Abstract
Study Objectives:
To study the effect of melatonin administration on glucose metabolism in humans in the morning and evening.
Design:
Placebo-controlled, single-blind design.
Setting:
Laboratory assessments.
Participants:
21 healthy women (24 ± 6 y; body mass index: 23.0 ± 3.3 kg/m2).
Interventions:
Glucose tolerance was assessed by oral glucose tolerance tests (OGTT; 75 g glucose) on 4 occasions: in the morning (9 AM), and evening (9 PM); each occurring 15 minutes after melatonin (5 mg) and placebo administration on 4 non-consecutive days.
Measurements and Results:
Melatonin administration impaired glucose tolerance. When administered in the morning, melatonin significantly increased the incremental area under the curve (AUC) and maximum concentration (Cmax) of plasma glucose following OGTT by 186% and 21%, respectively, as compared to placebo; while in the evening, melatonin significantly increased glucose AUC and Cmax by 54% and 27%, respectively. The effect of melatonin on the insulin response to the OGTT depended on the time of day (P < 0.05). In the morning, melatonin decreased glucose tolerance primarily by decreasing insulin release, while in the evening, by decreasing insulin sensitivity.
Conclusions:
Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. When administering melatonin, the proximity to meal timing may need to be considered, particularly in those at risk for glucose intolerance.
Citation:
Rubio-Sastre P, Scheer FA, Gómez-Abellán P, Madrid JA, Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. SLEEP 2014;37(10):1715-1719.
Keywords: glucose tolerance, insulin resistance, melatonin, metabolic syndrome, oral glucose tolerance test
INTRODUCTION
The recent discovery of MTNR1B as a novel type 2 diabetes (T2D) gene and the association between melatonin excretion and T2D risk sparked great interest in the role of melatonin in glucose metabolism.1–3 An estimated 5-12 million Americans use melatonin to treat sleeping problems, primarily without prescription and thus without medical supervision.4,5 This is of particular concern because the precise effect of exogenous melatonin on glucose metabolism in humans remains unknown. Indeed, previous studies are scarce and seem to be contradictory. For example, while Cagnacci et al.6 indicated that in aged women administration of 1 mg of melatonin reduces glucose tolerance and insulin sensitivity; Garfinkel et al.7 demonstrated that short-term use of prolonged-release melatonin improves sleep maintenance in type 2 diabetic patients with insomnia without affecting glucose and lipid metabolism. Moreover, to our knowledge no previous study has been performed to assess the influence of the time of day (morning or evening) of exogenous melatonin on glucose metabolism. For these reasons, the aim of the current investigation was to determine the effect of a clinical dose of melatonin (5 mg) on glucose tolerance and estimates of insulin resistance in young and healthy women at different times of the day (morning and evening) after an oral glucose tolerance tests (OGTT; 75 g), as compared to placebo administration.
METHODS
Participants
Twenty-one healthy women (24 ± 6 y; body mass index: 23.0 ± 3.3 kg/m2) completed the study in March 2013. Characteristics of the population and additional subject details are included in Table S1 (supplemental material). All procedures were in accordance with good clinical practice and were performed in accordance with the Helsinki Declaration of Human Studies and approved by the Ethical Committee of the University of Murcia. All subjects signed an informed consent form.
Melatonin/Placebo Administration and Oral Glucose Tolerance Test
To determine the influence of exogenous melatonin on glucose metabolism in the morning and evening, each participant underwent 4 OGTTs (75 g glucose). Melatonin (5 mg; 08:45 and 20:45) was administered 15 min before glucose load (09:00 and 21:00) on 2 non-consecutive days of the same week (Monday and Wednesday) in randomized order, and placebo was administered the following week using the same procedure, all in a single-blind fashion (Figure S1, supplemental material).
