Abstract
Background:
Sensory recovery of skin flaps is generally poor unless they are elevated as an innervated flap. The aim of this study was to elucidate if Schwann cell (SC)-like cells differentiated from adipose-derived stem cells (ASCs) could improve the cutaneous nerve regeneration in skin flaps.
Methods:
Microvascular island groin flaps were elevated bilaterally in 32 Lewis rats. On the right flap, the epigastric nerve was resected and ligated (noninnervated flap), and on the left flap, the nerve was crushed (innervated flap). ASCs, SC-like cells differentiated from ASCs (dASCs), SCs, or vehicle were simultaneously injected to the dermal and hypodermal layers of flap. After 20 weeks, the reinnervation pattern of flap was assessed immunohistochemically using a neuronal marker, PGP9.5.
Results:
dASC cultures produced significantly higher amount of nerve growth factor and brain-derived neurotrophic factor compared with ASC cultures (P < 0.01), and the level was comparable to that of SC cultures. Although a long-term survival of the transplanted cells was not found, dASCs and SCs significantly increased reinnervation density in the periphery of both types of flaps (P < 0.05), and this effect was more pronounced in noninnervated flaps. On the other hand, ASC transplantation showed no statistically significant effect on the peripheral reinnervation (P > 0.05). In the center of flap, there was no statistically significant increase in reinnervation density in all groups irrespective of flap innervation (P > 0.05).
Conclusions:
dASCs could improve flap reinnervation by 2 mechanisms: First, neurotrophic factors produced by dASCs facilitated regrowth of cutaneous axons from the surroundings of flap. Second, nerve growth factor released by dASCs induced the collateral sprouting of undamaged axons in adjacent tissues. In addition to the use of innervated flaps, dASC transplantation therapy could be a new approach to improve the sensory recovery of skin flaps.
With the advent of new concepts such as perforator flaps, tissue defects can be reconstructed with flaps from various sites, depending on the nature of the defects. As a next step, reconstruction with an emphasis on not only shape but also functionality, such as sensation, is desired. Sensory recovery of skin flaps is often very poor unless they are elevated as an innervated flap.1 Although many innervated flaps have been reported so far,2,3 not all flaps can be elevated as an innervated flap for anatomical reasons. In such cases, treatments with a different approach would be needed to further improve the sensation of transferred flaps.
Schwann cells (SCs) are one of the most potent supporting cells for nerve regeneration, which provide a bridge across the injured nerve stumps and a number of neurotrophic factors.4,5 For that reason, transplantation of SCs has been extensively investigated as a potent therapeutic option in various nervous disorders such as spinal cord injury.5 We therefore hypothesized that SC transplantation could be a therapeutic intervention to improve the cutaneous nerve regeneration into skin flaps. However, SCs have limited clinical application due to the need of a painful biopsy and a lengthy cell expansion in vitro. Therefore, alternative sources of easily accessible and rapidly expandable cells would be needed.
Over the past decade, human adipose-derived stem cells (ASCs) have been identified as a source of multipotent cells,6 which can be easily harvested from the abundant subcutaneous fat tissue by small biopsies or conventional liposuction procedures. Importantly, we recently reported that ASCs could also transdifferentiate into SC-like cells (dASCs) under specific conditions.7,8 The rat and human dASCs produce neurotrophic factors such as nerve growth factor (NGF) at a level comparable to or even greater than SCs.9–11 Furthermore, when these cells were transplanted into the peripheral nerve, they could take an active part in the axonal regeneration, thereby myelinating the regenerating axons.8,11 The aim of the present study was to elucidate if dASC and SC transplantation could improve the reinnervation of skin flaps. The effects of cell transplantation on the pattern of reinnervation were determined using noninnervated and innervated groin island flap models in rats. We used a well-established neuronal marker, protein gene product 9.5 (PGP9.5), for immunohistochemical analysis.12
MATERIALS AND METHODS
All procedures conformed to the Declaration of Helsinki and were approved by the Institutional Animal Care and Use Committee of Osaka University.
Isolation and Culture of ASCs
The Lewis based green fluorescent protein (GFP) rats [LEW-Tg(CAG-EGFP)1Ys]13 were provided by National Bio Resource Project-Rat with support, in part, by the National Bio Resource Project of the Ministry of Education, Culture, Sports, Science and Technology, Japan. ASCs were isolated as previously described.7 Briefly, inguinal subcutaneous fat was enzymatically dissociated for 60 minutes at 37°C using 0.15% collagenase type I (Invitrogen). The solution was passed through a 70-μm filter to remove undissociated tissue, neutralized by the addition of stem cell growth medium (minimal essential medium containing 10% fetal bovine serum) and centrifuged for 5 minutes. The stromal cell pellet was resuspended in growth medium. Cultures were maintained at subconfluent levels in an incubator at 37°C with 5% CO2.
Differentiation of ASCs into dASCs
ASCs were differentiated into dASCs as previously described7 using Her-β instead of glial growth factor 2. Briefly, subconfluent ASCs at passage 2 were cultured in growth medium containing 1 mM 2-mercaptoethanol (Sigma) for 24 hours. The cells were then washed and given fresh medium supplemented with 35 ng/ml all-trans-retinoic acid (Sigma). After 72 hours, the cells were washed and the medium was replaced with differentiation medium: growth medium supplemented with 5 ng/ml platelet-derived growth factor (PeproTech), 10 ng/ml basic fibroblast growth factor (PeproTech), 5.7 μg/ml forskolin (Sigma), and 200 ng/ml recombinant human heregulin-β1 (HRG-β1; R&D Systems). The cells were incubated for 14 days to achieve full differentiation while the medium was changed every 3 days.
