Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is commoner in patients with fluid-retaining states than in those without fluid retention, in men than in women, and worsens with aging. In men, OSA severity is related to the amount of fluid shifting out of the legs overnight, but a cause-effect relationship is not established. Our objective was to test the hypothesis that mimicking fluid overload during sleep would increase severity of OSA more in older (≥ 40 years) than in younger men (< 40 years).
Design:
Randomized, single-blind, double crossover study.
Setting:
Research sleep laboratory.
Patients or Participants:
Seven older and 10 younger men with non-severe or no sleep apnea, matched for body mass index.
Interventions:
During the control arm, normal saline was infused to keep the vein open. During intervention, subjects received an intravenous bolus of normal saline (22 mL/kg body weight) after sleep onset while they were wearing compression stockings to prevent fluid accumulation in the legs.
Measurements and Results:
Compared to younger men, infusion of similar amounts of saline in older men caused a greater increase in neck circumference (P < 0.05) and in the AHI (32.2 ± 22.1 vs. 2.2 ± 7.1, P = 0.002).
Conclusions:
Older men are more susceptible to the adverse effects of intravenous fluid loading on obstructive sleep apnea severity than younger men. This may be due to age-related differences in the amount of fluid accumulating in the neck or upper airway collapsibility in response to intravenous fluid loading. These possibilities remain to be tested in future studies.
Citation:
Yadollahi A, Gabriel JM, White LH, Taranto Montemurro L, Kasai T, Bradley TD. A randomized, double crossover study to investigate the influence of saline infusion on sleep apnea severity in men. SLEEP 2014;37(10):1699-1705.
Keywords: age, blood pressure, fluid overloading, male sex, obstructive sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA) is more common in men than in women, and more common in older men than in younger men.1 Although the key pathological feature of OSA is recurrent upper airway (UA) collapse during sleep, the cause of this collapse is not fully understood. Moreover, although continuous positive airway pressure alleviates OSA, many patients are intolerant of it.2 Thus, a more complete understanding of OSA pathogenesis could lead to better treatments. Since OSA is much more prevalent in patients with fluid-retaining states such as heart failure and renal failure than in the general population,3,4 the concept has emerged that overnight displacement of fluid from the legs into the neck can contribute to the pathogenesis of OSA.
The UA of patients with OSA is typically narrower and more collapsible while awake than in controls.5–8 UA collapse during sleep occurs when the normal withdrawal of UA dilator muscle activity at sleep onset is superimposed upon a narrow UA.5 UA collapsibility increases with aging in association with increased prevalence and severity of OSA.9,10 One cause of UA narrowing can be expansion of soft tissues and fluid in the neck that can increase peripharyngeal tissue pressure, impinge on the UA lumen, and predispose to OSA.11,12 For example, increases in fluid volumes of the jugular veins could displace the lateral walls of the pharynx inwardly and narrow the UA. In addition, increased hydrostatic capillary pressure in peripharyngeal tissues could cause transcapillary fluid filtration into the interstitial tissues of the neck and increase UA collapsibility and resistance.13
Previously, we showed that in non-obese healthy men, and in patients with heart failure, hypertension, and end-stage renal disease, severity of OSA, assessed by the frequency of apneas and hypopneas per hour of sleep (apnea-hypopnea index [AHI]), is strongly related to the amount of fluid displaced out of the legs overnight and to a reciprocal increase in neck circumference (NC).14–19 However, since these findings were observational, they did not establish a cause-effect relationship between fluid shifting into the neck and OSA. Therefore, we designed the present study to test the hypothesis that experimental induction of fluid overload via infusion of normal saline during sleep would cause a greater increase in severity of OSA in older men than in younger men.
METHODS
Subjects
Subjects were recruited by advertisement. Inclusion criteria were non-obese men, whom we divided into 2 groups: one group < 40 years (younger) and a second group ≥ 40 years of age (older), with a body mass index (BMI) < 30 kg/m2, a Berlin Questionnaire (BQ) score ≤ 1, and blood pressure (BP) ≤ 140/90 mm Hg. We chose a cutoff age 40 years because OSA is much more common in men > 40 than in men < 40 years of age, and because the peak prevalence of OSA in men is observed between the ages of 40 and 49 years.1 The exclusion criteria were a history of cardiovascular, renal, neurological, or respiratory diseases; taking any prescribed medication for these disorders; taking any over-the-counter medication that might influence fluid retention; a previous diagnosis of OSA; AHI ≥ 30 during the control arm of the protocol; or having less than one hour of sleep during either arm of the protocol.
