Abstract
Objectives:
We aimed to determine whether phasic burst duration and conventional REM sleep without atonia (RSWA) methods could accurately diagnose REM sleep behavior disorder (RBD) patients with comorbid OSA.
Design:
We visually analyzed RSWA phasic burst durations, phasic, “any,” and tonic muscle activity by 3-s mini-epochs, phasic activity by 30-s (AASM rules) epochs, and conducted automated REM atonia index (RAI) analysis. Group RSWA metrics were analyzed and regression models fit, with receiver operating characteristic (ROC) curves determining the best diagnostic cutoff thresholds for RBD. Both split-night and full-night polysomnographic studies were analyzed.
Setting:
N/A.
Participants:
Parkinson disease (PD)-RBD (n = 20) and matched controls with (n = 20) and without (n = 20) OSA.
Interventions:
N/A.
Measurements and Results:
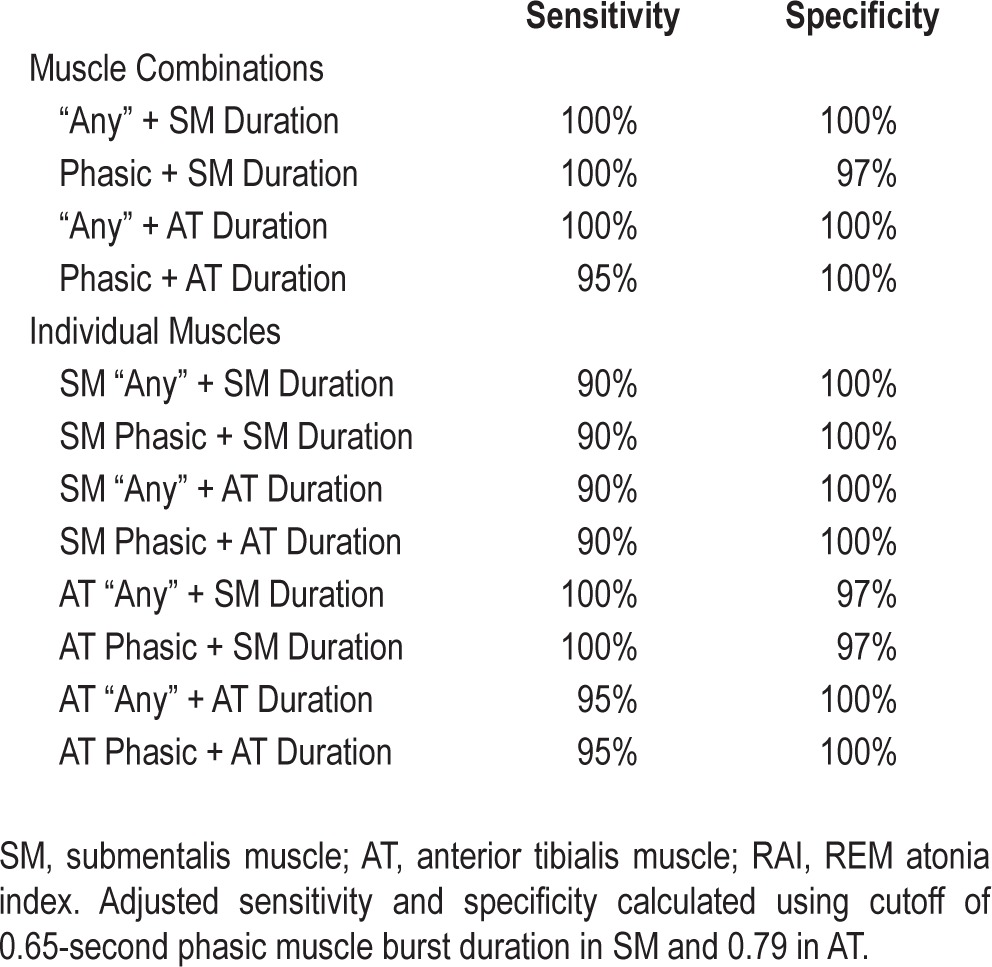
All mean RSWA phasic burst durations and muscle activities were higher in PD-RBD patients than controls (P < 0.0001), and RSWA associations with PD-RBD remained significant when adjusting for age, gender, and REM AHI (P < 0.0001). RSWA muscle activity (phasic, “any”) cutoffs for 3-s mini-epoch scorings were submentalis (SM) (15.5%, 21.6%), anterior tibialis (AT) (30.2%, 30.2%), and combined SM/AT (37.9%, 43.4%). Diagnostic cutoffs for 30-s epochs (AASM criteria) were SM 2.8%, AT 11.3%, and combined SM/AT 34.7%. Tonic muscle activity cutoff of 1.2% was 100% sensitive and specific, while RAI (SM) cutoff was 0.88. Phasic muscle burst duration cutoffs were: SM (0.65) and AT (0.79) seconds. Combining phasic burst durations with RSWA muscle activity improved sensitivity and specificity of RBD diagnosis.
Conclusions:
This study provides evidence for REM sleep without atonia diagnostic thresholds applicable in Parkinson disease-REM sleep behavior disorder (PD-RBD) patient populations with comorbid OSA that may be useful toward distinguishing PD-RBD in typical outpatient populations.
Citation:
McCarter SJ, St. Louis EK, Duwell EJ, Timm PC, Sandness DJ, Boeve BF, Silber MH. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. SLEEP 2014;37(10):1649-1662.
Keywords: REM sleep without atonia, REM sleep behavior disorder, Parkinson disease, obstructive sleep apnea, quantitative analysis, transient/ phasic muscle burst activity and duration, tonic muscle activity, diagnosis, threshold cutoffs, AASM
INTRODUCTION
REM sleep behavior disorder (RBD) is a potentially injurious parasomnia affecting up to 6% of individuals over the age of 70.1 RBD is characterized by dream enactment behaviors accompanied by polysomnographic REM sleep without atonia (RSWA).2 RSWA is of two types, either phasic/transient or tonic muscle activity. Several manual and automated RSWA scoring methods have been previously reported.3–16 Recently, the term “any” muscle activity, which comprises phasic, tonic, or duration of muscle activity that does not fall in either category has also been defined.17 Up to 82% of individuals with RBD eventually develop clinical symptoms of synucleinopathy neurodegeneration, primarily with Parkinson disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA) phenotypes.18–21 While the clinical significance of RSWA without accompanying RBD remains unknown, in selected cases RSWA may be seen prior to clinically overt RBD evolving, raising the question of whether apparently “incidental” RSWA identified during polysomnography may also signify an evolving synucleinopathy.20 Therefore, establishing diagnostic standards for RSWA that may eventually be applied outside of research settings in clinical polysomnography populations is an important public heath priority.
RSWA may be described either qualitatively or quantitatively.2,6,8,10-12,17,20,22-26 The first manual method for RSWA quantification developed by Lapierre and Montplaisir in 1992 proposed scoring two types of submentalis muscle activity using 2-s mini-epochs and 20-s epochs; phasic activity, with an amplitude greater than four times the background EMG and duration of 0.1-5 s, and tonic activity, which consisted of elongated muscle activity greater than double the background EMG lasting longer than 50% of a 20-s epoch.10 Several subsequent manual scoring methods have further adapted and refined the original method, with addition of a wider range of muscles sampled, different time standards, and varying definitions of phasic/transient muscle activity.4,5,7,8,12,17,27 Additionally, automated RSWA scoring methods have been used to identify RSWA in the submentalis muscle with high sensitivity and specificity for RBD, although automated limb muscle analyses methods have not yet been developed.3,6,9,11 Relative advantages and disadvantages of automated and manual methods of RSWA analysis can be found elsewhere.23 Current AASM definitions favor the term “transient muscle activity” rather than phasic activity; but for simplicity and familiarity throughout the remainder of this article, we will consider the terms “phasic” and “transient” muscle activity synonymous, and utilize the more familiar “phasic” for comparability with previously published works on RSWA quantitative analysis.8,12
Unfortunately, most previous RSWA research investigations have excluded patients with obstructive sleep apnea (OSA), a common comorbidity in RBD seen in up to 67% of patients, potentially limiting applicability of these prior quantitative RSWA analyses to the majority of RBD patients seen in clinical practice.28,29 Other methodological issues in previous RSWA research that may limit widespread application have included an emphasis on percentages derived from arbitrary mini-epochs of 2-3 s duration rather than direct measurement of abnormal phasic muscle bursts, and the time-consuming nature of existing manual quantitative RSWA analysis methods, suggesting the need for further evidence supporting the validity of automatic analysis methods. Previous manual RSWA scoring methods have not examined the temporal duration of phasic muscle activity bursts to determine whether there are differences in muscle activity duration between RBD patients and controls. We were interested in whether previously utilized manual and automated analyses methods had sufficient sensitivity and specificity to distinguish RBD patients with PD from controls with sleep disordered breathing in a naturalistic sleep laboratory sample commonly encountered in clinical practice. We analyzed PD-RBD patients for comparability with previous studies establishing cutoff values for RBD diagnosis.17,30
Our aims were (1) to analyze phasic muscle burst duration and conventional phasic and tonic muscle activity in PD-RBD patients and OSA controls without RBD who underwent split-night polysomnogram recordings, to determine RSWA metric diagnostic thresholds distinguishing PD-RBD patients from OSA controls; (2) to compare split-night versus full-night polysomnography RSWA metrics in PD-RBD patients and controls; and (3) to comparatively analyze established manual and automated methods for RSWA in these groups. We hypothesized that PDRBD patients would demonstrate significantly higher RSWA durations and higher muscle activity within both 3-s mini-epoch and 30-s AASM positive epoch visual scoring approaches, that direct measurement of phasic muscle burst duration would increase the diagnostic discrimination of RSWA metrics distinguishing RBD from controls, that split-night and full night polysomnograms would yield similar RSWA metrics in patient and control subjects, and that automated SM REM atonia index (RAI) would also distinguish RBD from controls in these groups.