Isolation and Culture of SCs
Sciatic nerves were harvested from the transgenic GFP rats. After removing the epineurium, the nerves were cut to 1-mm segments. The segments were cultured in a Petri dish with SC growth medium (Dulbecco's modified eagle medium containing 10% fetal bovine serum, 4.1 μg/ml forskolin, and 100 ng/ml HRG-β1) at 37°C with 5% CO2. Two weeks later, the medium was aspirated, and 0.0625% collagenase type IV and 0.585 U dispase were added to the dish. After 24-hour incubation, the cell suspension was filtered through a 70-μm cell strainer, followed by centrifugation for 5 minutes. Finally, the cell pellet was resuspended in SC growth medium and plated in 25 cm2 flask coated with poly-d-lysine. At confluence, the remaining fibroblasts were removed by an antibody complement method.14
Animal Experiments
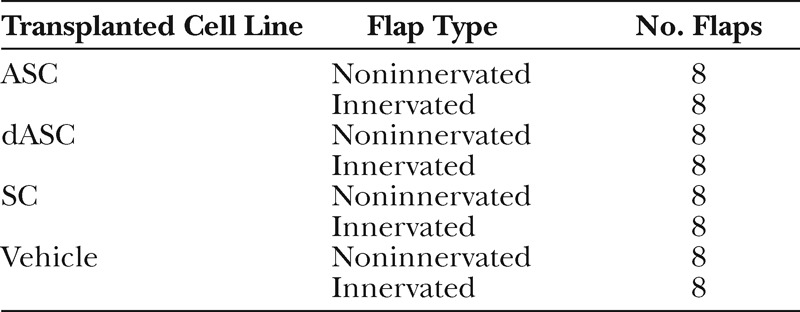
Thirty-two female Lewis rats (weighing 200–250 g) were anesthetized by intraperitoneal injection of a mixture of medetomidine (0.15 mg/kg), midazolam (2 mg/kg), and butorphanol (2.5 mg/kg). After the animals’ lower abdomens were shaved, oblique 3.5 cm × 2.5 cm groin island flaps based on superficial epigastric vessels were raised bilaterally. Under the operating microscope, the vascular trunk and the epigastric nerve were separated. On the right-hand side, a 1-cm segment of the nerve was resected and the proximal nerve stump was ligated with 9-0 nylon (Fig. 1A, left). On the contralateral side, the nerve was crushed with type 5 watchmaker forceps for 30 seconds at a point 1 cm proximal to the insertion to the flap and was left to regenerate (Fig. 1A, right). Immediately after the nerve injury, GFP-tagged ASCs, dASCs, or SCs (3 × 106 cells in 500 μl of serum-free medium) were transplanted to the periphery and the center of the bilateral flaps via intradermal and subcutaneous injection using a 26-gauge needle and 1-ml syringe (Fig. 1B). All flaps were then sutured back to their original sites with 4-0 nylon. Each group included 8 rats (ie, 8 noninnervated flaps and 8 innervated flaps for each cell type), and another 8 rats in which only medium was injected were used as control group. These experimental groups are summarized in Table 1.
Fig. 1.
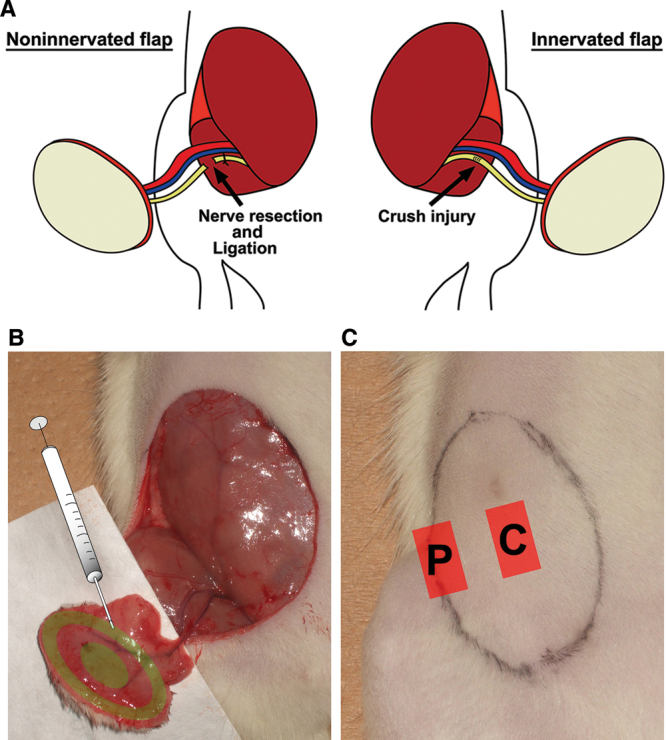
A, In bilateral microvascular groin island flaps, the epigastric nerve was resected and ligated proximally (noninnervated flap model, left) or crushed with microforceps (innervated flap model, right). B, GFP-labeled ASCs, dASCs, or SCs were injected to the flaps. C, After 20 wk, skin specimens were harvested from the periphery (“P” label) and the center (“C” label) of flaps.
Table 1.
Summary of the Experimental Groups
After 20 weeks, the animals were killed by a lethal dose of intraperitoneal pentobarbital, and skin specimens were harvested from a lateral peripheral site and a central site of the flap for immunohistochemistry (Fig. 1C). Unwounded abdominal skins were also taken from 8 rats as control specimens.
