The emergence of injectable fillers for breast enhancement has offered women several advantages, such as local anesthesia and short recovery times, among others, and the opportunity to more specifically choose breast size. Some fillers, however, have been associated with high complication rates and can be difficult to remove. This article describes the authors’ experience with a commercially available alternative technology that was initially developed for wrinkles and volume restoration, and has been approved for use in breast enhancement in some countries.
Keywords: Breast augmentation, Breast enhancement, Complications, Macrolane, NASHA gel, Treatment
Abstract
The authors report on their experience with using hyaluronic acid of non-animal origin manufactured using commercially available technology (Macrolane, Q-Med AB, Sweden) for breast enhancement in 4000 women treated since 2004 and describe the most common complications and their successful treatment. On average, 30 mL to 40 mL of Macrolane was injected into each breast. Of 274 women who returned to the clinic during 2007, <10% experienced local adverse events (eg, gel dislocation, Macrolane nodules and rare cases of infection). There were no serious systemic events and treatment was well tolerated. To prevent local complications, such as infection, an aseptic injection technique was required and early treatment of adverse events is recommended. While only small volumes of Macrolane were injected, it is comparatively easy and safe to perform breast enhancement of up to one cup size to correct asymmetry between breasts and to create fullness in the upper portion of the breast.
Abstract
Les auteurs présentent leur expérience de l’acide hyaluronique d’origine non animale fabriqué à l’aide d’une technologie commerciale (Macrolane, Q-Med AB, Suède) pour l’augmentation mammaire de 4 000 femmes traitées depuis 2004 et décrivent les principales complications et leur traitement réussi. En moyenne, ils ont injecté de 30 mL à 40 mL de Macrolane dans chaque sein. Parmi les 274 femmes qui sont retournées à la clinique en 2007, moins de 10 % ont présenté des effets secondaires (p. ex., dislocation du gel, nodules du Macrolane et rares cas d’infection). Il n’y a pas eu d’événements systémiques graves et le traitement a bien été toléré. Pour prévenir les complications locales, comme une infection, il fallait utiliser des techniques d’injection aseptiques. De plus, un traitement précoce des événements secondaires est recommandé. Même si un petit volume de Macrolane était injecté, il est comparativement facile et sécuritaire de procéder à une augmentation mammaire pouvant atteindre une taille de bonnet pour corriger une asymétrie entre les seins et créer une plénitude dans la partie supérieure des seins.
Breast enhancement using injectable fillers offers women the advantage of deciding on their desired size. It is performed under local anesthesia, and does not require hospitalization or have long recovery times. However, the selection of injectable filler is critical because many fillers in the past have been associated with high complication rates (1–4). Furthermore, nonabsorbent materials, such as polyacrylamide hydrogel, are difficult to remove, resulting in deformation of the breasts and scarring. Stabilized hyaluronic acid of non-animal origin manufactured using the patented NASHA™ technology (NASHA gel) has been used for the treatment of wrinkles and augmentation of soft tissue (5,6). NASHA gels (Restylane and Perlane, Q-Med AB, Sweden) are made of hyaluronic acid, which do not release harmful components (such as proteins or viruses) and are, therefore, rarely associated with infectious diseases or allergic reactions (7–10). These products have been approved in the United States but not in Japan. A further formulation of NASHA gel is Macrolane (Q-Med AB, Sweden), which was developed for volume restoration and contouring of body surfaces and has an excellent safety and efficacy profile (11). Until April 2012, the indication for Macrolane also included breast enhancement; however, Macrolane is currently not marketed for this indication due to an ongoing debate over issues with radiological imaging.
The authors report on their experience with Macrolane for breast enhancement in approximately 4000 women since 2004 (originally published in Japanese [12]). Of 4000 women, 395 (9.9%) received multiple injections of Macrolane. During that time, there were three cases of infection and four cases of gel dislocation. Of the 4000 treated women, 274 returned to the clinic in 2007 and were surveyed for additional complications. The present article describes these complications, which were predominantly implant site adverse events, and their subsequent management.