METHODS
Patient Selection
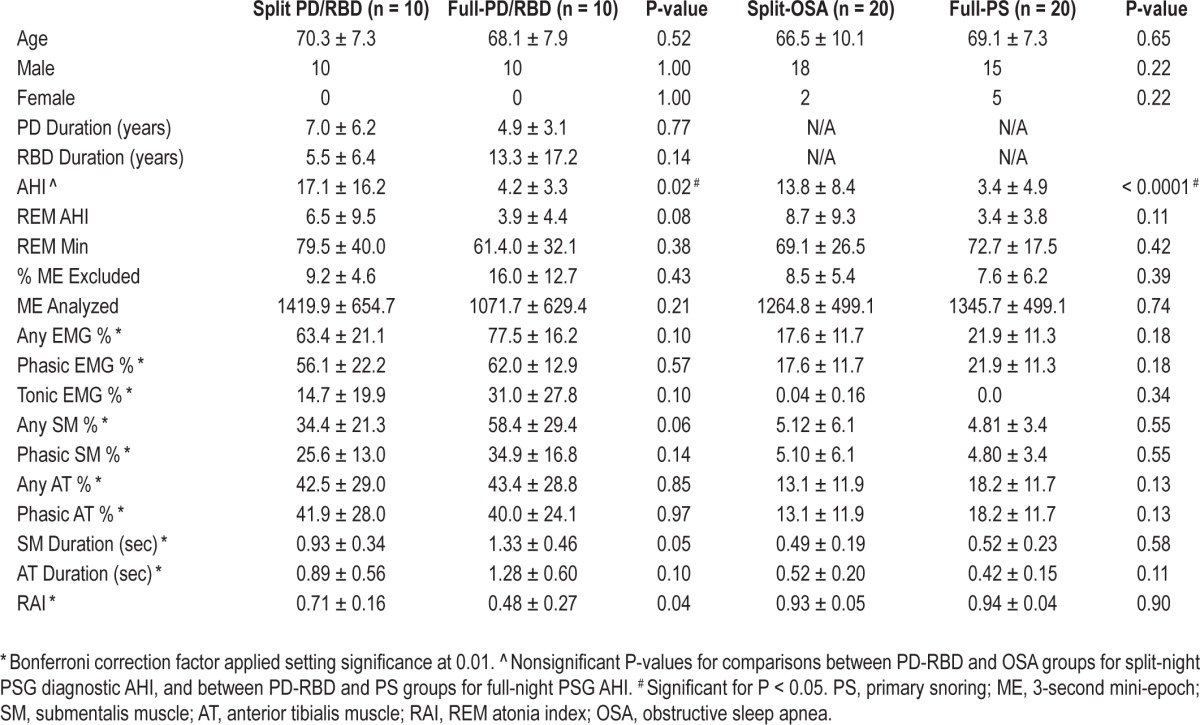
A total of 60 consecutive patients seen between 2008 and 2012 were identified for retrospective analysis of RSWA from the polysomnographic (PSG) database at the Mayo Clinic Center for Sleep Medicine. Patient subgroups included 10 Parkinson disease-RBD (PD-RBD) patients with full-night PSG and 10 split-night PD-RBD patients, as well as 20 full-night primary snorers (PS) and 20 split-night obstructive sleep apnea (OSA) patients without history of dream enactment. The 2 control groups were matched for REM AHI with the 2 PD-RBD groups. Chart review was performed for age, gender, REM sleep time, and other relevant clinical and demographic features. All patients with RBD and OSA met ICSD-2 diagnostic standards.2 Patients with Parkinson disease were diagnosed by board-certified Mayo Clinic neurologists according to United Kingdom PD Society Brain Bank criteria.31
Due to the significant amount of sleep disordered breathing required to warrant split-night polysomnography (a total sleep apnea-hypopnea index (AHI) ≥ 5/h), patients with a REM AHI of up to 30 were included in this study. AASM guidelines specify an AHI > 20-40/h during the first 2 h of sleep to warrant a split-night PSG.32 However, these parameters may exclude up to 60% of OSA cases, and an AHI of 5/h as used at our center is sufficient to rule in OSA.33 Patients and controls with a REM AHI > 30/h, total REM time < 5 min, and those using antidepressant medications, clonazepam, melatonin, or other central nervous system active drugs other than dopaminergic therapy were excluded from analysis. Twelve PD-RBD patients were using dopaminergic therapy and 2 were using anticholinesterase medications for mild cognitive impairment at time of PSG. The Mayo Clinic Institutional Review Board approved this study and oversaw study activities.
Polysomnographic Recordings
Video polysomnographic (PSG) recordings were conducted on a 16-channel Nicolet NicVue digital system with sensitivity at 5-7 μV/mm, EEG was bandpass filtered from 0.3–100 Hz (Cardinal Health Corporation, Madison, Wisconsin) and digitized at a sampling rate of 500 Hz. Electroencephalogram recordings were performed according to the International 10-20 system electrode placements (Fp1, Fp2, Fpz, Fz, Cz, C3, C4, O1, O2, Oz), including electrooculography (left and right outer canthus, LOC and ROC, placements), submentalis (SM) and bipolar linked anterior tibialis (AT) electromyography (EMG), and an electrocardiogram. Extensor digitorum communis (EDC) EMG leads were added for RBD patients only, but are not recorded routinely in patients who do not have a clinical suspicion for parasomnia in our center. Respirations were analyzed using an oronasal thermistor and nasal pressure sensor for airflow monitoring, with thoracoabdominal impedance plethysmography to monitor effort. Oxyhemoglobin saturation was evaluated by pulse oximetry. Standard 30-s epochs of PSG were used to score sleep in accordance with standard criteria.34 Because RSWA often does not allow scoring of REM sleep by established rules, the occurrence of the first REM in the electrooculographic channel was used to determine the onset of the REM sleep period.26 The end of the REM sleep period was determined when either no REMs were detected in 3 consecutive min or when an awakening, K complexes, or spindles were observed. SM and AT EMG channels were amplified at 5 μV/mm with low and high-frequency filters set at 10 and 70 Hz, respectively, with a sampling rate of 500 Hz.
Analysis of REM Sleep Muscle Activity
Background EMG amplitude was identified during REM sleep in all patients and varied from 0.5-2 μV in all subjects. Quantitative analysis of EMG activity was performed utilizing HypnoLab sleep scoring software (ATES Medica Labs, Verona, Italy). Overall tonic, phasic, and “any” (tonic, phasic, or both forms of muscle activity occurring within the same mini-epoch) percent muscle activity were visually determined and manually scored and calculated for each patient, similar to previously described methods.7,8,17,35 Phasic and “any” percent muscle activity were also calculated separately for submentalis (SM) and anterior tibialis (AT) muscles (Figure 1). In addition, each phasic muscle burst during REM sleep was directly measured, and those bursts fulfilling standards for scoring8,12,34 were individually recorded for each muscle, resulting in an overall average phasic muscle burst duration (Figures 2 and 3).
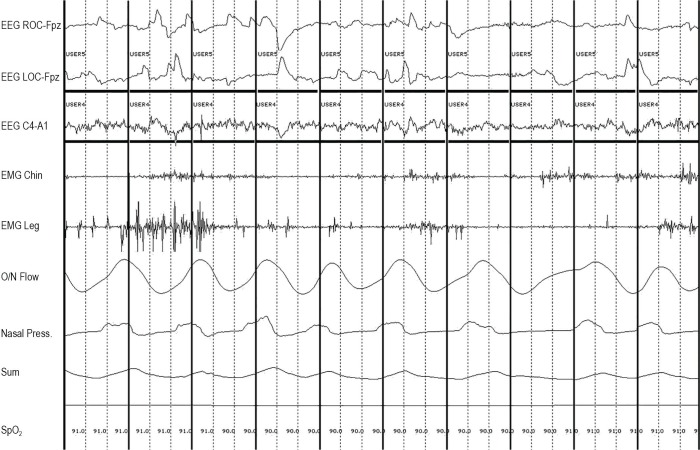
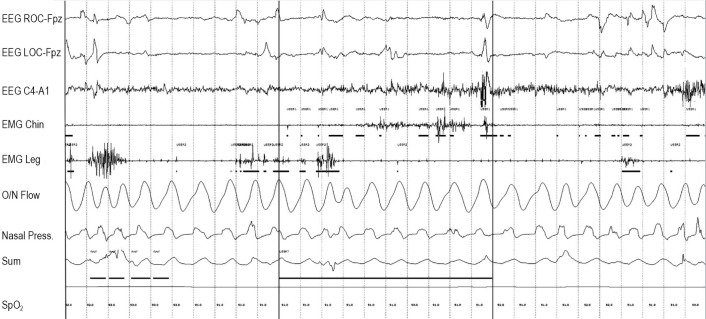
Figure 1.
Three-second mini-epoch scoring approach. The designation “User 4” (seen above the horizontal scoring bars placed within 3-s mini-epochs) indicates a specific type of a user-defined event, which marks a positive 3-s mini-epoch for phasic muscle activity in the submentalis muscle. “User 5” designation marks a positive 3-s mini-epoch for phasic muscle activity in the anterior tibialis muscles. Bold vertical lines indicate 3-s mini-epoch subdivisions.
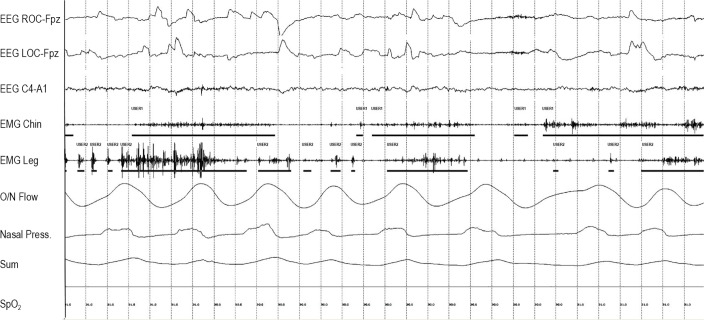
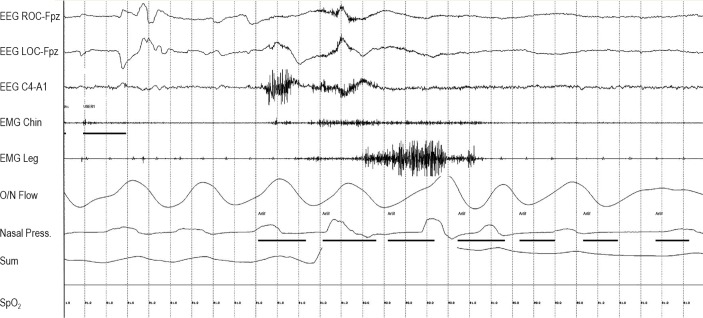
Figure 2.
Phasic muscle burst duration scoring. “User 1” marks the duration of each phasic muscle burst in the submentalis muscle. “User 2” marks the duration of each phasic muscle burst in the anterior tibiails muscle.
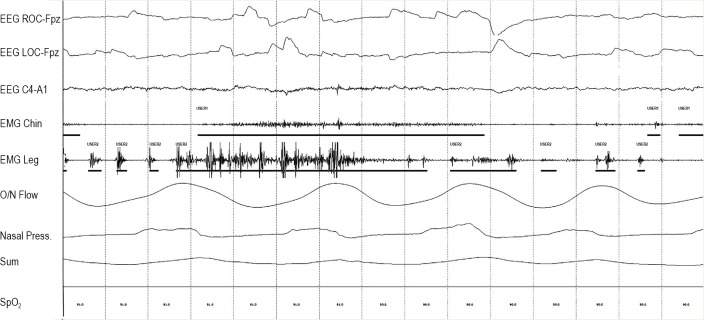
Figure 3.
Fifteen-second window of Epoch seen in Figure 2.
Thirty-s epochs were used to score tonic muscle activity in both the SM and AT muscles. An epoch was considered positive for tonic activity if > 50% of the epoch had any activity continuously greater than double the background EMG or ≥ 10 μV (Figures 4 and 5).8,10,12,34 Tonic percent muscle activity was calculated as the total number of positive 30-s epochs divided by the total number of analyzable 30-s REM sleep epochs. Tonic muscle activity has traditionally only been scored in the SM.7,8,10,12,13,17 However, there is no specific reason not to analyze tonic muscle activity in other muscles, and we also analyzed tonic activity in the AT to determine whether this may be of diagnostic benefit.
