Abstract
Summary:
The use of negative pressure wound therapy (NPWT) as a bolster for split-thickness skin grafts has been well documented in the literature. It facilitates the removal of transudate, which can result in the formation of seroma, and mitigates shear stress, which can detach the graft from the underlying wound bed. Its widespread use may be limited by factors such as increased cost and length of hospitalization. Recently, mechanically powered devices (Smart Negative Pressure; Spiracur, Inc., Sunnyvale, Calif.) have been reported as showing promise in healing wounds with outcomes surprisingly comparable to standard NPWT in the populations studied. We are unaware of any reports in the literature that have detailed the use of a mechanically powered NPWT device as a postoperative bolster for split-thickness skin grafts.
Up to 25% of people with diabetes will develop a foot ulcer,1 and mortality rates following a major amputation of the lower limb are worse than for most malignancies.2,3 Over the past 20 years negative pressure wound therapy (NPWT) has become a critical tool in limb salvage efforts around the world.
In one of the first multicenter, randomized clinical trials on NPWT, Armstrong and Lavery4 found that treatment with NPWT produced a higher number of healed wounds, as compared with standard moist wound therapy, and resulted in an increased rate of granulation tissue formation. In an even larger study in 2008, Blume et al5 reported that a greater proportion of diabetic foot ulcers achieved complete closure with NPWT and required fewer amputations.
Several devices, which allow for the safe and effective delivery of NPWT, are now available. One such device is the Smart Negative Pressure (SNaP) Wound Care System (Spiracur, Inc., Sunnyvale, Calif.), which is a novel, mechanically powered, ultraportable device that utilizes specialized springs to deliver NPWT (Fig. 1). Because it operates without a battery, SNaP does not require charging.
Fig. 1.

SNaP (Spiracur, Inc., Sunnyvale, Calif.) is roughly the size and weight of a cell phone. The device can deliver topical negative pressure at 75, 100, and 125 mm Hg.
The SNaP system is fully disposable and silent throughout its operation. It is readily available for “off-the-shelf” use; thus, the need for costly and time-consuming rental agreements, which are standard with many other NPWT devices, is obviated. The SNaP system consists of a cartridge, a hydrocolloid dressing layer with integrated nozzle and tubing, and a foam interface. It is roughly the size and weight of a cell phone, and it may be worn around a patient’s leg, hidden beneath their clothing. The cartridge, which doubles as the storage canister, has a 60-mL capacity. SNaP can deliver negative pressures of 75, 100, and 125 mm Hg, and the application process is generally simple and quick.6–8
Several studies focusing on the use of NPWT as a bolster for split-thickness skin grafts (STSG) have demonstrated promising results, but few studies have addressed many of the shortcomings, including the associated cost and length of hospital stay. We are unaware of any reports in the medical literature describing the use of a mechanically powered NPWT technology to bolster skin grafts. Therefore, the purpose of this manuscript is to describe the use of such a technique in the immediate postoperative period following application of STSG.
CASE REPORT
An 83-year-old woman, with type 2 diabetes and peripheral neuropathy, presented to our clinic with a large, painful wound on the medial aspect of her left ankle. The wound was composed of mixed granulation and fibrotic tissue, with moderate serous drainage, but it seemed clinically uninfected (Fig. 2). Because of exquisite tenderness related to the ulcer, the patient was reluctant to undergo sharp debridement; thus, maggot debridement therapy was initiated. As the proportion of granulation tissue increased, plans were made for skin grafting.
Fig. 2.
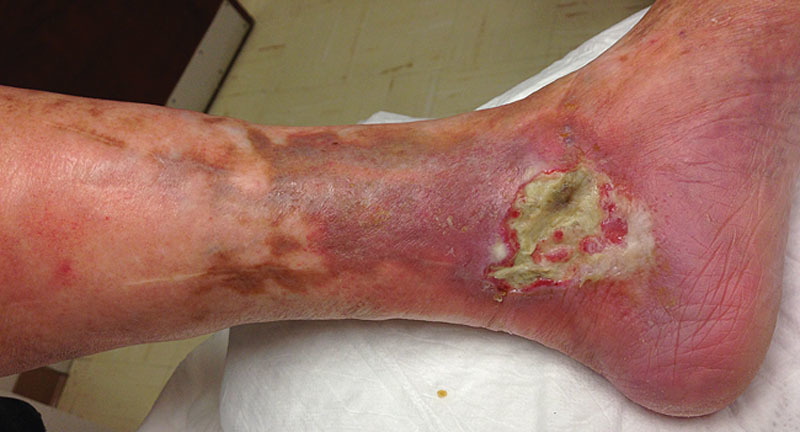
Initial presentation of the wound.