For short-term follow-up, 4 rats were additionally used for each cell type (12 rats in total) and were killed at 3, 7, 14, or 28 days postoperatively.
Immunohistochemistry
The harvested tissue was fixed in 4% paraformaldehyde and cryoprotected in 30% sucrose phosphate buffered saline. Then, the specimens were embedded in optimal cutting temperature compound (Tissue Tek) and cryosections (14 μm) were cut. Background staining was blocked using normal animal serum for 1 hour at room temperature and sections were then incubated at 4°C overnight with rabbit anti-PGP9.5 (1:500; DAKO, Denmark) and chicken anti-GFP (1:500; Molecular Probes, Invitrogen) antibodies.
The sections were washed 3 times, and Alexa fluor 555-labeled anti-rabbit IgG and Alexa fluor 488-labeled anti-chicken IgG antibodies (1:1000, Molecular Probes, Invitrogen for each) were added for 1 hour at room temperature. After washing 3 times, the cells were mounted and observed under a fluorescence microscope (Olympus BX51). For analysis of reinnervation density, images were captured from 3 serial sections using a digital camera (Olympus DP80) and analyzed using an automated method of quantifying the PGP9.5-positive area in each field of view with digital image analysis program ImageJ (public domain). Artifacts such as hair follicles were digitally deleted before analysis. Reinnervation density was calculated as percentage PGP9.5-positive area per total skin area, and the mean values of 3 sections were reported.
Western Blotting
After removing the culture medium, ASCs, dASCs, or SCs were washed once with phosphate buffered saline and lysed in tissue protein extraction reagent (Pierce) containing protease inhibitor cocktail (Roche Biochemicals). Then the lysates were clarified by centrifugation at 13,000g for 10 minutes at 4°C, and 25 μg protein was prepared per sample. The proteins were separated on SDS-polyaclylamidegel electrophoresis and transferred to polyvinylidine fluoride membranes by iBlot Dry Blotting System (iBlot device and iBlot Transfer Stack, Invitrogen). The membranes were incubated in tris buffered saline containing 5% skim milk and 0.05% Tween-20 for 60 minutes and blotted with primary antibodies at 4°C overnight. The primary antibodies used were rabbit anti-S100 (1:500; DAKO), rabbit anti-p75 (1:500; Promega), mouse anti-GFAP (glial fibrillary acidic protein) (1:200; Thermo Scientific, Japan), and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (1:1000; Santa-Cruz Biotechnology). Following 5 × 5 minute washes, the membranes were incubated for 1 hour with anti-mouse or anti-rabbit horseradish peroxidase-linked secondary antibody (1:2000, Cell Signaling Technology). The membranes were washed as described previously, and the reaction products were visualized by detection of chemiluminescence using enhanced chemiluminescence Western Blotting Detection System (GE Healthcare).
Enzyme-linked Immunosorbent Assay
ASCs, dASCs, or SCs were plated at 2 × 105 in 800-μl medium (n = 3) in a 12-well plate. At 48-hour culture, conditioned medium was aspirated and measured for NGF and brain-derived neurotrophic factor (BDNF) by the Emax ImmunoAssay System (Promega) according to the manufacturer’s instructions. The absorbance was measured at 450 nm using a microplate reader, and the amount of each neurotrophic factor (pg/ml) was calculated against a standard curve.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to test the statistical significance of group differences in the results of enzyme-linked immunosorbent assay (ELISA) and the reinnervation density in the periphery or in the center of flaps. Two-way ANOVA was used to test the significance of group differences in the overall reinnervation density. Post hoc pairwise comparisons between individual groups were made using the Tukey-Kramer test. A 0.05 significance level was used for statistical tests. All data are expressed as mean ± standard errors of the mean.
RESULTS
Characterization of Stem Cell Properties
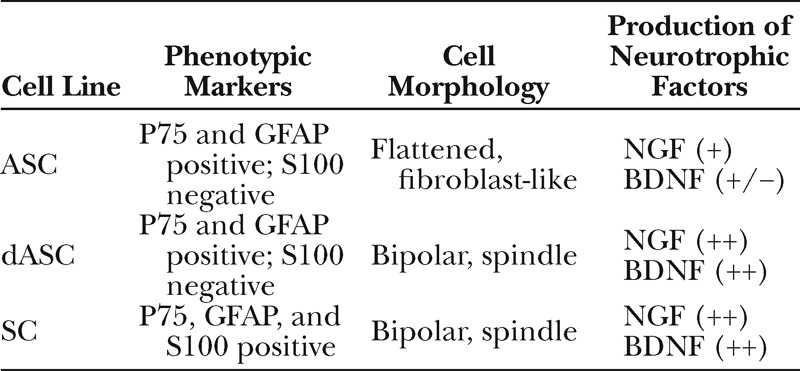
Rat subcutaneous adipose tissues were harvested and stromal cell fractions were isolated and cultured on flasks. Cultures were routinely tested for their multilineage differentiation potential as previously described.7 Cultures were then sequentially treated with 2-mercaptoethanol, retinoic acid, and a mixture of growth factors containing basic fibroblast growth factor, platelet-derived growth factor, and HRG-β1 plus forskolin, which has been used in the differentiation of the rat mesenchymal stem cells along a SC lineage.15 After addition of growth factors, fibroblast-like ASCs started to change to bipolar, spindle-shaped dASCs which were similar to SCs (Fig. 2A). Consistent with previous reports using rat and human cells,11,16 ASCs and dASCs both expressed SC marker p75 and GFAP, but they scarcely expressed S100 (Fig. 2B). A lower molecular weight band of GFAP (50 kDa) expressed in SCs was not detected in ASCs and dASCs. There was little difference in the expression levels of these phenotypic markers between ASCs and dASCs. On the other hand, ELISA using 48-hour conditioned culture medium showed that dASCs produced significantly higher amount of NGF and BDNF (258 ± 12 pg/ml and 18 ± 1.4 pg/ml, respectively) than ASCs (70 ± 6 pg/ml and 0.9 ± 0.4 pg/ml, respectively, P <0.01, one-way ANOVA followed by post hoc test) (Fig. 2C), and the levels were comparable to that of SCs (247 ± 16 pg/ml and 14 ± 0.8 pg/ml, respectively), indicative of potential trophic supports of dASCs for nerve regeneration. The results of phenotypic and functional characteristics of the 3 cell lines are summarized in Table 2.