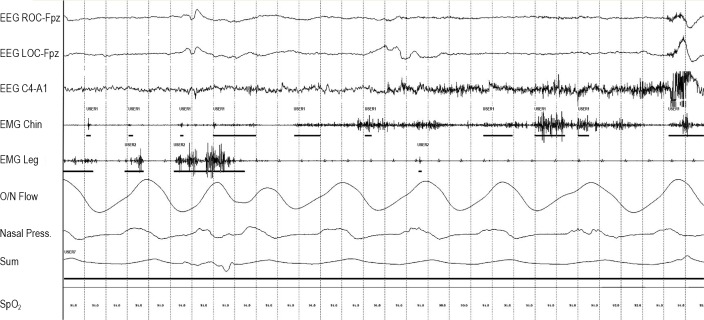
Figure 4.
Positive 30-second epoch for tonic muscle activity marked with “User 7.” “User 1” marks the duration of each phasic muscle burst in the submentalis muscle. “User 2” marks the duration of each phasic muscle burst in the anterior tibialis muscle.
Figure 5.
Ninety-second window showing tonic 30-s epoch surrounded by two 30-s epochs without tonic activity. “User 7” denotes a positive tonic epoch. “User 1” marks the duration of each phasic muscle burst in the submentalis muscle. “User 2” marks the duration of each phasic muscle burst in the anterior tibialis muscle. “Artif” marks 3-second mini-epoch with breathing or arousal event.
Each 30-s epoch was broken down into 3-s mini-epochs for analysis of phasic and “any” activity (according to SINBAR standards) in both the SM and AT muscles.7,8,17,23,35 Phasic and “any” activity was also calculated for EDC in RBD patients only. A 3-s mini-epoch was considered positive for phasic muscle activity with the presence of a phasic EMG burst that met measurement standards for scoring and negative if there was no muscle activity.7,8,10,12,17,23,35 Phasic muscle activity was defined as an EMG burst measuring > 4 times the background amplitude, with a duration lasting from 0.1 to 14.9 seconds.17,23 The longer time standard of 14.9 s was chosen because many phasic bursts are longer than the 5-s maximal duration established by the AASM and some investigators,8,23 yet are not as long as sustained tonic EMG activity (≥ 15 s, by definitions above). Our use of the term “phasic” muscle activity therefore overlaps with the term “any” muscle activity as defined by the SINBAR group, which included phasic bursts lasting longer than 5 s, but less than 14.9 seconds. The return of muscle activity to baseline for at least 200 msec was considered to be the end of a phasic burst.
A 3-s mini-epoch was considered to be positive for “any” muscle activity when either tonic or phasic muscle activity (or both) was present in either the SM or AT muscle (or both) within that mini-epoch. The “any” muscle activity term is primarily distinguished from “phasic” muscle activity by the inclusion of tonic muscle activity contained within a 3-s mini-epoch.7,8,17 Bursts of phasic activity occurring simultaneously with tonic activity were required to have an amplitude of twice the background tonic EMG activity within the same 3-s mini-epoch to be scored separately as phasic activity.17 Any 3-s mini-epoch containing either a breathing-related event or an arousal was scored as “artifact” and excluded from analysis (this was achieved for individual events on a mini-epoch basis, so as to preserve as many interpretable mini-epochs for scoring as possible; Figure 6). Overall phasic and “any” percent muscle activity was calculated as the total number of positive 3-s mini-epochs divided by the total number of analyzable 3-s mini-epochs. For the overall combined analysis, a 3-s mini-epoch was considered positive if there was phasic or “any” EMG activity in either the SM or AT muscles and negative if there was an absence of EMG activity in both muscles.
Figure 6.
Example of 30-second epoch containing an arousal. Each 3-s mini-epoch containing an arousal is excluded from analysis by the “Artif” (artifact) designation. “User 1” marks phasic muscle burst.
We included PLM-like muscle activity during REM sleep in our RSWA analysis, reasoning that REM PLM-like muscle activity is another manifestation of disinhibited REM motor control associated with RBD rather than true NREM PLMs (supplemental material).
Scorers of RSWA were blinded to patient group and had high inter-rater reliability with a k coefficient of 0.897. Kappa coefficients were calculated for both SM and AT muscles, as well as both muscles combined from a sample of 100 mini-epochs of different REM epochs of different patients, according to previously published methods.8
The REM atonia index (RAI) was calculated using computer-automated analysis performed by HypnoLab sleep scoring software to calculate the amount of abnormal muscle tone seen in only the SM EMG.6 In order to analyze SM muscle EMG tone, a 60-Hz notch filter was applied and the signal was rectified. Any 30-s epoch containing an arousal or breathing event was visually identified and excluded before RAI analysis was performed. The RAI was scored on a scale of 0-1, with a score of zero indicating complete absence of muscle atonia and one indicating completely preserved muscle atonia. Values < 0.8 are strongly indicative of abnormal muscle activity, values < 0.9 indicate likely abnormal muscle activity, while an RAI of > 0.9 indicates preserved muscle atonia.36 In patients with PD, an RAI of < 0.9 has been found to be sensitive to identifying the presence of RBD.30
Cutoff values for RBD diagnosis using phasic muscle activity were also calculated using the AASM criteria for excessive transient muscle activity, defined as one 30-s epoch containing ≥ 5 3-s mini-epochs containing phasic muscle activity.34 This standard was applied to each 30-s epoch of REM sleep, excluding those with breathing or arousal artifacts to generate AASM phasic percent muscle activity for the SM and AT muscles individually and combined.
Combined muscle activity cutoffs were also established for combinations of the SM and AT muscles for phasic, “any,” and tonic muscle activity, by considering the individual positive mini-epochs in SM and AT alone, as well as mini-epochs that contained muscle activity simultaneously in both the SM and AT (i.e., when muscle activity was present in both muscles, it was counted only a single time, without “double counting”).
Split-Night PSG vs. Full-Night PSG Comparison
Patients who demonstrated AHI ≥ 5/h over ≥ 2 h of total sleep time, were given a split-night therapeutic trial of CPAP. In order to determine whether RSWA differed between patients undergoing split-night PSG and those undergoing full-night PSG, overall phasic, tonic, and “any” percent muscle activity, as well as muscle specific percent muscle activities and average phasic muscle burst durations were calculated for both halves of the split-night PSG and then combined and compared to those patients who underwent full-night PSG. In addition, phasic, tonic, and “any” percent muscle activities were compared before and after CPAP therapy in patients who underwent split-night PSG, to determine whether or not CPAP treatment impacts RSWA. Similar analyses were performed for phasic muscle burst durations and RAI.
Statistical Analysis
Clinical, demographic, and PSG data are presented as means, standard deviations, and frequencies. Quantitative variables were analyzed using nonparametric Kruskal-Wallis and Mann-Whitney tests, while χ2 tests were used to analyze categorical variables using JMP statistical software (JMP, Version 9, SAS Institute Inc., Cary, NC). Relationships between clinical independent variables and dependent tonic, phasic, and “any” muscle activity, RAI, and phasic muscle burst duration were analyzed utilizing multivariable linear or logistic regression. Pre- and post-CPAP therapy analyses were performed using matched-pair Wilcoxon signed-rank tests. Significance was set at an α level of P < 0.05 for demographic data and PSG variable statistical tests, except for the Mann-Whitney test analyzing differences in RSWA indices between groups, in which a post hoc Bonferroni correction was applied for multiple tests, setting α level at P < 0.01. Receiver operating characteristic (ROC) curves were calculated for combined phasic, tonic, and “any” percent muscle activities as well as phasic and “any” percent muscle activities for SM and AT muscles. In addition, ROC curves were calculated for phasic muscle burst durations in both muscles. Area under the curve was calculated for each analysis, and cutoff diagnostic threshold values were chosen that yielded the highest combined sensitivity and specificity distinguishing RBD from OSA controls.
RESULTS
Clinical and Demographic Data
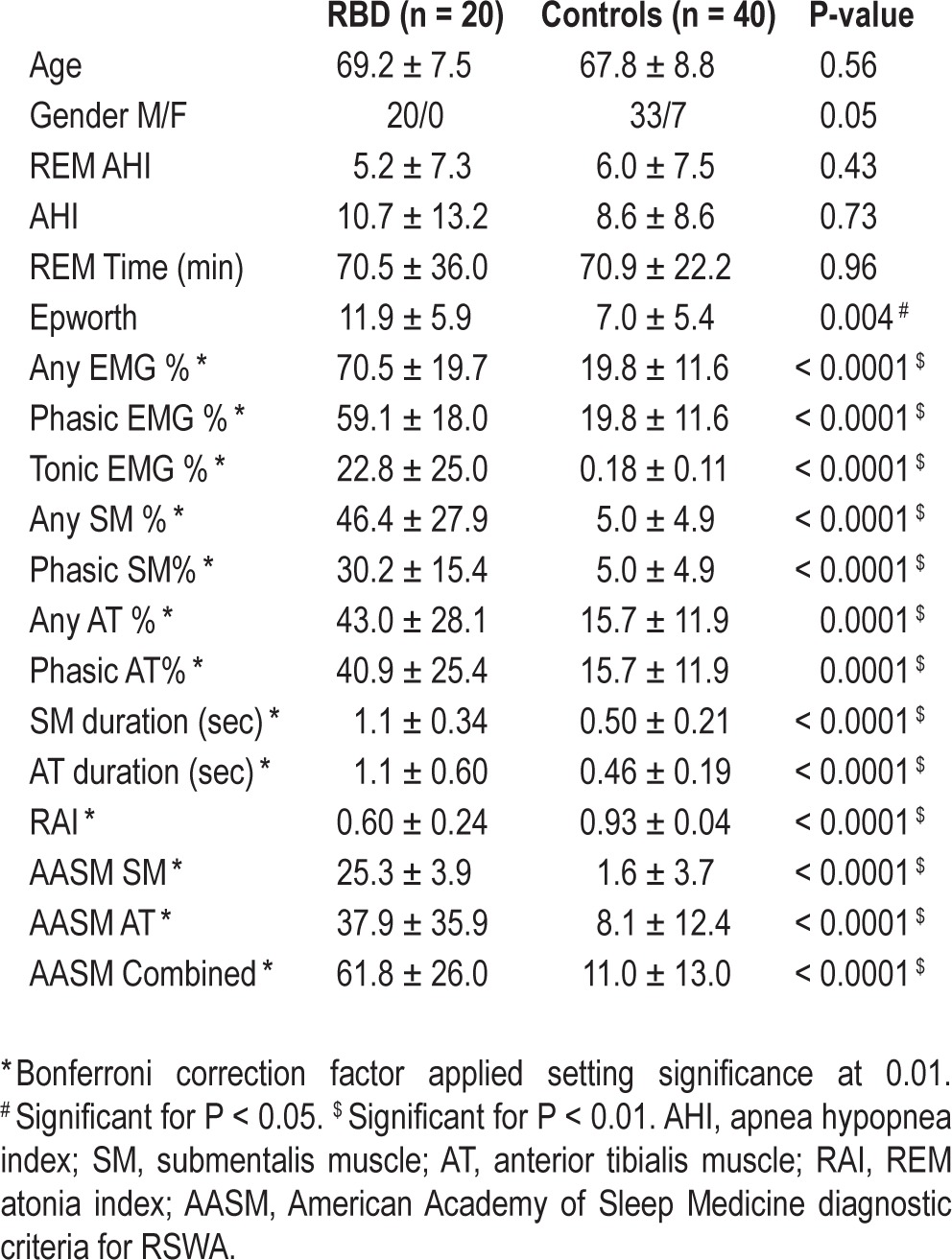
Of the 60 patients analyzed, 53 (88%) were male with a mean age of 68.2 ± 8.4 (range 48-82) years. There were no differences in age or gender between the 4 groups. PD-RBD patients had an average RBD symptom duration of 9.4 ± 13.2 (range 0.1-57) years and a mean PD symptom duration of 6.0 ± 5.0 (range 1-19) years. PD symptom duration was not associated with any measure of RSWA. Average levodopa dose equivalent was 829.3 ± 549.3 and was also unassociated with RSWA. Mean REM AHI for the entire cohort was 5.8 ± 7.4 (range 0-27) and did not differ between groups and was not associated with amount of RSWA. Baseline AHI did not differ between groups (Table 3). Sixteen control (40%) and 4 (20%) RBD subjects were on β-blocker therapy at the time of PSG; however, RSWA percent muscle activity, RAI, or phasic muscle burst duration did not differ between those using β-blockers and those who did not. Other clinical and demographic data did not differ between the 4 groups. A mean of 1285.4 ± 505.9 3-s mini-epochs were analyzed per patient.