RESULTS
Infection
The infection rate of 0.08% in the current study was lower than infection rates observed after permanent implants (13–15). Prophylactic antibiotics were not used before injection of Macrolane. There were two cases of suspected infection that improved after a course of intravenous antibiotics, while a third woman with symptoms of infection required more extensive treatment. This woman received 30 mL of Macrolane in each breast, followed by an additional 30 mL seven days later. Redness and swelling of the left breast, which was monitored by the woman at home, were evident 10 days after the second injection. Twenty-six days after the second injection, the woman presented to the clinic with a temperature of 38°C and had difficulty raising her left arm. These symptoms improved after intravenous antibiotics; however, after a few days, redness and swelling of the right breast appeared. Two weeks later, the woman was admitted because symptoms did not improve. The entire right breast was red and swollen (Figure 1A); therefore, a large volume of hyaluronic acid and pus was aspirated from the breast. Wound irrigation was performed (Penrose drain) and an additional round of intravenous antibiotics administered for four days. The patient had a white blood cell count of 8.6×109/L, a C-reactive protein level of 21 mg/L and bacterial cultures of the drained material showed methicillin-sensitive Staphylococcus aureus. The patient underwent an additional five days of antibiotics and the drain was removed on day 10. After treatment of the infection, there was a difference in the appearance of each breast due to the removal of the hyaluronic acid from the right breast (Figure 1B).
Figure 1).
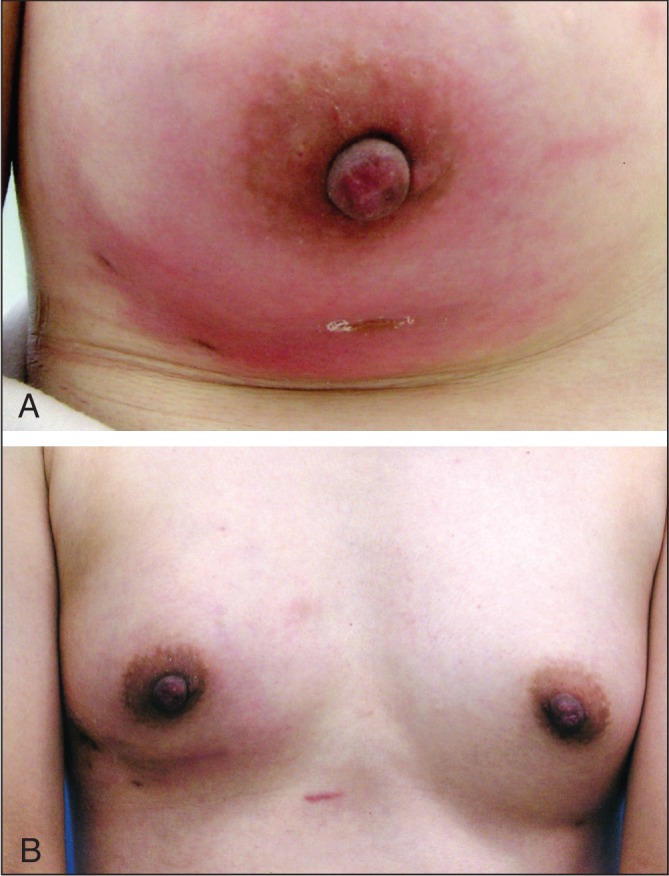
A 41-year-old woman. Seven days after the intial injection of hyaluronic acid 30/30 mL, an additional injection of 30/30mL was given. Two weeks later, right breast rubefaction and swelling appeared but a wait-and-watch approach was adopted for approximately two weeks with the patient staying at home. However, the patient presented to the hospital following worsening of her condition. The infection symptoms of the left breast subsequently improved. A Directly before incision and drainage. B Sixteen days after incision and drainage. A difference between the left and right breasts is apparent
Countermeasures:
Surgeons should make sure that Macrolane is injected under aseptic conditions to ensure sterility and devote special attention to the prevention of hematoma formations. Moreover, early treatment is required if infections do occur. The risk of infection should be explained to the patient and surgeons should stress the importance of returning to the clinic early if symptoms such as pain, redness and swelling occur.
Dislocation of injected Macrolane
Macrolane dislocation was observed in four cases, in which dislocation is the identification of a subcutaneous Macrolane nodule outside of the range of injection. Three cases involved dislocation to below the inframammary fold (Figure 2), and the final case involved dislocation to the precordium.