Sleep Studies
To facilitate daytime sleep, subjects underwent a single night of voluntary sleep restriction to < 4 h the night before each arm of the study to induce sleepiness. For their convenience, subjects underwent polysomnography during the daytime using standard techniques and criteria for scoring sleep stages and arousals. Thoracoabdominal motion was monitored by respiratory inductance plethysmography, nasal pressure by nasal cannulae, and arterial oxyhemoglobin saturation (SaO2) by oximetry.20 Apneas and hypopneas were defined and classified as obstructive or central as previously described.21 Subjects slept supine for the entire study period to eliminate any potential effect of postural changes on AHI and other variables. Sleep studies were scored by personnel blinded to randomization, and the AHI was calculated.
Saline Infusion
We inserted a 20 gauge intravenous catheter into a left forearm vein to infuse normal saline (0.9% NaCl in water), which we used because it is isotonic to plasma. During the control session, saline was infused at the minimal rate to keep the vein open. During the intervention session, saline was initially infused to keep the vein open, but once the subject entered stage 2 sleep, a bolus of 22 mL/kg body weight was infused over 30 minutes. This volume was chosen because it is safe and does not induce pulmonary edema in healthy subjects,22 and because it induces a transient state of fluid overload similar in degree to that in hemodialyzed renal failure patients between dialysis sessions (i.e., ≈ 2 L). Saline was heated to the body temperature by placing the bag in warm water at 37°C. None of the subjects complained about feeling cold due to saline infusion. To prevent saline accumulation in the legs, below-the-knee compression stockings were placed on subjects' legs and worn throughout the saline infusion arm.
Blood Pressure, Upper Airway Cross-Sectional Area, and Neck Circumference
At the beginning of the study prior to sleep, systolic, diastolic, and mean arterial BP (SBP, DBP, and MAP, respectively), and heart rate (HR) were measured using an automated sphygmomanometer. The measurements were repeated 3 times with 2-min intervals between successive measurements and were averaged to give a mean value. Then with subjects supine, upper airway cross-sectional area (UA-XSA) was assessed by acoustic pharyngometry,23 and NC by tape measure above the cricothyroid cartilage. A line was drawn at this level to ensure that measurements before and after sleep were made at the same level, as previously described.18,19 All measurements made prior to sleep were repeated following sleep.
Protocol
This was a randomized, single-blind, double crossover protocol. Subjects arrived in the sleep laboratory at noon following a night of sleep deprivation, and were instrumented for sleep studies. To reduce the effects of bias and confounders, subjects were randomized after they were set up to start the experiment. They were randomized into control or intervention arm using a computer-generated randomization table with unequal blocks of 2 and 4. Although subjects were blind to the intervention, experimenters were of necessity not blinded. However, personnel analyzing the results were kept blind to randomization. All subjects slept on a single foam pillow. Baseline measurements were then made, after which subjects were randomized to a control or intervention session. Once subjects woke up, baseline measurements were repeated. One week after the initial session, subjects crossed over to the other arm of the study. The protocol was approved by the Research Ethics Board of Toronto Rehabilitation Institute, and all subjects provided written consent prior to participation.
Data Analysis
During both control and saline infusion arms, changes (Δ) in NC, UA-XSA, HR, SBP, DBP, and MAP from pre-sleep to post-sleep were calculated. Characteristics of the younger and older groups were compared by Student t-tests or Mann-Whitney test. Within each group, we compared different variables during control and intervention periods by Student t-tests or Mann-Whitney test. We also compared the effects of saline infusion on different variables using Student t-tests or Mann-Whitney test. The change in AHI between the control and intervention periods between the 2 groups was compared by analysis of covariance (ANCOVA) to take into account potential variations during the control period. The primary outcome of this study was the difference in the change in AHI between the older and younger groups in response to saline infusion. The secondary outcomes were the differences in the changes in SBP, DBP, MBP, UA-XSA, and NC between the older and younger groups in response to saline infusion. Statistical analyses were performed by SAS 9.3, and a two-sided P-value < 0.05 was considered significant. Data are presented as mean ± SD.
RESULTS
Characteristics of the Subjects
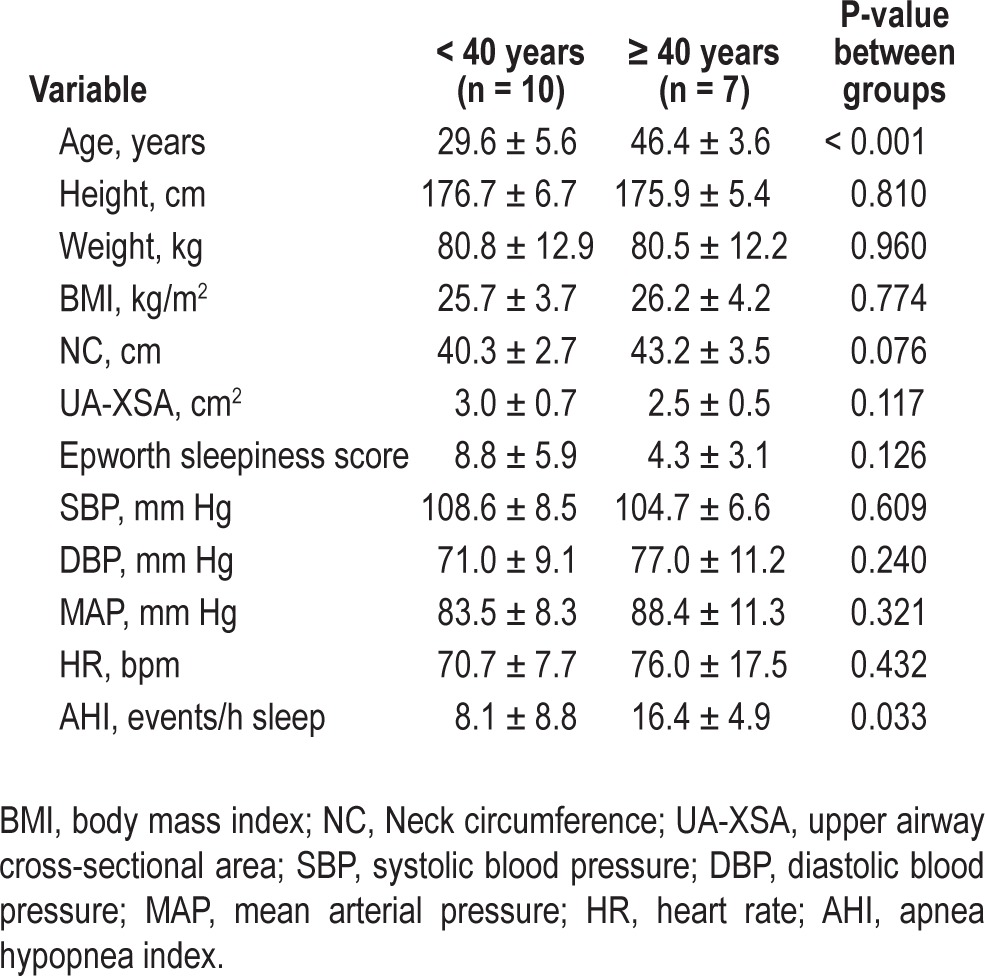
Thirty-seven men were screened for eligibility criteria, and 35 met screening criteria for the study. Eighteen subjects were excluded: 10 had AHI > 30 during control arm, 5 subjects could not fall sleep during the day, and 3 subjects were lost to follow-up. Recruitment flow diagram of the study is presented in Figure 1. Seventeen subjects (10 < 40 years and 7 ≥ 40 years of age) completed both arms of the study. Table 1 shows the baseline characteristics of the subjects in the 2 groups. Age was significantly greater in the older group by design. Height, weight, BMI, HR, and baseline BP were similar in both groups. There was a nonsignificant tendency in older men towards a greater NC and smaller UA-XSA than the younger group. The AHI during the control arm was significantly greater in the older group than in the younger group (P = 0.033).
Figure 1.
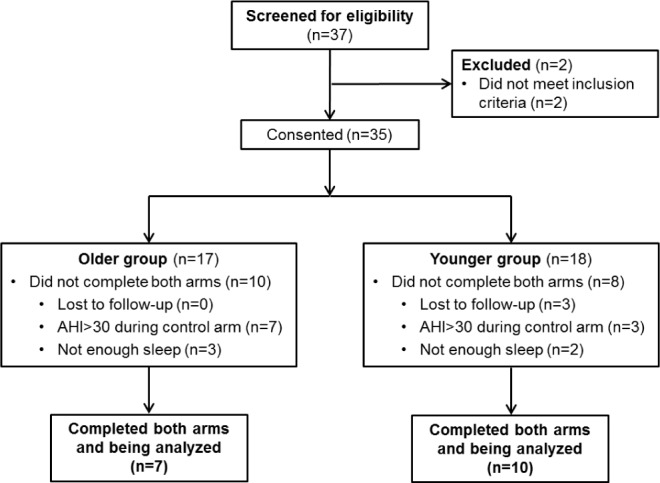
Recruitment flow diagram of the study.
Table 1.
Characteristics of the subjects.
Effects of Saline Infusion
Within Group Comparisons
Table 2 shows the influence of saline infusion within each age group. In the younger group, the volume of infused saline was significantly higher during intervention than control by design. There was no significant difference in total sleep time or the sleep structure between the 2 study arms. However, sleep efficiency was lower during intervention than during control (P = 0.01). There was no difference in ΔSBP, ΔDBP, ΔMAP, ΔHR, or ΔUA-XSA between the 2 arms. While during intervention, NC increased more than during the control arm (P = 0.008, Table 2), total, NREM and REM AHI, and minimum SaO2 did not change significantly compared to control (Figure 2 and Table 2, respectively).
Table 2.
Influence of intervention within the younger and older groups.
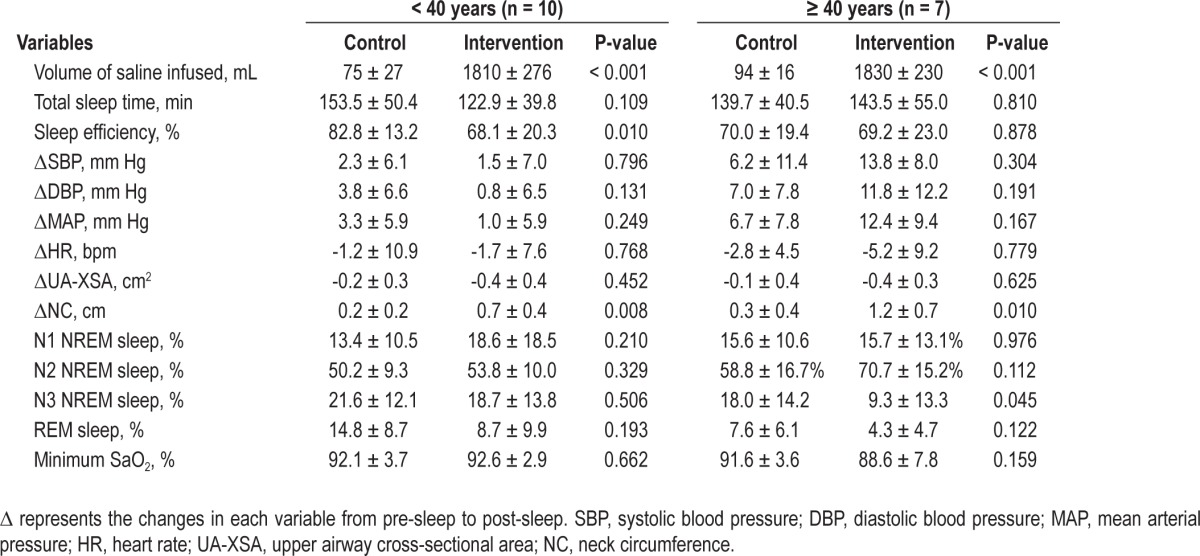
Figure 2.

Changes in the AHI during control and intervention arms for (A) total, (B) NREM, and (C) REM sleep. While all subjects had NREM sleep on both sleep studies, only 7 younger and 4 older subjects had REM sleep on both sleep studies. In the younger group, there was no significant change in the total, NREM, and REM AHI between the control and intervention. However, in the older group the total and NREM AHI increased significantly from control to intervention. The increase in the total and NREM AHI from control to intervention was significantly greater in the older than in the younger group.
In the older group, the volume of infused saline was higher during intervention than during control by design. There was no significant difference in total sleep time, sleep efficiency, time spent in N1 or N2 NREM sleep, ΔSBP, ΔDBP, ΔMAP, ΔHR, or ΔUA-XSA between the 2 study arms (Table 2). However, in the older group compared to the control arm, saline infusion caused a decrease in N3 NREM sleep but no change in REM. Similar to the younger group, NC increased more during intervention than during control (P = 0.010, Table 2). However, in contrast to the younger group, there was a highly significant 3-fold increase in the total AHI during intervention compared to control in the older group (P = 0.008, Figure 2A). This was due to a significant increase in NREM AHI, whereas REM AHI did not change (Figure 2B and 2C). There was no significant change in minimum SaO2 during intervention compared to control (Table 2).
Between Group Comparisons
Table 3 shows comparisons of the effects of intervention on variables between the older and younger groups. In both groups, similar amounts of saline were infused during intervention. There was no significant difference in total sleep time, sleep efficiency, N1 and N3 NREM sleep, and REM sleep during intervention between the 2 groups. However, older subjects had more N2 NREM sleep than younger subjects (P = 0.014). Compared to the younger group, the older group experienced greater increases in ΔSBP (P = 0.004), ΔDBP (P = 0.029), and ΔMAP (P = 0.008), but not in ΔHR during intervention. Intervention caused a greater increase in ΔNC (P = 0.048), but there was no difference in ΔUA-XSA between the older and younger group. There was a much greater increase in total and NREM AHI in the older than in the younger group in response to intervention (P = 0.002, and P = 0.003, respectively; Figure 2A and 2B), although REM AHI did not differ. In addition, for all subjects, ΔSBP, ΔDBP, and ΔMAP during intervention correlated significantly with the AHI (ΔSBP: R2 = 0.404, P = 0.006, ΔDBP: R2 = 0.414, P = 0.005, and ΔMAP: R2 = 0.494, P = 0.002).
Table 3.
Comparisons of effects of intervention on variables between the groups.

When considering age as a continuous variable, we found that there was a significant correlation between age and the increase in NC during saline infusion (r = 0.59, P = 0.013, Figure 3). The relationship remained significant even after controlling for baseline values of NC or the change in NC during the control arm of the study. We also found a significant correlation between age and change in AHI from control to intervention (r = 0.58, P = 0.015, Figure 4).
Figure 3.
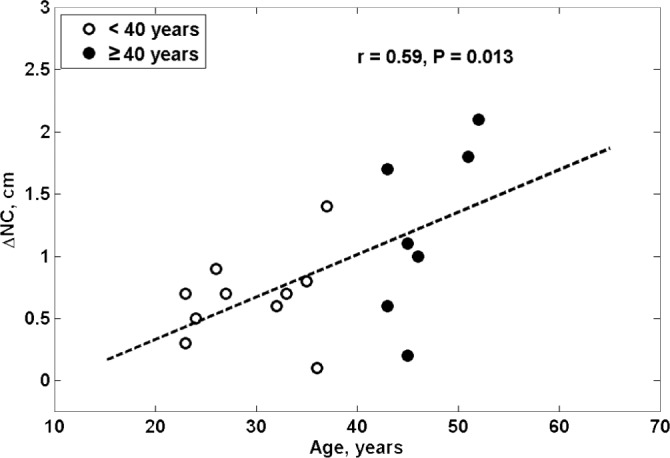
The relationship between age and change in NC during saline infusion.
Figure 4.
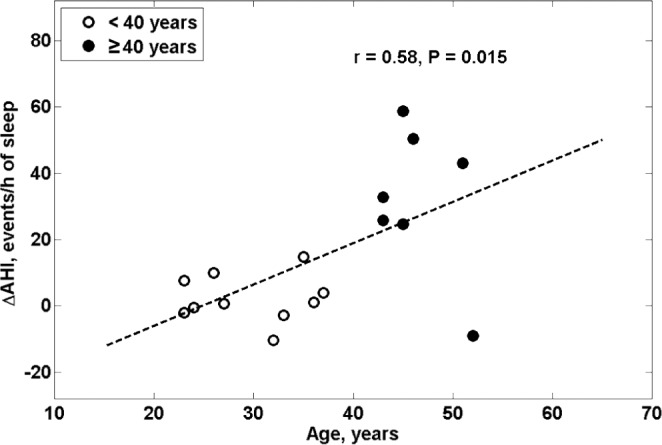
The relationship between age and change in AHI from control to the intervention arm (ΔAHI).
DISCUSSION
The most important finding of our study was that, compared to a control sleep session, infusion of normal saline while wearing compression stockings caused a highly significant 3-fold increase in the AHI in older, but not in younger men, despite a similar amount of saline infused in both groups. This difference remained significant even after controlling for differences in the AHI values during the control session. This increase in the AHI in the older subjects was associated with a greater increase in NC, suggesting that despite a similar amount of infused saline, neck fluid volume increased more in the older than the younger men. These results suggest that among men, age is a significant factor affecting both the amount of fluid accumulating in the neck and the severity of OSA in response to saline infusion, which could be one factor contributing to the higher prevalence of OSA in older than in younger men.
The greater increase in NC in older than in younger men and the linear relationship between change in NC and age during saline infusion was likely due to greater fluid accumulation in the jugular veins and/or interstitial spaces of the neck in the older subjects. This may have caused a greater increase in peripharyngeal tissue pressure that would increase UA collapsibility and resistance, and could have contributed to the greater increase in AHI in the older than in the younger men. The observation that the change in AHI during saline infusion is linearly related to age provides further support for this possibility and emphasizes that age could play an important role in susceptibility to the UA collapse during saline infusion.
Although the increase in neck fluid volume might be expected to decrease UA-XSA, there was no significant decrease in UAXSA in response to intervention compared to control in either group. This could be because we measured UA-XSA while the subjects were awake, because acoustic pharyngometry only measures UA-XSA below the level of the retro-palatal pharynx (and therefore would not be able to detect a reduction in retropalatal XSA), or that factors other than UA narrowing per se were involved in the greater increase in AHI in response to saline infusion in older than in younger men.
Several other factors may have contributed to the markedly greater increase in AHI in the older than in the younger men in response to saline infusion. One could be that fluid accumulation in the neck has more adverse effects in the older population due to the effects of aging on the properties of the UA. In support of this possibility, UA collapsibility and resistance during sleep increase with age.9,10,24 In addition, UA dilator reflexes may deteriorate with age, and ventilatory and genioglossus muscle responses to hypoxia are significantly lower in older (> 40 years) than in younger individuals.25 Thus, whereas younger individuals may have reflexively increased UA dilator muscle activity in response to peri-pharyngeal fluid accumulation sufficient to prevent UA collapse, older individuals may not. Another possibility is that the greater increase in AHI in response to saline infusion in the older group occurred because they started with a somewhat higher AHI during the control study than the younger group. However, this is very unlikely because after controlling for differences in baseline AHI by ANCOVA, the saline-induced increase in AHI in the older group was still much greater than in the younger group.
Our results show that while fluid loading caused a significant 3-fold increase in NREM AHI in the older, but not in the younger group, it had no effect on REM AHI in either group. The reason why saline infusion increased NREM but not REM AHI in older men is unclear. One possibility is that during REM sleep, muscle atonia is at its maximum and that UA mechanics cannot be further worsened by saline infusion. In contrast, during NREM sleep, pharyngeal muscle activity is higher than in REM sleep.26 Thus, there may be greater potential for nocturnal fluid shift into the peripharyngeal region to worsen UA mechanics in NREM than in REM sleep. However, one limitation to interpreting the reason for the observed difference between NREM and REM sleep AHI responses to saline infusion in older men is that only four of them had REM sleep on both sleep studies.
A second important finding was that saline infusion induced much greater increases in SBP, DBP and MAP in the older than in the younger men. This finding suggests that older men are more susceptible than younger men to the BP raising effects of intravascular fluid. However, this finding was unanticipated and the mechanisms involved were not identified. Several factors could have contributed to this greater BP increase in older men. First, arterial stiffness increases with aging and can begin as early as the third decade.27–29 Second, renal plasma flow and glomerular filtration rate decrease significantly, while renal vascular resistance increases and sodium homeostasis is impaired with aging.30–32 Consistent with those observations, a previous study showed that after fluid overloading, older men excreted less urine and reduced urine osmolality less than younger men.33 A third possibility, supported by the observation that the magnitude of blood pressure increase was directly related to the AHI on the saline infusion night, is that worsening of OSA induced by saline infusion raised BP possibly through apnea-induced, sympathetically mediated vasoconstriction.34 Based on these observations, BP could have risen more in older than in younger men in response to saline infusion because they had less compliant blood vessels, excreted less urine and sodium, or because their AHI increased more.
In relation to the results of the present study, we recently observed that in 16 older (age 60.0 ± 10.9 years) hypertensive patients with OSA, diuretics had the opposite effect on NC, AHI, and BP to saline infusion.35 Intensive diuretic therapy for two weeks reduced the overnight fluid shift from the legs and reduced the degree of the overnight increase in NC, in conjunction with reductions in the AHI and morning SBP and DBP. Thus, while on one hand, fluid loading during sleep in older men induced increases in NC, AHI, and BP, on the other hand, diuretic therapy in older hypertensive patients with OSA reduced NC, AHI, and BP. Taken together, these findings provide further evidence that fluid loading could contribute to the pathogenesis of OSA and nocturnal BP elevations, at least in older men.
Based on these results, it is possible that saline infusion for fluid resuscitation on medical wards and intensive care units, as well as fluid infusion during surgery may induce sleep apnea in older men. In this regard, Chung et al. investigated the factors that were associated with the postoperative exacerbation of sleep apnea.36 They showed that in 376 patients, compared to the preoperative AHI, there was a 50% increase in AHI on the third night after surgery. In addition, the postoperative increase in AHI was directly related to age. It is possible that fluid infusion and overload after surgery could have contributed to this postoperative worsening of OSA.
Our study was subject to some limitations. To avoid the potential confounding effects of obesity on OSA and fluid retention, we limited our study to non-obese subjects (BMI < 30). Therefore, our results may not be applicable to obese individuals. We have also limited our study to non-severe OSA (AHI < 30) because we wished to avoid a ceiling effect whereby OSA could not be made any worse by saline infusion than it already was, or to avoid inducing even worse OSA in such subjects. Our study was confined to men because previous studies showed that in patients with heart failure or renal failure, strong correlations between the overnight decrease in leg fluid volume and severity of OSA were observed in men, but not in women.17,37 Those results suggested that men are more susceptible to UA collapse in response to overnight rostral fluid shift than women. Further studies will be required to determine if men and women also differ in their AHI response to saline infusion during sleep.
For reasons of feasibility and convenience for subjects, sleep studies were performed during the day, and the findings may not be exactly the same if we had performed them at night. We restricted sleeping position to supine to minimize the effects of posture on AHI variability. The number of subjects was relatively small. Nevertheless, our study was a rigorously designed randomized, controlled, double crossover study with very large and highly significant differences in the AHI and BP responses to saline infusion between the younger and older age groups. We used changes in neck circumference as an index of changes in neck fluid volume because technical limitations prevented us from measuring neck fluid volume. Future studies will be required to measure the amount of fluid accumulating in the neck and how it relates to the changes in neck circumference and UA caliber in association with variations in the AHI.
In conclusion, our findings indicate that induction of fluid overload in older men can precipitate or worsen the severity of OSA. The reason why older men are more susceptible than younger men to this stimulus is unclear, but may involve differing effects of fluid accumulation on UA anatomy, collapsibility, and/or dilator reflexes. Further investigations could explore the possibilities that more fluid accumulates in peripharyngeal tissues, or that genioglossus activation or UA dilating reflexes in response to saline infusion are impaired in older compared to younger men, rendering their UAs more susceptible to collapse. The present findings, along with those of our previous studies, provide a strong rational for testing the effects of reducing nocturnal rostral fluid shift and fluid accumulation in the neck on OSA severity in randomized clinical trials, particularly in older men.
DISCLOSURE STATEMENT
This was not an industry supported study. The research was supported by funding from Canadian Institutes of Health Research (CIHR) operating grant MOP-82731. Dr. Yadollahi was supported by Fellowships from the Mitacs Elevate program and a CIHR Training Grant in Sleep and Biological Rhythms. Mr. Gabriel was supported by an Ontario Student Opportunity Trust Fund Awards from the Toronto Rehabilitation Institute and the Cardiovascular Sciences Collaborative Program of the University of Toronto. Dr. White was supported by the Sleep Country Canada Fellowship in Sleep Medicine. Dr. Montemurro was supported by fellowships from the Chair of Respiratory Medicine, University of Brescia, Brescia, Italy and from the Toronto Rehabilitation Institute. Dr. Kasai was supported by an unrestricted Research Fellowship from Fuji-Respironics Inc. Dr. Bradley was supported by the Clifford Nordal Chair in Sleep Apnea and Rehabilitation Research. There was no off-label or investigational use of a therapeutic product in this study. Experiments were performed in the Sleep Research Laboratory, Toronto Rehabilitation Institute, University Health Network, Toronto, Canada. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Mr. Mahmoud Azimaee for his help with the statistical analysis of data.
Footnotes
A commentary on this article appears in this issue on page 1587.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzaga CC, Gaddam KK, Ahmed MI, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6:363–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–7. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Bradley TD, Brown IG, Grossman RF, et al. Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med. 1986;315:1327–31. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- 6.Brown IG, Bradley TD, Phillipson EA, Zamel N, Hoffstein V. Pharyngeal compliance in snoring subjects with and without obstructive sleep apnea. Am Rev Respir Dis. 1985;132:211–5. doi: 10.1164/arrd.1985.132.2.211. [DOI] [PubMed] [Google Scholar]
- 7.Stauffer JL, Zwillich CW, Cadieux RJ, et al. Pharyngeal size and resistance in obstructive sleep apnea. Am Rev Respir Dis. 1987;136:623–7. doi: 10.1164/ajrccm/136.3.623. [DOI] [PubMed] [Google Scholar]
- 8.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 9.Eikermann M, Jordan AS, Chamberlin NL, et al. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131:1702–9. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray AD, Ogasa T, Magalang UJ, Krasney JA, Farkas GA. Aging increases upper airway collapsibility in Fischer 344 rats. J Appl Physiol. 2008;105:1471–6. doi: 10.1152/japplphysiol.00166.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. J Appl Physiol. 2005;99:2440–50. doi: 10.1152/japplphysiol.00772.2005. [DOI] [PubMed] [Google Scholar]
- 12.Schwab R, Gefter WB. Anatomical factors: insights from imaging studies. In: Pack AI, editor. Sleep apnea: pathogenesis, diagnosis and treatment. New York, NY: Marcel Dekker; 2002. pp. 1–30. [Google Scholar]
- 13.Elias RM, Chan CT, Paul N, et al. Relationship of pharyngeal water content and jugular volume with severity of obstructive sleep apnea in renal failure. Nephrol Dial Transplant. 2013;28:937–44. doi: 10.1093/ndt/gfs473. [DOI] [PubMed] [Google Scholar]
- 14.Elias RM, Bradley TD, Kasai T, Motwani SS, Chan CT. Rostral overnight fluid shift in end-stage renal disease: relationship with obstructive sleep apnea. Nephrol Dial Transplant. 2012;27:1569–73. doi: 10.1093/ndt/gfr605. [DOI] [PubMed] [Google Scholar]
- 15.Friedman O, Bradley TD, Ruttanaumpawan P, Logan AG. Independent association of drug-resistant hypertension to reduced sleep duration and efficiency. Am J Hypertens. 2010;23:174–9. doi: 10.1038/ajh.2009.220. [DOI] [PubMed] [Google Scholar]
- 16.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–27. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 17.Kasai T, Motwani SS, Yumino D, Mak S, Newton GE, Bradley TD. Differing relationship of nocturnal fluid shifts to sleep apnea in men and women with heart failure. Circ Heart Fail. 2012;5:467–74. doi: 10.1161/CIRCHEARTFAILURE.111.965814. [DOI] [PubMed] [Google Scholar]
- 18.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179:241–6. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 19.Yumino D, Redolfi S, Ruttanaumpawan P, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010;121:1598–605. doi: 10.1161/CIRCULATIONAHA.109.902452. [DOI] [PubMed] [Google Scholar]
- 20.Clark SA, Wilson CR, Satoh M, Pegelow D, Dempsey JA. Assessment of inspiratory flow limitation invasively and noninvasively during sleep. Am J Respir Crit Care Med. 1998;158:713–22. doi: 10.1164/ajrccm.158.3.9708056. [DOI] [PubMed] [Google Scholar]
- 21.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 22.Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986-2006) Eur J Appl Physiol. 2007;101:143–94. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- 23.Fredberg JJ, Wohl ME, Glass GM, Dorkin HL. Airway area by acoustic reflections measured at the mouth. J Appl Physiol. 1980;48:749–58. doi: 10.1152/jappl.1980.48.5.749. [DOI] [PubMed] [Google Scholar]
- 24.Fogel RB, White DP, Pierce RJ, et al. Control of upper airway muscle activity in younger versus older men during sleep onset. J Physiol. 2003;553:533–44. doi: 10.1113/jphysiol.2003.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klawe JJ, Tafil-Klawe M. Age-related response of the genioglossus muscle EMG-activity to hypoxia in humans. J Physiol Pharmacol. 2003;54(Suppl 1):14–9. [PubMed] [Google Scholar]
- 26.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135:957–64. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jani B, Rajkumar C. Ageing and vascular ageing. Postgrad Med J. 2006;82:357–62. doi: 10.1136/pgmj.2005.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redheuil A, Yu WC, Wu CO, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–26. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin JE, Sheaff MT. Renal ageing. J Pathol. 2007;211:198–205. doi: 10.1002/path.2111. [DOI] [PubMed] [Google Scholar]
- 31.Pollack JA, Skvorak JP, Nazian SJ, Landon CS, Dietz JR. Alterations in atrial natriuretic peptide (ANP) secretion and renal effects in aging. J Gerontol A Biol Sci Med Sci. 1997;52:B196–202. doi: 10.1093/gerona/52a.4.b196. [DOI] [PubMed] [Google Scholar]
- 32.Presta P, Lucisano G, Fuiano L, Fuiano G. The kidney and the elderly: why does the risk increase? Int Urol Nephrol. 2012;44:625–32. doi: 10.1007/s11255-011-0063-2. [DOI] [PubMed] [Google Scholar]
- 33.Crowe MJ, Forsling ML, Rolls BJ, Phillips PA, Ledingham JG, Smith RF. Altered water excretion in healthy elderly men. Age Ageing. 1987;16:285–93. doi: 10.1093/ageing/16.5.285. [DOI] [PubMed] [Google Scholar]
- 34.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 35.Kasai T, Bradley TD, Friedman O, Logan AG. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J Hypertens. 2014;32:673–80. doi: 10.1097/HJH.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 36.Chung F, Liao P, Elsaid H, Shapiro CM, Kang W. Factors associated with postoperative exacerbation of sleep-disordered breathing. Anesthesiology. 2014;120:299–311. doi: 10.1097/ALN.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 37.Su MC, Chiu KL, Ruttanaumpawan P, et al. Difference in upper airway collapsibility during wakefulness between men and women in response to lower-body positive pressure. Clin Sci (Lond) 2009;116:713–20. doi: 10.1042/CS20080321. [DOI] [PubMed] [Google Scholar]