Table 3.
RSWA comparison between RBD patients and controls.
RSWA Analysis
Split-Night PSG vs. Full-Night PSG
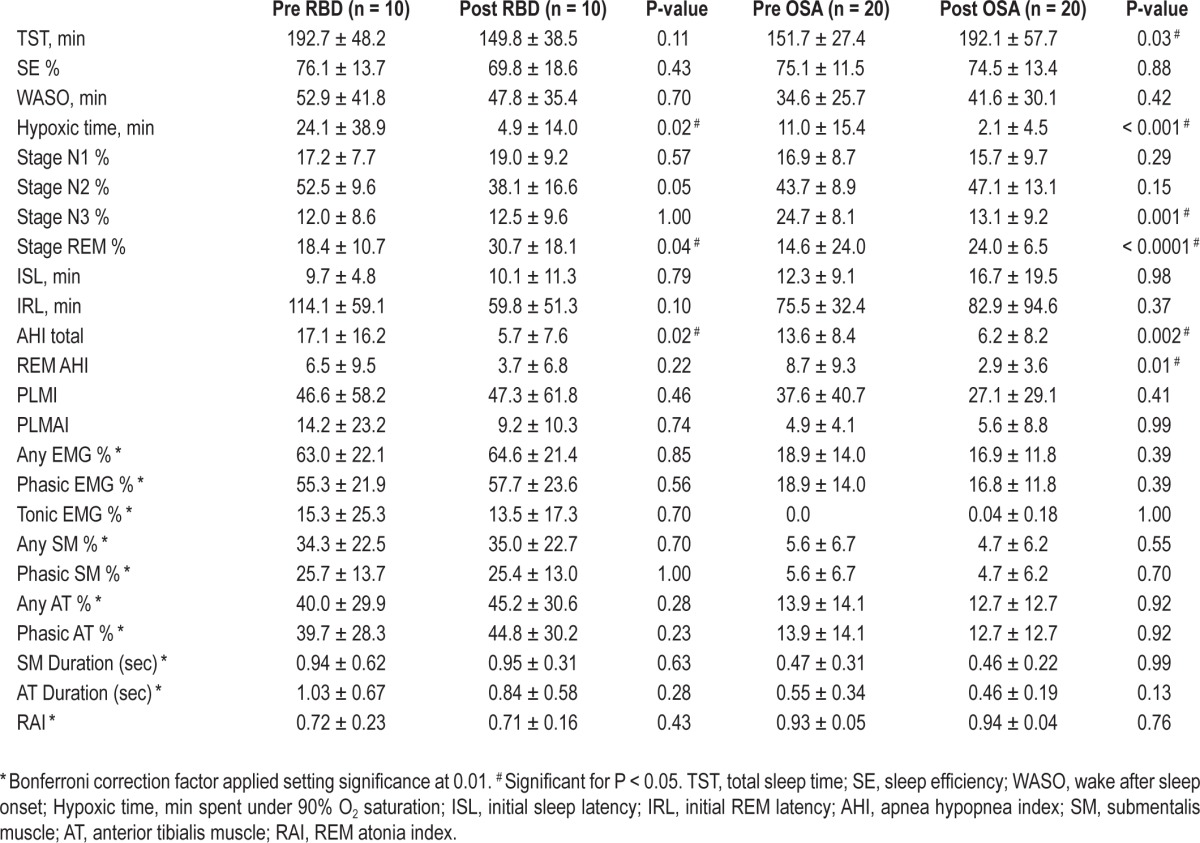
There were no significant differences in any RSWA measure between split-night compared with full-night PSG in either the RBD or OSA/PS control groups. Further results are shown in Table 1.
Table 1.
Pre- and post-CPAP therapy PSG variables for RBD and OSA groups.
Pre-CPAP vs. Post-CPAP Therapy
There did not appear to be an effect of CPAP therapy on any RSWA measure within either RBD or OSA control subjects undergoing split-night PSG (supplemental material and Table 2).
Table 2.
RSWA and clinical variables for split-night PSG and full-night PSG groups.
PD-RBD vs. Controls
Mean “any” muscle activity was 70.5 ± 19.7 for RBD patients compared to 19.8 ± 11.6 in controls (P < 0.0001; Table 3). Regression analyses demonstrated that only RBD group predicted RSWA as a dependent variable, adjusting for age, gender, and REM AHI (P < 0.0001). Phasic muscle activity was significantly higher in the RBD group (59.1 ± 18.0 vs. 19.8 ± 11.6, P < 0.0001). Controlling for gender, REM time, and REM AHI, only RBD remained associated with phasic muscle activity. In addition to group, older age was also associated with increased phasic muscle activity when groups were combined (P < 0.0001); however, this association was not significant within either the control or RBD groups when adjusting for gender, REM AHI, and REM time. Tonic muscle activity was significantly higher in the RBD group (22.8 ± 25.0 vs. 0.18 ± 0.11, P < 0.0001), and only RBD remained significantly associated with tonic muscle activity after controlling for age, gender, REM time, and REM AHI. RSWA values for the EDC muscle in RBD patients are shown in the supplemental material.
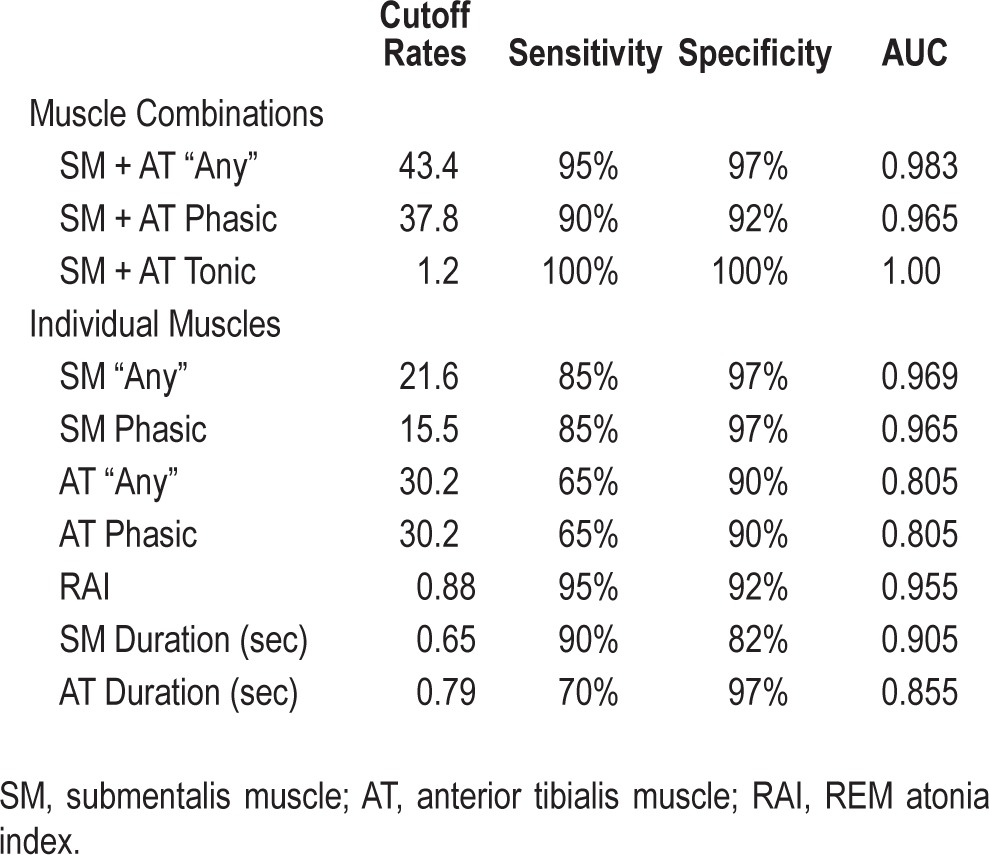
A cutoff value of 43.4% for combined SM and AT “any” percent muscle activity yielded 95% sensitivity and 97% specificity for RBD diagnosis (AUC 0.983), while a value of 37.9% for combined SM and AT phasic muscle activity yielded a sensitivity of 90% and specificity of 92% (AUC 0.965). A combined SM and AT tonic muscle activity of 1.2% was 100% sensitive and 100% specific for diagnosis of RBD (AUC 1.00; Table 4). SM tonic muscle activity was not different than the combined SM and AT tonic muscle activity.
Table 4.
Cutoff values for RBD diagnosis.
Both SM “any” muscle activity (46.4 ± 27.9 vs. 5.0 ± 4.9, P < 0.0001) and SM phasic muscle activity (30.2 ± 15.4 vs. 5.0 ± 4.9, P < 0.0001) were significantly higher in RBD patients, and both SM muscle activities remained significantly associated with RBD when adjusting for age, gender, REM time, and REM AHI. AT “any” muscle activity (43.0 ± 28.1 vs. 15.7 ± 11.9, P = 0.0001) and phasic muscle activity (40.9 ± 25.4 vs. 15.7 ± 11.9, P = 0.0001) were also higher in RBD patients. Increased AT “any” muscle activity (P < 0.0001) and phasic muscle activity (P < 0.0001) were associated with RBD as well as older age, after adjusting for gender, REM time, REM AHI, and PLMI. SM RAI was significantly lower in RBD patients compared to controls (0.60 ± 0.24 vs. 0.93 ± 0.04, P < 0.0001), and in regression analysis remained significantly associated with RBD when adjusting for age, gender, REM time, and REM AHI.
SM phasic muscle activity of 15.5% was 85% sensitive and 97% specific for RBD (AUC 0.965). A SM “any” percent muscle activity of 21.6% yielded 97% specificity and 85% sensitivity (AUC 0.969). SM automated RAI of 0.88 was 95% sensitive and 92% specific (AUC 0.955) for RBD. A cutoff of 30.2% in the AT yielded a sensitivity of 65% and specificity of 90% (AUC 0.805).
