, extracellular matrix. The biofilm provides a protective environment that helps shield cells from external stresses and facilitates community behaviors, such as interactions with a host organism and pathogenicity. Both the nature of the matrix and the regulatory mechanisms mediating its production appear to differ widely among bacterial species. Nonetheless, in most cases that have been studied in detail, the matrix consists of a combination of exopolysaccharide, a dedicated protein, and DNA (1, 2).
Staphylococcus aureus is an important human pathogen and frequently forms biofilms in clinical settings, most often on catheters and other implanted devices but also in chronic wounds (3, 4). S. aureus biofilm formation begins when cells bind to a surface or to host factors, such as fibrinogen and fibronectin (5). The cells then form a multilayered biofilm through intercellular interactions and the production of an extracellular matrix. Initially, it was thought that S. aureus biofilm formation relied solely on the production of an extracellular polysaccharide, polysaccharide intercellular adhesion (PIA), the product of genes of the ica operon (6). However, more recent work, including results presented here, indicates that in many strains PIA is dispensable for biofilm formation (7). Extracellular DNA (eDNA), released from cells through regulated autolysis, is thought to contribute to the structural stability of S. aureus biofilms (8). Finally, previously determined data indicate that proteins play an important role in biofilm formation, but a clear picture of the identity of the proteins that comprise the matrix has yet to emerge (2, 9).
Here we sought to identify, comprehensively, proteins that comprise the matrix in an unbiased approach based on the use of mass spectrometry. We report that the proteinaceous matrix is principally, if not exclusively, composed of cytoplasmic proteins that are recycled as components of the extracellular matrix during biofilm formation. We further show that the aggregation of these cytoplasmic proteins in the interstitial space around cells takes place in a manner that depends on decreasing pH during growth under biofilm-inducing conditions. We propose a model in which certain abundant cytoplasmic proteins moonlight during biofilm formation as components of the extracellular matrix that mediates cell-cell adherence.
RESULTS
Identification of biofilm-associated cell surface proteins.
S. aureus strain HG003 forms robust biofilms in tryptic soy broth (TSB) with 0.5% added glucose (here TSBG) (see Fig. S1A in the supplemental material). Both DNA and protein components of the matrix were found to be important for biofilm formation (see Fig. S1A). However, exopolysaccharide and the biofilm-implicated cell wall proteins, protein A (10) and fibrinogen-binding proteins A and B (11), did not contribute significantly (see Fig. S1B).
We took an unbiased approach to identify cell surface-associated proteins present under biofilm-inducing conditions by adapting a 14N/15N metabolic labeling procedure used in a previous proteomic analysis of S. aureus (12, 13). Cell surface-associated proteins were tagged by biotinylation, allowing their specific isolation and identification (13). The ratio of 14N (biofilm) to 15N (nonbiofilm) peptides for each protein gave an estimate of the abundance of a given protein under each growth condition (see Table S1 in the supplemental material).
Interestingly, despite our selecting specifically for extracellular proteins by biotinylation, most of the proteins identified were predicted cytoplasmic proteins. We established a cutoff value for proteins considered to be enriched during biofilm formation as those having a 14N/15N ratio greater than 2. These 11 proteins are characterized by roles in central metabolism, such as glycolysis and fermentation (14). Consistent with our findings, several of these proteins (for example, enolase, transketolase, acetolactate synthase, GAPDH [glyceraldehyde-3-phosphate dehydrogenase], and lactate dehydrogenase) were previously found to be abundant under biofilm-inducing conditions and in the biofilm exoproteome (15–17) in studies that did not investigate their role in biofilm formation. In addition to identifying proteins that were enriched under biofilm-inducing conditions, we also detected extracellular proteins that were either more abundant under the non-biofilm-inducing conditions or showed equal levels of abundance under the two sets of conditions (having a 14N/15N ratio of between 0.5 and 2) (see Table S1 in the supplemental material).
We focused on two of the identified proteins, enolase and GAPDH, and asked whether we could observe association of these proteins with the cell surface by a method that did not depend on biotinylation. Cells harvested from a mature biofilm were washed in phosphate-buffered saline (PBS) at 4°C for 1 h before the cells were removed by mild centrifugation. Among the proteins that were seen in the buffer fraction, by SDS-PAGE (Fig. 1A, lane 1), were enolase and GAPDH, as judged by immunoblot analysis (Fig. 1B).
FIG 1 .
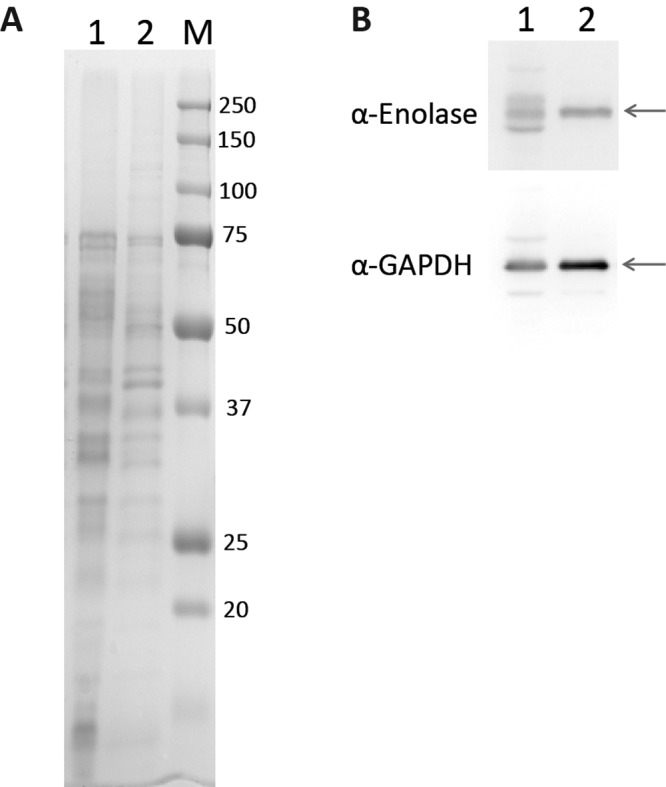
Cytoplasmic proteins are extracted from biofilm cells in PBS. HG003 biofilm cells grown in TSBG were incubated in PBS (pH 7.5). (A) SDS-PAGE analysis of buffer extract (lane 1) and cell lysate (lane 2). The size marker (M) is shown in kDa. (B) Western blots using anti-enolase (top panel) and anti-GAPDH (lower panel) antibodies. Arrows indicate the expected positions of enolase and GAPDH.
Do cell surface-associated, cytoplasmic proteins arise from cell lysis during sample preparation?