Fig. 2.
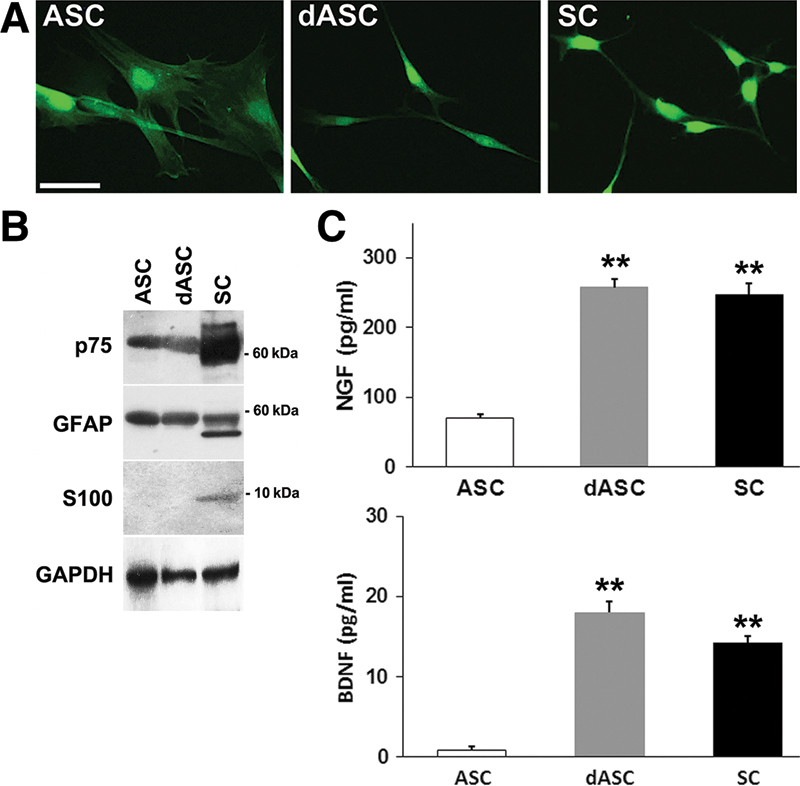
A, Fibroblast-like ASCs (left) changed to bipolar, spindle-shaped dASCs (middle), which were similar to SCs (right). Scale bar: 50 µm. B, Western blotting analysis of cell lysates showed that ASCs and dASCs both expressed SC markers p75 and GFAP but not S100. C, The amounts of NGF (above) and BDNF (below) in the conditioned medium from 48-h culture were measured by ELISA. The data are expressed as mean ± SEM, n = 3 experiments; **P < 0.01, one-way ANOVA followed by post hoc Tukey-Kramer test. SEM, standard errors of the mean.
Table 2.
Phenotypic Markers and Characteristics of the Cell Lines
Effect of Stem Cell Transplantation on Cutaneous Nerve Regeneration in Noninnervated and Innervated Flaps
All flaps survived without any problem. After undergoing some contraction during the first several weeks, the final flap sizes were comparable to those in the initial surgery. In the 3 cell-transplanted groups, no GFP-positive cells were detected in the skin sections from all rats, indicative of a significant cell loss during the period of 20 weeks. To see the time course of cell survival for each cell type, short-term follow-up models were additionally used. Three days after the cell transplantation, many GFP-positive cells were observed in the dermis and in the hypodermis in all flaps (Fig. 3, left). At 7 days postoperatively, the number of GFP-positive cells decreased considerably in the dASC-transplanted flaps, whereas a large number of GFP-positive cells were still observed in the ASC- and SC-transplanted flaps (Fig. 3, middle). At 14 days, no GFP-positive cell was detected in the dASC-transplanted flaps, and the number of GFP-positive cells in the ASC- and SC-transplanted flaps also dropped considerably (Fig. 3, right), which reached to zero at 28 days.
Fig. 3.