Following surgical debridement of the wound, a STSG was applied utilizing our standard technique, and SNaP was used as a postoperative bolster (Fig. 3). The device was set to 75 mm Hg and kept in place for 4 days. On the first postoperative visit, the graft appeared viable and well adhered to the underlying wound bed. At 4 weeks postoperative, the graft demonstrated near complete epithelialization, and by 12 weeks, the wound was closed (Fig. 4).
Fig. 3.
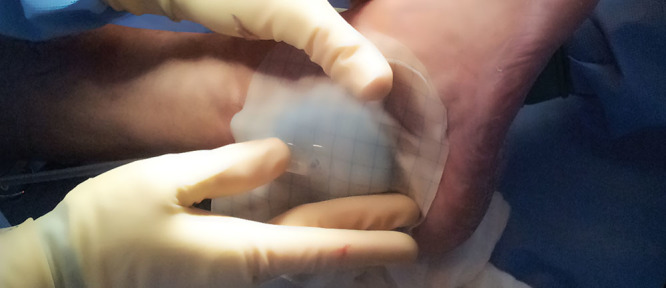
Application of SNaP following skin grafting. A nonadherent dressing layer is applied between the skin graft and foam interface. In this case, the device was set to 75 mm Hg and kept in place for 4 d.
Fig. 4.
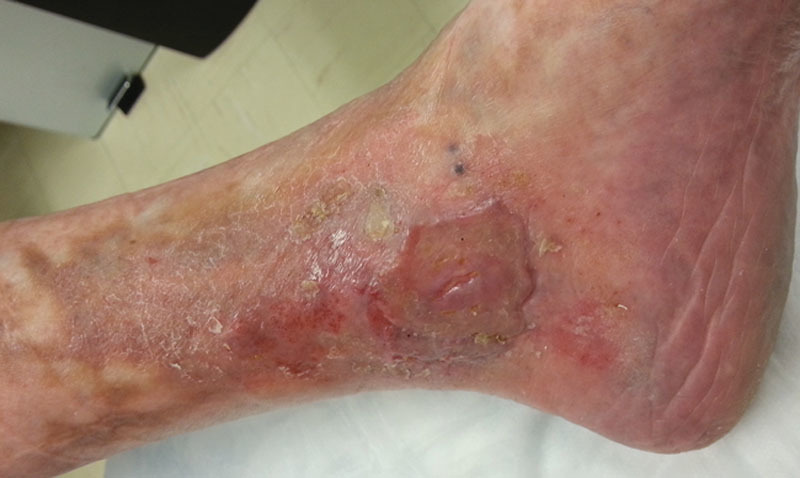
Wound closure is demonstrated at 12 wk postoperative.
DISCUSSION
In the first prospective, multicenter, randomized controlled comparative effectiveness study of its kind, Armstrong et al9 reported that SNaP-treated subjects demonstrated noninferiority, with respect to percent decrease in wound area, as compared with subjects treated with the Vacuum Assisted Closure device (V.A.C. Therapy; KCI, San Antonio, Tex.). The authors found that the mean application time for the SNaP was significantly shorter than that for V.A.C., and patients treated with the SNaP system reported improved activities of daily living with less interruption in sleep.
Argenta and Morykwas10 described the use of NPWT as a method for securing STSG in place over venous stasis ulcers. In their landmark study, the authors found that by using NPWT to bolster STSG, a uniform distribution of pressure could be maintained, while minimizing shear forces. Furthermore, in a prospective, blinded, randomized controlled trial conducted by Moisidis et al,11 graft take was found to be qualitatively better (50%) following the application of NPWT, as compared with standard bolster dressings.