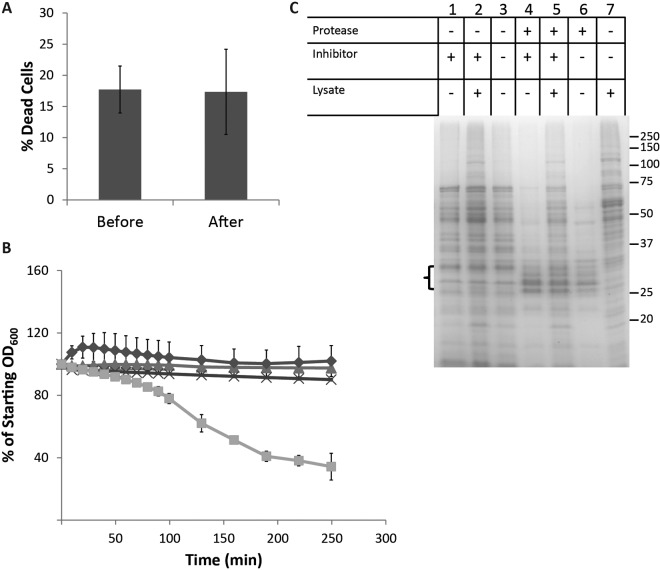
A possible explanation for the presence of cytoplasmic proteins in our cell surface samples was cell lysis or enhanced permeability during enrichment of surface-associated proteins from cells. We took three approaches to investigate this possibility. First, we asked whether cells become permeable upon harvesting from the biofilm and resuspension in PBS using LIVE/DEAD staining. This analysis indicated that the percentage of permeable cells was unaltered by incubation for 1 h in PBS (Fig. 2A).
FIG 2 .
Protein accumulation is not the result of cell lysis during sample processing. (A) Cells do not display increased permeability when resuspended in PBS. Biofilm cells were subjected to LIVE/DEAD staining before and after incubation in PBS (pH 7.5) for 1 h at 4°C. Quantification of the percentage of each population of cells (permeable to SYTO9) consisting of dead cells (permeable to propidium iodide) is shown, with error bars indicating the standard deviation. (B) The rate of autolysis of cells is unchanged by resuspension in PBS. HG003 was grown either under biofilm-forming conditions for 24 h or in the same growth medium with shaking (planktonic cells) for 2.5 h at 37°C. Cells were harvested by centrifugation and then resuspended in PBS or PBS containing 0.1% Triton X-100. Biofilm cells in PBS (Δ), planktonic cells in PBS (◊), biofilm cells in PBS plus Triton (x), and planktonic cells in PBS plus Triton (□) are shown. The percentage of starting OD600 is displayed as an average of the results of three independent experiments, with error bars representing the standard deviation. (C) Protein is not released in significant amounts after resuspension of biofilm cells in PBS. Proteins released into PBS buffer were analyzed by SDS-PAGE following treatment of biofilm cells with and without proteinase K (±protease). At 23 h, a protease inhibitor cocktail was added to certain samples (±inhibitor). Two test samples were spiked with HG003 cell-free lysate as a control (±lysate). An equal volume of PBS, not containing cells, was additionally spiked with HG003 cell-free lysate as a control (lane 7). Three bands expected to represent proteinase K (in those lanes where it was added) are indicated by the bracket on the left of the gel. A size marker is indicated on the right in kDa.
Second, we monitored cell lysis as the decrease in optical density at 600 nm (OD600) with time after resuspension in the presence of Triton X-100, a detergent that stimulates autolytic activity in S. aureus. Resuspension of cells from a mature biofilm in PBS had little to no effect on the rate of lysis, whether stimulated by Triton X-100 or not (Fig. 2B). The rate of stimulated autolysis (decrease in OD600 with time) was very low in cells harvested from the biofilm compared to cells from exponentially growing cultures in the same medium (Fig. 2B), suggesting that biofilm cells are not significantly prone to lysis and that extraction with PBS does not stimulate lysis.
Finally, we asked whether cell surface-associated cytoplasmic proteins arose during biofilm formation or from lysis in PBS. Proteinase K was added upon inoculation, and then a protease inhibitor was added 1 h prior to harvesting the biofilm cells. The results showed that only a very small amount of protein was detected in the PBS extract of biofilm cells (Fig. 2C). When we “spiked in” a cell lysate after the addition of inhibitor, proteins from the lysate were readily detected in the PBS extract, indicating the effectiveness of the inhibitor. The results are consistent with the idea that the cell surface-associated proteins were released from cells during biofilm formation when they accumulated on the outer side of cells and not during our sample preparation procedure. It should, however, be noted that since treatment with proteinase K inhibits biofilm formation (see Fig. S1A in the supplemental material), presumably through degradation of the protein matrix, it is possible that the treated cells could be physiologically different and thus less prone to subsequent cell lysis.
Protein retention at the cell surface reversibly depends on pH.
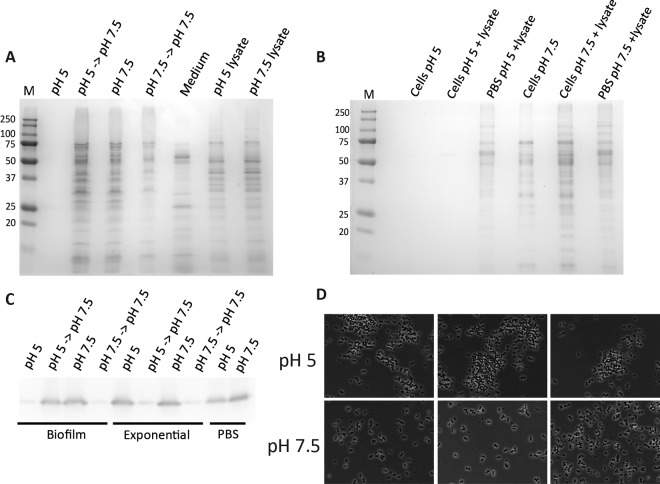
Next, we asked what property of PBS buffer might be responsible for the release of cell surface-associated proteins from cells from the biofilm into the surrounding milieu. We reasoned that a change in pH from the acidic conditions found within biofilms (11) to pH 7.5 in PBS might explain our results. In agreement with previous observations, we found that biofilm formation was accompanied by a significant decrease in growth medium pH which was dependent on the availability of glucose (see Fig. S2A in the supplemental material) and that a decrease in pH was required for biofilm formation (see Fig. S2B).
These results led us to hypothesize that cytoplasmic proteins associate with the cell surface to form a protein matrix in a manner that depends on low pH. To investigate this hypothesis further, we incubated biofilm cells in PBS buffer at pH 5. Little to no protein could be detected in the resulting extract, in contrast to the results seen when cells from the same biofilm sample were incubated at pH 7.5 (Fig. 3A, compare lanes 1 and 3). This was not due to protein degradation at pH 5 since proteins could still be extracted at pH 7.5 from biofilm cells that had been incubated at pH 5 for 1 h (Fig. 3A, compare lanes 1 and 2). Proteins remained cell associated at pH 5 but were released upon exposure to higher pH.