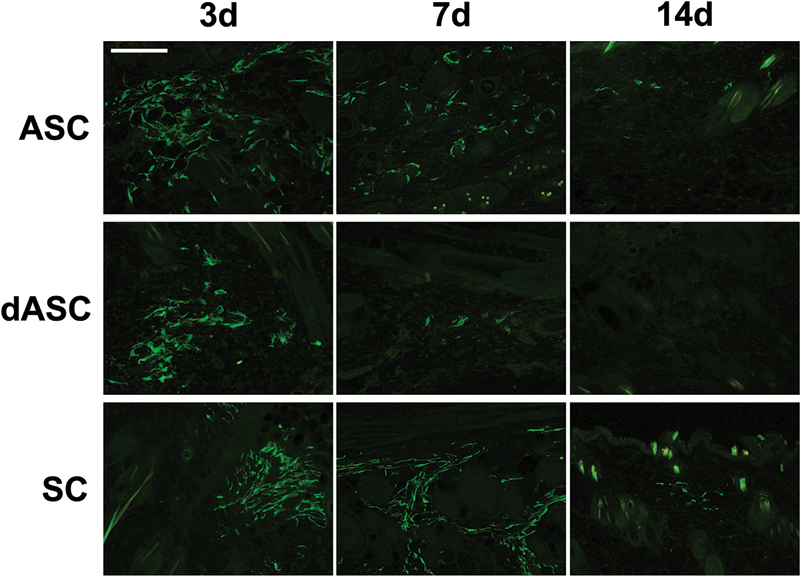
Images of GFP immunostaining of sections from the ASC-, dASC-, and SC-transplanted noninnervated flaps. The number of GFP-positive cells gradually decreased over time in each flap. Scale bar: 100 µm.
Staining of nerve tissue in skin sections was confirmed by PGP9.5-positive area in the dermis and in the hypodermis. In unwounded tissue, PGP9.5-positive axons typically arose from the hypodermis and then run through the dermis (Fig. 4). In the periphery of groin island flaps, more PGP9.5-positive axons were observed in the dermis than in the hypodermis irrespective of the type of flap innervation, and they tended to run parallel to the epidermis layer (Figs. 5 and 6, upper). On the other hand, in the center of flaps, the innervation pattern was rather similar to that of unwounded tissue although the nerve densities varied between noninnervated and innervated flaps (Figs. 5 and 6, lower).
Fig. 4.
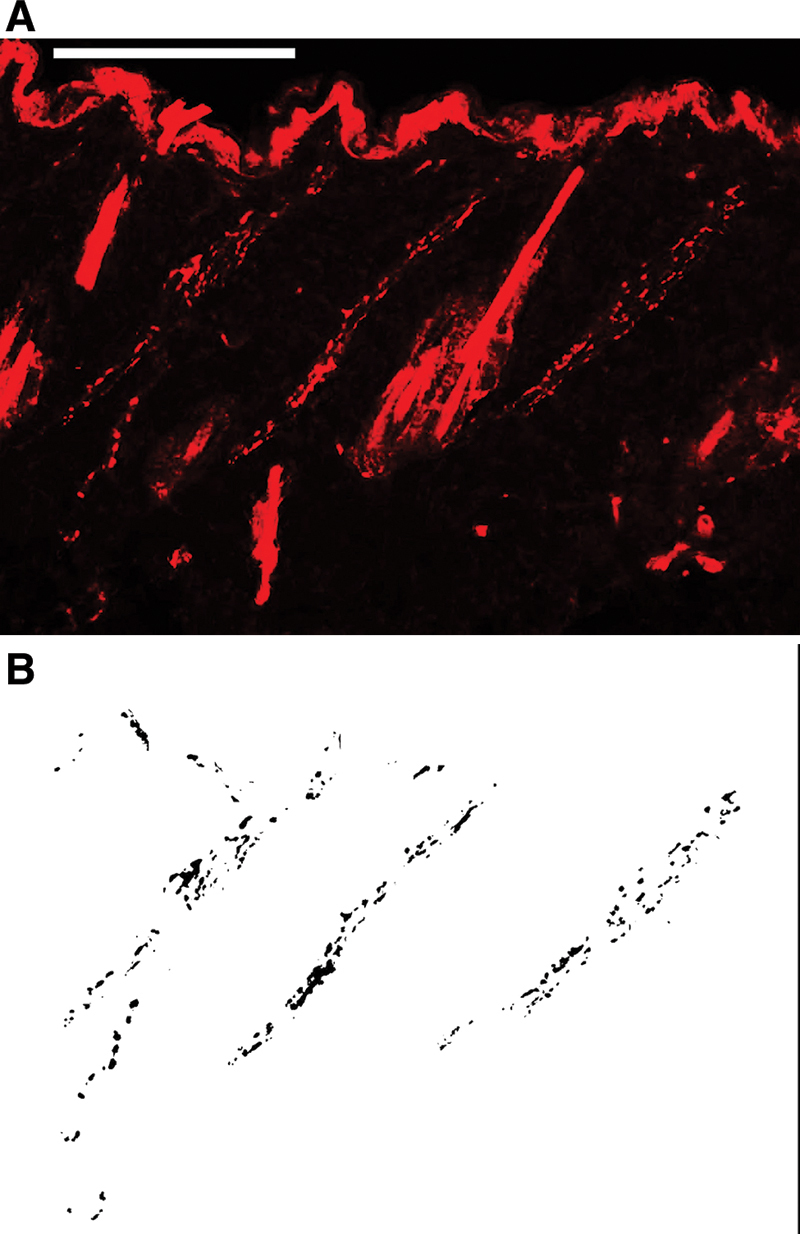
A, An image of PGP9.5 immunostaining of a section from unwounded skin. Scale bar: 100 µm. B, Images were analyzed using an automated method of quantifying the PGP9.5-positive area in each field of view with a digital image analysis program.
Fig. 5.