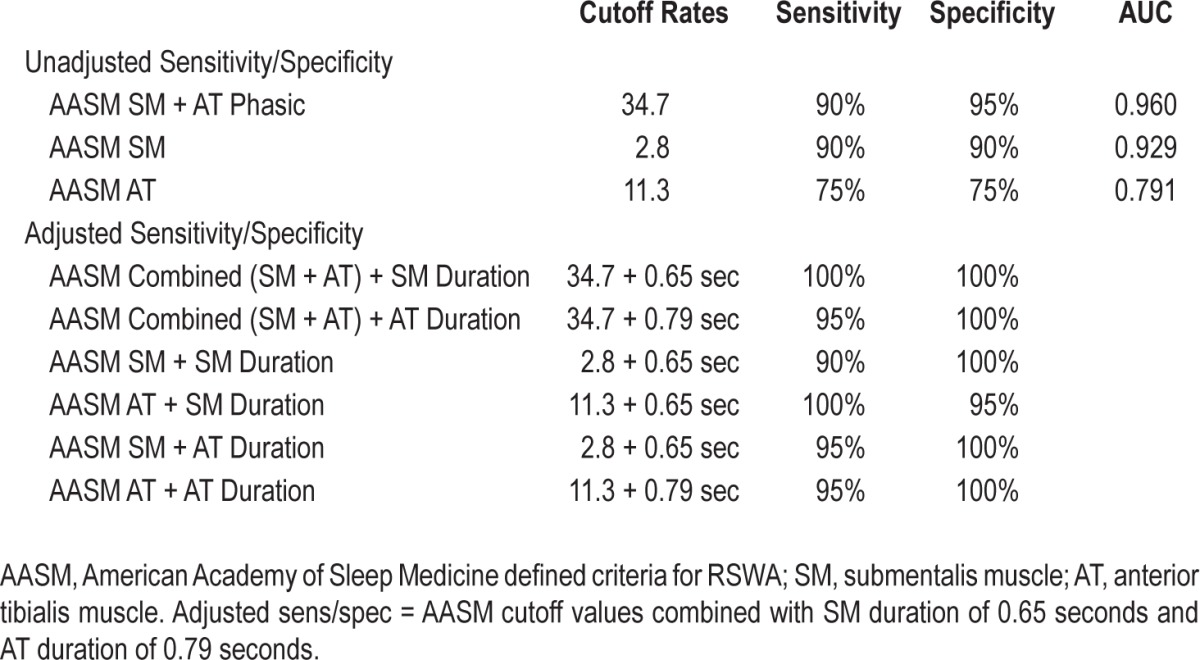
Using the AASM diagnostic standards for a diagnosis of RSWA resulted in a combined SM and AT phasic muscle activity cutoff of 34.7% with a sensitivity of 90% and specificity of 95% (AUC 0.960). A cutoff of 2.8% for AASM-defined SM phasic muscle activity was 90% sensitive and 90% specific for RBD diagnosis (AUC 0.929). An AASM AT percent muscle activity of 11.3% was 75% sensitive and 75% specific (AUC 0.791) for RBD diagnosis (Table 6).
Table 6.
American Academy of Sleep Medicine cutoff values for diagnosis of RSWA.
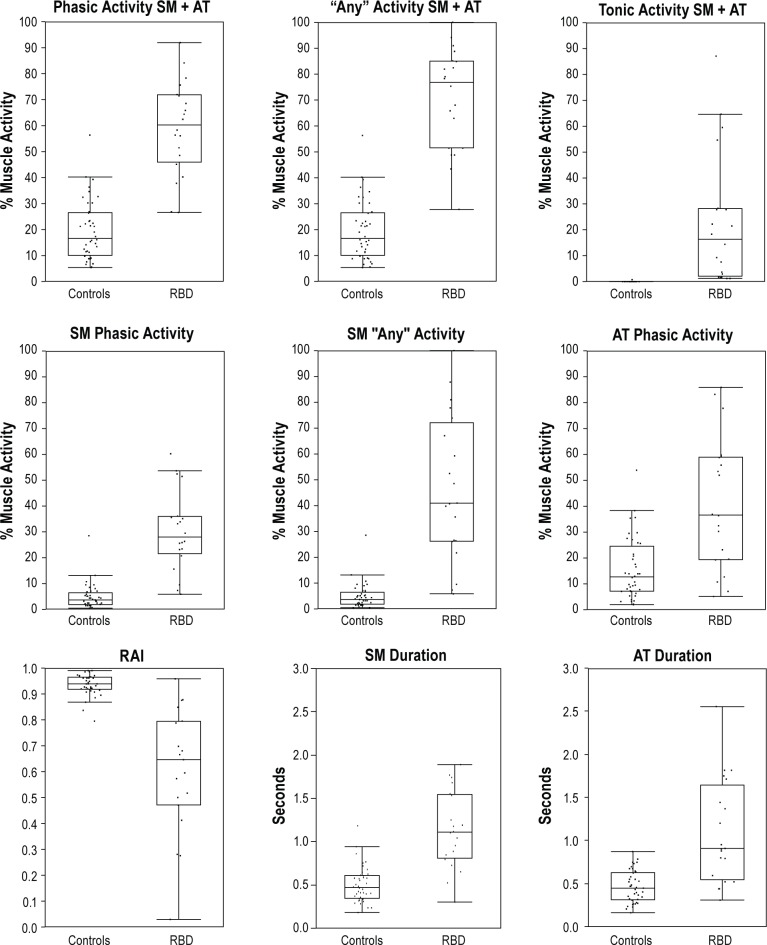
SM phasic muscle burst duration was significantly longer in RBD patients than controls (1.1 ± 0.34 vs. 0.50 ± 0.21, P < 0.0001). Longer SM phasic burst duration remained significantly associated with RBD when adjusting for age, gender, REM time, REM AHI, and β-blocker use. AT muscle burst duration was also significantly longer in PD-RBD patients (1.1 ± 0.60 vs. 0.46 ± 0.19, P < 0.0001), and longer phasic AT burst duration remained associated with RBD group after controlling for age, gender, REM time, and β-blocker use. SM and AT phasic muscle burst durations were not associated with age, levodopa drug use, LDE, or β-blocker use. Longer SM duration, (P = 0.03) as well as a decreased RAI (P = 0.04) and increased SM “any” muscle activity (P = 0.03) were each associated with longer duration of RBD symptoms in univariate analysis. However, these associations were not seen in multivariate analysis. Sensitivity was 90% with a specificity of 82% for an SM phasic muscle burst duration of 0.65 seconds (AUC 0.905), and 70% sensitive and 97% specific for AT phasic muscle burst duration of 0.79 seconds (AUC 0.855) for diagnosis of RBD. Boxplot representations of phasic, “any,” and tonic muscle activities as well as phasic muscle burst durations and RAI are shown in Figure 7 (3-s mini-epochs). Phasic muscle activity for 30-s (AASM) mini-epoch scoring is shown in Figure 8.
Figure 7.
Boxplot representations of phasic, “any,” and tonic percent muscle activity for combined montage as well as individual muscles in addition to RAI and phasic muscle burst durations for submentalis and anterior tibialis muscles. SM, submentalis muscle; AT, anterior tibialis muscle; RAI, REM atonia index.
Figure 8.
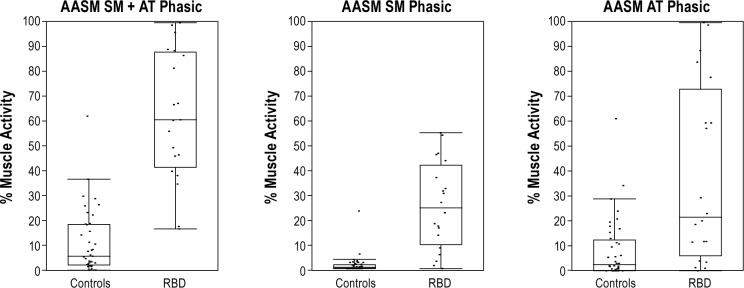
AASM-defined phasic muscle activities for combined, submentalis, and anterior tibialis muscles. AASM, American Academy of Sleep Medicine defined RSWA; SM, submentalis muscle; AT, anterior tibialis muscle.
To determine whether consideration of phasic muscle burst duration had any additive value in discriminating RBD diagnostic sensitivity and specificity, additional separate analyses were performed for patients meeting phasic and “any” percent muscle activity cutoffs for RBD diagnosis for combined montages, as well as individual muscles, based on whether they also exceeded cutoff values for phasic muscle burst duration in both the SM (0.65 s) and AT (0.79 s). We conducted these reclassification analyses for both the 3-s mini-epoch and 30-s (AASM positive) epoch scorings. For the 3-s mini-epoch reclassification analysis, 2 clinically diagnosed PD-RBD patients who did not meet standard RSWA percent muscle activity cutoffs, but who did meet RSWA burst duration cutoffs, were reclassified as having RSWA. Also, 2 control OSA subjects who did not have clinical dream enactment who met standard RSWA percent muscle activity cutoffs, but who did not meet RSWA burst duration cutoffs, were reclassified as not having RSWA. Similar reclassifications were performed for the 30-s (AASM positive) analyses, both for the combined SM/AT, SM, and AT muscles (as described in the supplemental material). With additional consideration of phasic muscle burst duration cutoff values together with phasic “any” and AASM muscle activity cutoff values, sensitivity, and specificity were greatly improved, both overall and in each individual muscle (Tables 5 and 6).
Table 5.
Adjusted sensitivity and specificity combining phasic and “any” percent muscle activity with phasic muscle duration.
DISCUSSION
Our method of RSWA analysis demonstrated similar cutoff values for phasic, tonic, and “any” muscle percent muscle activities as well as RAI cutoffs to those of previously published and validated methods,17,30 and demonstrates that similar techniques can be validly employed in clinical sleep laboratory populations, including patients with OSA, an extremely frequent comorbidity in RBD patients.28,29,37 A new finding in our study was that direct measurement of phasic muscle burst duration improves the diagnostic distinction of symptomatic RBD with comorbid OSA from OSA patients without dream enactment.