FIG 3 .
Protein retention at the cell surface reversibly depends on pH. (A) Proteins from the biofilm matrix were released from the cell surface in a pH-dependent manner. Data represent SDS-PAGE analysis of proteins from biofilm cells incubated in PBS at pH 5 (lane 1), pH 5 followed by pH 7.5 (lane 2), pH 7.5 (lane 3), and pH 7.5 followed by pH 7.5 (lane 4), proteins concentrated from biofilm growth medium (lane 5), and cell lysates from the cells incubated at pH 5 (lane 6) and pH 7.5 (lane 7). The size marker (M) is shown in kDa. (B) Accumulation of exogenously added proteins at the cell surface depends on low pH and on the presence of S. aureus biofilm cells. Data represent biofilm cells extracted in PBS at pH 5 (lane 1), biofilm cells spiked with cell-free lysate extracted at pH 5 (lane 2), PBS at pH 5 spiked with cell-free lysate (lane 3), biofilm cells extracted in PBS at pH 7.5 (lane 4), biofilm cells spiked with cell-free lysate extracted at pH 7.5 (lane 5), and PBS at pH 7.5 spiked with cell-free lysate (lane 6). (C) Exogenously added GFP interacts with the cell surface of biofilm but not exponential cells at pH 5. Lysate isolated from exponentially growing HG003 expressing GFP (LCF81) was added to PBS (pH 5 and pH 7.5). HG003 cells from biofilm-forming conditions and from exponential-growth-stage shaking cultures were incubated in the two buffers for 1 h at 4°C. Cells were then incubated in PBS (pH 7.5) for a further 1 h. The presence of GFP in each extraction was determined by immunoblotting using an anti-GFP antibody. (D) Cell clumping is reduced following resuspension at pH 7.5. Cells collected from biofilms were gently resuspended in PBS (pH 5 or pH 7.5) and observed by phase-contrast microscopy.
We found that proteins added exogenously (not originating from cells within the biofilm itself) could also be “captured” through an interaction with biofilm cells (Fig. 3B). Whole-cell lysate from HG003 cells was added to mature biofilm cell cultures, and the biofilm cells were then incubated at pH 5 and pH 7.5. Proteins (comprising those naturally occurring in the biofilm and those added in) (Fig. 3B, compare lanes 4 and 5) were released from the cell surface at pH 7.5 and not at pH 5 (Fig. 3B, compare lanes 2 and 5). Proteins from exogenously added cell-free lysate were equally recovered from PBS at pH 5 and pH 7.5 (Fig. 3B, lanes 3 and 6), indicating that, in the absence of biofilm cells, proteins do not precipitate at pH 5 but remain in aqueous solution. This suggests that, in the presence of cells from a biofilm, exogenously added lysate proteins interact with the cell surface at low pH and are then released at pH 7.5.
To investigate the specificity of protein interactions with the cell surface, we next determined whether a heterologous protein, green fluorescent protein (GFP), could interact with the surface of biofilm cells. Cells that had been grown under biofilm-forming conditions were incubated in PBS at pH 5 and pH 7.5 in the presence of lysate proteins isolated from a strain of HG003 that constitutively produces GFP (LCF81). After removal of the cells, GFP was detectable in PBS by immunoblotting with an anti-GFP antibody (Ab) at pH 7.5 but not at pH 5 (Fig. 3C). When cells from PBS at pH 5 were subsequently incubated in PBS at pH 7.5, GFP was released into the higher-pH buffer (Fig. 3C). In contrast, when cells already incubated at pH 7.5 were washed a second time at the same pH, no further GFP could be observed by immunoblot analysis, suggesting that GFP does not associate with cells at pH 7.5 (Fig. 3C). The pH-dependent interaction of GFP with the cell surface was specific to biofilm cells since GFP was detectable in PBS both at pH 5 and at pH 7.5 after incubation with HG003 cells grown to the exponential phase (Fig. 3C). This indicates that a specific feature of cells grown under biofilm-forming conditions is required to mediate the binding of exogenously added proteins to the cell surface.
In the course of conducting these experiments, we observed that cell pellets from biofilm cells formed as diffuse smears (directly from biofilm medium or in PBS at pH 5) at low pH but formed as compact pellets after incubation at pH 7.5 (data not shown). Cell pellets from a biofilm were also more easily resuspended to homogeneity at pH 7.5 than at pH 5. Consequently, we examined cells by phase-contrast microscopy following resuspension. Biofilm cells resuspended at pH 5 were frequently found in large cell clusters that were often associated with other material (Fig. 3D, upper panels). In contrast, cells resuspended at pH 7.5 were largely separate or in small clusters without this associated material (Fig. 3D, lower panels). We propose that low pH causes cytoplasmic proteins to aggregate at the cell surface and that this aggregate is a principal component of the extracellular matrix that holds cells together in the biofilm.
To assess whether the accumulation of cytoplasmic proteins on the cell surface of HG003 biofilm cells is a common feature of S. aureus, we repeated our analysis using four other strains commonly used for the study of biofilm formation. Although they did not make biofilms as robust as those made by HG003, strains Newman, UAMS1, MN8, and RN4220 all produced quantifiable levels of biofilm when grown in 96-well plates in TSBG (see Fig. S3A in the supplemental material); furthermore, the medium pH decreased during growth to around 4.5 to 5 in all cases (data not shown). Cells were harvested from each biofilm and incubated in PBS at pH 5 and pH 7.5 for 1 h at 4°C. As seen with HG003, it was possible to observe the release of proteins into PBS from three of the four strains at pH 7.5 but not at pH 5 (see Fig. S3B). Interestingly, RN4220 did not appear to release a significant amount of protein from the cell surface into PBS at pH 7.5 despite making a robust biofilm. This might suggest either that proteins adhere more tightly to RN4220 cells or that this strain utilizes a different mechanism for biofilm formation (7, 18). Taken together, these results indicate that accumulation of cytoplasmic proteins on the cell surface might be a frequent feature of biofilm formation in S. aureus.
Cytoplasmic proteins associate with the outer side of cells in intact biofilms.
If cytoplasmic proteins accumulate on the cell surface under biofilm-inducing conditions and constitute the extracellular matrix, it should be possible to detect them in intact biofilms that have not been manually disrupted. We used immunofluorescence microscopy to visualize only those proteins accessible to antibodies and thus external to cells. To eliminate nonspecific background caused by the IgG-binding activity of protein A, we utilized a protein A deletion mutant of HG003 (∆spa) which is unaffected in its ability to form biofilms (see Fig. S1B in the supplemental material). As expected, cells of the Δspa mutant did not exhibit binding to a non-specific IgG antibody, anti-Halotag (see Fig. S4A).