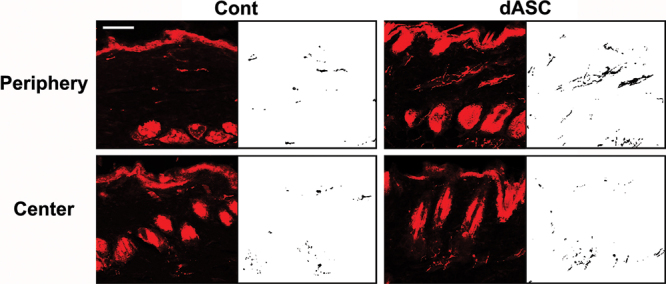
Images of PGP9.5 immunostainings of sections from noninnervated flaps. They were analyzed with a digital image analysis program. Note the dense PGP9.5-positive axons in the periphery of the dASC-transplanted flaps. Scale bar: 50 µm.
Fig. 6.
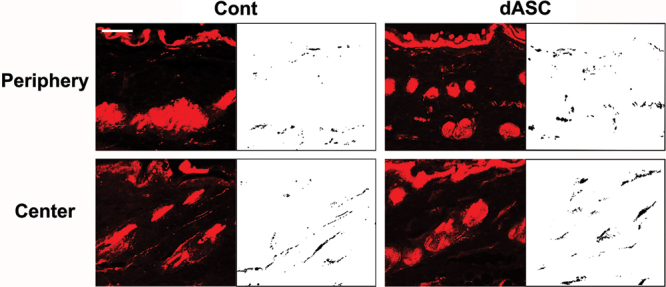
Images of PGP9.5 immunostainings of sections from innervated flaps. They were analyzed with a digital image analysis program. Note that axons tended to run parallel to the epidermis layer in the periphery of flap, whereas the innervation pattern in the center of flap was similar to that of unwounded skin. Scale bar: 50 µm.
In noninnervated flaps, two-way ANOVA revealed a significant difference in the reinnervation density between the periphery and the center of flaps (P < 0.01) (Fig. 7). Post hoc tests showed that dASC and SC transplantation significantly increased the overall reinnervation density (P < 0.01 and P < 0.05 vs control, respectively). One-way ANOVA followed by post hoc tests also revealed that the dASC- and SC-transplanted flaps showed significantly higher reinnervation density than control flaps in the periphery of flap (P < 0.01 and P < 0.05, respectively) (Fig. 7). ASC transplantation slightly increased the values, but it did not achieve statistical significance. On the other hand, cell transplantation did not affect the reinnervation density in the center of flaps in all the cell-transplanted groups.
Fig. 7.
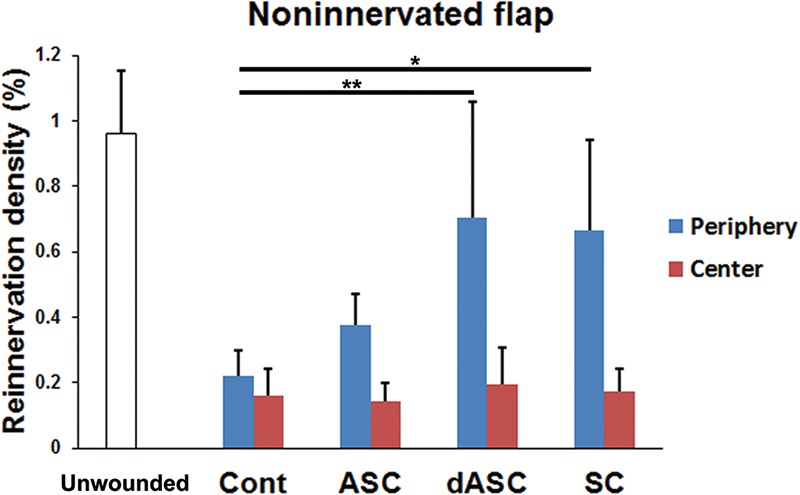
Reinnervation density in noninnervated flaps. The dASC-transplanted and SC-transplanted flaps showed significantly higher overall reinnervation density (peripheral plus central zones) than did the control group (P < 0.01 and P < 0.05, respectively, two-way ANOVA followed by post hoc Tukey-Kramer test). In the center of flaps, cell transplantation did not affect the reinnervation density. The data are expressed as mean ± SEM (n = 8 for each group). *P < 0.05, **P < 0.01 as indicated (one-way ANOVA followed by post hoc Tukey-Kramer test). SEM, standard errors of the mean.
In innervated flaps, two-way ANOVA revealed that there was no significant difference in the reinnervation density between the zones of flaps (Fig. 8). Post hoc tests showed that the overall reinnervation density was not affected by the transplantation of any type of cells. However, one-way ANOVA followed by post hoc tests showed that dASCs and SCs transplantation significantly increased the reinnervation density in the periphery of flaps (P < 0.01 vs control, respectively) as seen in noninnervated flaps, whereas ASC transplantation did not affect it (Fig. 8). Although cell transplantation did not affect the reinnervation density in the center of flap, the mean values were higher than those in noninnervated flaps in all groups (Figs. 7 and 8).
Fig. 8.