Our study highlights several important aspects of RSWA in a naturalistic clinic-based sample. First, average phasic muscle burst duration in both the SM and AT muscles is significantly longer in RBD patients than controls, a novel finding that could enhance discrimination of RBD from control patients. This may be especially useful in determining whether patients with “incidental RSWA” on PSG are at risk for future development of RBD and/or have an underlying synucleinopathy, possibly providing an even earlier time point for identifying at-risk patients allowing for the development and implementation of neuroprotective therapies. However, prospective studies with this method are needed to confirm this hypothesis. The significantly longer phasic burst durations seen in RBD patients compared to controls also suggests that further basic research involving animal RBD/RSWA models is necessary to refine understanding of which brainstem nuclei and neurotransmitter receptor mechanisms facilitate altered phasic muscle activity in RBD. REM atonia loss with resultant longer REM phasic burst duration appears to be particularly dependent on loss of medullary nucleus gigantocellularis metabotropic GABAB and ionotropic GABAA and glycinergic neurotransmission, but may also depend on loss of descending glutamatergic output from the subcoeruleus/sub-lateral dorsal nucleus or, alternatively, increased corticospinal glutamatergic excitatory output.38–40 Better understanding of mechanisms governing phasic motor control during REM could inform rationale for pharmacotherapeutic strategies and future neurostimulation approaches in RBD. Second, nasal CPAP therapy does not significantly impact RSWA, and therefore RSWA metrics of patients receiving split-night PSG with separate diagnostic and treatment phases can be combined into single “full-night” values, as these combined indices do not significantly differ from patients undergoing full-night diagnostic or treatment PSGs. Third, automated RAI analysis of SM muscle tone is a quick and efficient method with comparably high sensitivity and specificity to manual visual scoring methods for diagnosis of RBD. RAI may be particularly useful as a quick quantitative screening method for presence of RSWA in the clinical setting.
Our sample is similar to previously studied cohorts in terms of age and RBD duration.17,30 When compared with controls, RBD patients were significantly sleepier, an unsurprising result as excessive daytime sleepiness is associated with PD and often reported in RBD patients.29,41 In order to address the possible confounding factor of sleep disordered breathing causing increased RSWA, our controls were REM AHI matched to our RBD patients, and we selected patients having a REM AHI < 30/ hour to maximize analyzable REM sleep time and constrain rejection of mini-epochs involving disordered breathing events. Previous studies have reported decreased OSA in RBD patients; however, baseline total sleep AHI was no different between our combined groups.28 It is not clear whether this may be due to random variability between subjects or, more likely, because matching REM AHI resulted in more closely matched total sleep AHI or that including only mild or moderate REM-related OSA patients may have limited the severity of REM-related OSA in both groups. Since testing relationships between RBD and OSA severity was not a research hypothesis of this current work, we plan to conduct further studies of the relationship between OSA severity and RBD in idiopathic and symptomatic RBD patient populations.
Our method of calculating phasic, tonic, and “any” muscle activity resulted in similar sensitivity and specificity cutoff values for diagnosis of RBD in PD to previously published studies.17,30 For a combination of SM and AT muscles, an “any” percent muscle activity cutoff of 46.4% and phasic muscle activity cutoff of 44.2% was previously reported.17 Our cutoff values were 43.4% and 37.9% for combined “any” and phasic muscle activity, respectively. SM “any” muscle activity of 18.2%, phasic muscle activity of 16.3%, and tonic muscle activity of 9.6% have been reported,17 whereas our cutoffs for the SM were 21.6%, 15.5%, and 1.2% for “any,” phasic, and tonic percent muscle activity. We found very little tonic activity in AT, so differences in our tonic activity cutoffs and previously published values most likely reflect variability of RSWA between individuals. Phasic percent muscle activity in the left and right AT were reported as 22.4% and 24.8%, with a bilateral AT cut-off of 30.6% providing 100% specificity,17 comparatively close to our cutoff value using linked left and right AT muscles of 30.2%, which provided 90% specificity. Finally, a SM RAI of < 0.9 was reported to be 94% sensitive and 91% specific for diagnosis of RBD in PD patients, a finding that was corroborated in our study, which showed that an RAI of < 0.88 was 95% sensitive and 92% specific for RBD diagnosis in PD patients.
Interestingly, a cutoff value of 34.7% using AASM diagnostic standards for combined SM and AT phasic muscle activity was similar to both our own and previously reported 3-second mini-epoch scoring cutoff values.25 However, an AASM SM cutoff of 2.8% and an AT cutoff of 11.3% differed greatly from both our own and previously published 3-second mini-epoch scoring method cutoff values. In addition, our cutoff of AASM combined SM + AT (34.4% vs. 45.5%) and SM alone (2.8% vs. 14.5%) was quite different from previously published values using this method.17 These differences could potentially be explained by the more stringent requirement for AASM 30-second epoch scoring which must contain at least five 3-second mini-epochs having phasic muscle bursts for positive RSWA scoring, likely a less direct and less sensitive RSWA measurement leading to wider variation in RSWA percentage in contrast to the mini-epoch approach, which counts abnormal mini-epochs regardless of the overall macro-epoch scoring in which they occur. However, AASM SM cutoffs yielded 90% sensitivity and 80% specificity, and combined SM and AT cutoffs yielded 90% sensitivity and 85% specificity, supporting the use of this relatively expeditious method of RSWA determination in a clinical setting, since a reader could simply count the number of 30-second epochs containing five or more bursts of excessive phasic REM that are present either in SM, AT, or both, divided by the total REM epochs, to arrive at a percentage that can be easily and quickly compared to our overall combined AASM RSWA cutoff value of 34.7%.
Our diagnostic threshold cutoff values for RBD are similar to previously published figures in patients without OSA.17,30 Factors such as intra-individual night-to-night variability, geographical, and ethnic differences could account for some of the differences between our cutoff values and those published in previous studies.5,42 Our study also shows that both manual and automated methods of RSWA scoring the same series of subjects are valid and reliable for diagnosing RBD. In addition, this is one of the first studies demonstrating similar RSWA metrics in RBD patients with and without comorbid OSA, a common comorbidity in RBD. Our scoring approach using a linked-legs montage, a commonly used montage in clinical sleep laboratories, also suggests that our method could be useful for RBD diagnosis in clinical settings.
A strength of our study is the introduction of direct measurement of phasic muscle burst duration, which appears to improve diagnostic sensitivity and specificity for distinction of RBD from OSA subjects. RBD patients had longer SM and AT phasic muscle burst duration than controls with sleep apnea or primary snoring in our series, and combining phasic or “any” muscle activity cutoffs with cutoffs for SM and AT phasic muscle burst duration substantially increased sensitivity and specificity of RBD diagnosis. Future studies of phasic muscle burst duration are planned to determine whether this technique could aid diagnosis of RBD in patients who do not otherwise exceed usual phasic percent muscle activity cutoff values, and to explore whether more expeditious manual or automated analysis using direct phasic muscle burst duration instead of mini-epoch based techniques could be useful in RBD diagnosis. Additionally, prospective studies will be necessary to determine whether phasic muscle burst duration measurement could be useful for diagnosing incipient or preclinical RBD in patients with “incidental” RSWA without a history of dream enactment, potentially allowing for earlier diagnosis of RBD patients to enable initiation of treatment that could minimize injury, and application of future neuroprotective therapies for synucleinopathy neurodegeneration.29,43
Interestingly, older age was associated with higher RSWA muscle activity in AT in both the RBD and control groups, but not in SM. Previous studies have reported increased RSWA with older age in individuals without RBD; however, these studies only analyzed SM EMG tone.44–46 Phasic muscle activity in the AT was less sensitive and specific for diagnosis of RBD than SM phasic muscle activity. Additionally, phasic muscle burst duration in the AT was less sensitive and specific than muscle burst duration in the SM for RBD diagnosis. Neuronal dysfunction in the brainstem networks responsible for maintaining REM sleep atonia could be part of the normal aging process, with relative increases in AT RSWA measures being physiological, whereas SM RSWA could be a more specific indicator of evolving RBD and underlying synucleinopathy. Idiopathic RBD patients having increased tonic SM activity are at a greater risk of developing PD, lending support to the hypothesis that localized SM RSWA may be a more sensitive and specific marker for RBD.13 Further prospective studies are necessary to determine whether differences in the temporal evolution and distribution of RSWA may aid distinction of patients with synucleinopathy from those with normal aging.
This study has several limitations. As a retrospective analysis, we were unable to conduct split-night and full-night PSGs on the same patients, which would have been a preferable method for demonstrating stability and comparable RSWA metrics between split-night and full-night PSGs. However, even within the same subjects, night-to-night variability in the amount of recorded RSWA and REM sleep occurs.5,42 In addition, differences in RSWA metrics between split-night and full-night PSGs in RBD subjects were not significantly different, although trends toward differences in SM RSWA metrics were most likely due to inter-subject variability, since RSWA may increase with increasing age and RBD duration.11,35 Our full-night PSG subjects had longer RBD disease duration, and several SM RSWA metrics were associated with longer duration of RBD symptoms in univariate analysis. Therefore, differences between split-night and full-night RBD subjects were most likely due to differences in RBD characteristics, not PSG type. Twelve control subjects had periodic limb movements of sleep of unclear significance, although these did not appear to be a significant confounding factor since PLMs were not associated with any measure of RSWA in controls or RBD patients. The overall similarity between our RSWA cutoffs and previously published values supports our inclusion of PLM-like muscle activity in our RSWA analysis as valid and reasonable. We believe our practice of scoring REM PLM-like muscle activity as RSWA is an advantageous and more time-efficient approach given the practical and logistical difficulties in distinguishing possible true PLMs during REM (supplemental material). We plan to conduct future studies analyzing NREM PLMs and PLM-like movements during REM in RBD and control subjects, similar to a previous study.9 In addition, four PD-RBD patients and three control patients had RLS; however, RLS was not associated with increased RSWA. Another possible limitation of our study is the use of a bipolar linked AT montage, which could have increased our AT cutoff values by sampling muscle activity from both legs simultaneously, causing combined AT RSWA values to be higher than individually sampled AT muscles as was noted by the SINBAR group.17 Our EMG filter settings were different than previously published studies (10-70 Hz vs. 10-100 Hz)17 which could have influenced RSWA quantification. We used 10-70 Hz filter settings, the convention in our laboratory established during earlier analog PSG recordings. However, since our cutoff values were similar to those of previously published values, filter settings did not likely have a large influence on RSWA in our study.17
The majority of the patients in our study were men, which primarily reflects the demographics of patients with RBD1,19,20,22,29; however, this may have influenced our results, especially our control patients, as men with sleep disorders are more likely to manifest fragmentary myoclonus.47 Fragmentary myoclonus occurs in the limbs and is defined as muscle bursts lasting less than 150 ms47; however, mean duration of AT muscle bursts was 460 ms in controls, indicating that fragmentary myoclonus was not a significant contributor toward RSWA in our patients. Upper extremity EMG activity has been reported to increase sensitivity and specificity for RBD diagnosis by the SINBAR group.8,17 Unfortunately, we lacked controls with EDC EMG recordings for analysis of diagnostic EDC cutoffs. While β-blocker use has been reported to cause RBD, and several of our patients were on β-blocker therapy during polysomnography, RSWA indices and phasic muscle burst durations were no different between those receiving β-blockers and those who were not.48 Another limitation is that we deliberately selected patients having a REM AHI < 30/hour, since higher REM AHI values would very likely result in a large amount of artifact rejection, potentially compromising valid interpretation of RSWA. We therefore selected patients having only a mild or moderately severe REM AHI (i.e., REM AHI < 30/hour), which may limit generalizability of our results to RBD patients with comorbid OSA and severe REM-related OSA with REM AHI > 30/hour. Finally, since our sample only analyzed PD-RBD patients, our results may not apply to idiopathic RBD patients, who may display more variability in RSWA indices. We plan to conduct further analyses comparing the effects of OSA in idiopathic RBD patients.