Although the use of NPWT as a bolster dressing for skin grafts has been well documented, there are several limiting factors which may preclude its widespread use. In particular, the use of NPWT in such cases may represent a financial burden for the treating institution. Although there are several devices now available which provide safe and effective NPWT, it is by no means ubiquitous. Moreover, the increased length of hospitalization associated with many NPWT devices can have serious implications with respect to infection and deconditioning.
Sposato et al12 used a fully portable, battery-operated “Mini-VAC” as a bolster dressing for skin grafts and reported a successful outcome in 7 of 9 cases. The authors noted several advantages to using a portable device including an increase in patient activity and comfort and a reduction in nursing time, which could offset some of the associated costs. In contrast to the device used by Sposato et al12, SNaP is mechanically powered. It is available for off-the-shelf use and is fully disposable. Furthermore, it is lightweight, compact, and can be worn discretely.
CONCLUSIONS
Although there is no consensus in the literature regarding the optimum pressure needed to bolster these grafts and the amount of time these devices should be kept on, we used 75 mm Hg and kept the device in place for 4 days. Further studies are needed to address the efficacy of using NPWT as a bolster dressing for STSG. In particular, a comparative study with SNaP and standard bolster dressings or other NPWT devices may be useful. The use of SNaP as a bolster dressing following skin grafting could represent a groundbreaking way to improve graft take while maintaining patient comfort and reducing overall costs.
Footnotes
Presented, in part, at the 11th Meeting of the Diabetic Foot Study Group of the European Association for the Study of Diabetes, September 20–22, 2013, Sitges, Spain.
Disclosure: Dr. Armstrong has received research funding and has previously served on the scientific advisory board of Spiracur. Neither of the other authors has any financial disclosures. The Article Processing Charge was paid for by Spiracur, Inc. (Sunnyvale, Calif.).
REFERENCES
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4:286–287. doi: 10.1111/j.1742-481X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DG, Mills JL. Toward a change in syntax in diabetic foot care: prevention equals remission. J Am Podiatr Med Assoc. 2013;103:161–162. doi: 10.7547/1030161. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DG, Lavery LA Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704–1710. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]
- 5.Blume PA, Walters J, Payne W, et al. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2008;31:631–636. doi: 10.2337/dc07-2196. [DOI] [PubMed] [Google Scholar]
- 6.Fong KD, Hu D, Eichstadt S, et al. The SNaP system: biomechanical and animal model testing of a novel ultraportable negative-pressure wound therapy system. Plast Reconstr Surg. 2010;125:1362–1371. doi: 10.1097/PRS.0b013e3181d62b25. [DOI] [PubMed] [Google Scholar]
- 7.Lerman B, Oldenbrook L, Eichstadt SL, et al. Evaluation of chronic wound treatment with the SNaP wound care system versus modern dressing protocols. Plast Reconstr Surg. 2010;126:1253–1261. doi: 10.1097/PRS.0b013e3181ea4559. [DOI] [PubMed] [Google Scholar]
- 8.Lerman B, Oldenbrook L, Ryu J, et al. The SNaP Wound Care System: a case series using a novel ultraportable negative pressure wound therapy device for the treatment of diabetic lower extremity wounds. J Diabetes Sci Technol. 2010;4:825–830. doi: 10.1177/193229681000400409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong DG, Marston WA, Reyzelman AM, et al. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: a multicenter randomized controlled trial. Wound Repair Regen. 2012;20:332–341. doi: 10.1111/j.1524-475X.2012.00780.x. [DOI] [PubMed] [Google Scholar]
- 10.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563–576. discussion 577. [PubMed] [Google Scholar]
- 11.Moisidis E, Heath T, Boorer C, et al. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg. 2004;114:917–922. doi: 10.1097/01.prs.0000133168.57199.e1. [DOI] [PubMed] [Google Scholar]
- 12.Sposato G, Molea G, Di Caprio G, et al. Ambulant vacuum-assisted closure of skin-graft dressing in the lower limbs using a portable mini-VAC device. Br J Plast Surg. 2001;54:235–237. doi: 10.1054/bjps.2000.3537. [DOI] [PubMed] [Google Scholar]