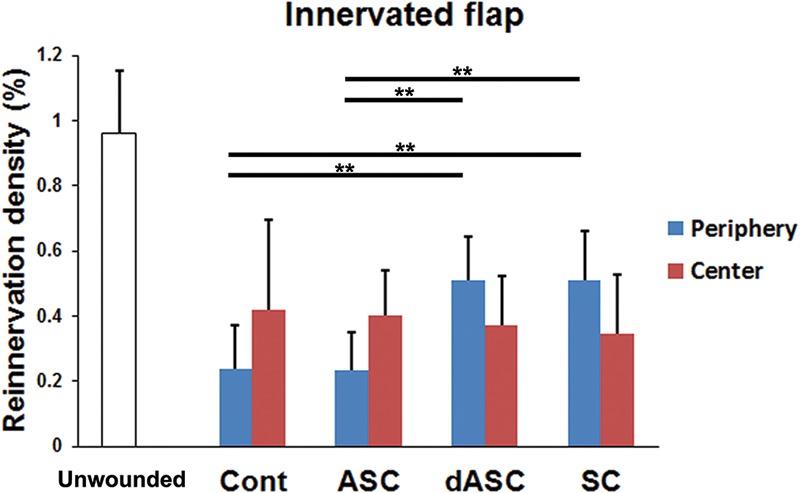
Reinnervation density in innervated flaps. There was no significant difference in the overall reinnervation density (peripheral plus central zones) between the treatment groups (two-way ANOVA followed by post hoc Tukey-Kramer test). In the periphery of flaps, however, dASC and SC transplantation increased the reinnervation density. These treatments did not affect the reinnervation density in the center of flaps. The data are expressed as mean ± SEM (n = 8 for each group). **P < 0.01 as indicated (one-way ANOVA followed by post hoc Tukey-Kramer test). SEM, standard errors of the mean.
DISCUSSION
In microvascular flap transfer such as perforator flaps, total degeneration of cutaneous nerves occurs within the flaps unless they are elevated as a pedicled, innervated flap. The initial reinnervation reportedly takes place mainly from the surrounding skin, and slightly later, from the base.12 Clinically, poor recovery of sensation in skin flaps attributes to fibrosis, scarring, and nerve injuries in the surroundings and the base of the flap.1,17 In addition, sensory recovery in thick flaps tends to be poor possibly because ingrowth of regenerating axons into adipose tissue could be retarded.18,19 To obtain better sensation, innervated flaps have been a promising approach. However, their applications are often limited due to the lack of dominant sensory nerves or recipient nerves. Moreover, normal sensation is hardly achieved even in innervated flaps.2,3,20
In this study, we asked if transplantation of SCs and SC-like cells, dASCs, can improve the cutaneous nerve reinnervation in rat island groin flaps. We also asked if these cell therapies can further improve the reinnervation of innervated flaps. Before transplantation, we phenotypically and functionally characterized ASCs and dASCs. Although the analysis of SC phenotypic markers revealed that differentiation of dASCs is not complete in vitro, dASCs produced significantly higher amount of NGF and BDNF than ASCs, and the levels were comparable to those of SCs. These results are consistent with our recent study using human cells where dASCs transplanted into peripheral nerves achieved a full glial commitment in vivo (ie, they myelinated regenerating axons).11
Our in vivo experiment demonstrated that both dASC and SC transplantation into the dermal and hypodermal layers significantly facilitated ingrowth of cutaneous axons from the periphery of flaps, and this effect was more pronounced in noninnervated flaps. It is conceivable that there are 3 types of mechanisms by which dASC and SC transplantation promoted the reinnervation in the periphery of flap. First, the transplanted cells migrated to form SC processes, navigating growing axons along them.21 Second, the transplanted cells produced neurotrophic factors, such as NGF and BDNF, which are known to enhance neurite outgrowth in primary sensory neurons, thereby promoting cutaneous nerve regrowth from the surrounding edges.4 Third, NGF produced by the transplanted cells promoted the collateral sprouting of undamaged axons in adjacent tissues, which has been shown to be completely dependent on NGF.22,23 In our models, it seems unlikely that the first mechanism contributed to the improved reinnervation, since if this is the case, at least some of the SCs forming processes would participate in the myelination process of regenerating axons. However, we detected no GFP-positive cells associated with PGP9.5-positive axons at 20 weeks postoperatively in the dASC-transplanted and in the SC-transplanted skin sections. On the other hand, the third mechanism has been known to occur in normal wounds as well where NGF is produced largely by proliferating keratinocytes,24 and reinnervation by the collateral sprouting is eventually replaced when the original nerve regenerates.22 This may explain the more pronounced effect of cell transplantation in noninnervated flaps in which a smaller number of original axons would regenerate as compared with innervated flaps.
In the center part of flaps, we observed no positive effect of cell transplantation on reinnervation in both types of flaps. This might be explained, in part, by the nonpermissive nature of adipose tissue for axonal ingrowth as mentioned above. In addition, the collateral sprouting from intact nerves unlikely occurs in this case because there would be few intact cutaneous nerves on the base of flap.
We detected no viable GFP-positive cells in all groups at 20 weeks after transplantation. Short-term follow-up models showed that the transplanted cells would have died within 2 weeks in the dASC-transplanted flaps and within 4 weeks in the ASC- and the SC-transplanted flaps. It is in contrast to the findings in our recent studies where a number of the transplanted dASCs and SCs survived and myelinated regenerating axons within the injured rat peripheral nerves, whereas few ASCs survived at 8 weeks after transplantation.11 These results suggest that the environment of dermis and hypodermis may not be suitable for the long-term survival of dASCs and SCs. Also, the in vivo differentiation of ASCs seems a rare event at least when transplanted to the skin flaps and to the injured peripheral nerve.