Figure 2).
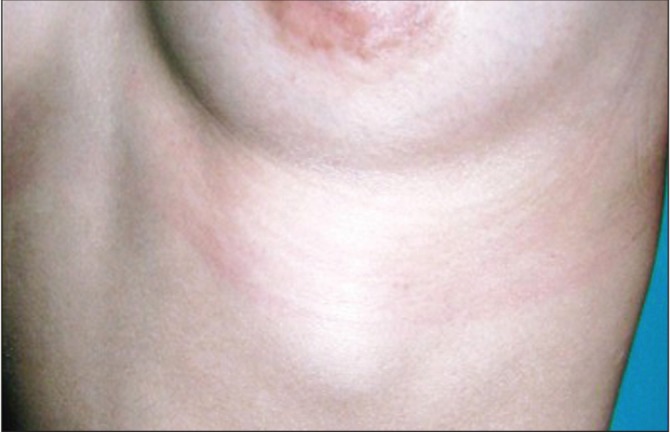
Migration of the injected material. The patient was a 39-year-old woman who was injected with hyaluronic acid 100/90 mL. One month after the injection, a subcutaneous mass appeared in the left hypochondrium
Countermeasures:
Aspiration of the dislocated Macrolane with an 18-gauge needle and injection of hyaluronidase should be performed to dissolve any remaining gel. In the authors’ experience, this resolved the dislocation, but there were reoccurrences to the same position in two women.
Other considerations
Of 274 women who returned to the clinic for retreatment or due to local complications during 2007, five experienced early degradation of the gel, six experienced abnormal firmness of the breasts and four had subcutaneous nodules of Macrolane.
Early degradation of gel
Previous studies have shown that Macrolane remains in the breast up to 18 months after injection with volumes of 80 mL to 100 mL per breast (16–20). Because the surgeons in the current study only injected 30 mL to 40 mL per breast, on average, a similar comparison is not possible. The women were advised that the aesthetic result may be expected to last approximately six to 12 months for a 40 mL injection volume per breast, and 12 to 18 months for >100 mL injection volume per breast. In three women who underwent magnetic resonance imaging, Macrolane was visible 12 months after injection (Figure 3). Additionally, over the entire study period, 24 women requested retreatment within six months of injection: two came to clinic complaining of early degradation; five clearly showed that their breasts had returned to pretreatment size within six months in photographs (Figures 4A and 4B); and the remaining 17 did not experience early degradation (based on photos) and had returned for further enlargement of their breasts.
Figure 3).
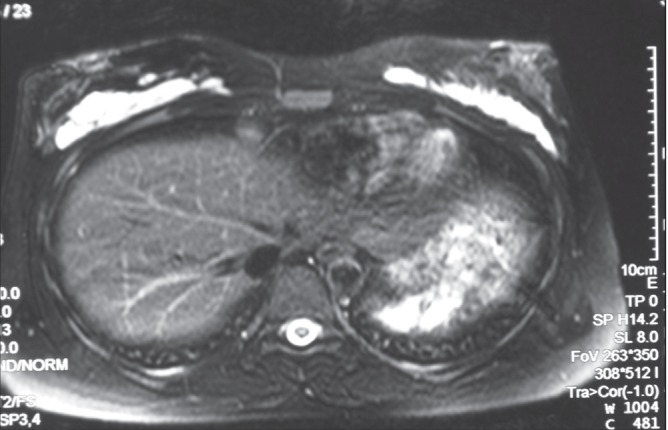
Magnetic resonance image taken 12 months after a 29-year-old woman received an 80 mL injection of hyaluronic acid to the right breast and a 90 mL injection to the left breast. The injected hyaluronic acid remained and no abnormalities were observed in mammary parenchyma
Figure 4).