CONCLUSIONS
Our method for direct measurement of phasic muscle burst duration and percent muscle activity measurements for quantitative RSWA analysis enables accurate RBD diagnosis in clinical sleep practice populations, including patients with comorbid OSA. Our data suggest that nasal CPAP treatment of sleep disordered breathing does not affect RSWA and that reduction in muscle tone does not occur in most patients with RBD when exposed to CPAP. We further validated the convenient and automated RAI as a rapid and reliable method for distinguishing between patients with and without RBD. Future studies of the phasic muscle burst duration method, alone and in combination with conventional mini-epoch based scoring methods, are planned to determine the value of this technique in distinction of idiopathic and symptomatic RBD patients from patients without dream enactment and to determine whether this technique may allow identification of patients who may have an underlying synucleinopathy.
DISCLOSURE STATEMENT
This was not an industry supported study. The project described was supported by a Mayo Clinic Alzheimer's Disease Research Center Grant Award from the National Institute on Aging (P50 AG016574), and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1 UL1 RR024150-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. St. Louis has received research support from the Mayo Clinic Center for Translational Science Activities (CTSA), supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1 UL1 RR024150-01. Dr. Boeve has been an investigator in clinical trials sponsored by Cephalon, Inc., Allon Pharmaceuticals, and GE Healthcare; has received royalties from the publication of a book entitled Behavioral Neurology of Dementia (Cambridge Medicine, 2009); and has received honoraria from the American Academy of Neurology. He receives research support from the National Institute on Aging [P50 AG16574 (Co-Investigator), U01 AG06786 (Co-Investigator), RO1 AG32306 (Co-Investigator)] and the Mangurian Foundation. The other authors have indicated no financial conflicts of interest. There was no off-label medication use.
ACKNOWLEDGMENT
The authors gratefully acknowledge Ms. Lori Lynn Reinstrom in the Mayo Clinic Department of Neurology for secretarial assistance with manuscript preparation and submission.
SUPPLEMENTAL MATERIAL
Reclassification Procedure and Impact on Analysis for the 30-Second (AASM Positive) Epoch Scorings
To determine whether consideration of phasic muscle burst duration had any additive value in discriminating RBD diagnostic sensitivity and specificity, a separate analysis was performed for patients meeting phasic and “any” percent muscle activity cutoffs for RBD diagnosis for combined montages, as well as individual muscles, based on whether they also exceeded cutoff values for phasic muscle burst duration in both the SM (0.65 seconds) and AT (0.79 seconds). We conducted these reclassification analyses for both the 3-s and 30-s (AASM positive) epoch scorings.
For the 30-s (AASM positive) epoch reclassification analysis of the combined SM/AT muscles, 2 clinically diagnosed PD-RBD patients who did not meet standard AASM defined cutoffs, but who did meet RSWA burst duration cutoff for the SM muscle, were reclassified as having RSWA. For the AASM combined SM/AT muscles, 2 control patients who met AASM defined cutoffs, but who did not meet SM muscle burst duration cutoffs, were reclassified as not having RSWA, yielding 100% sensitivity and 100% specificity for the combined SM/AT muscles with SM burst duration reclassification.
For the 30-s (AASM positive) epoch reclassification analysis of the combined SM/AT muscles, 1 of 2 clinically diagnosed PD-RBD patients who did not meet standard AASM defined cutoffs, but who did meet RSWA burst duration cutoff for the AT muscle, were reclassified as having RSWA. For the AASM combined SM/AT muscles, 2 clinically control patients who met AASM defined cutoffs, but who did not meet SM muscle burst duration cutoffs, were reclassified as not having RSWA, yielding 95% sensitivity and 100% specificity for the combined SM/AT muscles with AT burst duration reclassification.
For the 30-s (AASM positive) epoch reclassification analysis of the individual SM muscle, neither of 2 clinically diagnosed PD-RBD patients who did not meet standard AASM defined cutoffs, also did not meet RSWA burst duration cutoff for the SM muscle. For the AASM individual SM muscle, 1 of 2 control patients who met AASM defined cutoffs, but who did not meet SM muscle burst duration cutoffs, were reclassified as not having RSWA, yielding 90% sensitivity and 100% specificity for the individual SM muscle with SM burst duration reclassification. Reclassification analysis of the individual SM muscle with AT duration yielded 95% sensitivity and 100% specificity for RSWA diagnosis.
For the 30-s (AASM positive) epoch reclassification analysis of the individual AT muscle, 5 clinically diagnosed PD-RBD patients who did not meet standard AASM defined cutoffs, but who did meet RSWA burst duration cutoff for the SM muscle, were reclassified as having RSWA. For the AASM individual AT muscle, 9 of 11 control patients who met AASM defined cutoffs, but who did not meet AT muscle burst duration cutoffs, were reclassified as not having RSWA, yielding 100% sensitivity and 95% specificity for the individual AT muscle with SM burst duration reclassification. Four of 5 clinically diagnosed PD-RBD patients who did not meet standard AT AASM defined cutoffs, but who did meet RSWA burst duration cutoff for the AT muscle, were reclassified as having RSWA. Eleven control patients who met AASM defined cutoffs, but who did not meet AT muscle burst duration cutoffs, were reclassified as not having RSWA, yielding 95% sensitivity and 100% specificity for the individual AT muscle with AT burst duration reclassification.
With additional consideration of phasic muscle burst duration cutoff values together with phasic “any” and AASM percent muscle activities cutoff values, sensitivity and specificity were greatly improved, both overall and in each individual muscle (Table 6).
Rationale for Inclusion of PLM-like Activity
We included PLM-like muscle activity during REM sleep in our RSWA analysis, reasoning that REM PLM-like muscle activity is another manifestation of disinhibited REM motor control associated with RBD rather than true NREM PLMs per se, given that (1) during video review, REM PLM-like muscle activity most often resembles variable, rapid, distal limb phasic muscle jerks or twitches dissimilar to true NREM PLMs, which instead involve highly stereotyped slow toe extension and foot/ ankle dorsiflexion movements, often with associated proximal triple flexion movements of the hip and leg1–4; (2) REM PLM-like activity characteristics differ from true NREM PLMs by being less periodic and irregular5–7 and shorter in duration,5–9 in contrast to the more strictly periodic, rhythmic, and longer duration true PLMs3,6,8,9; (3) most neurologically normal individuals without RBD, incidental RSWA, narcolepsy, or restless legs syndrome (RLS) diagnoses suppress spinal alpha motor neuron pools during REM,2,9 thereby manifesting PLMs predominantly during NREM sleep,1,2,8 causing PLMs to either be absent or substantially less frequent during REM1–3,5–9; furthermore PLMs typically correspond to a preceding autonomic activation3,4 and the NREM sleep microarchitectural feature of cyclic alternating pattern (CAP) sleep2,9–11 which is absent during REM11,12; and (4) true PLMs most often have a characteristic circadian pattern of occurrence during the first half of the night, opposite to the prominent activation of phasic muscle activity in the second half of the night during high density REM sleep.2,3,8
RESULTS
Pre-CPAP vs. Post-CPAP Therapy
OSA control subjects had significantly increased total sleep time (TST) (P = 0.01), REM sleep percentage (P = 0.0001), and decreased hypoxic time (minutes spent < 90% O2 saturation) (P = 0.001), stage 3 sleep (P = 0.0002), and AHI (P = 0.0005) during administration of CPAP therapy. CPAP therapy significantly decreased hypoxic time (P = 0.04), initial REM latency (IRL) (P = 0.02), and AHI (P = 0.02) in RBD patients. Further comparisons of PSG and RSWA variables are shown in Table 2.
EDC Percent Muscle Activity
EDC phasic muscle activity for PD-RBD patients was 26.0 ± 12.8, while EDC “any” muscle activity for PD-RBD patient was 27.5 ± 16.4. EDC phasic burst duration was 1.3 ± 0.40 seconds. Tonic muscle activity was minimal in EDC.
DISCUSSION
Our data do not suggest there is a significant influence of CPAP on quantitative RSWA metrics in RBD patients with comorbid OSA or OSA patients without dream enactment (Table 1). Severe untreated OSA has been reported to mimic symptoms of RBD with subsequent treatment of OSA resulting in a decrease in RBD-like behaviors; however, none of these patients had RSWA on PSG, and RSWA indices comparing the pre-treatment and treatment PSGs were not reported.13 Another study demonstrated a similar RAI in patients with OSA compared to age-matched controls; however, subject numbers were very small.14 In our cohort, neither controls nor RBD subjects demonstrated a change in RSWA indices or phasic muscle burst duration when using nasal CPAP therapy. A previous study also reported that nasal CPAP therapy eliminated OSA but not DEB in a RBD patient, suggesting that treating sleep-disordered breathing itself may not directly impact the occurrence of RBD.15 Some patients report improvement in RBD symptoms when receiving nasal CPAP therapy.16 However, no direct polysomnographic evidence for improvement of RBD or decrease in RSWA has yet been demonstrated in these reports. Further study of the relationships between comorbid OSA in RBD and RSWA patient samples is necessary to establish the effect of nasal CPAP on RBD and RSWA.
While our study could be criticized for including REM PLM-like muscle activity in our RSWA analysis, we reasoned that REM PLM-like muscle activity was a manifestation of REM motor dyscontrol associated with RBD since REM PLM-like muscle activity is consistent with phasic leg muscle jerking during video review, usually appearing aperiodic,5–7 irregular, and shorter in duration3,5–7,9 in contrast to typically monomorphic, rhythmic, and relatively slow true PLMs; and PLMs are absent or much less frequent during REM in most normal individuals without RBD, RLS, or narcolepsy.1,3,5–7,9 We found that PLMs lacked association with any of our analyzed RSWA measures and excluded movement artifacts related to breathing and spontaneous arousals that can mimic or cause PLMs17–20 or RSWA.