CONCLUSIONS
dASCs transplanted to rat groin island flaps promoted the reinnervation of noninnervated flaps and innervated flaps. It could be explained by 2 mechanisms: First, neurotrophic factors such as NGF and BDNF produced by dASCs facilitated regrowth of cutaneous axons from the surroundings of flap. Second, NGF released by dASCs induced the collateral sprouting of undamaged axons in adjacent tissues. In addition to the use of innervated flaps, the dASC transplantation therapy could be a new approach to improve the sensory recovery of skin flaps.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This work was supported by grants from the Japanese Ministry of Education, Science, Sports and Culture (24659783), the Nakatomi Foundation, and the Casio Science Promotion Foundation. The Article Processing Charge was paid for by a grant from the Japanese Ministry of Education, Science, Sports and Culture (24659783).
REFERENCES
- 1.Hermanson A, Dalsgaard CJ, Arnander C, et al. Sensibility and cutaneous reinnervation in free flaps. Plast Reconstr Surg. 1987;79:422–427. doi: 10.1097/00006534-198703000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Yano K, Matsuo Y, Hosokawa K. Breast reconstruction by means of innervated rectus abdominis myocutaneous flap. Plast Reconstr Surg. 1998;102:1452–1460. doi: 10.1097/00006534-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Yano K, Hosokawa K, Takagi S, et al. Breast reconstruction using the sensate latissimus dorsi musculocutaneous flap. Plast Reconstr Surg. 2002;109:1897–1902; discussion 1903. doi: 10.1097/00006534-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194(Part 1):1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunge MB, Wood PM. Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury. Handb Clin Neurol. 2012;109:523–540. doi: 10.1016/B978-0-444-52137-8.00032-2. [DOI] [PubMed] [Google Scholar]
- 6.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kingham PJ, Kalbermatten DF, Mahay D, et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Tomita K, Madura T, Mantovani C, et al. Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J Neurosci Res. 2012;90:1392–1402. doi: 10.1002/jnr.23002. [DOI] [PubMed] [Google Scholar]
- 9.Kalbermatten DF, Schaakxs D, Kingham PJ, et al. Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res. 2011;344:251–260. doi: 10.1007/s00441-011-1142-5. [DOI] [PubMed] [Google Scholar]
- 10.Reid AJ, Sun M, Wiberg M, et al. Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience. 2011;199:515–522. doi: 10.1016/j.neuroscience.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 11.Tomita K, Madura T, Sakai Y, et al. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013;236:55–65. doi: 10.1016/j.neuroscience.2012.12.066. [DOI] [PubMed] [Google Scholar]
- 12.Manek S, Terenghi G, Shurey C, et al. Neovascularisation precedes neural changes in the rat groin skin flap following denervation: an immunohistochemical study. Br J Plast Surg. 1993;46:48–55. doi: 10.1016/0007-1226(93)90065-j. [DOI] [PubMed] [Google Scholar]
- 13.Inoue H, Ohsawa I, Murakami T, et al. Development of new inbred transgenic strains of rats with LacZ or GFP. Biochem Biophys Res Commun. 2005;329:288–295. doi: 10.1016/j.bbrc.2005.01.132. [DOI] [PubMed] [Google Scholar]
- 14.Caddick J, Kingham PJ, Gardiner NJ, et al. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006;54:840–849. doi: 10.1002/glia.20421. [DOI] [PubMed] [Google Scholar]
- 15.Dezawa M, Takahashi I, Esaki M, et al. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001;14:1771–1776. doi: 10.1046/j.0953-816x.2001.01814.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaewkhaw R, Scutt AM, Haycock JW. Anatomical site influences the differentiation of adipose-derived stem cells for Schwann-cell phenotype and function. Glia. 2011;59:734–749. doi: 10.1002/glia.21145. [DOI] [PubMed] [Google Scholar]
- 17.Lähteenmäki T, Waris T, Asko-Seljavaara S, et al. The return of sensitivity to cold, warmth and pain from excessive heat in free microvascular flaps. Scand J Plast Reconstr Surg Hand Surg. 1991;25:143–150. doi: 10.3109/02844319109111274. [DOI] [PubMed] [Google Scholar]
- 18.Weis J, Schröder JM. Differential effects of nerve, muscle, and fat tissue on regenerating nerve fibers in vivo. Muscle Nerve. 1989;12:723–734. doi: 10.1002/mus.880120905. [DOI] [PubMed] [Google Scholar]
- 19.Lutz BS, Ma SF, Chuang DC, et al. Interposition of a pedicle fat flap significantly improves specificity of reinnervation and motor recovery after repair of transected nerves in adjacency in rats. Plast Reconstr Surg. 2001;107:116–123. doi: 10.1097/00006534-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Shridharani SM, Magarakis M, Stapleton SM, et al. Breast sensation after breast reconstruction: a systematic review. J Reconstr Microsurg. 2010;26:303–310. doi: 10.1055/s-0030-1249313. [DOI] [PubMed] [Google Scholar]
- 21.Son YJ, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- 22.Devor M, Schonfeld D, Seltzer Z, et al. Two modes of cutaneous reinnervation following peripheral nerve injury. J Comp Neurol. 1979;185:211–220. doi: 10.1002/cne.901850113. [DOI] [PubMed] [Google Scholar]
- 23.Ansel JC, Kaynard AH, Armstrong CA, et al. Skin-nervous system interactions. J Invest Dermatol. 1996;106:198–204. doi: 10.1111/1523-1747.ep12330326. [DOI] [PubMed] [Google Scholar]
- 24.Di Marco E, Marchisio PC, Bondanza S, et al. Growth-regulated synthesis and secretion of biologically active nerve growth factor by human keratinocytes. J Biol Chem. 1991;266:21718–21722. [PubMed] [Google Scholar]