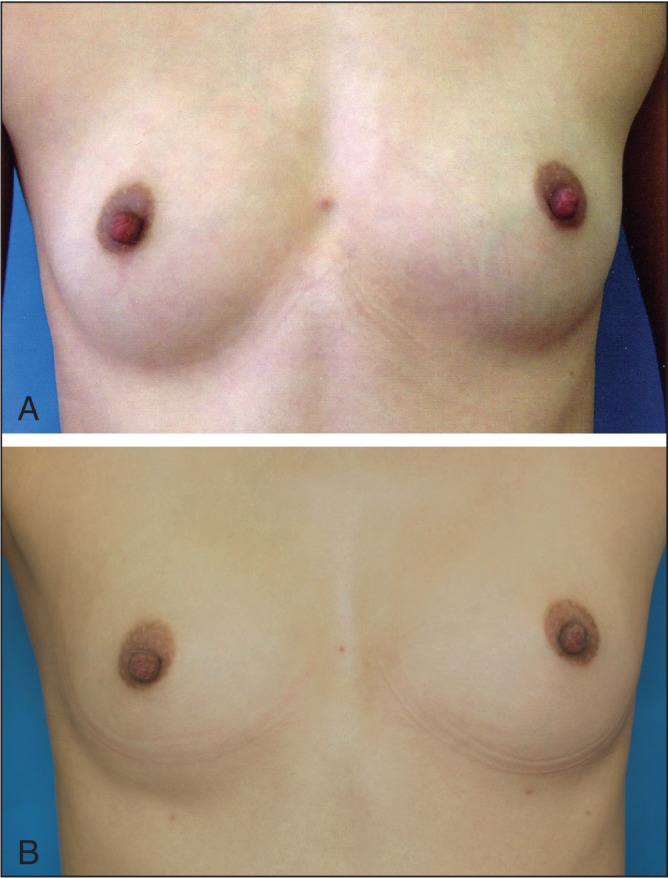
Early resorption. A 25-year-old woman, before surgery, 50/50 mL of hyaluronic acid was injected. B Three months after the injection. After approximately two to three months, the patient believed that the mass had returned, so she visited the hospital for an examination. The photograph shows that the external appearance had mostly returned
Countermeasures:
Because a thin capsule forms around the gel after injection (21), degradation is slower if the gel is injected into a single location rather than dispersed through the breast. Therefore, for women who desire larger breasts, Macrolane is injected as a single implant under the mammary gland positioned below the nipple. However, many women want to improve the fullness of the upper portion of the breast following breast feeding, which means the gel must be dispersed. This can result in a firm nodule because the mammary gland tissue is thin on the upper part of the breast. The potential for early degradation should also be explained to these women.
Firm breasts and nodules
Initially, the authors used Restylane SubQ (Q-Med AB, Sweden) for breast enhancement, but on switching to Macrolane, the number of women presenting with firm breasts or nodules declined. Of the 274 patients who returned to clinic, six complained of firmness of the breast and nodules.
Countermeasures:
Wearing surgical gloves, patients should monitor the firmness of their breasts as Macrolane is being injected. It should be explained that breast firmness and nodules are transient symptoms and should disappear within three to four months of injection. For example, among the six women with firmness or nodules, the symptoms disappeared naturally after three months for two, and three are still experiencing decreases in firmness or nodules, which are being monitored. One woman did not experience any improvement in her breast nodules within six months of treatment; therefore, hyaluronidase was injected, which then improved the result.
Visible subcutaneous nodules
Of the 274 surveyed women, four (1.5%) returned to clinic complaining of visible/palpable subcutaneous nodules (Figure 5).
Figure 5).