Fixed biofilms were probed for target proteins using primary rabbit antibodies and visualized using a secondary antibody conjugated to Alexa Fluor 488. The location of individual cells within the biofilm was observed by staining the chromosomal DNA with DAPI (4′,6-diamidino-2-phenylindole). Anti-enolase binding was visualized as a diffuse accumulation of fluorescent signal around and between cells within the biofilm (Fig. 4A). The detected proteins appeared to occupy areas between, and not overlapping with, regions of DAPI staining, suggesting an extracellular location. In contrast, a cytoplasmic protein (represented by fluorescence from GFP expressed constitutively) was seen to colocalize with regions of DAPI staining (see Fig. S4B in the supplemental material). The localization of enolase was distinct from that of a typical covalently attached cell wall protein, protein A, which was seen as clear rings around the cell periphery of wild-type cells (see Fig. S4C). Anti-GAPDH antibodies localized around and between cells within the biofilm in a honeycomb-like pattern, again clearly occupying a space distinct from the nuclei/cytoplasm (Fig. 4A). The proteins detected by the two antibodies were most apparent in certain areas and predominantly closer to the top of the biofilm. Fluorescent signal was not apparent using nonspecific antibodies such as an anti-GFP antibody (with cells not producing GFP) or a rabbit anti-mouse-FITC antibody (data not shown).
FIG 4 .
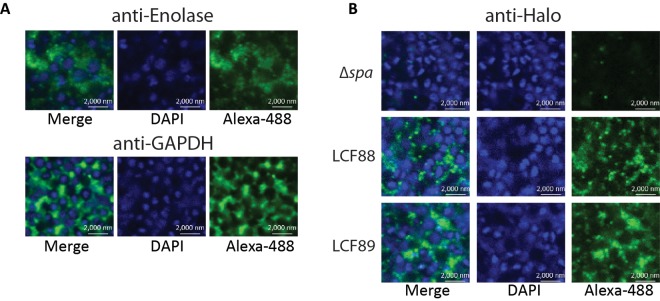
Immunofluorescence visualization of enolase and GAPDH in fixed HG003 biofilms. (A) Fixed HG003 Δspa biofilms were probed with primary rabbit anti-enolase or anti-GAPDH antibodies, followed by a secondary anti-rabbit antibody conjugated to Alexa Fluor 488 (green). Cell nuclei were stained with DAPI (blue). (B) Fixed HG003 Δspa, LCF88 (expressing enolase-HaloTag), and LCF89 (expressing GAPDH-HaloTag) biofilms were probed with primary rabbit anti-HaloTag antibodies, followed by a secondary anti-rabbit antibody conjugated to Alexa Fluor 488 (green). Cell nuclei were stained with DAPI (blue).
Since the genes encoding enolase and GAPDH are essential in S. aureus, we were unable to construct deletion mutants that would function as negative controls in immunofluorescence experiments. To confirm that our results were not due to nonspecific binding of anti-enolase and anti-GAPDH antibodies to other cell surface proteins, we constructed Δspa strains in which the native copy of each gene (encoding enolase or GAPDH) was replaced with a copy that would result in a fusion of the HaloTag with the C terminus of the respective protein. The resulting strains (LCF88 and LCF89) were viable and exhibited no significant changes in growth or biofilm formation compared to the ∆spa strain (see Fig. S5A in the supplemental material). Furthermore, LCF88 and LCF89 were found to produce bands of the expected size for the fusion products by immunoblotting using an anti-HaloTag antibody in cell lysates and in cell surface extracts (PBS at pH 7.5) of cells grown under biofilm conditions (see Fig. S5B). A commercial anti-HaloTag antibody did not react to proteins on the cell surface of the HG003 ∆spa mutant, indicating that the antibody did not display nonspecific binding (Fig. 4B). In contrast, in strains LCF88 and LCF89, respectively, the anti-HaloTag antibody revealed a pattern of protein localization similar to that seen with anti-enolase and anti-GAPDH antibodies (Fig. 4B).
DISCUSSION
A hallmark of biofilm formation in certain bacteria is the production of a dedicated protein component of the extracellular matrix, such as Curli in Escherichia coli or TasA in Bacillus subtilis (19). We failed to detect an S. aureus protein that is unique to the biofilm matrix, although it is possible that such a dedicated protein(s) contributes to the matrix under other conditions or in other strains. Instead, our evidence suggests a model in which cytoplasmic proteins attach to and assemble on the cell surface to form the extracellular matrix. Formation of the matrix is triggered by a decrease in pH during the post-exponential phase in biofilm-inducing medium. We further propose that the matrix thus formed causes cells in the biofilm to adhere to each other. Interestingly, the assembly process is reversible in that proteins can be solubilized from the cell surface, after manual disruption of the biofilm, by artificially increasing the pH. Finally, our evidence indicates that pH-driven association of proteins on the cell surface is a common feature of protein-dependent biofilm formation in diverse strains of S. aureus.
How are cytoplasmic proteins released into the surrounding milieu during biofilm growth? It has previously been reported that a variety of species of Gram-positive bacteria, including S. aureus, release cytoplasmic proteins into the external environment during stationary phase (20, 21). However, the mechanism by which proteins lacking signal peptides are exported from cells is unclear, and both specific (e.g., secretion) and nonspecific (e.g., cell lysis) mechanisms are possible (20–23). Biofilm formation by S. aureus has previously been suggested to be dependent on regulated autolysis for the accumulation of extracellular DNA (24). Conceivably, regulated autolysis may also be the basis for the extracellular presence of cytoplasmic proteins.
The idea that cytoplasmic proteins might accumulate extracellularly and perform a novel function in that location is not new (22, 25). Such “moonlighting” functions of proteins appear to be widespread. In particular, enzymes of glycolysis are commonly observed associating with the cell surface and mediating host factor attachment (26–28). For example, GAPDH and enolase were found to associate, at low pH, with the cell surface of L. crispatus cells and to mediate host attachment (29). Although we currently do not know what properties of proteins are important for their recruitment into the biofilm matrix, we observed that even a heterologous protein, GFP, could associate in a pH-dependent manner with cells when grown under biofilm-inducing conditions but not when grown under planktonic conditions, suggesting that some physical property of the cell surface under biofilm-inducing conditions mediates this interaction.
We observed that the presence of glucose, which leads to the accumulation of acidic byproducts from fermentation, is critical for the decrease in pH in biofilm-inducing medium. Interestingly, high glucose levels correlate with increased S. aureus biofilm formation in clinical settings and hyperglycemia is a major risk factor for nosocomial infections (30). It was found that the rate of nosocomial infection was 2.7-fold higher in patients with blood glucose levels of greater than 220 mg/dl (0.22%) (31), a concentration similar to that required to induce biofilm formation in vitro (0.25%) (data not shown). It is still unclear how much glucose is present in the microenvironment of a wound or in-dwelling-medical-device infection. However, many sites of S. aureus colonization have acidic pH (32) and it has frequently been reported that inflammation in the context of abscess infections is associated with a lowered-pH environment (33). Conceivably, low pH is a contributing factor for biofilm formation in physiological contexts. In further support of our model under physiological conditions, several cytosolic proteins were identified as biofilm cell wall antigens in a chronic osteomyelitis model in rabbits (15).