Subjects reported to the laboratory at 08:00 for the morning protocols and at 20:00 for the evening protocols, following a 7-7.5 h sleep duration: bedtime 23:30-24:00 and wake time ∼07:00 (sleep duration was self-reported), and after having refrained from exercise for 48 h. The OGTTs were started after an approximate 10-h fast on all 4 occasions. To achieve a 10-h fast, women were recommended to have a light dinner at 21:00 on the day before the morning protocols (12-h fast), and a similar meal before 11:00 on the day of the evening protocols (10-h fast). A catheter was placed in the antecubital vein by a qualified technician and was kept patent with a normal saline drip for each OGTT. All 4 OGTTs were performed while seated. Melatonin and placebo were administered in a liquid formulation instead of in tablet in order to increase the absorption rate. To avoid differential effect of melatonin solution (70% fructose), placebos were prepared in a same fructose solution. The total amount of fructose in both cases was < 1 g (0.63 g). By radioimmunoassay (IZASA, Barcelona, Spain) we confirmed that the melatonin concentration of the commercial solution (Melamil, Humana Pharma, Italy) was 5.5 mg/mL, requiring administration of 0.9 mL to the participants to obtain the desired dose of 5 mg. A venous blood sample was taken just before the administration of melatonin/placebo, approximately 15 min prior to the start of the OGTT (Figure S1) to determine fasting blood glucose level. OGTT was initiated 15 min after placebo or melatonin administration by ingestion of the 75-g glucose solution. Subsequent blood samples were taken at time 30, 60, 90, 120, and 180 min after consumption of the glucose load.
Calculations and Statistical Analysis
Areas under the curve above baseline (AUCs) for glucose and insulin for the first 120 min after OGTT were calculated with the trapezoid method.8 In order to assess whether melatonin influenced insulin sensitivity, we assessed the Insulin Sensitivity Index (ISI: 10,000/√[fasting glucose × fasting insulin × mean OGTT glucose × mean OGTT insulin]; mean OGTT across fasting-120 min).9 To estimate the effect of melatonin on β-cell function, we determined the corrected insulin response (CIR: 100 × insulin at 30 min/[glucose at 30 min × glucose 30 min-3.89]).10 Finally, the disposition index was calculated as a measure of insulin secretion in relation to insulin sensitivity (DI = CIR × ISI).11
The effect of melatonin was calculated as the values following melatonin administration minus the values following placebo administration (paired t-test). Data were also analyzed for time and treatment effects using a two-way ANOVA with repeated measurements. When ANOVA was significant, paired t-test was used to evaluate times in which variations were different. Results are presented as means ± SEM. Statistical analyses were performed using SPSS 15.0 software (SPSS).
RESULTS
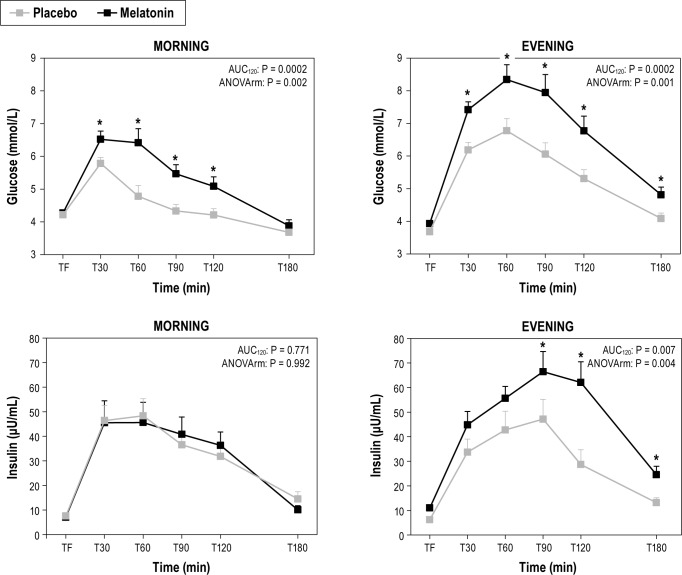
Melatonin administration significantly impaired glucose tolerance, both when administered in the morning and in the evening, as compared to placebo (Figure 1 and Table 1). In the morning, melatonin caused a significant increase in the area under the curve above baseline (AUC) and maximum plasma concentration (Cmax) of glucose by 186% (P < 0.001) and 21% (P < 0.001), respectively; while in the evening, melatonin caused a significant increase in the AUC and Cmax for glucose by 54% (P < 0.001) and 27% (P < 0.001), respectively. The elevated postprandial glucose concentrations after melatonin administration were concurrent with either no decrease (morning) or even an increase in insulin AUC (evening; 45%; P = 0.002), suggesting that melatonin decreased insulin sensitivity and resulted in insufficient β-cell compensation.
Figure 1.
Comparison between the effects of placebo and melatonin administrations on plasma glucose and insulin concentrations in response to an oral load of glucose (75 g) performed in the morning (09:00 and evening (21:00). TF, time fasting; T30, 60, 90, 120, and 180, time after OGTT (min); AUC120, paired t-test for AUC (melatonin and placebo) calculated with 120 min; ANOVArm, two-way ANOVA for time and treatment effects with repeated measurements. When ANOVA was significant, paired t-test was used to evaluate times in which variations were different. *Different from placebo at that time, P < 0.05.
Table 1.
Differences in effects of melatonin and placebo administration on glucose metabolism assessed by oral glucose tolerance tests (OGTT) (morning and evening).
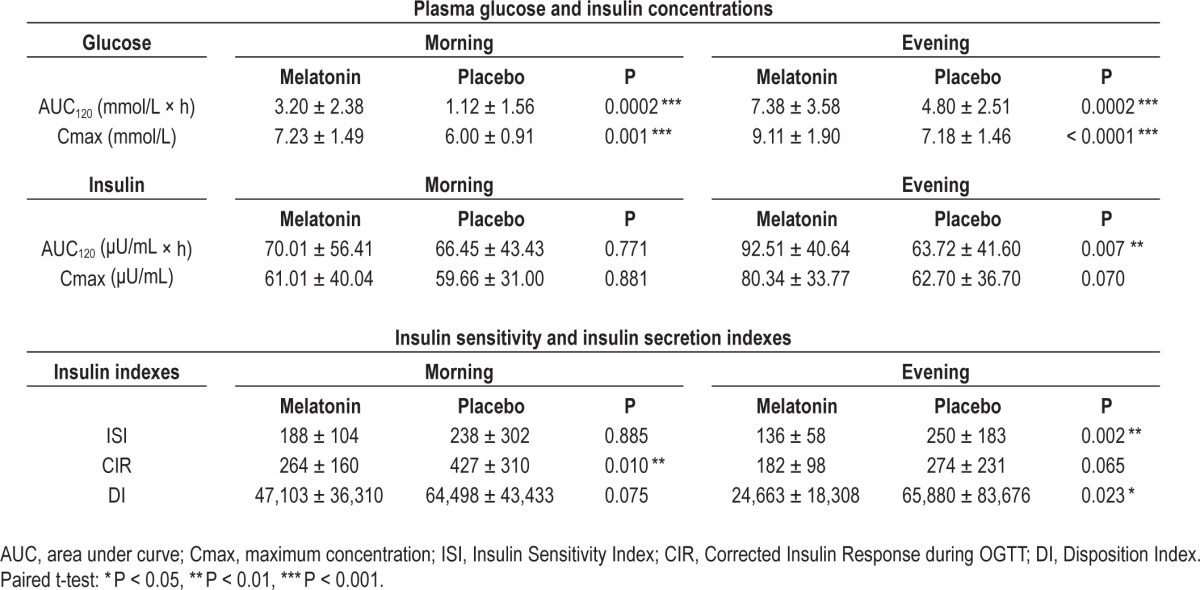
To test whether melatonin influenced glucose tolerance differently dependent on the time-of-day (morning vs. evening), we compared the effect of melatonin (AUC melatonin-AUC placebo and Cmax melatonin-Cmax placebo) between the morning and the evening. The effect of melatonin on glucose AUC was not different between the morning (mean ± SEM: 2.07 ± 0.46) and evening (2.58 ± 0.58) (P = 0.094). However, the effect of melatonin on insulin AUC was significantly greater in the evening (28.78 ± 9.53) than the morning (3.55 ± 12.6) (P = 0.004), suggesting decreased insulin sensitivity especially in the evening and decreased β-cell compensation especially in the morning. These results were in agreement with ISI that was significantly decreased by 46% only in the evening (P = 0.007), while this change did not reach significance in the morning. Similar results were obtained for the Disposition Index with a significant reduction in the evening only, by 63% (P = 0.023). In contrast, there was a significant decrease in CIR by 38% in the morning (P = 0.010), without significant change in the evening (Table 1).
DISCUSSION
The current data indicate that acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Furthermore OGTT results suggest that melatonin impairs glucose tolerance primarily by decreasing insulin sensitivity concurrent with insufficient β-cell compensation in the evening, and primarily by decreased insulin release in the morning. These results are in agreement with the substantial body of evidence indicating that melatonin inhibits β-cell function and stimulates α-cell function.12
However, our results are opposite to those obtained in studies of nocturnal animals, which have shown that melatonin ameliorates glucose and insulin resistance.13,14 In nocturnal mammals, feeding occurs mainly during the dark phase when melatonin levels are high, while in humans, feeding typically occurs during the light phase when melatonin levels are low. This raises the question whether in nocturnal mammals, melatonin may augment the physiological response to food, while exogenous melatonin administration during the daytime in humans interferes with the physiological response to food intake, leading to carbohydrate intolerance, which would make such administration inadvisable.
Moreover, our data appear to contradict the findings of Mc-Mullan et al.,15 which showed an inverse relationship between urinary 6-sulfatoxy melatonin excretion and risk for developing T2D in the Nurses' Health Study cohort. This apparent contradiction may be due to the fact that the study by McMullan relates melatonin production during the overnight fast to diabetes risk, whereas the present data pertain to the impact of melatonin during the daytime on glucose tolerance. Moreover, the work by McMullan is an observational study, and thus the direction of causality cannot be established.16 Indeed, because this cohort was exposed to a significant amount of shift work it has been questioned to what degree the changes in melatonin excretion alter T2D risk versus whether suppressed urinary 6-sulphatoxy melatonin is a reflection of circadian disruption which has been independently associated with T2D risk.17
To our knowledge, this is the first study to analyze the effect of melatonin on glucose tolerance in humans using an OGTT, after a therapeutic dose of melatonin (5 mg), not only in the morning but also in the evening. Results are consistent with a previous study performed in the morning in postmenopausal women, in which 1 mg melatonin administration resulted in decreased glucose tolerance as assessed by OGTT (increase glucose AUC), without significant change in plasma insulin concentrations.6 An intravenous glucose tolerance test confirmed decreased glucose tolerance without significant change in insulin concentrations and indicated a decrease in insulin sensitivity. The current study extends this observation by looking at the effect of time of the day and by studying premenopausal and healthy women without hormone replacement therapy. The novel finding of a significant reduction in glucose tolerance, insulin sensitivity, and disposition index following melatonin administration in the evening is particularly relevant because this is the habitual time of melatonin administration in the treatment of sleep or in order to phase advance the circadian system in the treatment of delayed sleep phase syndrome (DSPS) and non-24-hour sleep-wake disorder (“Non-24h”).18
The timing of melatonin administration in the treatment of sleeping problems is typically within an hour before bedtime, and thus adverse effects of the elevated melatonin concentrations on glucose tolerance would affect primarily people eating late at night. However, the most effective timing of melatonin administration in the treatment of DSPS is approximately 5-7 h prior to habitual bedtime, thus greatly increasing the likelihood of adverse concurrence of elevated melatonin concentrations and food intake, e.g., dinner.19
A previous study using nightly melatonin administration in diabetic patients found no adverse effects on glucose and lipid metabolism or other routine biochemical tests.7 A difference of that study compared to ours is that these patients were treated with prolonged-release melatonin for three weeks as opposed to a single dose of immediate release melatonin.
Another potential explanation for the lack of detrimental effects in that study is that beneficial effects on glucose control caused by improved sleep could have counteracted the adverse effects of melatonin.20 Indeed, in Garfinkel et al.,7 significant improvements in several sleep parameters were found for prolonged-release melatonin compared with placebo, while it has been established that sleep restriction results in metabolic and endocrine alterations, including decreased glucose tolerance and decreased insulin sensitivity.21,22 Because our OGTTs occurred immediately after melatonin administration, without any intervening sleep opportunity, our results were not affected by any indirect influences through sleep.
Other differences between the current data and previous works could be due to varying doses of melatonin, drug formulation (fast- or prolonged-release), time of day or night of administration, differences in study population, or the coincidence (or lack of coincidence) of food intake and melatonin administration.23 Because melatonin and placebo were administered after the fasting sample of the OGTT, we could not assess effects of melatonin on fasting glucose or insulin. Cagnacci and colleagues reported that morning melatonin administration also slightly increased fasting glucose concentrations.6 Future studies are needed to investigate the time-of-day dependent effects of melatonin on fasting glucose and insulin control.
In summary, although more research is needed to determine the impact of acute melatonin in populations with impaired glucose tolerances such as T2D, the current data show that a clinical dose (5 mg) of melatonin negatively impacts glucose tolerance. Therefore, the present results suggest that one may need to consider the proximity of melatonin administration to meal timing, particularly in those at risk for glucose intolerance or T2D, the optimum time should be at least 2 hours after the last meal to allow partial recovery of normal plasma glucose values before melatonin administration and therefore to avoid the associated impaired glucose tolerance. Although, these results are obtained in normal healthy women, data suggest that duration between a meal and melatonin administration may need to be even longer in patients with impaired glucose tolerance and T2D. Indeed in patients with T2D glucose concentrations may be elevated for 4 hours after a glucose load.24
One limitation of this study is that it was designed to determine basic physiological effects in a sample of healthy women (a homogeneous group with low variability). Future studies are needed to test this in men and in vulnerable populations, such as obese, diabetic, and older populations. Moreover, although OGTT is a good representation of what happens during the real life after ingestion of carbohydrate rich food/beverages, it has a higher variability in absorption rates and can be less reproducible than other techniques like IVGTT. An additional limitation is that the current design did not allow us to determine if there was an order effect in the treatment (placebo/melatonin); although subjects were given instructions to keep sleep and wake times constant, this could not be confirmed by objective measures and thus, it may be possible that differences in sleep prior to study visits may have influenced study findings.
In conclusion, acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. When administering melatonin, the proximity to meal timing may need to be considered, particularly in those at risk for glucose intolerance.
DISCLOSURE STATEMENT
This study was supported by grants from the TomásPascual and Pilar Gómez-Cuétara Foundations, the Spanish Government of Science and Innovation (BFU2011-24720 and BFU2010-21945-C02-01) and the Séneca Foundation from the Government of Murcia (15123/PI/10). Dr. Scheer was supported by grant NHLBI R01 HL094806. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Patricia Rubio-Sastre performed recruitment, laboratory assays and conducted the study, Purificación Gómez-Abellán performed laboratory assays, conducted the study, analyzed data, and wrote the paper; Frank A.J.L. Scheer discussed data and wrote the paper; Juan A. Madrid designed the study and wrote the paper. Marta Garaulet designed the study, wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript.
ABBREVIATIONS
- AUC
area under the curve above baseline
- Cmax
maximum concentration
- DI
Disposition Index
- DSPS
delayed sleep phase syndrome
- ISI
Insulin Sensitivity Index
- OGTT
oral glucose tolerance test
- T2D
type 2 diabetes
SUPPLEMENTAL MATERIAL
Protocol in the morning and evening. M/P, melatonin or placebo administration; OGTT, oral glucose tolerance test; BS, blood sample extraction; T, time; TF, time fasting. * Time after OGTT (min).
General characteristics of the population studied.
REFERENCES
- 1.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 2.Lyssenko V, Nagorny CL, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–8. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prokopenko I, Langenberg C, Florez JC, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamberg L. Melatonin potentially useful but safety, efficacy remain uncertain. JAMA. 1996;276:1011–4. [PubMed] [Google Scholar]
- 5.National Sleep Foundation. Summary of findings. 2005 Sleep in America Poll. Available at: http://www.sleepfoundation.org/sites/default/files/2005_summary_of_findings.pdf.
- 6.Cagnacci A, Arangino S, Renzi A, et al. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin Endocrinol. 2001;54:339–46. doi: 10.1046/j.1365-2265.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- 7.Garfinkel D, Zorin M, Wainstein J, Matas Z, Laudon M, Zisapel N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metab Syndr Obes. 2011;4:307–13. doi: 10.2147/DMSO.S23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–50. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 10.Sluiter WJ, Erkelens DW, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach I. Assay of the beta-cell response after oral glucose loading. Diabetes. 1976;25:241–4. doi: 10.2337/diab.25.4.241. [DOI] [PubMed] [Google Scholar]
- 11.Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–8. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 12.Peschke E, Bahr I, Muhlbauer E. Melatonin and Pancreatic islets: interrelationships between melatonin, insulin and glucagon. Int J Mol Sci. 2013;14:6981–7015. doi: 10.3390/ijms14046981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitagawa A, Ohta Y, Ohashi K. Melatonin improves metabolic syndrome induced by high fructose intake in rats. J Pineal Res. 2012;52:403–13. doi: 10.1111/j.1600-079X.2011.00955.x. [DOI] [PubMed] [Google Scholar]
- 14.Agil A, Rosado I, Ruiz R, Figueroa A, Zen N, Fernandez-Vazquez G. Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J Pineal Res. 2012;52:203–10. doi: 10.1111/j.1600-079X.2011.00928.x. [DOI] [PubMed] [Google Scholar]
- 15.McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309:1388–96. doi: 10.1001/jama.2013.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMullan CJ, Schernhammer ES, Forman JP. Melatonin level and risk for type 2 diabetes--in reply. JAMA. 2013;310:537. doi: 10.1001/jama.2013.7655. [DOI] [PubMed] [Google Scholar]
- 17.Abbott SM, Zee PC. Melatonin level and risk for type 2 diabetes. JAMA. 2013;310:536–7. doi: 10.1001/jama.2013.7649. [DOI] [PubMed] [Google Scholar]
- 18.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30:1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab. 2010;95:3325–31. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–61. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nedeltcheva AV, Scheer FA. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21:293–8. doi: 10.1097/MED.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 23.Hussain SA, Khadim HM, Khalaf BH, Ismail SH, Hussein KI, Sahib AS. Effects of melatonin and zinc on glycemic control in type 2 diabetic patients poorly controlled with metformin. Saudi Med J. 2006;27:1483–8. [PubMed] [Google Scholar]
- 24.Basu A, Dalla Man C, Basu R, Toffolo G, Cobelli C, Rizza RA. Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care. 2009;32:866–72. doi: 10.2337/dc08-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garaulet M, Hernandez-Morante JJ, Tebar FJ, Zamora S, Canteras M. Two-dimensional predictive equation to classify visceral obesity in clinical practice. Obesity (Silver Spring) 2006;14:1181–91. doi: 10.1038/oby.2006.135. [DOI] [PubMed] [Google Scholar]
- 26.Diabetes care. Introduction. Diabetes Care. 2013;36(Suppl 1):S1–2. doi: 10.2337/dc13-S001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol in the morning and evening. M/P, melatonin or placebo administration; OGTT, oral glucose tolerance test; BS, blood sample extraction; T, time; TF, time fasting. * Time after OGTT (min).
General characteristics of the population studied.