REFERENCES
- 1.Fantini ML, Michaud M, Gosselin N, Lavigne G, Montplaisir J. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology. 2002;59:1889–94. doi: 10.1212/01.wnl.0000038348.94399.f6. [DOI] [PubMed] [Google Scholar]
- 2.Hening W. The clinical neurophysiology of the restless legs syndrome and periodic limb movements. Part I: diagnosis, assessment, and characterization. Clin Neurophysiol. 2004;115:1965–74. doi: 10.1016/j.clinph.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 3.Sforza E, Jouny C, Ibanez V. Time course of arousal response during periodic leg movements in patients with periodic leg movements and restless legs syndrome. Clin Neurophysiol. 2003;114:1116–24. doi: 10.1016/s1388-2457(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 4.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 5.Ferri R, Gschliesser V, Frauscher B, Poewe W, Hogl B. Periodic leg movements during sleep and periodic limb movement disorder in patients presenting with unexplained insomnia. Clin Neurophysiol. 2009;120:257–63. doi: 10.1016/j.clinph.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Manconi M, Ferri R, Zucconi M, Fantini ML, Plazzi G, Ferini-Strambi L. Time structure analysis of leg movements during sleep in REM sleep behavior disorder. Sleep. 2007;30:1779–85. doi: 10.1093/sleep/30.12.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollmacher T, Schulz H. Periodic leg movements (PLM): their relationship to sleep stages. Sleep. 1993;16:572–7. [PubMed] [Google Scholar]
- 8.Boehm G, Wetter TC, Trenkwalder C. Periodic leg movements in RLS patients as compared to controls: Are there differences beyond the PLM index? Sleep Med. 2009;10:566–71. doi: 10.1016/j.sleep.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Nicolas A, Michaud M, Lavigne G, Montplaisir J. The influence of sex, age and sleep/wake state on characteristics of periodic leg movements in restless legs syndrome patients. Clin Neurophysiol. 1999;110:1168–74. doi: 10.1016/s1388-2457(99)00033-4. [DOI] [PubMed] [Google Scholar]
- 10.Parrino L, Boselli M, Buccino GP, Spaggiari MC, Di Giovanni G, Terzano MG. The cyclic alternating pattern plays a gate-control on periodic limb movements during non-rapid eye movement sleep. J Clin Neurophysiol. 1996;13:314–23. doi: 10.1097/00004691-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Parrino L, Ferri R, Bruni O, Terzano MG. Cyclic alternating pattern (CAP): the marker of sleep instability. Sleep Med Rev. 2012;16:27–45. doi: 10.1016/j.smrv.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Parrino L, Halasz P, Tassinari CA, Terzano MG. CAP, epilepsy and motor events during sleep: the unifying role of arousal. Sleep Med Rev. 2006;10:267–85. doi: 10.1016/j.smrv.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–6. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 14.Ferri R, Rundo F, Manconi M, et al. Improved computation of the atonia index in normal controls and patients with REM sleep behavior disorder. Sleep Med. 2010;11:947–9. doi: 10.1016/j.sleep.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Chiu HFK, Wing YK, Lam LCW, et al. Sleep-related injury in the elderly—an epidemiological study in Hong Kong. Sleep. 2000;23:513–7. [PubMed] [Google Scholar]
- 16.Mery V, Lafontaine A, Robinson A, Pinto L, Benedetti A, Kimoff R, Kaminska M. Improvement of REM sleep behaviour disorder symptoms with treatment of obstructive sleep apnea in patients with Parkinson's disease. Sleep. 2013;36:A234. (Abstract Suppl) [Google Scholar]
- 17.Baran AS, Richert AC, Douglass AB, May W, Ansarin K. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep. 2003;26:717–20. doi: 10.1093/sleep/26.6.717. [DOI] [PubMed] [Google Scholar]
- 18.Exar EN, Collop NA. The association of upper airway resistance with periodic limb movements. Sleep. 2001;24:188–92. doi: 10.1093/sleep/24.2.188. [DOI] [PubMed] [Google Scholar]
- 19.Warnes H, Dinner DS, Kotagal P, Burgess RC. Periodic limb movements and sleep apnoea. J Sleep Res. 1993;2:38–44. doi: 10.1111/j.1365-2869.1993.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamashiro Y, Kryger MH. Acute effect of nasal CPAP on periodic limb movements associated with breathing disorders during sleep. Sleep. 1994;17:172–5. doi: 10.1093/sleep/17.2.172. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Boot BP, Boeve BF, Roberts RO, et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol. 2012;71:49–56. doi: 10.1002/ana.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders, 2nd ed: diagnostic and coding manual. [Google Scholar]
- 3.Burns JW CF, Little RJ, Angell KJ, Gilman S, Chervin RD. EMG variance during polysomnography as an assessment for REM sleep behavior disorder. Sleep. 2007;30:1771–8. doi: 10.1093/sleep/30.12.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consens FB, Chervin RD, Koeppe RA, et al. Validation of a polysomnographic score for REM sleep behavior disorder. Sleep. 2005;28:993–7. doi: 10.1093/sleep/28.8.993. [DOI] [PubMed] [Google Scholar]
- 5.Cygan F, Oudiette D, Leclair-Visonneau L, Leu-Semenescu S, Arnulf I. Night-to-night variability of muscle tone, movements, and vocalizations in patients with REM sleep behavior disorder. J Clin Sleep Med. 2010;6:551–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Ferri R, Manconi M, Plazzi G, et al. A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder. J Sleep Res. 2008;17:89–100. doi: 10.1111/j.1365-2869.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 7.Frauscher B, Iranzo A, Hogl B, et al. Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31:724–31. doi: 10.1093/sleep/31.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iranzo A, Frauscher B, Santos H, et al. Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med. 2011;12:284–8. doi: 10.1016/j.sleep.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Kempfner J, Jennum P, Nikolic M, Christensen JA, Sorensen HB. Automatic detection of REM sleep in subjects without atonia. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5777–80. doi: 10.1109/EMBC.2012.6346903. [DOI] [PubMed] [Google Scholar]
- 10.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–4. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 11.Mayer G, Kesper K, Ploch T, et al. Quantification of tonic and phasic muscle activity in REM sleep behavior disorder. J Clin Neurophysiol. 2008;25:48–55. doi: 10.1097/WNP.0b013e318162acd7. [DOI] [PubMed] [Google Scholar]
- 12.Montplaisir J, Gagnon JF, Fantini ML, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010;25:2044–51. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 13.Postuma RB, Gagnon JF, Rompre S, Montplaisir JY. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology. 2010;74:239–44. doi: 10.1212/WNL.0b013e3181ca0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bliwise DL, Rye DB. Elevated PEM (phasic electromyographic metric) rates identify rapid eye movement behavior disorder patients on nights without behavioral abnormalities. Sleep. 2008;31:853–7. doi: 10.1093/sleep/31.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dauvilliers Y, Rompre S, Gagnon JF, Vendette M, Petit D, Montplaisir J. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep. 2007;30:844–9. doi: 10.1093/sleep/30.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dauvilliers Y, Pennestri MH, Petit D, Dang-Vu T, Lavigne G, Montplaisir J. Periodic leg movements during sleep and wakefulness in narcolepsy. J Sleep Res. 2007;16:333–9. doi: 10.1111/j.1365-2869.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 17.Frauscher B, Iranzo A, Gaig C, et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35:835–47. doi: 10.5665/sleep.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–53. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 19.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older males initially diagnosed with idiopathic REM sleep behavior disorder (RBD): 16-year update on a previously reported series. Sleep Med. 2013;14:744–8. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 20.McCarter SJ, St Louis EK, Boeve BF. REM sleep behavior disorder and REM sleep without atonia as an early manifestation of degenerative neurological disease. Curr Neurol Neurosci Rep. 2012;12:182–92. doi: 10.1007/s11910-012-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarter SJ, St Louis EK, Boeve BF. Is rapid eye movement sleep behavior disorder in Parkinson disease a specific disease subtype? Sleep Med. 2013;14:931–3. doi: 10.1016/j.sleep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frauscher B, Ehrmann L, Hogl B. Defining muscle activities for assessment of REM sleep behavior disorder: From a qualitative to a quantitative diagnostic level. Sleep Med. 2013;14:729–33. doi: 10.1016/j.sleep.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 25.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 26.Mahowald MW SC. REM sleep parasomnias. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier Saunders Company; 2005. pp. 897–916. [Google Scholar]
- 27.Zhang J, Lam SP, Ho CK, et al. Diagnosis of REM sleep behavior disorder by video-polysomnographic study: is one night enough? Sleep. 2008;31:1179–85. [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J, Zhang J, Lam SP, et al. Amelioration of obstructive sleep apnea in REM sleep behavior disorder: implications for the neuromuscular control of OSA. Sleep. 2011;34:909–15. doi: 10.5665/SLEEP.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarter SJ, Boswell CL, St Louis EK, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013;14:237–42. doi: 10.1016/j.sleep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferri R, Fulda S, Cosentino FI, Pizza F, Plazzi G. A preliminary quantitative analysis of REM sleep chin EMG in Parkinson's disease with or without REM sleep behavior disorder. Sleep Med. 2012;13:707–13. doi: 10.1016/j.sleep.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 33.Khawaja IS, Olson EJ, van der Walt C, et al. Diagnostic accuracy of split-night polysomnograms. J Clin Sleep Med. 2010;6:357–62. [PMC free article] [PubMed] [Google Scholar]
- 34.Iber C Ancoli-Israel S, Chesson AJ, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specification. [Google Scholar]
- 35.Iranzo A, Ratti PL, Casanova-Molla J, Serradell M, Vilaseca I, Santamaria J. Excessive muscle activity increases over time in idiopathic REM sleep behavior disorder. Sleep. 2009;32:1149–53. doi: 10.1093/sleep/32.9.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferri R, Rundo F, Manconi M, et al. Improved computation of the atonia index in normal controls and patients with REM sleep behavior disorder. Sleep Med. 2010;11:947–9. doi: 10.1016/j.sleep.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–6. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 38.Brooks PL, Peever JH. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J Neurosci. 2012;32:9785–95. doi: 10.1523/JNEUROSCI.0482-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 40.Luppi PH, Clement O, Valencia Garcia S, Brischoux F, Fort P. New aspects in the pathophysiology of rapid eye movement sleep behavior disorder: the potential role of glutamate, gamma-aminobutyric acid, and glycine. Sleep Med. 2013;14:714–8. doi: 10.1016/j.sleep.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR. Pre-motor features of Parkinson's disease: the Honolulu-Asia Aging Study experience. Parkinsonism Relat Disord. 2012;18(Suppl 1):S199–202. doi: 10.1016/S1353-8020(11)70062-1. [DOI] [PubMed] [Google Scholar]
- 42.Ferri R, Marelli S, Cosentino FI, Rundo F, Ferini-Strambi L, Zucconi M. Night-to-night variability of automatic quantitative parameters of the chin EMG amplitude (Atonia Index) in REM sleep behavior disorder. J Clin Sleep Med. 2013;9:253–8. doi: 10.5664/jcsm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenck C, Montplaisir J, Frauscher B, et al. REM sleep behavior disorder (RBD): devising controlled active treatment studies for symptomatic and neuroprotective therapy—a consensus statement by the International RBD Study Group. Sleep Med. 2013;14:795–806. doi: 10.1016/j.sleep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferri R, Bruni O, Fulda S, Zucconi M, Plazzi G. A quantitative analysis of the submentalis muscle electromyographic amplitude during rapid eye movement sleep across the lifespan. J Sleep Res. 2012;21:257–63. doi: 10.1111/j.1365-2869.2011.00958.x. [DOI] [PubMed] [Google Scholar]
- 45.Kohyama J. REM sleep atonia: from basic background to clinical application. J Med Dent Sci. 2001;48:29–39. [PubMed] [Google Scholar]
- 46.Winkelman JW, James L. Serotonergic antidepressants are associated with REM sleep without atonia. Sleep. 2004;27:317–21. doi: 10.1093/sleep/27.2.317. [DOI] [PubMed] [Google Scholar]
- 47.Frauscher B, Kunz A, Brandauer E, Ulmer H, Poewe W, Hogl B. Fragmentary myoclonus in sleep revisited: a polysomnographic study in 62 patients. Sleep Med. 2011;12:410–5. doi: 10.1016/j.sleep.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Iranzo A, Santamaria J. Bisoprolol-induced rapid eye movement sleep behavior disorder. Am J Med. 1999;107:390–2. doi: 10.1016/s0002-9343(99)00245-4. [DOI] [PubMed] [Google Scholar]