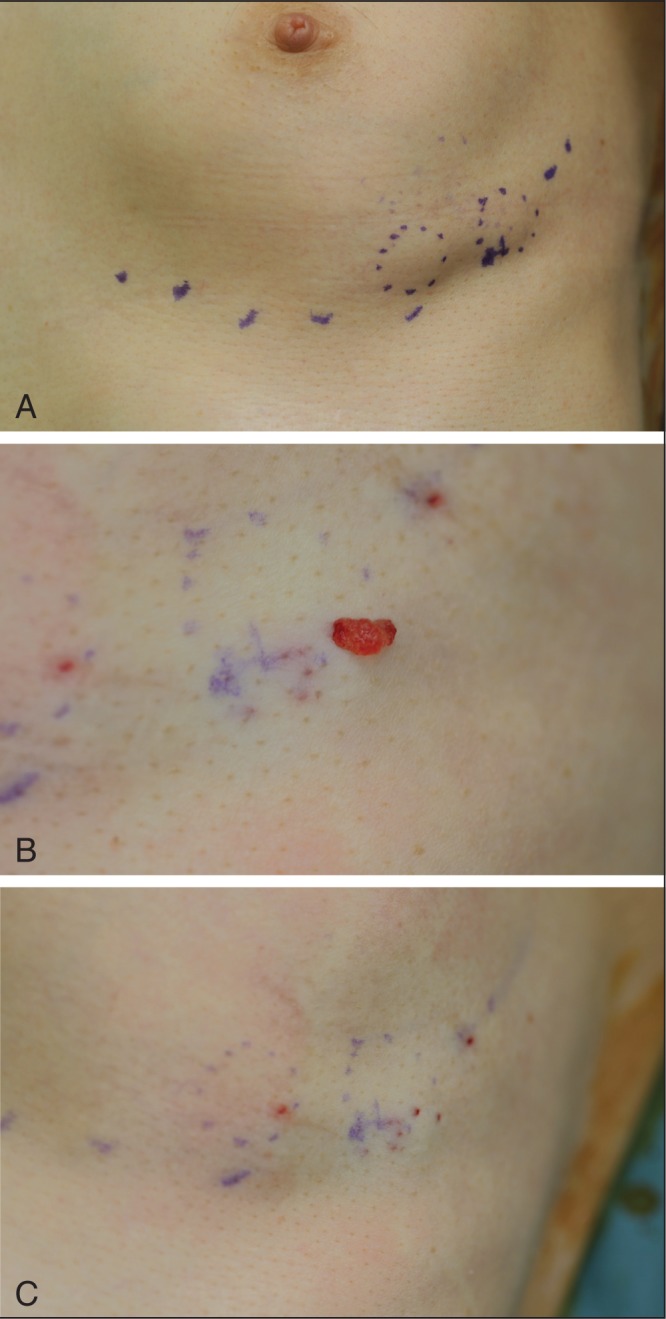
Subcutaneous mass. A A 57-year-old woman in whom subcutaneous masses were observed in two sites, 50/50 mL of hyaluronic acid was injected. Although more than one year had passed, the mass still remained. B Using an 18-gauge needle, a puncture was made directly above the mass and hyaluronic acid was expelled. C After expulsion
Countermeasures:
Among these four women, one case was resolved by manual compression breaking the capsule that forms around the injected material. Manual compression is the simplest and easiest method of improving subcutaneous nodules (located in the superficial subcutaneous layer) that occur several days after injection. For the remaining three women in whom nodules could not be broken manually because they were located in the deep subcutaneous tissue, there was improvement following aspiration of the gel using an 18-gauge needle under local anesthesia (Figures 5B and 5C). If there is no improvement after aspiration, hyaluronidase injection should be used to degrade the nodule.
SUMMARY
Among the 4000 women treated in the clinic, no serious systemic complications occurred. To prevent local adverse events, such as infection, sterile treatment is required. Early treatment of local adverse events is also recommended. While only small volumes (30 mL to 40 mL per breast) of Macrolane were injected in the present study, it was comparatively easy and safe to perform breast enhancement up to one cup size to correct differences between breasts and to create fullness in the upper portion of the breast.