We postulate that cell surface-associated cytoplasmic proteins contribute to biofilm formation in S. aureus. However, genetic evidence for this model will be difficult to obtain because the genes for many of the cytoplasmic proteins we identified, for example, GAPDH (gapA) and enolase (eno), are essential. Additionally, since we postulate that many cytoplasmic proteins can contribute to the matrix, elimination of just one such protein might have little effect on matrix formation. We therefore anticipate that further investigation of the role of cytoplasmic proteins in biofilm formation will require an understanding of the mechanism by which cytoplasmic proteins are released from stationary-phase cells and then aggregate on the cell surface in response to a drop in pH. It is also tempting to speculate about a possible role within the context of this model for extracellular DNA (eDNA), which, along with protein, is required for biofilm formation. We suggest that eDNA might serve as an electrostatic net linking protein-coated cells in the biofilm. We note that at pH 4.5 to 5, these surface-associated, cytoplasmic proteins are likely to carry a net positive charge, facilitating their interaction with the proposed eDNA net. Finally, our findings are of course limited to an in vitro, static biofilm model at a specific biofilm growth stage. It remains to be seen whether these findings will be applicable to other in vitro biofilm models (e.g., flow cells) and to physiologically relevant biofilms.
The repurposing of abundant cytoplasmic proteins as matrix components offers S. aureus the advantage of not investing in the production of dedicated matrix proteins during biofilm formation. Conceivably, other Gram-positive bacteria employ a similar moonlighting strategy in making biofilms, and the use of conserved cytoplasmic proteins in the biofilm matrix might facilitate interspecies interactions. S. aureus has been found in chronic wounds (such as diabetic ulcers) with multiple other bacterial species, and 60% of such wounds were observed to contain biofilms (4). S. aureus might form multispecies biofilms without the necessity to specifically recognize the dedicated matrix components of the other species. The recycling of cytoplasmic proteins could therefore represent an efficient and versatile strategy for building multicellular communities. It will be interesting to see in future work whether cytoplasmic proteins that moonlight as matrix components represent a widespread strategy for biofilm formation.
MATERIALS AND METHODS
Strains and growth conditions.
S. aureus strains are listed in Table S2 in the supplemental material. S. aureus was cultured in tryptic soy broth (TSB; EMD Millipore) and on LB agar (BD) at 37°C. Other growth media were brain heart infusion (BHI; EMD Millipore), BioExpress 1000 (Cambridge Isotope Laboratories), and TSB without glucose (peptone from casein [BD], 17 g/liter; peptone from soymeal [Amresco], 3 g/liter; NaCl [Sigma], 5 g/liter; dipotassium hydrogen phosphate [Macron], 2.5 g/liter). E. coli DH5α was cultured in LB and on LB agar. Where appropriate, S. aureus was selected on 3 µg/ml tetracycline and E. coli on 50 µg/ml ampicillin. The pH of biofilm cultures was measured by spotting 20 to 30 µl on pH strips (range, 4.5 to 10; VWR).
S. aureus quantitative biofilm assays.
For biofilm growth, an overnight culture of HG003 was diluted at 1 in 1,000 into fresh medium (typically TSB supplemented with 0.5% glucose [TSBG] or as specified in the text) and 200 µl was divided into aliquots and introduced into a Nunc MicroWell 96-well microplate (catalog. no. 167008; Thermo/Fisher Scientific). The starting OD600 was recorded using a Bio-Tek Synergy II plate reader (BioTek Instruments). Plates were incubated statically at 37°C for 24 h. The medium in each well was removed to a new 96-well plate. The biofilms were washed twice with 100 µl phosphate-buffered saline (PBS) (pH 7.5), and the two washes were amalgamated into a new 96-well microtiter plate. The biofilms were resuspended in 200 µl PBS. The OD600 of each fraction was recorded using a Bio-Tek Synergy II plate reader. The starting OD600 was subtracted from that for each medium sample, and the absorbance of PBS was subtracted from the values for the wash and biofilm samples. Results from replicate wells (≥4) were averaged, and a standard deviation was calculated. Proteinase K (Omega Bio-Tek) was used at 0.1 mg/ml. DNase I (Qiagen) was used at 28 U/ml.
Construction of unmarked deletion mutants in HG003.
In-frame, unmarked deletions of specific regions of the HG003 genome were constructed essentially as described in reference 34. Deletion constructs were generated in pKFT using Gibson assembly (35) and primers shown in Tables S3 and S4. Constructs were transferred to HG003 and double-crossover recombinants selected as described in reference 34. For a detailed description, see the supplemental material.
Enrichment for cell-associated proteins from biofilm cells.
HG003 was inoculated at 1 in 1,000 into TSBG and BioExpress 1000 15N in 6-well tissue culture plates (Falcon 353046) for each of three biological replicates. Plates were incubated statically at 37°C for 24 h. Cells and growth media were harvested using a well scraper at 4°C, and cells were pelleted by centrifugation at 7,500 × g for 5 min. Cells grown in TSBG for each replicate were resuspended in 5 ml PBS (pH 7.5 to 8) containing 1 mM phenylmethylsulfonyl fluoride (PMSF), and this was used to resuspend cells grown in BioExpress (equal numbers of OD600 units). Surface-associated proteins were enriched by biotinylation as described in reference 13. Proteins were separated by SDS-PAGE and stained with Coomassie brilliant blue R-250. A complete protein separation lane was cut into 5 equal gel slices. In-gel digestion was performed with trypsin (Promega) (36).
All peptides obtained from an in-gel digestion were separated by liquid chromatography and measured online by electrospray ionization (ESI) mass spectrometry. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses were performed using an Easy-nLC II high-pressure liquid chromatography (HPLC) system (Thermo/Fisher Scientific) coupled directly to an LTQ Orbitrap Velos mass spectrometer (Thermo/Fisher Scientific). All MS analysis and protein identification and quantification procedures were performed at the Harvard Mass Spectrometry and Proteomics Resource Laboratory, FAS Center for Systems Biology, Cambridge, MA.
Analysis of proteins released into PBS.
Biofilm cells grown in 6-well plates were pelleted by centrifugation at 5,500 × g for 10 min. Proteins were precipitated from growth medium using trichloroacetic acid (TCA) precipitation. Biofilm cells were gently resuspended in 5 ml PBS (pH 7.5 to 8 or pH 5 [pH decreased by the addition of HCl]) containing cOmplete Protease Inhibitor Cocktail (Roche) (according to manufacturer’s instruction) by gently swirling the 50-ml tube. Cells were incubated at 4°C with gentle rotation for 1 h. Cells were pelleted by centrifugation at 5,500 × g for 10 min, and the supernatant was passed over a 0.2-µm-pore-size filter. Proteins were concentrated for gel electrophoresis by TCA precipitation. Whole-cell lysates were produced by FastPrep disruption (MP Biomedicals) (speed, 6.5; three 40-s steps) followed by centrifugation at 18,000 × g and 4°C for 15 min to pellet cell debris. Proteins were routinely separated on 12.5% SDS-PAGE gels and stained with GelCode Blue Stain reagent (Thermo/Fisher Scientific).
For proteinase K experiments, 0.1 mg/ml proteinase K was added at the time of inoculation to appropriate wells. Protease activity was inhibited by adding ProteaseArrest solution (G-Biosciences) to biofilm wells at a 3× final concentration and incubating for 1 h at 37°C. Where applicable, whole-cell lysate from HG003 or LCF81 grown to the stationary phase in TSB (passed over a 0.2-µm-pore-size filter to remove intact cells) (approximately 25 µg total) was added either directly to biofilm cultures after removal of cells from the biofilm growth plate or in the PBS extraction buffer, as indicated.
Western blot analysis.
Proteins separated by SDS-PAGE were transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked for 2 h at room temperature in 5% milk powder–5% goat serum (Sigma-Aldrich)–Tris-buffered saline–Tween 20 (TBST; 150 mM NaCl, 50 mM Tris-HCl, 0.05% Tween 20). Membranes were probed with primary antibodies diluted in 5% milk–TBST. Rabbit anti-enolase antibody (against B. subtilis enolase; kind gift of Jörg Stülke) was used at a dilution of 1:5,000, rabbit anti-GAPDH (37) was used at a dilution of 1:10,000, rabbit anti-GFP (38) was used at a dilution of 1:4,000, and rabbit anti-HaloTag polyclonal Ab (pAb) (Promega) was used at a dilution of 1:5,000. Antibody binding was detected with a goat anti-rabbit antibody conjugated to horseradish peroxidase (Bio-Rad Laboratories) and SuperSignal West Dura chemiluminescent substrate (Thermo/Fisher Scientific).
LIVE/DEAD staining.
A 1-µl volume of propidium iodide (diluted 1/10 in PBS) and a 1-µl volume of SYTO 9 (1/10 in PBS) (LIVE/DEAD BacLight bacterial viability kit; Life Technologies) were added per ml of cells. Cells were incubated with the dyes at room temperature for 15 min. Cells were washed once in PBS, resuspended in 150 µl PBS, and observed on a phase-contrast microscope fitted with a 63× objective. Images were taken using phase (500-ms exposure), GFP (100-ms exposure), and mCherry (100-ms exposure) filters. Live (green) and dead (red) cells were counted manually, and data were averaged from a minimum of 1,000 cells across at least three fields of view in three replicate experiments.
Autolysis assay.
Triton X-100 induced autolysis assays were performed essentially as described in reference 39. Briefly, cells were grown in TSBG under biofilm-inducing conditions for 24 h or under shaking conditions to the exponential phase (2 h). Cells were harvested by centrifugation as described and then resuspended in PBS to an OD600 of 2. An equal volume of PBS or PBS containing 0.1% Triton X-100 was then added, and 100 µl of cells was introduced into each of eight replicate wells in a 96-well microtiter plate. The OD600 was monitored every 10 min for 1.5 h and every 30 min thereafter. The OD600 at each time point was plotted as a percentage of the starting OD600.
Immunofluorescence.
To carry out immunofluorescence assays of S. aureus biofilms, we adapted a method for visualizing cell surface proteins on planktonic cells (10) (for details, see the supplemental material). To detect antibody binding, samples were exposed to goat anti-rabbit IgG conjugated to Alexa Fluor 488 dye (Life Technologies) diluted 1:1,000 in PBS and chromosomal DNA was stained with DAPI (7.5 µg/ml final concentration). Biofilms were visualized by confocal laser scanning microscopy on a Zeiss LSM 700 microscope fitted with a 63× oil objective. Alexa Fluor 488 was excited with a 488-nm laser and DAPI with a 405-nm laser. Images were processed and analyzed using ZEN lite 2012 (Blue edition; Zeiss).
Construction of gene-HaloTag fusions at native loci.
Replacement of the eno and gapA genes, at their native loci in the genome, with copies of the respective ORFs fused to a sequence encoding the HaloTag (Promega) was carried out as described in the supplemental material. Subsequently, to allow the use of these strains for immunofluorescence, the spa gene was deleted by homologous recombination using construct pLF048.
SUPPLEMENTAL MATERIAL
Supplemental Materials and Methods. Download
Factors affecting in vitro biofilm formation by S. aureus HG003. (A) The following treatments were added or not at the time of inoculation of HG003 biofilm cultures in TSBG: no treatment, 0.1 mg/ml proteinase K (PK), and 28 U/ml DNase I. Biofilm formation was quantified spectrophotometrically after 24 h. (B) Each HG003 deletion mutant strain was grown under biofilm conditions, and biofilm formation was quantified after 24 h. Download
Biofilm formation by S. aureus HG003 is characterized by and dependent on a decrease in growth medium pH. (A) The pH of biofilm growth medium measured with time. (B) Quantification of biofilm formation in different media. (C) The correlation between the final pH of a given growth medium and the percentage of biomass found in the biofilm fraction. A range of different growth conditions (not listed) were assayed. Download
Comparison of the matrix protein accumulation profiles of different S. aureus strains. (A) Quantification of biofilm formation by strains HG003, Newman, UAMS1, MN8, and RN4220. (B) SDS-PAGE analysis of proteins released from biofilm cells incubated in PBS (pH 5 or pH 7.5) for 1 h at 4°C. The size marker (M) is shown in kDa. Download
Protein localization in S. aureus. (A) Deletion of spa in HG003 removes background binding of rabbit IgG to the cell wall. Fixed biofilms of the HG003 wild type and ∆spa mutant were probed with rabbit anti-HaloTag antibodies followed by goat anti-rabbit Alexa 488 (green). Nuclei were stained with DAPI (blue). (B) A cytoplasmic protein (GFP) colocalizes with DAPI-stained nuclei. A fixed biofilm of HG003 ∆spa pCM29 (constitutive GFP expression) was observed by confocal laser scanning microscopy (CLSM). Cytoplasmic GFP (green) and nuclei stained with DAPI (blue) are shown. (C) A typical covalently attached cell wall protein is observed as rings around the cell periphery. Fixed biofilms of HG003 wild type were probed with rabbit anti-HaloTag antibodies followed by goat anti-rabbit Alexa 488 (green). Nuclei were stained with DAPI (blue). Download
Expression of HaloTag fusion proteins in the HG003 ∆spa mutant. (A) Quantification of biofilm formation by Halo fusion strains LCF88 (enolase-Halo) and LCF89 (GAPDH-Halo). (B) Detection of enolase-Halo (approximately 81 kDa) produced by strain LCF88 and GAPDH-Halo (approximately 70 kDa by LCF89) by immunoblotting using an anti-HaloTag antibody. Lane 1, pH 5 extract; lane 2, pH 7.5 extract; lane 3, cell lysate. The size marker (M) is shown in kDa. Red arrows on the right of the image indicate the bands of expected size for the two halo fusion proteins (Eno-Halo and GAPDH-Halo). Download
A summary of all “extracellular” proteins identified by quantitative proteomics and by enrichment through biotinylation.
Strains used in this study.
Constructs used to create S. aureus HG003 unmarked mutations.
Primers used in this study.
ACKNOWLEDGMENTS
We thank D. Heinrichs (University of Western Ontario), J. Stülke (University of Gӧttingen), M. Sugai (Hiroshima University), A. Horswill (University of Iowa), and M. Gilmore and F. Ausubel (Harvard University) for the gift of S. aureus strains and reagents, and the Bauer Core Mass Spectrometry Facility and Biological Imaging Centre at Harvard University. We thank members of the Losick laboratory for helpful discussion and comments on the manuscript.
Funding for this work was provided by the NIH (PO1-AI083214).
Footnotes
CitationFoulston L, Elsholz AKW, DeFrancesco AS, Losick R. 2014. The extracellular matrix of Staphylococcus aureus biofilms comprises cytoplasmic proteins that associate with the cell surface in response to decreasing pH. mBio 5(5):e01667-14. doi:10.1128/mBio.01667-14.
REFERENCES
- 1. Lemon KP, Earl AM, Vlamakis HC, Aguilar C, Kolter R. 2008. Biofilm development with an emphasis on Bacillus subtilis. Curr. Top. Microbiol. Immunol. 322:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joo HS, Otto M. 2012. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem. Biol. 19:1503–1513. 10.1016/j.chembiol.2012.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otto M. 2008. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 322:207–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. James G, Swogger E, Wolcott R, de Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. 2008. Biofilms in chronic wounds. Wound Repair Regen. 16:37–44. 10.1111/j.1524-475X.2007.00321.x [DOI] [PubMed] [Google Scholar]
- 5. Clarke SR, Foster SJ. 2006. Surface adhesins of Staphylococcus aureus. Adv. Microb. Physiol. 51:187–224. 10.1016/S0065-2911(06)51004-5 [DOI] [PubMed] [Google Scholar]
- 6. Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fitzpatrick F, Humphreys H, O’Gara JP. 2005. Evidence for icaADBC-independent biofilm development mechanism in methicillin-resistant Staphylococcus aureus clinical isolates. J. Clin. Microbiol. 43:1973–1976. 10.1128/JCM.43.4.1973-1976.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 104:8113–8118. 10.1073/pnas.0610226104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boles BR, Thoendel M, Roth AJ, Horswill AR. 2010. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5:e10146. 10.1371/journal.pone.0010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penadés JR, Lasa I. 2009. Protein A-mediated multicellular behavior in Staphylococcus aureus. J. Bacteriol. 191:832–843. 10.1128/JB.01222-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, Loughman A, Foster TJ, O’Gara JP. 2008. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 190:3835–3850. 10.1128/JB.00167-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Becher D, Hempel K, Sievers S, Zühlke D, Pané-Farré J, Otto A, Fuchs S, Albrecht D, Bernhardt J, Engelmann S, Völker U, van Dijl JM, Hecker M. 2009. A proteomic view of an important human pathogen-towards the quantification of the entire Staphylococcus aureus proteome. PLoS One 4:e8176. 10.1371/journal.pone.0008176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hempel K, Pané-Farré J, Otto A, Sievers S, Hecker M, Becher D, Pane J. 2010. Quantitative cell surface proteome profiling for SigB-dependent protein expression in the human pathogen Staphylococcus aureus via biotinylation approach. J. Proteome Res. 9:1579–1590. 10.1021/pr901143a [DOI] [PubMed] [Google Scholar]
- 14. Zhu Y, Weiss EC, Otto M, Fey PD, Smeltzer MS, Somerville GA. 2007. Staphylococcus aureus biofilm metabolism and the influence of arginine on polysaccharide intercellular adhesin synthesis, biofilm formation, and pathogenesis. Infect. Immun. 75:4219–4226. 10.1128/IAI.00509-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brady RA, Leid JG, Camper AK, Costerton JW, Shirtliff ME. 2006. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect. Immun. 74:3415–3426. 10.1128/IAI.00392-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Resch A, Leicht S, Saric M, Pásztor L, Jakob A, Götz F, Nordheim A. 2006. Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics 6:1867–1877. 10.1002/pmic.200500531 [DOI] [PubMed] [Google Scholar]
- 17. Gil C, Solano C, Burgui S, Latasa C, García B, Toledo-Arana A, Lasa I, Valle J. 2014. Biofilm matrix exoproteins induce a protective immune response against Staphylococcus aureus biofilm infection. Infect. Immun. 82:1017–1029. 10.1128/IAI.01419-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vergara-Irigaray M, Valle J, Merino N, Latasa C, García B, Ruiz de los Mozos I, Solano C, Toledo-Arana A, Penadés JR, Lasa I. 2009. Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect. Immun. 77:3978–3991. 10.1128/IAI.00616-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. López D, Vlamakis H, Kolter R. 2010. Biofilms. Cold Spring Harb. Perspect. Biol. 2:a000398. 10.1101/cshperspect.a000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pasztor L, Ziebandt AK, Nega M, Schlag M, Haase S, Franz-Wachtel M, Madlung J, Nordheim A, Heinrichs DE, Götz F. 2010. Staphylococcal major autolysin (Atl) is involved in excretion of cytoplasmic proteins. J. Biol. Chem. 285:36794–36803. 10.1074/jbc.M110.167312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang CK, Ewis HE, Zhang X, Lu CD, Hu HJ, Pan Y, Abdelal AT, Tai PC. 2011. Nonclassical protein secretion by Bacillus subtilis in the stationary phase is not due to cell lysis. J. Bacteriol. 193:5607–5615. 10.1128/JB.05897-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boël G, Jin H, Pancholi V. 2005. Inhibition of cell surface export of group A streptococcal anchorless surface dehydrogenase affects bacterial adherence and antiphagocytic properties. Infect. Immun. 73:6237–6248. 10.1128/IAI.73.10.6237-6248.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boël G, Pichereau V, Mijakovic I, Mazé A, Poncet S, Gillet S, Giard JC, Hartke A, Auffray Y, Deutscher J. 2004. Is 2-phosphoglycerate-dependent automodification of bacterial enolases implicated in their export? J. Mol. Biol. 337:485–496. 10.1016/j.jmb.2003.12.082 [DOI] [PubMed] [Google Scholar]
- 24. Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW. 2009. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4:e5822. 10.1371/journal.pone.0005822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huberts DH, van der Klei IJ. 2010. Moonlighting proteins: an intriguing mode of multitasking. Biochim. Biophys. Acta 1803:520–525. 10.1016/j.bbamcr.2010.01.022 [DOI] [PubMed] [Google Scholar]
- 26. Henderson B, Martin A. 2013. Bacterial moonlighting proteins and bacterial virulence. Curr. Top. Microbiol. Immunol. 358:155–23. 10.1007/82_2011_188 [DOI] [PubMed] [Google Scholar]
- 27. Carneiro CR, Postol E, Nomizo R, Reis LF, Brentani RR. 2004. Identification of enolase as a laminin-binding protein on the surface of Staphylococcus aureus. Microbes Infect. 6:604–608. 10.1016/j.micinf.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 28. Yamaguchi M, Ikeda R, Nishimura M, Kawamoto S. 2010. Localization by scanning immunoelectron microscopy of triosephosphate isomerase, the molecules responsible for contact-mediated killing of Cryptococcus, on the surface of Staphylococcus. Microbiol. Immunol. 54:368–370. 10.1111/j.1348-0421.2010.00225.x [DOI] [PubMed] [Google Scholar]
- 29. Antikainen J, Kuparinen V, Kupannen V, Lähteenmäki K, Korhonen TK. 2007. pH-dependent association of enolase and glyceraldehyde-3-phosphate dehydrogenase of Lactobacillus crispatus with the cell wall and lipoteichoic acids. J. Bacteriol. 189:4539–4543. 10.1128/JB.00378-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waldrop R, McLaren A, Calara F, McLemore R. 6 March 2014. Biofilm growth has a threshold response to glucose in vitro. Clin. Orthop. Relat. Res. 10.1007/s11999-014-3538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pomposelli JJ, Baxter JK, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, Bistrian BR. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J. Parenter. Enteral Nutr. 22:77–81. 10.1177/014860719802200277 [DOI] [PubMed] [Google Scholar]
- 32. Weinrick B, Dunman PM, Mcaleese F, Projan SJ, Fang Y, Novick RP, Murphy E. 2004. Effect of mild acid on gene expression in Staphylococcus aureus. J. Bacteriol. 186:8407–8423. 10.1128/JB.186.24.8407-8423.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lardner A. 2001. The effects of extracellular pH on immune function. J. Leukoc. Biol. 69:522–530 [PubMed] [Google Scholar]
- 34. Kato F, Sugai M. 2011. A simple method of markerless gene deletion in Staphylococcus aureus. J. Microbiol. Methods 87:76–81. 10.1016/j.mimet.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 35. Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6:343–345. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- 36. Eymann C, Dreisbach A, Albrecht D, Bernhardt J, Becher D, Gentner S, Tam LT, Büttner K, Buurman G, Scharf C, Venz S, Völker U, Hecker M. 2004. A comprehensive proteome map of growing Bacillus subtilis cells. Proteomics 4:2849–2876. 10.1002/pmic.200400907 [DOI] [PubMed] [Google Scholar]
- 37. Taylor JM, Heinrichs DE. 2002. Transferrin binding in Staphylococcus aureus: involvement of a cell wall-anchored protein. Mol. Microbiol. 43:1603–1614. 10.1046/j.1365-2958.2002.02850.x [DOI] [PubMed] [Google Scholar]
- 38. Rudner DZ, Losick R. 2002. A sporulation membrane protein tethers the pro-sigmaK processing enzyme to its inhibitor and dictates its subcellular localization. Genes Dev. 16:1007–1018. 10.1101/gad.977702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mani N, Tobin P, Jayaswal RK. 1993. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J. Bacteriol. 175:1493–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials and Methods. Download
Factors affecting in vitro biofilm formation by S. aureus HG003. (A) The following treatments were added or not at the time of inoculation of HG003 biofilm cultures in TSBG: no treatment, 0.1 mg/ml proteinase K (PK), and 28 U/ml DNase I. Biofilm formation was quantified spectrophotometrically after 24 h. (B) Each HG003 deletion mutant strain was grown under biofilm conditions, and biofilm formation was quantified after 24 h. Download
Biofilm formation by S. aureus HG003 is characterized by and dependent on a decrease in growth medium pH. (A) The pH of biofilm growth medium measured with time. (B) Quantification of biofilm formation in different media. (C) The correlation between the final pH of a given growth medium and the percentage of biomass found in the biofilm fraction. A range of different growth conditions (not listed) were assayed. Download
Comparison of the matrix protein accumulation profiles of different S. aureus strains. (A) Quantification of biofilm formation by strains HG003, Newman, UAMS1, MN8, and RN4220. (B) SDS-PAGE analysis of proteins released from biofilm cells incubated in PBS (pH 5 or pH 7.5) for 1 h at 4°C. The size marker (M) is shown in kDa. Download
Protein localization in S. aureus. (A) Deletion of spa in HG003 removes background binding of rabbit IgG to the cell wall. Fixed biofilms of the HG003 wild type and ∆spa mutant were probed with rabbit anti-HaloTag antibodies followed by goat anti-rabbit Alexa 488 (green). Nuclei were stained with DAPI (blue). (B) A cytoplasmic protein (GFP) colocalizes with DAPI-stained nuclei. A fixed biofilm of HG003 ∆spa pCM29 (constitutive GFP expression) was observed by confocal laser scanning microscopy (CLSM). Cytoplasmic GFP (green) and nuclei stained with DAPI (blue) are shown. (C) A typical covalently attached cell wall protein is observed as rings around the cell periphery. Fixed biofilms of HG003 wild type were probed with rabbit anti-HaloTag antibodies followed by goat anti-rabbit Alexa 488 (green). Nuclei were stained with DAPI (blue). Download
Expression of HaloTag fusion proteins in the HG003 ∆spa mutant. (A) Quantification of biofilm formation by Halo fusion strains LCF88 (enolase-Halo) and LCF89 (GAPDH-Halo). (B) Detection of enolase-Halo (approximately 81 kDa) produced by strain LCF88 and GAPDH-Halo (approximately 70 kDa by LCF89) by immunoblotting using an anti-HaloTag antibody. Lane 1, pH 5 extract; lane 2, pH 7.5 extract; lane 3, cell lysate. The size marker (M) is shown in kDa. Red arrows on the right of the image indicate the bands of expected size for the two halo fusion proteins (Eno-Halo and GAPDH-Halo). Download
A summary of all “extracellular” proteins identified by quantitative proteomics and by enrichment through biotinylation.
Strains used in this study.
Constructs used to create S. aureus HG003 unmarked mutations.
Primers used in this study.