Abstract
Reduced levels of HDL cholesterol (HDL-C) are a strong independent predictor of coronary artery disease (CAD) risk. The major anti-atherogenic function of HDL is to mediate reverse cholesterol transport. This response is highly dependent on apoA-I and apoE, protein components of HDL. Randomized clinical trials have assessed effects of several classes of drugs on plasma cholesterol levels in CAD patients. Agents including cholestyramine, fibrates, niacin, and statins significantly lower LDL cholesterol (LDL-C) and induce modest increases in HDL-C, but tolerance issues and undesirable side effects are common. Additionally, residual risk may be present in patients with persistently low HDL-C and other complications despite a reduction in LDL-C. These observations have fueled interest in the development of new pharmacotherapies that positively impact circulating lipoproteins. The goal of this review is to discuss the therapeutic potential of synthetic apolipoprotein mimetic peptides. These include apoA-I mimetic peptides that have undergone initial clinical assessment. We also discuss newer apoE mimetics that mediate the clearance of atherogenic lipids from the circulation and possess anti-inflammatory properties. One of these (AEM-28) has recently been given orphan drug status and is undergoing clinical trials.
Keywords: apolipoprotein E, apolipoprotein A-I, high density lipoprotein, low density lipoprotein, mimetic peptides, atherosclerosis, inflammation
The American College of Cardiology (ACC) and the American Heart Association (AHA) provide current treatment guidelines for primary and secondary coronary artery disease (CAD) prevention (1). ACC/AHA recommendations call for the use of HMG-CoA reductase inhibitors (statins) in high-risk patient groups, including those with LDL cholesterol (LDL-C) values >190 mg/dl (1). A significant number of CAD patients, however, display resistance to statin therapy. A meta-analysis of seven randomized trials indicates that up to 50% of patients receiving intensive statin therapy fail to achieve LDL-C goals (2). This phenomenon has been linked to nucleotide polymorphisms in ABCA1 and apoE ε3 alleles (3). Other data suggest that cytokines and adipokines induce statin resistance by disrupting LDL-receptor feedback mechanisms (4, 5). Significant residual risk may also be present in CAD patients who have reduced LDL-C levels with statin therapy, but have elevated triglycerides and low HDL cholesterol (HDL-C) (6, 7).
Results of the Framingham Study showed a strong inverse correlation between HDL-C levels and CAD risk, even when LDL-C levels were normal (8, 9). HDL possesses anti-inflammatory and anti-oxidant properties, but its principal anti-atherogenic function is thought to be due to its ability to mediate reverse cholesterol transport (RCT). While macrophages possess a passive diffusion mechanism for cholesterol removal, the association of apoA-I with ABCA1 allows lipid-poor HDL to act as an active acceptor for cholesterol (10). Mature α-HDL particles also mediate cholesterol efflux via an interaction with ABCG1 (10). At this point, cholesteryl ester transfer protein (CETP) can mediate the exchange of HDL-associated cholesteryl esters for triglycerides derived from apoB-containing lipoproteins (11). The terminal step in RCT is characterized by the binding of the apoE component of HDL to one of multiple LDL receptor subtypes, resulting in the uptake and metabolism of cholesterol and lipids (12). Hepatic scavenger receptor class B member 1 (SRB1) also mediates the uptake of HDL-C and rerelease of preβ-HDL particles.
The concept that HDL may become dysfunctional in some disease states is gaining acceptance, and it has been suggested that the anti-inflammatory status of HDL may be of greater predictive value for CAD risk than HDL-C levels per se (11, 13). Thus, therapeutic approaches that increase the functional properties of HDL may be superior to simply raising HDL-C levels. In this review, we discuss recent therapeutic approaches for the management of plasma lipids that involve the application of apolipoproteins and synthetic apolipoprotein mimetic peptides. The apoA-I and apoE mimetic peptides are functionally similar to native apolipoproteins, but possess unique structural properties. A feature common to these peptides is that they have a positive impact on HDL quality and function. The apoA-I mimetic peptide 4F inhibits atherosclerotic lesion formation in murine models of atherosclerosis by inducing the formation of preβ-HDL particles that are enriched in apoA-I and the anti-oxidant enzyme paraoxonase-1 (PON-1) (14). 4F also possesses anti-inflammatory and anti-oxidant properties that are independent of its effect on HDL quality per se. Newer apoE mimetics similarly improve HDL quality but have the added benefit that they rapidly clear atherogenic lipids from the circulation.
CLINICAL TRIALS OF HDL-ELEVATING DRUGS
Multiple drug classes (fibrates, niacin, statins) exert beneficial effects on serum HDL-C concentration by stimulating hepatic apoA-I synthesis (15–20). Widespread use of these agents as HDL-raising drugs has been limited due to a modest stimulatory effect on circulating levels of the lipoprotein. In addition, compliance may be limited by unwanted side effects and adverse reactions. The AIM-HIGH trial was designed to test the effects of Niaspan (extended release niacin) on outcomes in subjects with atherogenic dyslipidemia (21). Results of this study showed that combined niacin/statin therapy reduced triglycerides and raised HDL-C levels; however, outcomes (myocardial infarction, stroke) were not reduced compared with statin treatment alone (21). The HPS2-THRIVE study assessed the effects of extended release niacin combined with an anti-flushing agent in patients receiving statins. This combination modestly increased HDL-C levels, but did not reduce the risk of major vascular events (22). In fact, the risk for development of diabetic complications was significantly increased (22). Recent efforts have also focused on the development of CETP inhibitors. The CETP inhibitor torcetrapib was shown to increase HDL-C levels in animal models and in human subjects with low HDL-C (23, 24). The ILLUMINATE trial subsequently tested effects of torcetrapib on HDL-C and outcomes in high-risk patients, but was terminated early due to an increase in mortality related to off-target effects (25). A subsequent trial showed that dalcetrapib increased HDL-C in CAD patients by approximately 30%, but did not improve cardiovascular (CV) outcomes (26). Further, dalcetrapib administration was associated with a significant increase in C-reactive protein and systolic blood pressure. Newer CETP inhibitors (anacetrapib and evacetrapib) are currently undergoing phase III clinical evaluation (27).
ANTI-ATHEROGENIC EFFECTS OF RECONSTITUTED HDL IN ANIMALS
Modified forms of apoA-I have been developed as therapeutic tools. In the presence of phospholipids, apoA-I forms discoidal preβ-like HDL particles that incorporate cholesterol to yield mature α-HDL particles (28). apoA-IMilano is a variant of apoA-I that was originally identified in carriers who were at low risk for development of CAD despite possessing low circulating HDL-C levels (29). Laboratory studies showed that recombinant apoA-IMilano reduced the lipid and macrophage content of arteries and reduced lesion formation in mouse and rabbit models of atherosclerosis (30, 31). While apoA-IMilano and wild-type apoA-I were equally effective in mediating ABCA1-dependent cholesterol efflux, the anti-oxidant properties of apoA-IMilano were found to be superior to native apoA-I (32).
Recent studies have examined the HDL mimetic properties of CSL-111 and CSL-112 (CSL Behring, Inc.) (33, 34). These are complexes of apoA-I (purified from human plasma) and phosphatidylcholine formed by the cholate dialysis method (34). Administration of CSL-111 to C57BL/6 mice resulted in an increase in serum preβ-HDL and cholesterol content. Ex vivo analyses showed that serum isolated from CSL-111-treated mice possessed enhanced macrophage cholesterol efflux capacity compared with serum from vehicle-treated controls (33). CSL-112 treatment in rabbits induced a similar increase in plasma-mediated ABCA1-dependent cholesterol efflux (34). CER-001 (Cerenis Therapeutics, Inc.) is another HDL mimetic that is composed of recombinant human apoA-I complexed with phospholipids (35). It is a potent inducer of cholesterol efflux and possesses anti-inflammatory properties. Administration of CER-001 to LDL receptor-null (LDLR−/−) mice was shown to reduce both atherosclerotic plaque size and vascular lipid content (35).
RECONSTITUTED HDL AND ATHEROMA REGRESSION IN HUMANS
Infusion of recombinant human proapoA-I/phospholipid complexes has been shown to transiently increase plasma apoA-I and HDL-C levels and stimulate cholesterol excretion in humans (36). Accordingly, several studies have tested the effects of reconstituted HDL (rHDL) on lesion regression in patients with acute coronary syndrome (ACS). Brewer and colleagues showed that delipidation of human plasma effectively converts αHDL to a preβ-like HDL particle (37). Using a plasmapheresis approach, they tested the effects of infusion of delipidated and unmodified HDL on fatty lesions in 26 ACS patients. Atheroma volume was measured at baseline by intravascular ultrasound (IVUS), followed by seven weekly infusions of delipidated or control plasma (37). Analysis of lipid profiles showed no change in total cholesterol, HDL-C, LDL-C, or VLDL cholesterol (VLDL-C) in either group over the course of the study. IVUS measurements at the end of the study period showed a trend for a decrease in atheroma volume in patients receiving delipidated HDL, but this was not significantly reduced compared with control plasma infusion (37).
The CHI-SQUARE trial subsequently tested the effects of CER-001 on coronary atherosclerosis in ACS patients (38). Atheroma volume was measured at baseline by IVUS, followed by six weekly infusions of placebo or CER-001 (3–12 mg/kg). At the end of the study, no difference in atheroma volume was noted between patients treated with CER-001 or placebo (38). The ERASE trial was similarly designed to test the effects of CSL-111 on lesion regression (39). ACS patients received four weekly infusions of placebo, CSL-111 (40 mg/kg), or CSL-111 (80 mg/kg) (39). The latter group was discontinued due to liver function abnormalities. At the end of the study, IVUS measurements showed no difference in atheroma volume between placebo- and CSL-111-treated subjects (39). Plaque volume, however, was reduced in the CSL-111 group compared with baseline (39). Recent studies show that CSL-112 administration in normal humans significantly increases plasma apoA-I and HDL-C, suggesting an increase in cholesterol efflux into the plasma compartment (40, 41). apoA-I levels in CSL-112-treated patients remained elevated above baseline for 3 days after treatment (41). At this time, effects of CSL-112 on atheroma regression have not been reported.
An important difference of apoA-IMilano:phospholipid complex administration in humans compared with animal studies is that it interacts with human apoA-II (a Cys-containing dimer) to form heterodimers of apoA-IMilano and apoA-II (42). In all other species, apoA-II is a monomer, does not contain a Cys residue, and thus does not form a heterodimer with apoA-IMilano (43). The low levels of circulating HDL-C in human carriers of apoA-IMilano may be related to an increased susceptibility of the apoA-IMilano-apoA-II heterodimer to proteolysis and increased clearance of apoA-II, which has been shown to be pro-atherogenic (42, 44, 45).
Effects of recombinant apoA-IMilano on plaque regression have been tested in ACS patients (46). Atheroma volume was measured at baseline by IVUS, followed by five weekly infusions of apoA-IMilano (15–45 mg/kg) or placebo. At the end of the study, atheroma volume was reduced 4.2% in patients receiving apoA-IMilano compared with baseline (46). While these results show promise for apoA-IMilano as a therapeutic agent, the study required intravenous (IV) administration of the drug and use of a relatively large amount of protein in the form of a protein-lipid complex (46). Surprisingly, published results from most studies of rHDL administration did not include analyses of plasma lipoproteins at study completion. A common consensus is that additional trials are required that target an elevation in HDL-C levels and which have defined clinical endpoints.
A PEPTIDE-BASED APPROACH TO HDL THERAPY
The observation that apolipoproteins possess lipid binding domains with a common structure initiated studies of peptides containing this structure (47, 48). The class A amphipathic helix is a common structural motif present in exchangeable apolipoproteins and is defined as an α-helix with opposing polar and nonpolar faces that are hydrophilic and hydrophobic, respectively (49). This sidedness of the helix forms a structure complementary to that of phospholipids, thus facilitating the formation of protein:lipid complexes (49). During the 1980s, several laboratories initiated studies to test the amphipathic helix hypothesis using short peptides containing unique sequences that were unrelated to those found in any of the exchangeable apolipoproteins. Yokoyama et al. (50) designed a 22-residue amphiphilic peptide composed of Lys, Leu, and Glu residues. This peptide associated with phospholipids and was shown to modestly activate the enzyme LCAT. The Baylor group subsequently designed a group of peptides called lipid-associating peptides (LAPs) that were composed of a 15 amino acid peptide sequence bound to acyl chains of variable length (51). Several of the LAP peptides were also shown to associate with phospholipids and activate LCAT. For unknown reasons, research on these two groups of peptides was not continued.
In order to understand the features of amphipathic helical domains that associate with phospholipids, we designed a model peptide with the sequence DWLKAFYDKVAEKLKEAF that possessed characteristics of a class A amphipathic helix (52). This peptide, designated 18A, has no sequence homology to any of the naturally occurring protein or peptide sequences. Despite its small size, addition of the peptide to a suspension of dimyristoyl phosphatidylcholine resulted in the formation a clear solution and (analogous to apoA-I) formed discoidal complexes (52, 53). In addition to forming small HDL-like particles, 18A was also able to activate LCAT (54). The model peptide 18A mimicked many of the properties of apoA-I and was therefore designated as an apoA-I mimetic peptide. We additionally found that capping the N terminus with an acetyl group and the carboxyl terminus with an amide group (Ac-18A-NH2) increased the helicity and lipid binding affinity of the peptide compared with unblocked 18A (55).
We subsequently developed a family of apoA-I mimetic peptides that were structural variants of 18A. It was found that sequential substitution of aliphatic amino acids (Leu, Val) on the nonpolar face of 18A with phenylalanine (F) resulted in the formation of class A peptides with increased hydrophobicity and lipid binding affinity (3F, 4F, 5F, 6F, 7F) (56). The phospholipid binding capacity was found to be greatest with end-capped 4F and 5F, both of which formed discoidal HDL-like structures when complexed with phospholipids (56). A unique feature of these peptide analogs is that while apoA-I (in the absence of cholate) cannot solubilize POPC vesicles, the peptides instantly solubilize turbid vesicular suspensions of this lipid to form peptide:lipid complexes (47, 57).
DEMONSTRATION OF ANTI-ATHEROGENIC EFFECTS OF apoA-I MIMETIC PEPTIDES IN ANIMAL MODELS OF ATHEROSCLEROSIS
Despite publications on the in vitro properties of apoA-I mimetic peptides, the field did not evolve for another decade when the first atherosclerosis inhibition study was performed. Garber et al. (58) showed that daily intraperitoneal (IP) injection with 5F in C57BL/6J mice fed an atherogenic diet significantly reduced aortic lesion formation compared with treatment with either saline or mouse apoA-I. 5F administration was also more effective than mouse apoA-I in reducing plasma lipid hydroperoxide content and monocyte chemotactic activity (58). The observation that total plasma cholesterol levels and lipoprotein profiles were not significantly different between the treated and control groups, suggested that protective effects of 5F were independent of these factors (58).
Once it was demonstrated that small peptides have the potential to inhibit atherosclerosis, several laboratories initiated the design of peptide analogs that exhibit properties of apoA-I (e.g., activation of LCAT and PON-1; cholesterol efflux activity). In 1990, we reported that a 22mer synthetic peptide analog (based on the consensus sequence domain of amphipathic helical repeats in apoA-I) rivaled native apoA-I in its ability to induce the activation of LCAT (59). Subsequently, smaller peptides, including KRES and FREL, were developed that were shown to increase HDL-C and PON-1 activity and reduce atherosclerotic lesion formation in apoE−/− mice (60). A 10 amino acid peptide from the apoJ sequence was also shown to increase plasma cholesterol efflux capacity in apoE−/− mice and improve HDL anti-inflammatory properties (61). Other groups set out to improve the properties of existing peptide sequences (62, 63) or to design entirely new sequences (64–67). Starting with the design of 18A and its analogs, examples of these apolipoprotein mimetic peptides are summarized in Table 1.
TABLE 1.
Apolipoprotein mimetic peptides studied in different laboratories
| Peptide | Sequence | Properties | Reference |
| Analogs of 18A | |||
| 18A | DWLKAFYDKVAEKLKEAF | Shortest peptide shown to clear phospholipid | 52, 74 |
| Ac-18A-NH2 | Ac-DWLKAFYDKVAEKLKEAF-NH2 | Significantly enhanced lipid-associating properties | 55–56 |
| 3F-1 | Ac-DKLKAFYDKVFEWAKEAF-NH2 | Inhibits monocyte chemotaxis and lesion | 56 |
| 3F-2 | Ac-DKWKAVYDKFAEAFKEFL-NH2 | Inhibits monocyte chemotaxis and lesion | 56 |
| 3F3 | Ac-DWFKAFYDKVAEKLKEAF-NH2 | Inactive to inhibit monocyte chemotaxis and lesion | 56 |
| 3F14 | Ac-DWLKAFYDKVAEKFKEAF-NH2 | Inactive to inhibit monocyte chemotaxis and lesion | 56 |
| 4F | Ac-DWFKAFYDKVAEKFKEAF-NH2 | Extensively studied-inhibits lesion and inflammation | 14, 68–73, 75, |
| D-4F is orally active | 78–79 | ||
| 5F | Ac-DWLKAFYDKFFEKFKEFF-NH2 | First apoA-I mimetic used to demonstrate lesion inhibition | 56, 58 |
| 6F | DWLKAFYDKFFEKFKEFF | Transgenic 6F tomato extract reduces lesion in mice | 56, 81–82 |
| AP-peptide | PKLEELKEKLKELLEKLKEKLA | Associates with lipid to form complexes-modest LCAT activation | 50 |
| LAP | X-SSLKEYWSSLKESES [where X = C8-CO to C16-CO-] | Associates with lipid vesicles-modest LCAT activation | 51 |
| Pro-linked dimers | |||
| 37pA | 18A-P-18A | Effluxes cellular cholesterol via ABCA1 and microsolubilization | 63, 74, 83–84 |
| 5A | 18A-P-DWAKAAYDKAAEKAKEAA | Specific for ABCA1-mediated cellular cholesterol efflux-inhibits lesion | 84–87 |
| 4F-P-4F | DWFKAFYDKVAEKFKEAF-P- DWFKAFYDKVAEKFKEAF | A more effective mimic of native apoA-I properties than the 4F monomer | 62, 78 |
| ELK-2K2A2E | EKLKAKLEELKAKLEELL-P- EKLKAKLEELKAKLEELL | More effective than apoA-I in capacity and specificity of cholesterol efflux | 64, 87 |
| apoA-I consensus peptide | |||
| A-I consensus sequence | PVLDEFREKLNEXLEALKQKEK [where X = E,A,R,H] | Monomers and P-linked dimers were synthesized. The dimer peptide with X = E was as active as apoA-I in LCAT activation | 59 |
| Peptides derived from apoA-I | |||
| apoA-I [44-65] and [220-241] | Native sequences [44-65] and [220-241] | Only the N-terminal and C-terminal monomers and no other 22mers associate with phospholipid | 88 |
| ApoA-I [44-87] and [209-241] | Native sequences [44-87] and [209-241] | Only N-terminal and C-terminal 44 and 33 residue tandem dimers associate with lipid | 67 |
| Helix 10 analog | CGVLESFKASFLSALEEWTKKLQ-NH2 | Modified helix 10 [221-241] sequence. Incorporation of monomer or trimer sequence in nanoparticles inhibits lesion formation in LDLR−/− mice | 66, 89 |
| Peptide derived from apoE lipid-associating sequence | |||
| ATI-5261 | EVRSKLEEWFAAFREFAEEFLARLKS | Mediates ABCA1-dependent cellular cholesterol efflux and inhibits lesion | 65, 127 |
| Shorter peptides studied | |||
| apoJ [113-122] | Ac-LVGRQLEEFL-NH2 | An orally active 10-residue peptide that inhibits lesion | 61 |
| FREL | Boc-FREL-oBut | Shortest apoA-I mimetic peptide that inhibits lesion when administered orally | 60 |
| apoE mimetic peptides | |||
| Ac-hE18A-NH2 | Ac-LRKLRKRLLR-18A-NH2 | Rapidly decreases cholesterol in animal models and inhibits lesion | 93, 122, 131–133, 135–136 |
| Ac-[R]hE18A-NH2 | Ac-LRRLRRRLLR-18A-NH2 | More effective than Ac-hE18A-NH2 in cellular uptake of LDL and VLDL, remains to be studied for lesion inhibition | 93 |
| mR18L | Ac-GFRRFLGSWARIYRAFVG-NH2 | Rapidly decreases cholesterol but is less effective than Ac-hE18A-NH2 in reducing lesion | 133, 144 |
| apoE [141-155] dimer | Ac-YLRKLRKRLLRDADDL- LRKLRKRLLRDADDL | Acetylated dimer increases plasma lipoprotein binding and clearance | 125 |
apoA-I mimetic sequences which appear in patents but no paper is published or papers in which peptide sequence is not given are not covered in this review and therefore, are not part of this table.
Due to its superior anti-oxidative effects, peptide 4F was most extensively studied in several different laboratories within and outside the US (68–73). A comparative study showed that 4F had improved solubility properties and was more effective in inhibiting LDL-induced monocyte chemotaxis than 5F (56). For these reasons, subsequent studies largely focused on an examination of atherosclerosis-protective mechanisms of 4F in cell culture and animal models. Based on our observation that 18A synthesized using D-amino acids (D-18A) was resistant to metabolism in vivo and found intact in the urine of rats, we tested to determine whether synthesis of the 4F peptide using D-amino acids (D-4F) yielded an apoA-I mimetic that could be orally administered (74). Preliminary studies in apoE−/− mice showed that the plasma concentration of D-4F reached ∼138 ng/ml 20 min after administration of 500 μg peptide by gavage (14). Subsequent studies showed that D-4F could be detected in the circulation of LDLR−/− mice after oral administration and was resistant to proteolytic degradation (75). In contrast, peptide was undetectable in plasma after oral administration of 4F synthesized from L-amino acids (75). D-4F reduced atherogenic lesions in LDLR−/− mice, but did not significantly reduce plasma or HDL-C (75). D-4F treatment was, however, associated with an improvement in the functional properties of HDL. Under ex vivo conditions, HDL isolated from D-4F-treated mice was more effective in inhibiting LDL oxidation than was HDL isolated from saline-treated mice (75). Similar anti-atherogenic effects of D-4F were observed in hyperlipidemic rabbits (76). These results are in agreement with the proposal by Ansell et al. (77) and suggest that HDL quality and function are better predictors of atherogenic lesion formation than circulating HDL concentration per se.
Subsequent studies showed that D-4F treatment induced the formation of preβ-HDL in the plasma of apoE−/− mice that was enriched in apoA-I and possessed PON-1 activity (14). Further, the lipid hydroperoxide content of apoB-containing lipoproteins was significantly reduced (14). These results suggested that D-4F induces the formation of HDL particles possessing a high capacity for cholesterol uptake (14). The timing of 4F administration may be a critical determinant of its anti-atherogenic effect. While most studies have shown that 4F administration prevents the development of atherogenic lesions, regression of established lesions appears to require adjunct cholesterol-lowering therapy (78, 79).
RECENT INSIGHTS INTO THE MECHANISM OF ACTION OF 4F
Initial studies suggested that the anti-atherogenic mechanism of apoA-I mimetic peptide action involved the remodeling of HDL particles in plasma. Recent studies, however, suggest that the small intestine may be a critical site of action for 4F and structurally related peptides (80). D-4F was administered either orally or subcutaneously (SQ) to apoE−/− mice. Plasma levels of peptide achieved by SQ treatment were approximately 1,000-fold higher compared with oral administration of the same dose (80). Despite significant differences in plasma levels of the peptide achieved by oral or SQ administration, plasma serum amyloid A (SAA), the HDL inflammatory index, and atherogenic lesion formation were similarly reduced. Further, the amount of peptide measured in feces was similar (80). The authors concluded that anti-atherogenic responses to 4F administration are dependent on the dosage of the peptide rather than the plasma levels achieved and that the intestine may be a major site of 4F action (80). A recent study shows that the N- and C-uncapped peptide 6F (DWLKAFYDKFFEKFKEFF) exerts similar anti-atherogenic effects as capped 4F (Ac-DWFKAFYDKVAEKFKEAF-NH2) (81, 82). Oral administration of 6F resulted in its localization to the small intestine of LDLR−/− mice (81). This was associated with an increase in circulating HDL-C and PON-1 activity and a reduction in total cholesterol, SAA, intestinal lipid peroxide content, and aortic lesion formation (81). It was proposed that a major mechanism of action of 6F was to reduce intestinal and circulating levels of lysophosphatidic acid, an important mediator of the systemic inflammatory response (81, 82). The 6F peptide used in these studies was derived from genetically engineered tomatoes that expressed the uncapped peptide. Mice fed the lyophilized tomato powder from plants expressing peptide 6F (mixed with mouse chow) had reduced lesion formation. In contrast, mice fed freeze-dried tomatoes containing vector did not show any effect (81). While the approach of administering an apoA-I mimetic peptide as a dietary product is promising, the quantity of ingested tomatoes required for therapeutic efficacy in humans (3.15 g/70 kg) may be impractical. Thus, additional research is needed to improve dosing regimens.
DIMERIC apoA-I MIMETIC PEPTIDES
Because apoA-I contains tandem repeating amphipathic helical domains, most of them punctuated by a Pro residue, several laboratories have developed and tested the effects of apoA-I mimetic peptide dimers on atherosclerotic lesion formation (62, 78, 83). A 4F dimer, created via proline residue linkage, was shown to avidly associate with HDL particles in vivo while the 4F monomer did not. Further, the 4F dimer displayed stronger anti-inflammatory effects in vivo, as revealed by a significant reduction in SAA levels in apoE−/− mice (62, 78). It was suggested that dimeric 4F was a more effective mimic of native apoA-I properties than the monomer (62, 78). Other studies have shown that the peptides L-37pA and D-37pA, bihelical analogs of 18A (18A-Pro-18A), form HDL-like particles that are similar to rHDL (63, 84). These peptides promoted macrophage cholesterol efflux by both ABCA1-dependent and -independent mechanisms (63). In the presence of POPC, the size of the particles formed and the α-helical content of L-37pA, D-37pA, and apoA-I were similar (83). L-37pA and D-37pA exerted similar protective effects as rHDL by reducing adhesion molecule expression in TNF-α-stimulated endothelial cells and by preventing cardiac dysfunction in a model of ischemia-reperfusion injury (83). 5A is a bihelical amphipathic peptide derived from 37pA (63). This peptide is obtained by the substitution of Ala for five existing hydrophobic residues in one of the 18A helices. The asymmetry in the lipid binding capacity of the helices was associated with a reduction in cytotoxicity compared with 37pA and enhancement of ABCA1-dependent cholesterol efflux from macrophages (63). 5A was also shown to reduce lesion formation in apoE−/− mice, ameliorate inflammation in a carotid artery injury model in the rabbit, and attenuate airway inflammation in a murine model of asthma (63, 85, 86).
ELK-2A2K2E is another bihelical peptide that induces the formation of small dense HDL particles in the plasma of apoE−/− mice and demonstrates highly efficient cholesterol efflux activity and a reduction in aortic lesion content (64). In contrast to other apoA-I mimetic peptides, ELK-2A2K2E significantly reduced plasma lipoprotein and triglyceride levels (64). D’Souza et al. (87) have assessed the structure/function relationships of 22 bihelical apoA-I mimetic peptides (including ELK-2A2K2E) with their putative anti-atherogenic functions. Parameters that were analyzed included the capacity/specificity of peptides to mediate cholesterol efflux, efficacy in inhibiting inflammatory responses, and anti-oxidant properties (87). None of the peptides were equally effective in inhibiting each of the parameters of HDL function. The authors suggested that different structural motifs in HDL inhibit atherogenesis by different mechanisms, thus underscoring the difficulty in designing an ideal HDL mimetic.
The synthesis and application of HDL-like nanolipid particles is a recent development in the field of HDL therapy. Earlier, it was shown that, among the 22mer helices of apoA-I, only the N- and C-terminal helices were able to associate with phospholipids while the other regions (either as 22mers or as 44mers) did not (67, 88). These studies also showed that amino acid residues 221-241 of apoA-I play an important role in lipid binding and cholesterol efflux (67, 88). Zhao et al. (66) modified this sequence and appended a Cys-Gly dipeptide to generate a 23 amino acid α-helical peptide. Peptide-containing nanoparticles (9–15 nm) were generated by addition of dimyristoyl phosphatidylcholine multilamellar vesicles (66). Under in vitro conditions, nanoparticles incorporated into HDL converted the native lipoprotein into a lipid-poor HDL particle and were effective mediators of macrophage cholesterol efflux (66). A trimeric structure was subsequently generated by linking three copies of the peptide to an organic scaffold, followed by incorporation into nanoparticles (89). Administration of these HDL nanoparticles (either orally or by IP administration) increased HDL-C, while reducing VLDL-C/LDL-C and aortic lesion area in LDLR−/− mice (89). Surprisingly, monomeric and trimeric peptides (synthesized using L-amino acids) were orally active but could not be detected in plasma (89). It was suggested that, similar to 4F/6F, the intestine is a critical site of HDL nanoparticle action (89).
CLINICAL EVALUATION OF THE apoA-I MIMETIC PEPTIDE 4F
Because a large body of information had been published for the apoA-I mimetic peptide 4F, an initial clinical evaluation of D-4F was performed in high-risk CAD patients. Subjects received a single oral dose of D-4F (30, 100, 300, or 500 mg) or placebo (90). The study showed that D-4F was safe and well-tolerated but had a low bioavailability. A major finding of this study was that D-4F administration significantly reduced the HDL inflammatory index in patients receiving the 300 or 500 mg dosage (90). Plasma samples, however, were not analyzed to determine whether preβ-HDL was formed, analogous to observations in murine models. In a subsequent study, Watson et al. (91) tested the effects of IV or SQ administered L-4F (3–100 mg) on CV risk factors in CAD patients. The goal of this study was to test whether comparable effects could be elicited by IV/SQ administration of a low dose of the peptide that resulted in a higher plasma concentration. The HDL inflammatory index was not improved by either route of administration (91). It was subsequently proposed that L-4F levels in intestine may not reach a level required for therapeutic efficacy when administered by IV/SQ route (92). This may explain the lack of effect of low dose L-4F on the HDL inflammatory index in the Watson study (91). While it is not clear why the second clinical study was not performed using D-4F (despite encouraging results), it is possible that the sponsors of clinical studies wanted to avoid the possible accumulation of metabolically resistant D-4F in tissues (74). A drawback in the development of apoA-I mimetic technology has been the identification of an acceptable and easily reproducible biomarker which can be monitored during the course of treatment. HDL-C levels are not suitable for this purpose because they are not altered by 4F treatment. Due to the heterogeneity of HDL-associated proteins, the identification of a specific protein that is altered by 4F treatment has also been challenging. In the absence of such an appropriate biomarker, the anti-atherogenic efficacy of apoA-I mimetic treatment will be difficult to assess. In addition, it is important to compare the efficacy of different peptide analogs in the same set of experiments in order to determine specific apoA-I mimetic property(ies) that is/are important for inhibiting not only atherosclerosis but also several other lipid-mediated disorders.
ANTI-ATHEROGENIC MECHANISMS OF apoE ACTION
apoE is an exchangeable apolipoprotein that is associated with HDL, VLDL, and LDL remnant particles. It is synthesized primarily by hepatocytes and macrophages and plays a critical role in regulating plasma cholesterol levels. apoE contains a lipid-associating domain and a globular domain containing the LDL receptor binding site (93). The presence of the LDL receptor binding domain facilitates the hepatic clearance of cholesteryl esters, resulting in a significant decrease in plasma cholesterol (94). Unlike LDL, which is cleared by the LDL receptor, the apoE-containing lipoproteins can also be cleared by alternative receptors such as LDL receptor-related proteins (LRPs) and heparan sulfate proteoglycans (HSPGs) (95). Thus, apoE plays a prominent role in clearing apoB-containing lipoproteins such as chylomicrons, VLDL, and remnant lipoproteins, all of which can be pro-atherogenic.
Several studies highlight the efficiency of apoE and show that only a small amount of the apolipoprotein is required to reduce plasma cholesterol and atherosclerotic lesion formation (96, 97). Transgenic apoE−/−hTgE+/0 mice have been generated that express the human apoE3 gene in macrophages (96). Plasma apoE levels in these mice are approximately 7–10% of those in wild-type mice. Despite the low level expression of apoE, apoE-containing lipoproteins were formed in plasma of apoE−/−hTgE+/0 mice that were as effective as lipoproteins from wild-type mice in mediating cholesterol efflux from fibroblasts (96). In contrast, cholesterol efflux mediated by lipoproteins from apoE−/− mice was negligible (96). In another study, bone marrow (BM) from wild-type mice was injected into irradiated apoE−/− mice. After 4 weeks, serum apoE levels reached 12.5% of levels measured in normal C57BL/6 mice (97). This level of apoE was associated with a 74% reduction in plasma cholesterol compared with baseline cholesterol measurement prior to transplantation (97). Further, aortic lesions were significantly reduced in these mice 3 months after initiating feeding with a Western diet (97).
Protective effects of apoE have been observed in the context of coagulation, macrophage function, oxidative processes, central nervous system physiology, inflammation, and cell signaling (98, 99). Data suggest that anti-inflammatory effects of the apolipoprotein are independent of its cholesterol-lowering property (99–102). Polymorphisms in the apoE gene are associated with enhancement of the inflammatory response and an increase in mortality in animal models and patients with sepsis (103, 104). Deletion of the apoE gene also induces these responses in mice treated with either bacterial lipopolysaccharide (LPS) or infected with bacterial pathogens (101, 102, 105). In apoE-expressing mice, the apolipoprotein has been shown to directly interact with and neutralize endotoxin, resulting in a reduction in plasma cytokine levels and mortality (100–106). Further, adoptive transfer of apoE-replete BM has been shown to attenuate the type I inflammatory response in apoE−/− mice (100).
Monocytes and macrophages are important targets for apoE action. apoE-containing HDL subspecies were shown to reduce circulating Ly6hi and increase Ly6lo monocytes, resulting in decreased monocyte adhesion to the endothelium (99). Transduction of apoE in macrophages of atherosclerotic apoE−/− mice significantly reduces lesion formation without affecting plasma cholesterol levels (107–109). Several studies suggest that increasing apoE expression in monocytes induces an anti-inflammatory phenotype and reduces the recruitment of these cells to sites of injury (110–113). Leukocytosis (monocytosis and neutrophilia) increases tissue injury in models of sepsis and atherosclerosis (114, 115). This process is regulated by apoE. In the absence of apoE, hematopoietic stem cell proliferation, mobilization, and the production of monocytes and neutrophils are significantly increased (115). It follows that transplantation of BM containing apoE reduced leukocytosis (115). Leukocytosis was also induced by a reduction in HDL concentration in mice and humans in a manner that was attenuated by raising apoE and HDL levels (115–118). The protective role of apoE under these conditions was associated with apoE binding to leukocyte HSPG and an interaction with ABCA1/ABCG1 to reduce cellular cholesterol content (115, 118). HDL-associated apoE thus plays a critical role in ameliorating inflammatory cell injury. On the basis of these observations, we set out to design small peptide structures which not only possess anti-inflammatory properties (analogous to apoA-I mimetic peptides) but also reduce plasma cholesterol levels by an LDL receptor-independent mechanism.
apoE MIMETICS: A NEW DIRECTION IN PEPTIDE-BASED THERAPIES
One of the milestones in the study of cholesterol metabolism is the discovery of receptor-mediated uptake and clearance of LDL-C, which was recognized by the award of the Nobel prize to Drs. Goldstein and Brown (119). While the ligand for LDL is apoB with its receptor binding domain being positively charged [RLKRGLK (residues 359-367)], the ligand for clearing nonLDL apoB-containing particles is apoE via its positively charged receptor binding domain [LRKLRKRLLR (residues 141-150)]. These lipoproteins possess one or two copies of such receptor binding domains because the apolipoproteins are large (4,536 and 299 amino acids for apoB-100 and apoE, respectively). Additionally, while LDL clearance is highly regulated via regulation of LDL receptor synthesis, the receptors for apoE (LRP and HSPG) are not. Earlier research by Mahley and Ji (120) demonstrated that apoE-containing atherogenic particles are cleared via HSPG in the space of Disse. It was also demonstrated that both the lipid-associating and receptor binding domains of apoE are required for lipoprotein binding and clearance. Because apoE has a long amphipathic helical lipid-associating domain, we hypothesized that direct linkage of the apoE putative receptor binding domain to a short peptide (such as 18A) capable of associating with lipid would produce a dual domain peptide which could associate with atherogenic apoB-containing lipoproteins to enhance their binding and clearance. Further, such a structure would allow several molecules of the peptide to associate with and clear atherogenic lipoproteins rapidly. This formed the basis for the design of a dual-domain apoE mimetic peptide that will be discussed later.
Numerous synthetic peptides based on the structure of apoE have been designed (121). apoE contains a lipid-associating domain (residues 203-266) and a globular domain (residues 1-191) containing the LDL receptor binding site (93, 122). Several peptides spanning the 130-169 region of apoE have been synthesized in order to test lipoprotein binding and clearance (123, 124). Curtiss and colleagues studied the effects of modified apoE peptides on LDL receptor binding (123). A dimeric peptide containing residues 141-155 of apoE was shown to bind to the LDL receptor while the 141-155 monomer was ineffective (123). It was subsequently reported that acetylation of the N-terminal domain of the 141-155 dimer increased plasma lipoprotein binding and clearance (125).
Bielicki and colleagues have identified a region in the C-terminal domain of apoE that plays a critical role in ABCA1-dependent cholesterol efflux and HDL particle assembly (126). C-terminal residues 238-270 were structurally modified to yield the single helix model peptide ATI-5261 (65). Biophysical studies showed that the peptide possessed a high aqueous solubility that dissociated to trimers, dimers, and monomers upon dilution (127). The efficiency of ATI-5261 in mediating macrophage cholesterol efflux was similar to that of full-length apoA-I and apoE (65). Further, cholesterol efflux was increased by treatment of cells with peptide in either a lipid-free form or as a phospholipid complex (65). Chronic treatment with ATI-5261 reduced fatty lesions in aortae of LDLR−/− and apoE−/− mice (65). Results of these studies suggested that less frequent dosing with ATI-5261 was required for inhibition of atherogenic lesions compared with apoA-I mimetic peptides (65). Other investigators have incorporated α-aminoisobutyric acid groups within residues 133-149 of apoE to generate chimeric peptides with increased helicity (128, 129). These peptides, designated cogniscience apoE mimetic peptides (COGs), were shown to exert prominent anti-inflammatory effects in the central nervous system (128, 129). COG133 and COG1410 reduce inflammatory injury in murine models of multiple sclerosis and traumatic brain injury, respectively, and are thought to mediate their effects via binding to LRP (128–130).
In light of the importance of apoE not only in reducing levels of atherogenic lipoproteins but also in exerting cholesterol-independent atheroprotective effects, our laboratory initiated the development of synthetic dual-domain apolipoprotein mimetic peptides which are structurally and functionally similar to apoA-I and apoE but possess unique properties analogous to apoE (93, 122). The synthetic peptide 18A, similar to apoA-I, possesses a class A amphipathic helical structure and mimics some properties of apoA-I (53). Several class A peptides exert prominent anti-inflammatory effects by reducing lesion formation in murine models of atherosclerosis but do not reduce plasma cholesterol levels (58). Based on the dual-domain structural concept present in apoE, we developed a synthetic peptide that mimicked the cholesterol-lowering properties of apoE. A peptide encoding an arginine-rich region (residues 141-150, LRKLRKRLLR) of the putative LDL receptor binding sequence of apoE was linked to 18A. The peptide was acetylated and amidated at the N and C termini, respectively, to yield the stabilized peptide Ac-hE18A-NH2.
ANTI-ATHEROGENIC AND ANTI-INFLAMMATORY EFFECTS OF Ac-hE18A-NH2
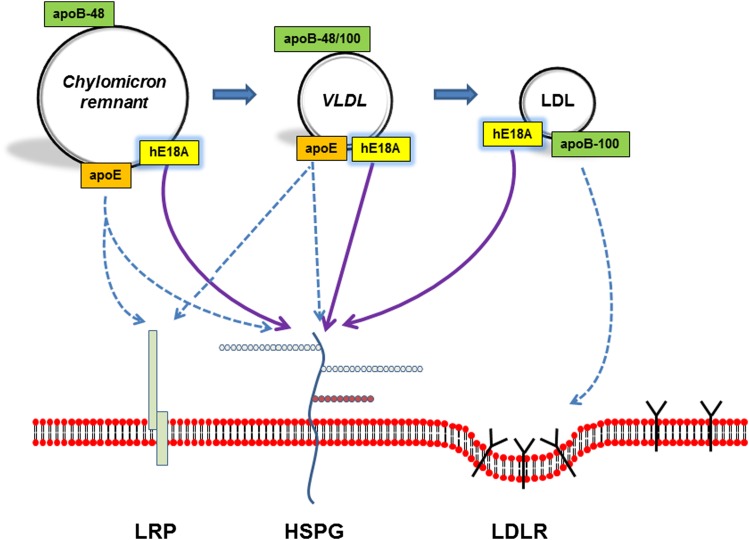
Preliminary studies demonstrated that Ac-hE18A-NH2 associates with chylomicron remnants, VLDL-C, and LDL-C, and targets them to hepatocytes for clearance via binding to HSPG (Fig. 1) (93, 122). The peptide was able to mediate cholesterol efflux independently of ABCA1 and had the additional advantage of facilitating cholesterol clearance due to the presence of the receptor binding domain (131). In vivo administration of Ac-hE18A-NH2 has been shown to reduce plasma cholesterol in mouse and rabbit models of atherosclerosis (122, 132, 133). In Watanabe heritable hyperlipidemic (WHHL) rabbits, plasma cholesterol reduction was associated with an increase in plasma PON-1 activity (132). The observation that Ac-hE18A-NH2 reduces plasma cholesterol in WHHL rabbits and LDLR−/− mice fed a Western diet supports the conclusion that the peptide clears atherogenic lipoproteins via a LDL receptor-independent mechanism. Candidates for clearance include HSPG and LRP. In another study, we found that pretreatment of apoE−/− mice with heparinase, followed by apoE mimetic administration, did not reduce plasma cholesterol (134). This result strongly supports that Ac-hE18A-NH2 clears atherogenic lipoproteins via interaction with HSPGs. Agarose gel electrophoresis of lipoproteins in Ac-hE18A-NH2-treated animals shows that the mobility of apoB-containing lipoproteins is retarded (132). This suggests that the peptide readily associates with apoB-containing lipoproteins and that, by enhancing surface positive charge, it facilitates the clearance of these surface-modified lipoproteins via the HSPG pathway.
Fig. 1.
High efficiency clearance of atherogenic lipoproteins by Ac-hE18A-NH2. apoE plays an important role in the hepatic uptake of chylomicron remnants and VLDL particles via binding to LRPs and/or HSPGs. apoB-100 mediates the interaction of LDL particles with LDL receptors. These lipoprotein uptake mechanisms are denoted by dashed lines. Ac-hE18A-NH2 (hE18A) associates with each lipoprotein class and mediates enhanced uptake by binding to HSPGs (solid lines).
Administration of Ac-hE18A-NH2 in WHHL rabbits also improved endothelial function, a response that was associated with a reduction in tissue superoxide anion formation (132). While Ac-hE18A-NH2 is rapidly cleared from the circulation after IV administration, it continues to exert significant beneficial effects on aortic lesions for up to a month after suspending peptide administration in mouse models of atherosclerosis (135). Subsequent studies revealed that Ac-hE18A-NH2 induces the release of PON-1 and lipid-poor preβ-HDL particles from hepatocytes and also promotes apoE secretion from hepatocytes and macrophages (131, 133, 136, 137). These studies further showed that Ac-hE18A-NH2 is recycled by cells, thus enabling the potentiation and prolongation of its anti-atherogenic and anti-inflammatory effects (131). These results suggest that the apoE mimetic peptide Ac-hE18A-NH2, analogous to apoE, exerts cytoprotective effects that are independent of effects on plasma cholesterol (138–140). In support of this, a recent study shows that administration of Ac-hE18A-NH2 in APP/PS1ΔE9 transgenic mice, a murine model of Alzheimer’s disease, inhibits amyloid plaque deposition and improves cognitive function (137).
The anti-inflammatory properties of Ac-hE18A-NH2 were found to be superior to those of the class A peptide 4F (141, 142). Specifically, Ac-hE18A-NH2 was more effective than 4F in reducing atherogenic lesion formation in apoE−/− mice (141). In this study, the anti-inflammatory response to Ac-hE18A-NH2 was associated with an increase in HDL-associated PON-1 activity and a reduction in SAA (141). Another study showed that Ac-hE18A-NH2 was more effective than 4F in reducing respiratory burst activity in human leukocytes treated with LPS (142). Further, Ac-hE18A-NH2 displayed a greater inhibitory effect on cytokine secretion in THP-1 macrophages (142). Treatment of cells with LPS isolated from one of three different Escherichia coli strains or Pseudomonas aeruginosa significantly increased TNF-α and IL-6 release (142). While pretreatment with 4F reduced cytokine release, the inhibitory response to Ac-hE18A-NH2 was greater in all cases (142).
Several analogs of Ac-hE18A-NH2 were designed in order to understand the importance of positively charged residues and the role of the hydrophobic residues in the receptor binding domain of the peptide (93). Ac-LRRLRRRLLR-18A-NH2 [Ac-hE(R)18A-NH2] and Ac-LRKMRKRLMR-18A-NH2 (Ac-mE18A-NH2) contained an extended hydrophobic face, including the receptor binding region, while Ac-RRRRRRRRRR-18A-NH2 [Ac-R(10)18A-NH2] did not. A scrambled control peptide, Ac-LRLLRKLKRR-18A-NH2 [Ac-hE(Sc)18A-NH2], had the same amino acid residues as Ac-hE18A-NH2, but these were scrambled to disrupt the extended hydrophobic face. Despite identifying Ac-hE(R)18A-NH2 as more effective than Ac-hE18A-NH2 in reducing plasma cholesterol, extensive in vivo studies have not been performed on other peptides. It is also possible that the lipid-associating domain 18A can be changed to other apoA-I mimetic peptides. With additional studies on future analogs, we hope to develop apoE mimetic peptides that are active when administered orally or by inhalation. With proper modification of the sequence, it is also possible to genetically engineer an apoE sequence that can be produced in fruits and vegetables, as has been accomplished for the apoA-I mimetic peptide 6F (82).
DEVELOPMENT OF AN ORALLY ACTIVE apoE MIMETIC PEPTIDE
Our studies to date have shown that Ac-hE18A-NH2 bioactivity is significantly reduced by oral or IP administration; thus, parenteral administration is required for therapeutic efficacy. Recent efforts have focused on the development of an orally active peptide that possesses both cholesterol-reducing and anti-inflammatory properties. To accomplish this, the structure of a helical class L peptide (18L) was modified by incorporating aromatic amino acids at the center of the nonpolar face (143). This resulted in the formation of a modified 18L (m18L) with increased capacity to bind to oxidized lipids. The peptide m18L was further modified by substituting Lys residues with Arg to yield mR18L (Ac-GFRRFLGSWARIYRAFVG-NH2) (144). The incorporation of Arg residues in this peptide enhanced the uptake of LDL by HepG2 cells (144). Further, oral administration of mR18L, but not m18L, reduced plasma cholesterol and atherosclerotic lesion formation in apoE−/− mice (144). Plasma from m18L-treated mice promoted the adhesion of monocytes to cultured bovine aortic endothelial cells. This response was not observed with plasma from mR18L-treated mice (144). These results suggested that mR18L possessed similar properties as Ac-hE18A-NH2, but possessed the advantage of oral bioavailability.
A head-to-head comparison of the anti-atherogenic effects of Ac-hE18A-NH2 and mR18L has been recently performed in LDLR−/− mice (133). Multiple retro-orbital administrations of each peptide (100 μg/mouse, twice weekly for 8 weeks) resulted in similar reductions in plasma cholesterol (133). Plasma levels of reactive oxygen species were significantly reduced in mice treated with Ac-hE18A-NH2, but not mR18L. While atherosclerotic lesion formation was reduced by administration of both peptides, Ac-hE18A-NH2 was significantly more effective in reducing lesion area and plaque macrophage content (133). Under in vitro conditions, Ac-hE18A-NH2 also induced apoE secretion from HepG2 cells and THP-1 macrophages, whereas mR18L was without effect (133). These observations, as well as the observation that Ac-hE18A-NH2 reduces lesions in mice even one month post cessation of peptide treatment, strongly support plasma cholesterol-independent effects of this peptide (135). The additional benefit of Ac-hE18A-NH2 may be due to its ability to promote apoE secretion from hepatocytes and macrophages.
CURRENT STATUS OF APOLIPOPROTEIN MIMETIC THERAPY
The search for a drug that lowers both circulating levels of atherogenic lipoproteins and increases HDL levels and/or function is ongoing. The potential of apoA-I mimetic peptide therapy in reducing CAD risk has not been fulfilled. Results of clinical studies using D-4F and L-4F suggest that a relatively high dose of the peptide is required for efficacy in CAD patients. The future development of 4F as a therapeutic agent is therefore uncertain. While genetic production of apoA-I mimetics in fruits and vegetables is a novel concept, the technology has to be further developed to make it practical. Almost all of the apoA-I mimetic peptides tested thus far appear to inhibit atherosclerosis by promoting RCT and/or by inhibiting inflammation. Plasma cholesterol reduction is not a consistent response to these peptides. This holds true for recently described nanoparticle formulations of monomeric and trimeric apoA-I mimetic peptides which reduce lesions in LDLR−/− mice (89). As discussed by Wool and colleagues, lipid lowering does not appear to be a critical component of the atheroprotective response to apoA-I mimetics (145). While current data point to the intestine as a critical site of apoA-I mimetic peptide action, underlying mechanisms have not been defined. A common and practical biomarker that can be inexpensively adapted in any laboratory for assessing efficacy of different apoA-I mimetic peptides is critically required for the advancement of this technology.
ACC/AHA guidelines recommend the use of statins to reduce CV risk (1). As noted previously, statin resistance may arise in response to gene polymorphisms and/or upregulation of proprotein convertase subtilisin/kexin type 9 (PCSK9) (3, 4, 146). In addition, statin withdrawal has been shown to cause rebound pro-inflammatory effects (147). Monoclonal antibodies directed against the catalytic domain of PCSK9 represent a new class of cholesterol-reducing drug (148, 149). These agents facilitate the LDL clearance by preventing LDL receptor degradation and show strong potential to reduce coronary risk in patients who are statin-resistant. Recent data also show that PCSK9 inhibitors reduce circulating levels of pro-atherogenic lipoprotein(a) (150). A potential limitation of this form of therapy may be a lack of effect on the clearance of nonLDL atherogenic lipoproteins. Some subjects with homozygous familial hypercholesterolemia have reduced LDL receptor function and show a limited response to statin or PCSK9 inhibitor therapy (151–153). Treatment options for these individuals are limited. A recent study shows that type III hyperlipoproteinemia is induced by mutations in the LDL receptor binding domain of apoE and is characterized by inhibition of both remnant lipoprotein clearance and HDL biogenesis (154).
apoE mimetic peptide therapy may be ideally suited for treatment of hypercholesterolemia associated with defects in LDL receptor function. apoE mimetics may provide additional benefits compared with the PCSK9 inhibitor strategy including: 1) the capacity to reduce plasma levels of chylomicrons, β-VLDL-C, VLDL-C, and VLDL remnants; 2) the ability to improve HDL function; 3) improved anti-oxidant properties; and 4) cholesterol-independent anti-atherogenic effects. These factors may contribute to the sustained response to apoE mimetic peptide treatment seen in mouse and rabbit studies (133, 135). Because plasma cholesterol reducing ability is robust, it is anticipated that this technology will be useful in acute treatment to stabilize lesions via a rapid plasma cholesterol reduction. Depending on the initial plasma cholesterol levels in patients, the dosage can be adjusted to achieve required cholesterol reductions. Ac-hE18A-NH2 has recently been assigned orphan drug status and licensed under the trade name AEM-28 by LipimetiX, LLC and Capstone Therapeutics, Inc. It is currently undergoing initial clinical evaluation. Future clinical trials are required in order to determine the dosage needed to achieve targeted levels of cholesterol and stabilization or regression of lesion. It is possible that, once lesions are stabilized, adjunct therapy with apoA-I mimetics can be employed for chronic treatment of lipid-mediated inflammatory diseases.
Footnotes
Abbreviations:
- ACC
- American College of Cardiology
- ACS
- acute coronary syndrome
- AHA
- American Heart Association
- BM
- bone marrow
- CAD
- coronary artery disease
- CETP
- cholesteryl ester transfer protein
- COG
- cogniscience apoE mimetic peptide
- CV
- cardiovascular
- HDL-C
- HDL cholesterol
- HSPG
- heparan sulfate proteoglycan
- IP
- intraperitoneal
- IV
- intravenous
- IVUS
- intravascular ultrasound
- LAP
- lipid-associating peptide
- LDL-C
- LDL cholesterol
- LDLR−/−
- LDL receptor-null
- LRP
- LDL receptor-related protein
- LPS
- bacterial lipopolysaccharide
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- PON-1
- paraoxonase-1
- RCT
- reverse cholesterol transport
- rHDL
- reconstituted HDL
- SAA
- serum amyloid A
- SQ
- subcutaneous
- VLDL-C
- VLDL cholesterol
- WHHL
- Watanabe heritable hyperlipidemic
This work was supported in part by National Institutes of Health Grants R01 HL090803, HL34343, and R01 GM082952. G.M.A. is a Principal in Bruin Pharma, Inc. and holds shares in LipimetiX, LLC. C.R.W. and D.W.G. have intellectual property on the apoE mimetic peptides.
REFERENCES
- 1.Stone N. J., Robinson J. G., Lichtenstein A. H., Bairey Merz C. N., Blum C. B., Eckel R. H., Goldberg A. C., Gordon D., Levy D., Lloyd-Jones D. M., et al. 2014. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 129: S1–S45. [DOI] [PubMed] [Google Scholar]
- 2.Josan K., Majumdar S. R., McAlister F. A. 2008. The efficacy and safety of intensive statin therapy: a meta-analysis of randomized trials. CMAJ. 178: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voora D., Shah S. H., Reed C. R., Zhai J., Crosslin D. R., Messer C., Salisbury B. A., Ginsburg G. S. 2008. Pharmacogenetic predictors of statin-mediated low-density lipoprotein cholesterol reduction and dose response. Circ Cardiovasc Genet. 1: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Ruan X. Z., Li Q., Huang A., Moorhead J. F., Powis S. H., Varghese Z. 2007. Inflammatory cytokines disrupt LDL-receptor feedback regulation and cause statin resistance: a comparative study in human hepatic cells and mesangial cells. Am. J. Physiol. Renal Physiol. 293: F680–F687. [DOI] [PubMed] [Google Scholar]
- 5.Melone M., Wilsie L., Palyha O., Strack A., Rashid S. 2012. Discovery of a new role of human resistin in hepatocyte low-density lipoprotein receptor suppression mediated in part by proprotein convertase subtilisin/kexin type 9. J. Am. Coll. Cardiol. 59: 1697–1705. [DOI] [PubMed] [Google Scholar]
- 6.Campbell C. Y., Rivera J. J., Blumenthal R. S. 2007. Residual risk in statin-treated patients: future therapeutic options. Curr. Cardiol. Rep. 9: 499–505. [DOI] [PubMed] [Google Scholar]
- 7.Fruchart J. C., Sacks F. M., Hermans M. P., Assmann G., Brown W. V., Ceska R., Chapman M. J., Dodson P. M., Fioretto P., Ginsberg H. N., et al. ; Residual Risk Reduction Initiative (R3I). 2008. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Diab. Vasc. Dis. Res. 5: 319–335. [DOI] [PubMed] [Google Scholar]
- 8.Gordon T., Kannel W. B., Castelli W. P., Dawber T. R. 1981. Lipoproteins, cardiovascular disease, and death. The Framingham study. Arch. Intern. Med. 141: 1128–1131. [PubMed] [Google Scholar]
- 9.Castelli W. P. 1988. Cholesterol and lipids in the risk of coronary heart disease: the Framingham Heart Study. Can. J. Cardiol. 4: 5A–10A. [PubMed] [Google Scholar]
- 10.Yvan-Charvet L., Wang N., Tall A. R. 2010. The role of HDL, ABCA1 and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 30: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lüscher T. F., Landmesser U., von Eckardstein A., Fogelman A. M. 2014. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ. Res. 114: 171–182. [DOI] [PubMed] [Google Scholar]
- 12.Getz G. S., Reardon C. A. 2009. Apoprotein E as a lipid transport and signaling protein in the blood, liver, and artery wall. J. Lipid Res. 50: S156–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.deGoma E. M., deGoma R. L., Rader D. J. 2008. Beyond high-density lipoprotein cholesterol levels. Evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J. Am. Coll. Cardiol. 51: 2199–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Wagner A. C., Frank J. S., Datta G., Garber D., et al. 2004. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 109: 3215–3220. [DOI] [PubMed] [Google Scholar]
- 15.Belalcazar L. M., Ballantyne C. M. 1998. Defining specific goals of therapy in treating dyslipidemia in the patient with low high-density lipoprotein cholesterol. Prog. Cardiovasc. Dis. 41: 151–174. [DOI] [PubMed] [Google Scholar]
- 16.Gnasso A., Lehner B., Haberbosch W., Leiss O., von Bergmann K., Augustin J. 1986. Effect of gemfibrozil on lipids, apoproteins, and postheparin lipolytic activities in normolipidemic subjects. Metabolism. 35: 387–393. [DOI] [PubMed] [Google Scholar]
- 17.Wierzbicki A. S., Mikhailidis D. P., Wray R., Schacter M., Cramb R., Simpson W. G., Byrne C. B. 2003. Statin-fibrate combination: therapy for hyperlipidemia: a review. Curr. Med. Res. Opin. 19: 155–168. [DOI] [PubMed] [Google Scholar]
- 18.Malik S., Kashyap M. L. 2003. Niacin, lipids, and heart disease. Curr. Cardiol. Rep. 5: 470–476. [DOI] [PubMed] [Google Scholar]
- 19.Maron D. J., Fazio S., Linton M. F. 2000. Current perspectives on statins. Circulation. 101: 207–213. [DOI] [PubMed] [Google Scholar]
- 20.Chapman M. J. 2004. Are the effects of statins on HDL-cholesterol clinically relevant? Eur. Heart J. 6: C58–C63. [Google Scholar]
- 21.Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W.; AIM-HIGH Investigators. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
- 22.Landray M. J., Haynes R., Hopewell J. C., Parish S., Aung T., Tomson J., Wallendszus K., Craig M., Jiang L., Jiang L., Collins R., et al. ; HPS2-THRIVE Collaborative Group. 2014. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 371: 203–212. [DOI] [PubMed] [Google Scholar]
- 23.Morehouse L. A., Sugarman E. D., Bourassa P-A., Sand T. M., Zimetti F., Gao F., Rothblat G. H., Milici A. J. 2007. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in NZW rabbits. J. Lipid Res. 48: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 24.Brousseau M. E., Schaefer E. J., Wolfe M. L., Bloedon L. T., Digenio A. G., Clark R. W., Mancuso J. P., Rader D. J. 2004. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N. Engl. J. Med. 350: 1505–1515. [DOI] [PubMed] [Google Scholar]
- 25.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. ; ILLUMINATE Investigators. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. ; dal-OUTCOMES Investigators. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 27.Mohammadpour A. H., Akhlaghi F. 2013. Future of cholesteryl ester transfer protein (CETP) inhibitors: a pharmacological perspective. Clin. Pharmacokinet. 52: 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirz R., Scanu A. M. 1970. Reassembly in vitro of a serum high density lipoprotein. Biochim. Biophys. Acta. 207: 364–367. [DOI] [PubMed] [Google Scholar]
- 29.Franceschini G., Sirtori C. R., Capurso A., Weisgraber K. H., Mahley R. W. 1980. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J. Clin. Invest. 66: 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah P. K., Yano J., Reyes O., Chyu K. Y., Kaul S., Bisgaier C. L., Drake S., Cercek B. 2001. High-dose recombinant apolipoprotein A-IMilano mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein E-deficient mice: potential implications for acute plaque stabilization. Circulation. 103: 3047–3050. [DOI] [PubMed] [Google Scholar]
- 31.Parolini C., Marchesi M., Lorenzon P., Castano M., Balconi E., Miragoli L., Chaabane L., Morisetti A., Lorusso V., Martin B. J., et al. 2008. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-IMilano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques. In vivo assessment by intravascular ultrasound and magnetic resonance imaging. J. Am. Coll. Cardiol. 51: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 32.Bielicki J. K., Oda M. N. 2002. Apolipoprotein A-IMilano and apolipoprotein A-IParis. exhibit an antioxidant activity distinct from that of wild-type apolipoprotein A-I. Biochemistry. 41: 2089–2096. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z., O’Neill E. A., Meurer R. D., Gagen K., Luell S., Wang S. P., Ichetovkin M., Frantz-Wattley B., Eveland S., Strack A. M., et al. 2012. Reconstituted HDL elicits marked changes in plasma lipids following single-dose injection in C57Bl/6 mice. J. Cardiovasc. Pharmacol. Ther. 17: 315–323. [DOI] [PubMed] [Google Scholar]
- 34.Diditchenko S., Gille A., Pragst I., Stadler D., Waelchli M., Hamilton R., Leis A., Wright S. D. 2013. Novel formulation of a reconstituted high-density lipoprotein (CSL112) dramatically enhances ABCA1-dependent cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 33: 2202–2211. [DOI] [PubMed] [Google Scholar]
- 35.Tardy C., Goffinet M., Boubekeur N., Ackermann R., Sy G., Bluteau A., Cholez G., Keyserling C., Lalwani N., Paolini J. F., et al. 2014. CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis. 232: 110–118. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson M., Carlson L. A., Miettinen T. A., Angelin B. 1999. Stimulation of fecal steroid excretion after infusion of recombinant proapolipoprotein A-I. Potential reverse cholesterol transport in humans. Circulation. 100: 594–598. [DOI] [PubMed] [Google Scholar]
- 37.Waksman R., Torguson R., Kent K. M., Pichard A. D., Suddath W. O., Satler L. F., Martin B. D., Perlman T. J., Maltais J. A., Weissman N. J., et al. 2010. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 55: 2727–2735. [DOI] [PubMed] [Google Scholar]
- 38.Tardif J. C., Ballantyne C. M., Barter P., Dasseux J. L., Fayad Z. A., Guertin M. C., Kastelein J. J. P., Keyserling C., Klepp H., Koenig W., et al. ; for the Can HDL Infusions Significantly Quicken Atherosclerosis Regression (CHI-SQUARE) Investigators. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur. Heart J. Epub ahead of print. April 29, 2014; 10.1093/eurheartj/ehu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tardif J. C., Grégoire J., L’Allier P. L., Ibrahim R., Lespérance J., Heinonen T. M., Kouz S., Berry C., Basser R., Lavoie M. A., et al. ; Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators. 2007. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 297: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 40.Gille A., Wright S., Easton R., Shear C. 2013. Infusion of CSL112, a novel formulation of human apolipoprotein A-I, in healthy subjects removes tissue cholesterol and directs its clearance. Eur. Heart J. 34(suppl 1): 10.1093/eurheartj/eht308.1947. [Google Scholar]
- 41.Easton R., Gille A., D’Andrea D., Davis R., Wright S. D., Shear C. 2014. A multiple ascending dose study of CSL112, an infused formulation of ApoA-I. J. Clin. Pharmacol. 54: 301–310. [DOI] [PubMed] [Google Scholar]
- 42.Rocco A. G., Mollica L., Gianazza E., Calabresi L., Franceschini G., Sirtori C. R., Eberini I. 2006. A model structure for the heterodimer apoA-IMilano-apoA-II supports its peculiar susceptibility to proteolysis. Biophys. J. 91: 3043–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong E. L., Stoltzfus L. J., Brion C. M., Murugesh D., Rubin E. M. 1996. Contrasting in vivo effects of murine and human apolipoprotein A-II: role of monomer versus dimer. J. Biol. Chem. 271: 5984–5987. [DOI] [PubMed] [Google Scholar]
- 44.Escolà-Gil J. C., Marzal-Casacuberta À., Julve-Gil J., Ishida B. Y., Ordóñez-Llanos J., Chan L., Gonzãlez-Sastre F., Blanco-Vaca F. 1998. Human apolipoprotein A-II is a pro-atherogenic molecule when it is expressed in transgenic mice at a level similar to that in humans: evidence of a potentially relevant species-specific interaction with diet. J. Lipid Res. 39: 457–462. [PubMed] [Google Scholar]
- 45.Yi D. W., Jeong D. W., Lee S. Y., Son S. M., Kang Y. H. 2012. The association between apolipoprotein A-II and metabolic syndrome in Korean adults: a comparison study of apolipoprotein A-I and apolipoprotein B. Diabetes Metab. J. 36: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nissen S. E., Tsunoda T., Tuzcu E. M., Schoenhagen P., Cooper C. J., Yasin M., Eaton G. M., Lauer M. A., Sheldon W. S., Grines C. L., et al. 2003. Effect of recombinant apoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 290: 2292–2300. [DOI] [PubMed] [Google Scholar]
- 47.Chung B. H., Anatharamaiah G. M., Brouillette C. G., Nishida T., Segrest J. P. 1985. Studies of synthetic peptide analogs of the amphipathic helix. Correlation of structure with function. J. Biol. Chem. 260: 10256–10262. [PubMed] [Google Scholar]
- 48.Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr 1974. A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 38: 247–258. [DOI] [PubMed] [Google Scholar]
- 49.Segrest J. P., Garber D. W., Brouillette C. G., Harvey S. C., Anantharamaiah G. M. 1994. The amphipathic α helix: A multifunctional structural motif in plasma apolipoproteins. Adv. Protein Chem. 45: 303–369. [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama S., Fukushima D., Kupferberg J. P., Kézdy F. J., Kaiser E. T. 1980. The mechanism of activation of lecithin:cholesterol acyltransferase by apolipoprotein A-I and an amphiphilic peptide. J. Biol. Chem. 255: 7333–7339. [PubMed] [Google Scholar]
- 51.Ponsin G., Strong K., Gotto A. M., Jr, Sparrow J. T., Pownall H. J. 1984. In vitro binding of synthetic acylated lipid-associating peptides to high-density lipoproteins: effect of hydrophobicity. Biochemistry. 23: 5337–5342. [DOI] [PubMed] [Google Scholar]
- 52.Anantharamaiah G. M., Jones J. L., Brouillette C. G., Schmidt C. F., Chung B. H., Hughes T. A., Bhown A. S., Segrest J. P. 1985. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J. Biol. Chem. 260: 10248–10255. [PubMed] [Google Scholar]
- 53.Mendez A. J., Anantharamaiah G. M., Segrest J. P., Oram J. F. 1994. Synthetic amphipathic helical peptides that mimic apolipoprotein A-I in clearing cellular cholesterol. J. Clin. Invest. 94: 1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Epand R. M., Gawish A., Iqbal M., Gupta K. B., Chen C. H., Segrest J. P., Anantharamaiah G. M. 1987. Studies of synthetic peptide analogs of the amphipathic helix. Effect of charge distribution, hydrophobicity, and secondary structure on lipid association and lecithin:cholesterol acyltransferase activation. J. Biol. Chem. 262: 9389–9396. [PubMed] [Google Scholar]
- 55.Tang C., Vaughan A. M., Anantharamaiah G. M., Oram J. F. 2006. Janus kinase 2 modulates the lipid-removing but not protein-stabilizing interactions of amphipathic helices with ABCA1. J. Lipid Res. 47: 107–114. [DOI] [PubMed] [Google Scholar]
- 56.Datta G., Chaddha M., Hama S., Navab M., Fogelman A. M., Garber D. W., Mishra V. K., Epand R. M., Epand R. F., Lund-Katz S., et al. 2001. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J. Lipid Res. 42: 1096–1104. [PubMed] [Google Scholar]
- 57.Jonas A. 1986. Reconstitution of high-density lipoproteins. Methods Enzymol. 128: 553–582. [DOI] [PubMed] [Google Scholar]
- 58.Garber D. W., Datta G., Chaddha M., Palgunachari M. N., Hama S. Y., Navab M., Fogelman A. M., Segrest J. P., Anantharamaiah G. M. 2001. A new synthetic class A amphipathic peptide analogue protects mice from diet-induced atherosclerosis. J. Lipid Res. 42: 545–552. [PubMed] [Google Scholar]
- 59.Anantharamaiah G. M., Venkatachalapathi Y. V., Brouillette C. G., Segrest J. P. 1990. Use of synthetic peptide analogues to localize lecithin:cholesterol acyltransferase activating domain in apolipoprotein A-I. Arteriosclerosis. 10: 95–105. [DOI] [PubMed] [Google Scholar]
- 60.Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Frank J. S., Grijalva V. R., Ganesh V. K., Mishra V. K., Palgunachari M. N., et al. 2005. Oral small peptides render HDL antiinflammatory in mice and monkeys and reduce atherosclerosis in ApoE null mice. Circ. Res. 97: 524–532. [DOI] [PubMed] [Google Scholar]
- 61.Navab M., Anantharamaiah G. M., Reddy S. T., Van Lenten B. J., Wagner A. C., Hama S., Hough G., Bachini E., Garber D. W., Mishra V. K., et al. 2005. An oral apoJ peptide renders HDL antiinflammatory in mice and monkeys and dramatically reduces atherosclerosis in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 25: 1932–1937. [DOI] [PubMed] [Google Scholar]
- 62.Wool G. D., Vaisar T., Reardon C. A., Getz G. S. 2009. An apoA-I mimetic peptide containing a proline residue has greater in vivo HDL binding and anti-inflammatory ability than the 4F peptide. J. Lipid Res. 50: 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Remaley A. T., Thomas F., Stonik J. A., Demosky S. J., Bark S. E., Neufeld E. B., Bocharov A. V., Vishnyakova T. G., Patterson A. P., Eggerman T. L., et al. 2003. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J. Lipid Res. 44: 828–836. [DOI] [PubMed] [Google Scholar]
- 64.Ditiatkovski M., D’Souza W., Kesani R., Chin-Dusting J., de Haan J. B., Remaley A., Sviridov D. 2013. An apolipoprotein A-I mimetic peptide designed with a reductionist approach stimulates reverse cholesterol transport and reduces atherosclerosis in mice. PLoS ONE. 8: e68802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bielicki J. K., Zhang H., Cortez Y., Zheng Y., Narayanaswami V., Patel A., Johansson J., Azhar S. 2010. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J. Lipid Res. 51: 1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y., Imura T., Leman L. J., Curtiss L. K., Maryanoff B. E., Ghadiri M. R. 2013. Mimicry of high-density lipoprotein: functional peptide-lipid nanoparticles based on multivalent peptide constructs. J. Am. Chem. Soc. 135: 13414–13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palgunachari M. N., Mishra V. K., Lund-Katz S., Phillips M. C., Adeyeye S. O., Alluri S., Anantharamaiah G. M., Segrest J. P. 1996. Only the two end helixes of eight tandem amphipathic helical domains of human apo A-I have significant lipid affinity. Implications for HDL assembly. Arterioscler. Thromb. Vasc. Biol. 16: 328–338. [DOI] [PubMed] [Google Scholar]
- 68.Van Lenten B. J., Wagner A. C., Jung C. L., Ruchala P., Waring A. J., Lehrer R. I., Watson A. D., Hama S., Navab M., Anantharamaiah G. M., et al. 2008. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J. Lipid Res. 49: 2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ou J., Ou Z., Jones D. W., Holzhauer S., Hatoum O. A., Ackerman A. W., Weihrauch D. W., Gutterman D. D., Guice K., Oldham K. T., et al. 2003. L-4F, an apolipoprotein A-1 mimetic, dramatically improves vasodilation in hypercholesterolemia and sickle cell disease. Circulation. 107: 2337–2341. [DOI] [PubMed] [Google Scholar]
- 70.Peterson S. J., Drummond G., Kim D. H., Li M., Kruger A. L., Ikehara S., Abraham N. G. 2008. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J. Lipid Res. 49: 1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vecoli C., Cao J., Neglia D., Inoue K., Sodhi K., Vanella L., Gabrielson K. K., Bedja D., Paolocci N., L'abbate A., et al. 2011. Apolipoprotein A-I mimetic peptide L-4F prevents myocardial and coronary dysfunction in diabetic mice. J. Cell. Biochem. 112: 2616–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morgantini C., Imaizumi S., Grijalva V., Navab M., Fogelman A. M., Reddy S. T. 2010. Apolipoprotein A-I mimetic peptides prevent atherosclerosis development and reduce plaque inflammation in a murine model of diabetes. Diabetes. 59: 3223–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie Q., Zhao S. P., Li F. 2010. D-4F, an apolipoprotein A-I mimetic peptide, promotes cholesterol efflux from macrophages via ATP-binding cassette transporter A1. Tohoku J. Exp. Med. 220: 223–228. [DOI] [PubMed] [Google Scholar]
- 74.Garber D. W., Venkatachalapathi Y. V., Gupta K. B., Ibdah J., Phillips M. C., Hazelrig J. B., Segrest J. P., Anantharamaiah G. M. 1992. Turnover of synthetic class A amphipathic peptide analogues of exchangeable apolipoproteins in rats. Correlation with physical properties. Arterioscler. Thromb. 12: 886–894. [DOI] [PubMed] [Google Scholar]
- 75.Navab M., Anantharamaiah G. M., Hama S., Garber D. W., Chaddha M., Hough G., Lallone R., Fogelman A. M. 2002. Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 105: 290–292. [DOI] [PubMed] [Google Scholar]
- 76.Van Lenten B. J., Wagner A. C., Navab M., Anantharamaiah G. M., Hama S., Reddy S. T., Fogelman A. M. 2007. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J. Lipid Res. 48: 2344–2353. [DOI] [PubMed] [Google Scholar]
- 77.Ansell B. J., Navab M., Hama S., Kamranpour N., Fonarow G., Hough G., Rahmani S., Mottahedeh R., Dave R., Srinivasa S. T., et al. 2003. Inflammatory/anti-inflammatory properties of high density lipoprotein distinguish patients from control subjects better than high density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 108: 2751–2756. [DOI] [PubMed] [Google Scholar]
- 78.Wool G. D., Cabana V. G., Lukens J., Shaw P. X., Binder C. J., Witztum J. L., Reardon C. A., Getz G. S. 2011. 4F Peptide reduces nascent atherosclerosis and induces natural antibody production in apolipoprotein E-null mice. FASEB J. 25: 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Navab M., Anantharamaiah G. M., Hama S., Hough G., Reddy S. T., Frank J. S., Garber D. W., Handattu S., Fogelman A. M. 2005. D-4F and statins synergize to render HDL anti-inflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 25: 1426–1432. [DOI] [PubMed] [Google Scholar]
- 80.Navab M., Reddy S. T., Anantharamaiah G. M., Imaizumi S., Hough G., Hama S., Fogelman A. M. 2011. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J. Lipid Res. 52: 1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chattopadhyay A., Navab M., Hough G., Gao F., Meriwether D., Grijalva V., Springstead J. R., Palgnachari M. N., Namiri-Kalantari R., Su F., et al. 2013. A novel approach to oral apoA-I mimetic therapy. J. Lipid Res. 54: 995–1010. [Erratum. 2013. J. Lipid Res. 54: 3220.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Navab M., Hough G., Buga G. M., Su F., Wagner A. C., Meriwether D., Chattopadhyay A., Gao F., Grijalva V., Danciger J. S., et al. 2013. Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid. J. Lipid Res. 54: 3403–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gomaraschi M., Calabresi L., Rossoni G., Iametti S., Franceschini G., Stonik J. A., Remaley A. T. 2008. Anti-inflammatory and cardioprotective activities of synthetic high-density lipoprotein containing apolipoprotein A-I mimetic peptides. J. Pharmacol. Exp. Ther. 324: 776–783. [DOI] [PubMed] [Google Scholar]
- 84.Sethi A. A., Stonik J. A., Thomas F., Demosky S. J., Amar M., Neufeld E., Brewer H. B., Davidson W. S., D’Souza W., Sviridov D., et al. 2008. Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides. J. Biol. Chem. 283: 32273–32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tabet F., Remaley A. T., Segaliny A. I., Millet J., Yan L., Nakhla S., Barter P. J., Rye K. A., Lambert G. 2010. The 5A apolipoprotein A-I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler. Thromb. Vasc. Biol. 30: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yao X., Dai C., Fredriksson K., Dagur P. K., McCoy J. P., Qu X., Yu Z. X., Keeran K. J., Zywicke G. J., Amar M. J., et al. 2011. 5A, an apolipoprotein A-I mimetic peptide, attenuates the induction of house dust mite-induced asthma. J. Immunol. 186: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.D'Souza W., Stonik J. A., Murphy A., Demosky S. J., Sethi A. A., Moore X. L., Chin-Dusting J., Remaley A. T., Sviridov D. 2010. Structure/function relationships of apolipoprotein A-I mimetic peptides: implications for antiatherogenic activities of high-density lipoprotein. Circ. Res. 107: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mishra V. K., Palgunachari M. N., Lund-Katz S., Phillips M. C., Segrest J. P., Anantharamaiah G. M. 1995. Effect of the arrangement of tandem repeating units of class A amphipathic alpha-helixes on lipid interaction. J. Biol. Chem. 270: 1602–1611. [DOI] [PubMed] [Google Scholar]
- 89.Zhao Y., Black A. S., Bonnet D. J., Maryanoff B. E., Curtiss L. K., Leman L. J., Ghadiri M.R. In vivo efficacy of HDL-like nanolipid particles containing multivalent peptide mimetics of apolipoprotein A-I. J. Lipid Res. Epub ahead of print. June 29, 2014; 10.1194/jlr.M049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bloedon L. T., Dunbar R., Duffy D., Pinell-Salles P., Norris R., DeGroot B. J., Movva R., Navab M., Fogelman A. M., Rader D. J. 2008. Safety, pharmacokinetics and pharmacodynamics of a single dose of oral apolipoprotein A-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 49: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watson C. E., Weissbach N., Kjems L., Ayalasomayajula S., Zhang Y., Chang I., Navab M., Hama S., Hough G., Reddy S. T., et al. 2011. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 52: 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reddy S. T., Navab M., Anantharamaiah G. M., Fogelman A. M. 2014. Searching for a successful HDL-based treatment strategy. Biochim. Biophys. Acta. 1841: 162–167. [PubMed] [Google Scholar]
- 93.Datta G., Chaddha M., Garber D. W., Chung B. H., Tytler E. M., Dashti N., Bradley W. A., Gianturco S. H., Anantharamaiah G. M. 2000. The receptor binding domain of apolipoprotein E, linked to a model class A amphipathic helix, enhances internalization and degradation of LDL by fibroblasts. Biochemistry. 39: 213–220. [DOI] [PubMed] [Google Scholar]
- 94.Segall M. L., Dhanasekaran P., Baldwin F., Anantharamaiah G. M., Weisgraber K. H., Phillips M. C., Lund-Katz S. 2002. Influence of apoE domain structure and polymorphism on the kinetics of phospholipid vesicle solubilization. J. Lipid Res. 43: 1688–1700. [DOI] [PubMed] [Google Scholar]
- 95.Gonzales J. C., Gordts P. L., Foley E. M., Esko J. D. 2013. Apolipoproteins E and AV mediate lipoprotein clearance by hepatic proteoglycans. J. Clin. Invest. 123: 2742–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu Y., Bellosta S., Langer C., Bernini F., Pitas R. E., Mahley R. W., Assmann G., von Eckardstein A. 1998. Low-dose expression of a human apolipoprotein E transgene in macrophages restores cholesterol efflux capacity of apolipoprotein E-deficient mouse plasma. Proc. Natl. Acad. Sci. USA. 95: 7585–7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Linton M. F., Atkinson J. B., Fazio S. 1995. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 267: 1034–1037. [DOI] [PubMed] [Google Scholar]
- 98.Davignon J. 2005. Apolipoprotein E and atherosclerosis-beyond lipid effect. Arterioscler. Thromb. Vasc. Biol. 25: 267–269. [DOI] [PubMed] [Google Scholar]
- 99.Gaudreault N., Kumar N., Posada J. M., Stephens K. B., Reyes de Mochel N. S., Eberle D., Olivas V. R., Kim R. Y., Harms M. J., Johnson S., et al. 2012. ApoE suppresses atherosclerosis by reducing lipid accumulation in circulating monocytes and the expression of inflammatory monocytes on monocytes and vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 32: 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ali K., Middleton M., Pure E., Rader D. J. 2005. Apolipoprotein E suppresses the type I inflammatory response in vivo. Circ. Res. 97: 922–927. [DOI] [PubMed] [Google Scholar]
- 101.Van Oosten M., Rensen P. C. N., Van Amersfoort E. S., Van Eck M., Van Dami A. M., Breve J. J. P., Vogel T., Panet A., Van Berkel T. J. C., Kuiper J. 2001. Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. J. Biol. Chem. 276: 8820–8824. [DOI] [PubMed] [Google Scholar]
- 102.de Bont N., Netea M. G., Demacker P. N. M., Kullberg B. J., van der Meer J. W., Stalenhoef A. F. 2000. Apolipoprotein E-deficient mice have an impaired immune response to Klebsiella pneumoniae. Eur. J. Clin. Invest. 30: 818–822. [DOI] [PubMed] [Google Scholar]
- 103.Wang H., Christensen D. J., Vitek M. P., Sullivan P. M., Laskowitz D. T. 2009. APOE genotype affects outcome in a murine model of sepsis: implications for a new treatment strategy. Anaesth. Intensive Care. 37: 38–45. [DOI] [PubMed] [Google Scholar]
- 104.Moretti E. W., Morris R. W., Podgoreanu M., Schwinn D. A., Newman M. F., Bennett E., Moulin V. G., Mba U. U., Laskowitz D. T. 2005. APOE polymorphism is associated with risk of severe sepsis in surgical patients. