…in critically ill patients receiving RRT, elevated concentrations of inflammatory and apoptosis biomarkers are stigmata of death for both kidneys and patients…
Keywords: acute kidney injury, biomarkers, mortality, renal recovery, renal replacement therapy
Abstract
Background
Survivors of critical illness complicated by acute kidney injury requiring renal replacement therapy (RRT) are at an increased risk of dialysis dependence and death but the mechanisms are unknown.
Methods
In a multicenter, prospective, cohort study of 817 critically ill patients receiving RRT, we examined association between Day 1 plasma inflammatory [interleukin (IL)-1β, IL-6, IL-8, IL-10 and IL-18; macrophage migration inhibitory factor (MIF) and tumor necrosis factor]; apoptosis [tumor necrosis factor receptor (TNFR)-I and TNFR-II and death receptor (DR)-5]; and growth factor (granulocyte macrophage colony stimulating factor) biomarkers and renal recovery and mortality at Day 60. Renal recovery was defined as alive and RRT independent.
Results
Of 817 participants, 36.5% were RRT independent and 50.8% died. After adjusting for differences in demographics, comorbid conditions; premorbid creatinine; nephrotoxins; sepsis; oliguria; mechanical ventilation; RRT dosing; and severity of illness, increased concentrations of plasma IL-8 and IL-18 and TNFR-I were independently associated with slower renal recovery [adjusted hazard ratio (AHR) range for all markers, 0.70–0.87]. Higher concentrations of IL-6, IL-8, IL-10 and IL-18; MIF; TNFR-I and DR-5 were associated with mortality (AHR range, 1.16–1.47). In an analysis of multiple markers simultaneously, increased IL-8 [AHR, 0.80, 95% confidence interval (95% CI) 0.70–0.91, P < 0.001] and TNFR-I (AHR, 0.63, 95% CI 0.50–0.79, P < 0.001) were associated with slower recovery, and increased IL-8 (AHR, 1.26, 95% CI 1.14–1.39, P < 0.001); MIF (AHR, 1.18, 95% CI 1.08–1.28, P < 0.001) and TNFR-I (AHR, 1.26, 95% CI 1.02–1.56, P < 0.03) were associated with mortality.
Conclusions
Elevated plasma concentrations of inflammatory and apoptosis biomarkers are associated with RRT dependence and death. Our data suggest that future interventions should investigate broad-spectrum immune-modulation to improve outcomes.
INTRODUCTION
Individuals surviving critical illness with acute kidney injury (AKI) requiring renal replacement therapy (RRT) are at an increased risk for RRT dependence and death in the subsequent months [1–3]. More than one-half of patients die, and of survivors, 25% of patients are dependent on RRT at 2 months following acute illness [1, 4]. This increased risk persists long after resolution of acute illness [5–8] and is not fully explained by differences in underlying demographic characteristics; premorbid renal function; comorbid disease burden or severity of illness [5, 9–11]. If acute illness plays a causal role in nonrecovery of renal function, the mechanisms are unclear.
Critically ill patients with AKI have higher circulating plasma concentrations of inflammatory [12, 13] and apoptosis [14–16] biomarkers compared with patients without AKI. For instance, there is a 1.5-fold increase in circulating proinflammatory marker interleukin (IL)-6 [13, 16] and apoptosis markers soluble tumor necrosis factor receptor (TNFR)-I and TNFR-II [16] in patients with AKI compared with those without AKI. These concentrations are 5-fold higher than in patients with end-stage renal disease and 10-fold higher than in healthy controls [12]. While these markers have been implicated in susceptibility to AKI [13, 16, 17], and subsequent risk of death in patients with AKI [12], the relationship of these biologic processes to renal recovery following acute illness has not been examined.
We conducted a cohort study entitled Biological Markers of Recovery for the Kidney (BioMaRK) as an ancillary study to the Veterans Affairs (VA)/National Institute of Health (NIH) acute renal failure trial network (ATN) [1] study of patients receiving RRT and examined associations between a set of candidate molecules defined a priori, and renal recovery and mortality. Molecules were selected in three implicated pathways: inflammatory [IL-6, IL-8, IL-10, IL-18, IL-1β, macrophage migration inhibitory factor (MIF), tumor necrosis factor (TNF)]; [12, 17–19] apoptosis [TNFR-I, TNFR-II and death receptor (DR)-5]; [15, 20] and growth factors (granulocyte-macrophage colony-stimulating factor [GM-CSF]) [21].
We examined the hypothesis that lower baseline concentrations of plasma inflammatory and apoptosis markers, and higher concentrations of growth factors are associated with renal recovery and survival. Our goal was not to examine biomarker prediction of outcomes, but rather, the relative contribution of each marker to the overall association with the outcomes of interest. In order to examine whether failure to recover kidney function is not confounded by death, we performed sensitivity analyses using five mutually exclusive categories of renal recovery.
MATERIALS AND METHODS
Study design and selection of participants
The BioMaRK study was a multicenter, prospective, nested, observational cohort study conducted as an ancillary study to the VA/NIH ATN clinical trial. The ATN study was a multicenter, randomized clinical trial (n = 1124) comparing intensive and less-intensive RRT strategies in critically ill patients and is described in detail elsewhere [1, 22]. As per the primary ATN trial, patients with chronic kidney disease (defined as premorbid serum creatinine >2 mg/dL in men and >1.5 mg/dL in women) or prior kidney transplantation were excluded. The ATN trial found no difference in 60-day mortality and renal recovery between the two RRT strategies. The BioMaRK study included all participants in the ATN study who gave additional written consent to blood collections for sample banking. We obtained approval from the institutional review boards of the University of Pittsburgh and all other participating sites.
Blood sample collection
Blood samples were collected on Day 1 of enrollment in ATN and BioMaRK studies. Since the ATN study protocol allowed for participants to be enrolled in the trial if they had received no more than one session of intermittent hemodialysis or sustained low-efficiency dialysis or received continuous renal-replacement therapy less than 24 h before randomization, 68.5% of participants (n = 560) had received RRT at the time of enrollment in BioMaRK. Of participants who were receiving RRT, blood samples were drawn prior to RRT initiation in case of intermittent hemodialysis. Of participants receiving continuous RRT, samples were collected prior to protocolized RRT dosing. Of participants who did not receive any RRT, blood samples were collected before first protocolized RRT initiation. Details of biomarker assays are provided in Supplementary material, Item S1 and intra-assay and interassay coefficients of variation for each marker are shown in Supplementary material, Table S1.