Acknowledgments
The authors thank Dr Elizabeth Hutchinson of Fishawack Communications, who provided editorial support, funded by Galderma.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Cheng NX, Wang YL, Wang JH, Zhang XM, Zhong H. Complications of breast augmentation with injected hydrophilic polyacrylamide gel. Aesthetic Plast Surg. 2002;26:375–82. doi: 10.1007/s00266-002-2052-4. [DOI] [PubMed] [Google Scholar]
- 2.Cheng NX, Xu SL, Deng H, et al. Migration of implants: A problem with injectable polyacrylamide gel in aesthetic plastic surgery. Aesthetic Plast Surg. 2006;30:215–25. doi: 10.1007/s00266-005-0081-5. [DOI] [PubMed] [Google Scholar]
- 3.Christensen LH, Breiting VB, Aasted A, Jorgensen A, Kebuladze I. Long-term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr Surg. 2003;111:1883–90. doi: 10.1097/01.PRS.0000056873.87165.5A. [DOI] [PubMed] [Google Scholar]
- 4.Rubin JP, Yaremchuk MJ. Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: A comprehensive review of the literature. Plast Reconstr Surg. 1997;100:1336–53. doi: 10.1097/00006534-199710000-00043. [DOI] [PubMed] [Google Scholar]
- 5.Carruthers A, Carruthers J. Non-animal-based hyaluronic acid fillers: Scientific and technical considerations. Plast Reconstr Surg. 2007;120(6 Suppl):33S–40S. doi: 10.1097/01.prs.0000248808.75700.5f. [DOI] [PubMed] [Google Scholar]
- 6.Verpaele A, Strand A. Restylane SubQ, a non-animal stabilized hyaluronic acid gel for soft tissue augmentation of the mid- and lower face. Aesthet Surg J. 2006;26(1S):S10–7. doi: 10.1016/j.asj.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Alam M, Dover JS. Management of complications and sequelae with temporary injectable fillers. Plast Reconstr Surg. 2007;120(6 Suppl):98S–105S. doi: 10.1097/01.prs.0000248859.14788.60. [DOI] [PubMed] [Google Scholar]
- 8.Lin K, Bartlett SP, Matsuo K, et al. Hyaluronic acid-filled mammary implants: An experimental study. Plast Reconstr Surg. 1994;94:306–15. [PubMed] [Google Scholar]
- 9.Matarasso SL, Carruthers JD, Jewell ML. Consensus recommendations for soft-tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane) Plast Reconstr Surg. 2006;117(3 Suppl):3S–34S. doi: 10.1097/01.prs.0000204759.76865.39. [DOI] [PubMed] [Google Scholar]
- 10.Price RD, Berry MG, Navsaria HA. Hyaluronic acid: The scientific and clinical evidence. J Plast Reconstr Aesthet Surg. 2007;60:1110–9. doi: 10.1016/j.bjps.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Inami F, Handa T, Handa K. Mammoplasty with fillers. Jap J Plast Reconstr Surg (Keisei Geka) 2006;49:1335–41. [Google Scholar]
- 12.Ishii H, Sakata K. Complications of breast enhancement with hyaluronic acid and their management. Jap J Plast Reconstr Surg (Keisei Geka) 2009;52:1311–7. [Google Scholar]
- 13.Brand KG. Infection of mammary prostheses: A survey and the question of prevention. Ann Plast Surg. 1993;30:289–95. doi: 10.1097/00000637-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Handel N, Cordray T, Gutierrez J, Jensen JA. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg. 2006;117:757–67. doi: 10.1097/01.prs.0000201457.00772.1d. [DOI] [PubMed] [Google Scholar]
- 15.Brown MH, Shenker R, Silver SA. Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg. 2005;116:768–79. doi: 10.1097/01.prs.0000176259.66948.e7. [DOI] [PubMed] [Google Scholar]
- 16.Buck DW, Alam M, Kim JY. Injectable fillers for facial rejuvenation: A review. J Plast Reconstr Aesthet Surg. 2009;62:11–8. doi: 10.1016/j.bjps.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 17.Heden P, Sellman G, von Wachenfeldt M, Olenius M, Fagrell D. Body shaping and volume restoration: The role of hyaluronic acid. Aesthetic Plast Surg. 2009;33:274–82. doi: 10.1007/s00266-008-9303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanchwala SK, Holloway L, Bucky LP. Reliable soft tissue augmentation: A clinical comparison of injectable soft-tissue fillers for facial-volume augmentation. Ann Plast Surg. 2005;55:30–5. doi: 10.1097/01.sap.0000168292.69753.73. [DOI] [PubMed] [Google Scholar]
- 19.Narins RS, Bowman PH. Injectable skin fillers. Clin Plast Surg. 2005;32:151–62. doi: 10.1016/j.cps.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (Restylane) in facial cosmetic surgery: Review and technical considerations. Plast Reconstr Surg. 2007;120(6 Suppl):41S–54S. doi: 10.1097/01.prs.0000248794.63898.0f. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Cossio S, Castano-Oreja MT. Biocompatibility of two novel dermal fillers: Histological evaluation of implants of a hyaluronic acid filler and a polyacrylamide filler. Plast Reconstr Surg. 2006;117:1789–96. doi: 10.1097/01.prs.0000214656.07273.b0. [DOI] [PubMed] [Google Scholar]