Data collection
We prospectively ascertained baseline characteristics including demographic; cause of AKI; other clinical, physiologic and laboratory data at the time of entry into the ATN study. We collected individual comorbid illnesses and assessed comorbidity using the Charlson comorbidity score [23]. Severity of illness was ascertained at enrollment using the acute physiology and chronic health evaluation (APACHE)-II [24], and the Cleveland Clinic intensive care unit acute renal failure score [25]. We defined acute organ dysfunction as a new sequential organ failure assessment score of ≥3 in any of six organ systems [26]. All participants were followed daily until hospital discharge, death or Day 28 after randomization, whichever occurred first.
Outcome ascertainment
Our primary outcomes were renal recovery and mortality at Day 60, and corresponding time to event outcomes. Renal recovery specified a priori was defined as being alive and independent from RRT by Day 60 irrespective of the participant's discharge location. Outcomes were ascertained daily during hospitalization, and at Days 28 and 60 using telephone and/or mail follow up. Time to recovery was defined as time to dialysis independence as in ATN study. Survival data on patients who could not be contacted was ascertained using the VA beneficiary identification and records locator system, the National Center for Health Statistics National Death Index database or the Social Security Administration's Death Master File [1].
Statistical analysis
We first performed an outcome-stratified analysis comparing baseline characteristics by renal recovery and mortality. Continuous data were compared using Student's t-test or Wilcoxon rank-sum test and categorical data using the χ2 test or Fisher's exact test. Left-censored biomarker data were imputed using the lower limit of detection. Plasma biomarker data were log-transformed and analyzed in natural logarithm scale. For participants with no record of comorbid condition (n = 94), we assumed that the condition was absent. For participants with missing data elements for calculating the APACHE II score (n = 44), SOFA score (n = 201) and Cleveland CLINIC ICU acute renal failure score (n = 146), we imputed the score using regression-based maximum likelihood method as previously published using the same dataset [27].
As our goal was to examine association (and not risk prediction), we built models with individual biomarker concentration and outcome in a cohort of 682 subjects without missing data. We fitted logistic regression models and computed risk-adjusted odds ratios (ORs). We examined the time to renal recovery and mortality using Cox proportional hazards regression and computed risk-adjusted hazard ratios (HRs). For time to renal recovery analyses, time to independence from RRT was modeled only among survivors at Day 60. Participants who became independent of RRT but died before Day 60 were treated as nonrecovery throughout the study period. For all analyses, ORs and HRs were calculated for each natural log-unit increase in biomarker concentration. We plotted Kaplan–Meier failure curves stratified by quartiles of biomarker concentrations and compared differences across quartiles using the log-rank test.
In order to examine the relative contribution of multiple biomarkers, we included markers simultaneously in the regression analysis, while adjusting for all baseline covariates and other biomarkers. For sensitivity analyses, we fitted multinomial logistic regression using all five categories of renal recovery. Statistical analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC), with statistical significance set at P < 0.05.
Sensitivity analysis
To ascertain that the association between individual biomarkers and renal recovery is not confounded by death, we performed sensitivity analyses by varying the definitions of renal recovery and using five mutually exclusive categories that were specified a priori. We defined renal recovery as complete; partial; dependent on RRT; death on RRT and death without RRT. Complete recovery was defined as independence from RRT and return of serum creatinine to no more than 150% of baseline level before death or hospital discharge, as proposed by the Acute Dialysis Quality Initiative [28]. Partial renal recovery was defined as independence from RRT with final serum creatinine higher than 150% of baseline level before death or hospital discharge [28].
RESULTS
Characteristics of study participants
Participants in the BioMaRK and ATN cohorts were similar (Supplementary material, Table S2). Of 817 participants, 298 (36.5%) were alive and free of RRT and 415 (50.8%) died by Day 60. Participants who recovered renal function and who survived were younger and had lower comorbid disease burden (Table 1). Among those who recovered renal function, there was a lower prevalence of chronic liver disease; and, among survivors, there was a higher prevalence of immunocompromised state and lower prevalence of chronic hypoxemia compared with nonsurvivors. Participants who had renal recovery and who were survivors were generally less severely ill.
Table 1.
Baseline characteristics of participants stratified by renal recovery and mortality
| Characteristic | No (%) |
|||||
|---|---|---|---|---|---|---|
| Recovery (n = 298) | Nonrecovery (n = 519) | P-value | Survivors (n = 402) | Nonsurvivors (n = 415) | P-value | |
| Age, years, mean (SD) | 56 (16.3) | 62.9 (14.3) | <0.001 | 57.3 (15.6) | 63.3 (14.7) | <0.001 |
| Male gender | 207 (69.5) | 360 (69.4) | 0.98 | 274 (68.2) | 293 (70.6) | 0.45 |
| Race | ||||||
| White | 225 (75.5) | 401 (77.3) | 0.87 | 300 (74.6) | 326 (78.6) | 0.60 |
| Black | 46 (15.4) | 78 (15) | 67 (16.7) | 57 (13.7) | ||
| Hispanic | 19 (6.4) | 30 (5.8) | 26 (6.5) | 23 (5.5) | ||
| Other | 8 (2.7) | 10 (1.9) | 9 (2.2) | 9 (2.2) | ||
| Comorbid condition | ||||||
| Charlson comorbidity index, mean (SD)a | 2.2 (2.4) | 2.6 (2.4) | 0.02 | 2.3 (2.4) | 2.7 (2.5) | 0.03 |
| Cardiovascular disease | 112 (37.6) | 197 (38) | 0.92 | 144 (35.8) | 165 (39.8) | 0.25 |
| Chronic hypoxia | 22 (7.4) | 58 (11.2) | 0.08 | 31 (7.7) | 49 (11.8) | 0.049 |
| Liver disease | 23 (7.7) | 71 (13.7) | 0.01 | 38 (9.4) | 56 (13.5) | 0.07 |
| Diabetes | 89 (29.9) | 149 (28.7) | 0.73 | 124 (30.8) | 114 (27.5) | 0.29 |
| Malignancy | 55 (18.5) | 102 (19.6) | 0.68 | 69 (17.2) | 88 (21.2) | 0.14 |
| Immunocompromised | 53 (17.8) | 70 (13.5) | 0.10 | 71 (17.7) | 52 (12.5) | 0.04 |
| Severity of illness | ||||||
| APACHE-II score, mean (SD)b | 23.7 (6.8) | 27.7 (6.8) | <0.001 | 23.8 (6.6) | 28.5 (6.8) | <0.001 |
| Cleveland Clinic ICU Acute Renal Failure score, mean (SD)c | 10.8 (3.4) | 12.3 (3.1) | <0.001 | 10.9 (3.3) | 12.6 (2.9) | <0.001 |
| SOFA score, mean (SD)d | 12.3 (3.7) | 14.6 (3.7) | <0.001 | 12.4 (3.7) | 15.1 (3.6) | <0.001 |
| Renal function prior to onset of AKI | ||||||
| Premorbid serum creatininee | 1.1 (0.4) | 1.1 (0.3) | 0.20 | 1.1 (0.4) | 1.1 (0.3) | 0.67 |
| Estimated GFR, mL/min/1.73 m2e,f | ||||||
| ≥60 | 182 (67.9) | 312 (62.5) | 0.33 | 238 (64.8) | 256 (59.9) | 0.56 |
| 45–59 | 55 (20.5) | 121 (24.2) | 79 (21.5) | 97 (24.2) | ||
| 30–44 | 31 (11.6) | 66 (13.2) | 50 (13.6) | 47 (11.8) | ||
| Serum creatinine before RRT initiation | 4.5 (2) | 3.7 (1.5) | <0.001 | 4.4 (2) | 3.7 (1.5) | <0.001 |
| Presence of oliguria | 208 (69.8) | 446 (85.9) | <0.001 | 300 (74.6) | 354 (85.3) | <0.001 |
| Use of mechanical ventilation | 209 (70.1) | 451 (86.9) | <0.001 | 292 (72.6) | 368 (88.7) | <0.001 |
| Diagnosis of sepsis | 189 (63.4) | 340 (65.5) | 0.55 | 254 (63.2) | 275 (66.3) | 0.36 |
| Cause of AKI | ||||||
| Ischemia | 235 (78.9) | 414 (79.9) | 0.72 | 319 (79.4) | 330 (79.7) | 0.90 |
| Nephrotoxins | 90 (30.2) | 108 (20.8) | 0.003 | 116 (28.9) | 82 (19.8) | 0.003 |
| Sepsis | 153 (51.3) | 271 (52.3) | 0.79 | 206 (51.2) | 218 (52.7) | 0.69 |
| Multifactorial causes | 159 (53.4) | 281 (54.2) | 0.81 | 221 (55) | 219 (52.9) | 0.55 |
aAccording to Charlson et al. [23] without the age.
bAcute physiology and chronic health evaluation includes initial values of 12 routine physiologic measurements, age and previous health status ranging from 1 to 71; An increasing score is closely correlated with the subsequent risk of hospital death [24].
cThe Cleveland clinic intensive care unit acute renal failure score can range from 1 to 20, with higher scores predictive of increased risk of death [25].
dSequential Organ Failure Assessment score includes six organ systems with scores ranging from 0 to 4 for each organ system (with the renal system score included in the calculation of the total SOFA score); higher scores indicate more severe organ dysfunction [26].
ePremorbid serum creatinine was available only in 683 of the 817 (83.6%) subjects.
fEstimated GFR was calculated using four-variable Modification of Diet in Renal Disease estimation equation [29].
SD, standard deviation; ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; RRT, renal replacement therapy; GFR, glomerular filtration rate; AKI, acute kidney injury.
Despite similar premorbid renal function, those who recovered renal function and survivors had higher serum creatinine prior to initiation of RRT. Patients receiving RRT at lower creatinine also tended to be more oliguric (mean serum creatinine in those with and without oliguria, 3.79 ± 1.63 versus 5.01 ± 1.87 mg/dL, P < 0.001). Use of mechanical ventilation was higher among those who did not recover and in nonsurvivors. Nephrotoxin exposure was more frequently noted as the cause of AKI in those who had renal recovery. The hospital length of stay was longer among survivors [median(interquartile range, IQR), 33(19–54) versus 9(3–20), P < 0.001] and among those who recovered kidney function [30(17–48) versus 13(4–29), P < 0.001], the latter primarily due to higher mortality among those who did not recover. Premorbid serum creatinine data were available only in 683 subjects (83.6%). There was no difference in missing premorbid creatinine values between those who did and did not recover (18.8 versus 15%, P = 0.16) and survivors and nonsurvivors (16.1 versus 16.6%, P = 0.84).
Association of biomarker concentration with renal recovery
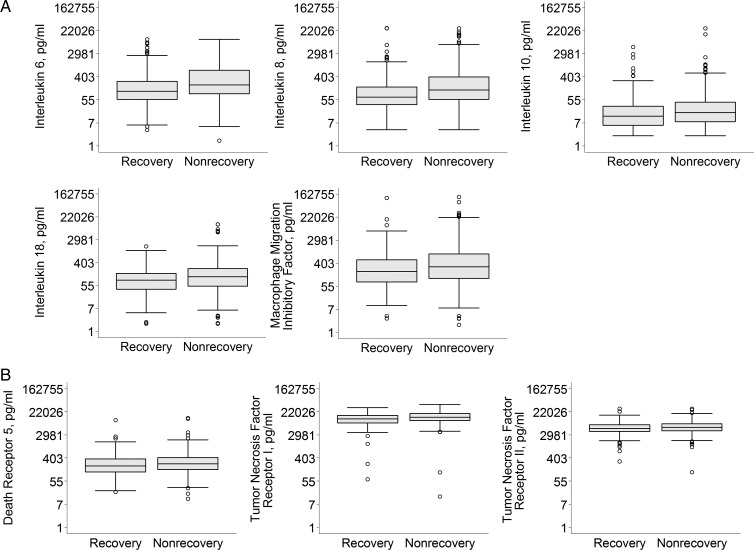
Figure 1 shows median biomarker concentrations, stratified by renal recovery and Table 2 shows raw biomarker concentration. Of 11 biomarkers, 3 had moderate to heavy proportion of measurements censored below detection thresholds: IL-1β (62.7%), TNF (63.7%) and GM-CSF (25.3%). At baseline, concentrations of plasma inflammatory (IL-6, IL-8, IL-10, IL-18 and MIF) and apoptosis (TNFR-I, TNFR-II and DR-5) biomarkers were modestly elevated in those who did not recover renal function compared with those who recovered. We found no difference in biomarker concentration between those who did and did not receive RRT within the previous 24 h prior to enrollment in BioMaRK (Supplementary material, Table S3).
FIGURE 1:
Plasma inflammatory and apoptosis biomarker concentrations in subjects receiving RRT, stratified by renal recovery. Boxplot summaries of plasma inflammatory (A) and apoptosis (B) biomarker concentrations are displayed in natural logarithm scale and labeled with their corresponding biomarker concentration in picograms/milliliter. The vertical box represents the 25th percentile (bottom line), median (middle line) and 75th percentile (top line) values. The lowest datum (lower whisker) represents 1.5 times the interquartile range of the lower quartile and the highest datum (upper whisker) represent 1.5 times the interquartile range of the upper quartile. The open circles represent the outliers. *P < 0.05.
Table 2.
Biomarker concentration stratified by renal recovery and mortality
| Biomarker | Median (IQR) |
|||
|---|---|---|---|---|
| Recovery | Nonrecovery | Survivors | Nonsurvivors | |
| Inflammation | ||||
| IL-6 | 114 (56.6–272) | 197 (91.6–710)** | 124 (56.6–280) | 230 (103–911)** |
| IL-8 | 69.5 (36–166) | 128 (57.3–393)** | 71.5 (35.8–164) | 146 (63–505)** |
| IL-10 | 13.3 (6–31.4) | 17.9 (8.2–44.3)** | 13.4 (6.2–29.5) | 19.2 (8.5–56)** |
| IL-18 | 87.9 (39.1–157) | 118 (50.7–238)** | 86.6 (39.3–155) | 127 (52.7–254)** |
| IL-1βa | 22.7 (22.7–34.3) | 22.7 (22.7–45.7) | 22.7 (22.7–32.6) | 22.7 (22.7–47.9)** |
| MIF | 196 (78.8–540) | 298 (106–891)** | 184 (70.7–531) | 337 (139–1003)** |
| TNFa | 2.4 (2.4–3.7) | 2.4 (2.4–3.4) | 2.4 (2.4–3.6) | 2.4 (2.4–3.5) |
| Apoptosis | ||||
| TNFR-I | 11 919 (8294–15 958) | 13 698 (10 167–18 805)** | 12 173 (8630–16 247) | 13 779 (10 312–19 147)** |
| TNFR-II | 5179 (3939–7194) | 5700 (4254–7775)* | 5285 (4005–7217) | 5729 (4300–8084)* |
| DR-5 | 201 (120–370) | 240 (147–417)** | 200 (120–357) | 261 (154–448)** |
| Growth factor | ||||
| GM-CSFa | 8.5 (3.1–20.6) | 8.8 (3.1–20) | 7.9 (3.1–19) | 9.4 (3.9–21.6) |
a25.3% of GM-CSF values, 62.7% of IL-1β and 63.7% of TNF values were below the detection thresholds and were censored.
*P < 0.05; **P < 0.01.
IQR, interquartile range. Median biomarker concentrations are expressed in picograms/milliliter. All biomarkers were measured in 817 subjects except DR-5, which was measured in 816 subjects.
Supplementary material, Table S4 shows the unadjusted and Table 3 shows the adjusted ORs (AORs) and adjusted hazard ratios (AHRs) for association between individual biomarker concentration and renal recovery, and time to renal recovery. When adjusted for differences in age; sex; race; Charlson comorbidity score without age; premorbid serum creatinine; history of chronic hypoxemia, liver disease and immunocompromised state; nephrotoxin exposure; diagnosis of sepsis; presence of oliguria prior to RRT; use of mechanical ventilation, APACHE-II score and intensity of RRT, each natural log unit increase in plasma IL-8 [AOR, 0.74, 95% confidence interval (95% CI) 0.65–0.85], IL-18 (AOR, 0.82, 95% CI 0.70–0.96), MIF (AOR, 0.86, 95%CI 0.76–0.97) and TNFR-I (AOR, 0.62, 95% CI 0.46–0.84) concentrations were associated with lower odds of renal recovery (Table 3).
Table 3.
Association between individual biomarker concentration and renal recovery and mortality
| Biomarker | (95% CI) |
|||
|---|---|---|---|---|
| Renal recovery |
Mortality |
|||
| OR for renal recovery | HR for time to renal recovery | OR for death | HR for time to death | |
| Inflammation | ||||
| IL-6 | 0.91 (0.81–1.01) | 0.93 (0.86–1.01) | 1.19 (1.07–1.32)* | 1.19 (1.12–1.27)* |
| IL-8 | 0.74 (0.65–0.85)* | 0.81 (0.73–0.89)* | 1.50 (1.32–1.71)* | 1.36 (1.27–1.46)* |
| IL-10 | 0.88 (0.77–1.01) | 0.92 (0.83–1.03) | 1.30 (1.14–1.49)* | 1.24 (1.16–1.33)* |
| IL-18 | 0.82 (0.70–0.96)*** | 0.87 (0.78–0.97)*** | 1.35 (1.16–1.58)* | 1.27 (1.15–1.40)* |
| MIF | 0.86 (0.76–0.97)*** | 0.92 (0.85–1.01) | 1.37 (1.22–1.54)* | 1.26 (1.17–1.35)* |
| Apoptosis | ||||
| TNFR-I | 0.62 (0.46–0.84)** | 0.70 (0.60–0.82)* | 1.61 (1.16–2.24)** | 1.47 (1.16–1.86)** |
| TNFR-II | 0.88 (0.64–1.23) | 0.90 (0.70–1.16) | 1.17 (0.85–1.60) | 1.16 (0.95–1.43) |
| DR-5 | 0.91 (0.74–1.12) | 0.91 (0.78–1.06) | 1.36 (1.10–1.67)** | 1.24 (1.09–1.41)$ |
Multivariable logistic regression models with corresponding OR were constructed to examine association between each individual biomarker concentration (per natural log unit) and renal recovery and mortality. All models were adjusted for differences in age; race; sex; Charlson comorbidity score without age; history of chronic hypoxemia, liver disease and immunocompromised state; premorbid serum creatinine; nephrotoxic cause of AKI; diagnosis of sepsis; presence of oliguria at initiation of RRT; use of mechanical ventilation; APACHE-II score, and intensity of RRT. Models were not adjusted for other biomarkers.
For the recovery model, an OR of >1 indicates that higher biomarker concentration is associated with increased renal recovery, and an OR <1 indicates nonrecovery. For the mortality model, OR >1 indicates that higher biomarker concentration is associated with increased mortality and OR <1 indicates lower mortality.
Multivariable Cox models with corresponding HR were constructed to examine association between individual biomarker concentration and time to event outcomes. Models were adjusted for baseline covariates as above. For time to renal recovery model, a HR >1 indicates that higher marker concentration is associated with faster recovery and <1 indicates slower recovery. For time to death, a HR of >1 indicates that higher marker concentration is associated with shorter time to death and <1 indicates longer time to death. The models included 682 subjects due to missing premorbid creatinine data in 134 subjects and DR-5 marker levels in 1 subject. Models were not constructed for IL-1 β, TNF, and GM-CSF due to high censoring of biomarker data.
*P < 0.001; **P < 0.01; ***P < 0.05; $P = 0.001.
IL, interleukin; MIF, macrophage migration inhibitory factor; TNFR, tumor necrosis factor receptor; DR, death receptor.
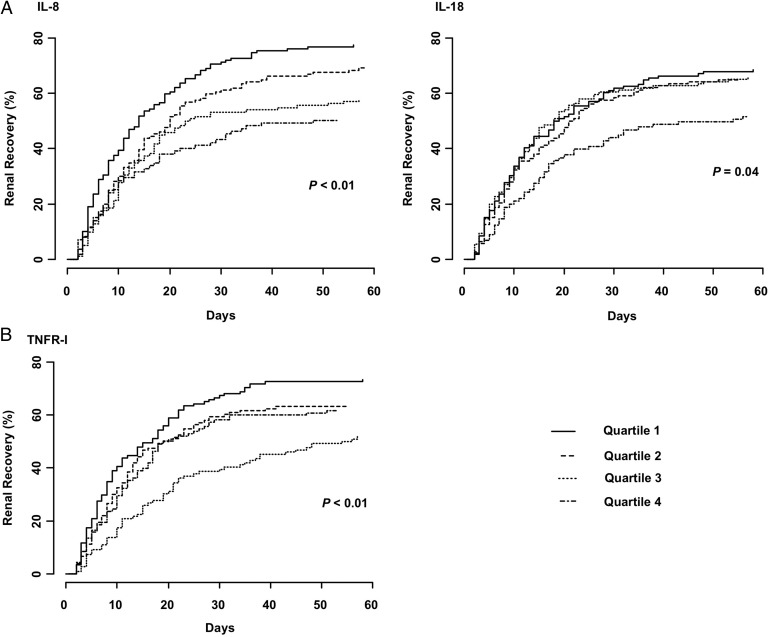
Figure 2 shows Kaplan–Meier failure plots for time to renal recovery stratified by quartiles of biomarker concentration. Higher biomarker concentrations were associated with lower probability of renal recovery for plasma inflammatory (IL-8 and IL-18) and apoptosis (TNFR-I) markers. From Cox models, we found that higher concentrations of plasma IL-8 (AHR, 0.81, 95% CI 0.73–0.89), IL-18 (AHR, 0.87, 95% CI 0.78–0.97) and TNFR-I (AHR, 0.70, 95% CI 0.60–0.82) were associated with increased time to renal recovery (Table 3).
FIGURE 2:
Kaplan–Meier failure plots showing time to renal recovery stratified by quartiles of plasma inflammatory (A) and apoptosis (B) biomarker concentrations. Markers were compared across quartiles for trend using a log-rank test for ordered survival curves. Higher plasma IL-8, IL-18 and TNFR-I concentrations are associated with slower renal recovery.
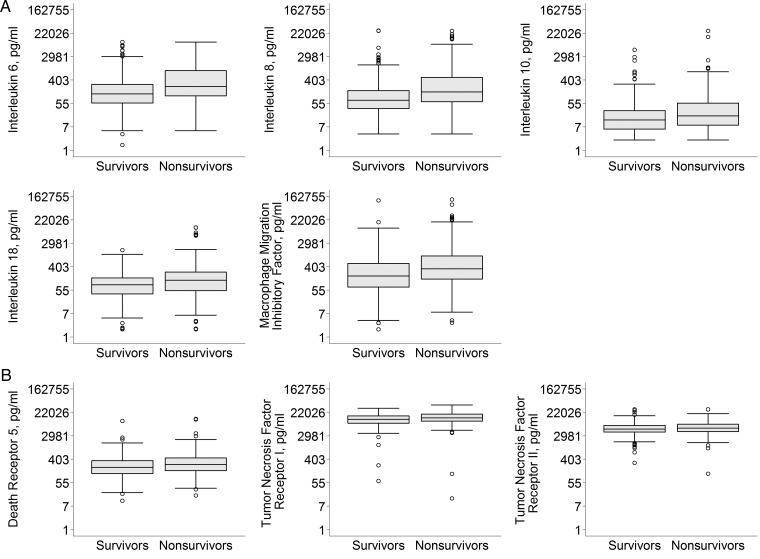
Association of biomarker concentration with mortality
Plasma inflammatory (IL-6, IL-8, IL-10, IL-18 and MIF) and apoptosis (TNFR-I, TNFR-II and DR-5) biomarkers were modestly elevated in nonsurvivors compared with survivors (Figure 3). When adjusted for differences in age; sex; race; Charlson comorbidity score; premorbid creatinine; history of chronic hypoxemia, liver disease and immunocompromised state; nephrotoxin exposure; diagnosis of sepsis; oliguria prior to RRT; use of mechanical ventilation, APACHE-II score, and intensity of RRT, per natural log increase in plasma inflammatory (IL-6, IL-8, IL-10, IL-18 and MIF: AOR range for all markers, 1.19–1.50) and apoptosis marker (DR-5: AOR, 1.36, 95% CI 1.10–1.67; and TNFR-I: AOR, 1.61, 95% CI 1.16–2.24) concentrations were associated with increased mortality (Table 3).
FIGURE 3:
Plasma inflammatory and apoptosis biomarker concentrations stratified by 60-day mortality. Boxplot summaries of plasma inflammatory (A) and apoptosis (B), biomarker concentrations are displayed in natural logarithm scale and labeled with their corresponding biomarker concentration in picograms/milliliter. The vertical box represents the 25th percentile (bottom line), median (middle line) and 75th percentile (top line) values. The lowest datum (lower whisker) represents 1.5 times the interquartile range of the lower quartile and the highest datum (upper whisker) represents 1.5 times the interquartile range of the upper quartile. The open circles represent the outliers. *P < 0.05.
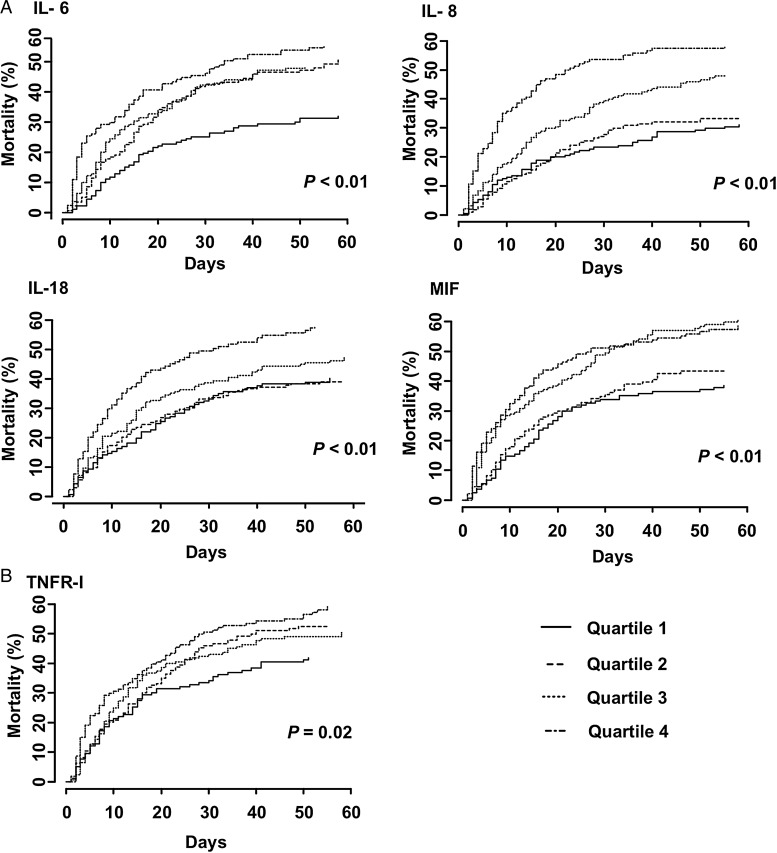
Per quartile increase in plasma inflammatory (IL-6, IL-8, IL-18 and MIF) and apoptosis (TNFR-I) marker concentrations were associated with decreased time to death (Figure 4). Using a Cox model after adjustment for baseline covariates, higher plasma inflammatory marker (IL-6, IL-8, IL-10, IL-18 and MIF: AHR range, 1.19–1.36) and apoptosis marker concentrations (TNFR-I: AHR, 1.47, 95% CI 1.16–1.86; and DR-5: AHR, 1.24, 95% CI 1.09–1.41) were associated with an increased hazard of death (Table 3).
FIGURE 4:
Kaplan–Meier failure plots showing time to death stratified by quartiles of plasma inflammatory (A) and apoptosis (B) biomarker concentrations. Markers were compared across quartiles for trend using a log-rank test for ordered survival curves. Higher plasma IL-6, IL-8, IL-18, MIF and TNFR-I concentrations are associated with faster time to death.
Multimarker models of renal recovery and mortality
Table 4 shows the multivariable analyses of multiple makers to examine whether specific markers are independently associated with clinical outcomes. When adjusted for differences in baseline covariates, higher plasma concentrations of IL-8 (AHR, 0.80, 95% CI 0.70–0.91, P < 0.001) and TNFR-I (AHR, 0.63, 95% CI 0.50–0.79, P < 0.001) were associated with lower probability of recovery of kidney function. Higher plasma concentrations of IL-8, MIF and TNFR-I (AHR, 1.26, 1.18 and 1.26, respectively) were associated with increased risk of death.
Table 4.
Multivariable analyses of independent effects of multiple biomarkers on clinical outcomes
| Biomarker | Adjusted HR (95% CI) | P-value |
|---|---|---|
| Renal recovery | ||
| IL-8 | 0.80 (0.70–0.91) | <0.001 |
| TNFR-I | 0.63 (0.50–0.79) | <0.001 |
| Mortality | ||
| IL-8 | 1.26 (1.14–1.39) | <0.001 |
| MIF | 1.18 (1.08–1.28) | <0.001 |
| TNFR-I | 1.26 (1.02–1.56) | 0.03 |
Only significant results for time to renal recovery and mortality from Cox models are shown. All models were adjusted for differences in age; race; sex; Charlson comorbidity score without age; history of chronic hypoxemia, liver disease and immunocompromised state; premorbid serum creatinine; nephrotoxic cause of AKI; diagnosis of sepsis; presence of oliguria at initiation of RRT; use of mechanical ventilation, intensity of RRT and other biomarkers. Models were not adjusted for APACHE II score due to multicolinearity between APACHE II score and biomarkers.
For time to renal recovery model, a HR >1 indicates that per natural log increase in biomarker concentration is associated with faster recovery and <1 indicates slower recovery. For time to death, a HR of >1 indicates that per natural log increase in biomarker concentration is associated with shorter time to death and <1 indicates longer time to death.
The models included 682 subjects due to missing premorbid creatinine data in 134 subjects and DR-5 marker levels in 1 subject. Models were not constructed for IL-1β, TNF and GM-CSF due to high censoring of biomarker data.
IL, interleukin; MIF, macrophage migration inhibitory factor; TNFR-I, tumor necrosis factor receptor-I.
Sensitivity analyses
Of the 817 participants, 217 (26.5%) had complete and 81 (9.9%) had partial renal recovery; 107 (13.1%) were RRT dependent; 327 (39.9%) died on RRT and 87 (10.6%) died without RRT. Compared with complete recovery (as the reference category), higher plasma IL-6 was associated with lower odds of partial renal recovery (OR, 0.79, 95% CI 0.66–0.95) (Supplementary material, Table S5). We also found that higher plasma inflammatory (IL-6, IL-8, IL-10, IL-18 and MIF: OR range, 1.26–1.45) and apoptosis markers (TNFR- I, TNFR-II and DR-5: OR range, 1.26–2.02) were associated with increased risk of RRT dependence. While higher concentrations of plasma IL-6 (OR, 1.17, 95% CI 10.1–1.36) and IL-8 (OR, 1.34, 95% CI 1.12–1.59) were associated with increased risk of death on RRT, increased concentrations of MIF were associated with lower risk of death without RRT (OR, 0.81, 95% CI 0.69–0.95). Subgroup analyses of biomarker concentrations by etiology of AKI did not change our results (data not shown).
DISCUSSION
Among critically ill individuals with AKI requiring RRT, we found increased circulating concentrations of plasma inflammatory and apoptotic biomarkers at initiation of renal support in those who subsequently did not recover kidney function and among those who died. When adjusted for differences in participant characteristics and severity of illness, higher plasma concentrations of IL-8, IL-18 and TNFR-I were associated with subsequent RRT dependence, while plasma IL-6, IL-8, IL-10, IL-18, MIF, TNFR-I and DR-5 were associated with increased risk of death. When adjusted for baseline characteristics and ‘other markers’, plasma IL-8 and TNFR-I were independent predictors of RRT dependence and plasma IL-8, MIF and TNFR-I were predictors of mortality. This study, to the best of our knowledge, is the first large-scale investigation of the association between immune and apoptosis biomarkers and the patient-centered outcomes of independence from RRT and mortality in patients with AKI. The results of our study have important implications for designing interventions to enhance renal recovery and to lower mortality in patients with AKI.
Higher cytokine concentrations observed at baseline in patients ultimately dependent on RRT and nonsurvivors could be due to elevations before occurrence of acute illness due to chronic health conditions, or due to an interaction between poor chronic health status and acute illness. We speculate that the latter is likely because circulating cytokine concentrations were lower than we observed in prior studies, even in individuals with end-stage renal disease [12, 30]. Our findings were not confounded by severity of illness because the association between increased biomarker concentration and RRT dependence persisted after adjusting for APACHE score. The significant association between various biomarkers and RRT dependence in the sensitivity analysis also suggests that our findings were not confounded by death. Furthermore, the magnitude of association and the dose–response relationship (Figures 2 and 4) between individual biomarkers and outcomes suggest that these markers are unlikely to be just surrogates or an epiphenomenon in severe illness.
In our study, the risk of RRT dependence and death appeared to be greatest for increased IL-8 concentrations. IL-8, a chemokine, is an important mediator of innate and adaptive immunity and has been implicated in the pathogenesis of AKI [31–33]. Higher IL-8 concentrations may reflect a persistent proinflammatory milieu among renal tubular cells impairing renal recovery. IL-18 is produced by macrophages and other cell types present in the kidney during ischemia–reperfusion injury [34]. Higher serum IL-18 concentrations have been associated with hospital mortality in patients receiving RRT [35].
IL-6 is a pleiotropic cytokine and higher concentrations have been associated with increased susceptibility [13] and mortality in patients with AKI [12]. The cytokine, MIF, exerts a variety of biologic responses including macrophage activation, enhancement of adherence and phagocytosis, and higher MIF concentrations have been associated with severe AKI [36]. Stimulation of Fas/TNFR receptor family by ligands triggers cell apoptosis. Higher concentrations of TNFR have been associated with susceptibility to AKI and mortality [15].
Our data extend the findings of other studies in which pro- and anti-inflammatory cytokines are associated with poor outcomes [12, 16, 37]. As several molecules were associated with adverse outcomes in our study, immunomodulation strategies that include inhibition of single molecules are unlikely to be successful and that broad-spectrum modulation of multiple molecules is essential to improve outcomes. Furthermore, since associations persisted after adjusting for baseline clinical variables, patient selection for immune modulation therapies will require monitoring of biomarker concentrations.
Our study has several important limitations. Firstly, being an observational study, our findings are hypotheses generating and cannot prove cause and effect between biomarkers and adverse outcomes. Secondly, we did not examine biomarker concentrations prior to onset of severe illness for practical reasons. Larger differences in immune response may have occurred during this period, which might have increased susceptibility to critical illness, which may represent a surrogate for unknown underlying illness. Thirdly, we did not examine local (i.e. renal tissue or urinary) concentrations of these markers, which could have been different from serum levels. Fourthly, three of the biomarkers studied were below the detection thresholds and were thus censored confounding interpretation. Finally, we made a conscious decision not to adjust any of our analyses for multiple comparisons—as recommended in studies of natural phenomena like ours [38]. We recognize that this approach increases risk of type I error for null associations but at the same time decreases the risk of type II error for those associations that are not null.
Our study has several major strengths. Firstly, being a large multicenter prospective cohort study, our finding of association between high concentrations of immune and apoptosis markers and adverse outcomes in critically ill patients receiving RRT is highly generalizable. Secondly, we measured biomarkers that have been implicated in renal injury and recovery and thus able to gain insight on mechanisms and long-term outcomes. Thirdly, we were able to compare the marker concentrations in patients who are at risk for nonrecovery unlike other case–control studies that compared markers in critically ill patients with AKI to that of healthy controls or end-stage renal disease [12]. Finally, by varying definitions of renal recovery, we were able to examine the influence of biomarkers on various degrees of clinically meaningful outcomes.
In summary, our results show that in critically ill patients receiving RRT, elevated concentrations of inflammatory and apoptosis biomarkers are associated with nonrecovery of kidney function and continued dependence on RRT and risk of death. Future studies should examine whether broad-spectrum immune modulation of inflammatory and apoptosis markers improves outcomes. The importance of the chemokine IL-8, as a marker of nonrecovery and death, is also notable.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
FUNDING
BioMaRK was supported by a grant (R01DK070910) from the National Institute of Diabetes and Digestive, and Kidney Diseases (NIDDK). Additional support for J.K., X.W. and F.P. was provided by grant (R01DK083961) and for R.M. from the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR000146. The VA/NIH ATN study was supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development (CSP #530) and by NIDDK by interagency agreement Y1-DK-3508.
CONFLICT OF INTEREST STATEMENT
None declared. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of NIDDK or NIH.
Supplementary Material
ACKNOWLEDGEMENTS
The BioMaRK study was conducted by the BioMaRK investigators. The primary data and samples from the parent trial, ‘A Comparison of Intensive versus Conventional Renal Support in Acute Renal Failure’ (ATN study), from which the analyses reported here were performed, were supplied by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development (CSP #530) and by the NIDDK Central Repositories. This manuscript does not necessarily reflect the opinions or views of the ATN study, NIDDK Central Repositories or the NIDDK. We are indebted to the nurses, respiratory therapists, phlebotomists, physicians and other health-care professionals who participated in the ATN and BioMaRK studies, as well as the patients and their families.
REFERENCES
- 1.Palevsky PM, Zhang JH, O'Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. doi:10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. doi:10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 3.Hamel MB, Phillips RS, Davis RB, et al. Outcomes and cost-effectiveness of initiating dialysis and continuing aggressive care in seriously ill hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1997;127:195–202. doi: 10.7326/0003-4819-127-3-199708010-00003. doi:10.7326/0003-4819-127-3-199708010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Palevsky PM, O'Connor TZ, Chertow GM, et al. Intensity of renal replacement therapy in acute kidney injury: perspective from within the Acute Renal Failure Trial Network Study. Crit Care. 2009;13:310. doi: 10.1186/cc7901. doi:10.1186/cc7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng KP, Chanouzas D, Fallouh B, et al. Short and long-term outcome of patients with severe acute kidney injury requiring renal replacement therapy. QJM. 2012;105:33–39. doi: 10.1093/qjmed/hcr133. doi:10.1093/qjmed/hcr133. [DOI] [PubMed] [Google Scholar]
- 6.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. doi:10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 7.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. doi: 10.1097/SLA.0b013e3181a40a0b. doi:10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 8.Elseviers MM, Lins RL, Van der NP, et al. Renal replacement therapy is an independent risk factor for mortality in critically ill patients with acute kidney injury. Crit Care. 2010;14:R221. doi: 10.1186/cc9355. doi:10.1186/cc9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–2058. doi: 10.1097/00003246-200209000-00016. doi:10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Morgera S, Kraft AK, Siebert G, et al. Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis. 2002;40:275–279. doi: 10.1053/ajkd.2002.34505. doi:10.1053/ajkd.2002.34505. [DOI] [PubMed] [Google Scholar]
- 11.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. doi:10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 12.Simmons EM, Himmelfarb J, Sezer MT, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–1365. doi: 10.1111/j.1523-1755.2004.00512.x. doi:10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 13.Chawla LS, Seneff MG, Nelson DR, et al. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol. 2007;2:22–30. doi: 10.2215/CJN.02510706. doi:10.2215/CJN.02510706. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Tao LJ, Ning JP, et al. [Effects of high-volume hemofiltration on serum levels of tumor necrosis factor and its receptors in patients with multiple organ dysfunction syndromes] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004;16:81–84. [PubMed] [Google Scholar]
- 15.Iglesias J, Marik PE, Levine JS. Elevated serum levels of the type I and type II receptors for tumor necrosis factor-alpha as predictive factors for ARF in patients with septic shock. Am J Kidney Dis. 2003;41:62–75. doi: 10.1053/ajkd.2003.50024. doi:10.1053/ajkd.2003.50024. [DOI] [PubMed] [Google Scholar]
- 16.Liu KD, Glidden DV, Eisner MD, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35:2755–2761. [PMC free article] [PubMed] [Google Scholar]
- 17.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. doi: 10.1038/ki.2009.502. doi:10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangemi S, Mallamace A, Minciullo PL, et al. Involvement of interleukin-18 in patients on maintenance haemodialysis. Am J Nephrol. 2002;22:417–421. doi: 10.1159/000065269. doi:10.1159/000065269. [DOI] [PubMed] [Google Scholar]
- 19.Bacher M, Metz CN, Calandra T, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci USA. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. doi:10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuruya K, Ninomiya T, Tokumoto M, et al. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003;63:72–82. doi: 10.1046/j.1523-1755.2003.00709.x. doi:10.1046/j.1523-1755.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- 21.Nishida M, Fujimoto S, Toiyama K, et al. Effect of hematopoietic cytokines on renal function in cisplatin-induced ARF in mice. Biochem Biophys Res Commun. 2004;324:341–347. doi: 10.1016/j.bbrc.2004.09.051. doi:10.1016/j.bbrc.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 22.Palevsky PM, O'Connor T, Zhang JH, et al. Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. Clin Trials. 2005;2:423–435. doi: 10.1191/1740774505cn116oa. doi:10.1191/1740774505cn116oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. doi:10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi:10.1097/00003246-198510000-00009. [PubMed] [Google Scholar]
- 25.Paganini EP, Halstenberg WK, Goormastic M. Risk modeling in acute renal failure requiring dialysis: the introduction of a new model. Clin Nephrol. 1996;46:206–211. [PubMed] [Google Scholar]
- 26.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. doi:10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 27.Demirjian S, Chertow GM, Zhang JH, et al. Model to predict mortality in critically ill adults with acute kidney injury. Clin J Am Soc Nephrol. 2011;6:2114–2120. doi: 10.2215/CJN.02900311. doi:10.2215/CJN.02900311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. doi:10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;13:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. doi:10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 30.Hasuike Y, Nonoguchi H, Ito K, et al. Interleukin-6 is a predictor of mortality in stable hemodialysis patients. Am J Nephrol. 2009;30:389–398. doi: 10.1159/000235687. doi:10.1159/000235687. [DOI] [PubMed] [Google Scholar]
- 31.Liu KD, Altmann C, Smits G, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care. 2009;13:R104. doi: 10.1186/cc7940. doi:10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liangos O, Kolyada A, Tighiouart H, et al. Interleukin-8 and acute kidney injury following cardiopulmonary bypass: a prospective cohort study. Nephron Clin Pract. 2009;113:c148–c154. doi: 10.1159/000232595. doi:10.1159/000232595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon O, Molitoris BA, Pescovitz M, et al. Urinary actin, interleukin-6, and interleukin-8 may predict sustained ARF after ischemic injury in renal allografts. Am J Kidney Dis. 2003;41:1074–1087. doi: 10.1016/s0272-6386(03)00206-3. doi:10.1016/S0272-6386(03)00206-3. [DOI] [PubMed] [Google Scholar]
- 34.Leslie JA, Meldrum KK. The role of interleukin-18 in renal injury. J Surg Res. 2008;145:170–175. doi: 10.1016/j.jss.2007.03.037. doi:10.1016/j.jss.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 35.Lin C-Y, Chang C-H, Fan P-C, et al. Serum interleukin-18 at commencement of renal replacement therapy predicts short-term prognosis in critically ill patients with acute kidney injury. PLoS One. 2013;8:e66028. doi: 10.1371/journal.pone.0066028. doi:10.1371/journal.pone.0066028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payen D, Lukaszewicz AC, Legrand M, et al. A multicentre study of acute kidney injury in severe sepsis and septic shock: association with inflammatory phenotype and HLA genotype. PLoS One. 2012;7:e35838. doi: 10.1371/journal.pone.0035838. doi:10.1371/journal.pone.0035838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. doi:10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–316. doi:10.1097/00001648-199001000-00010. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.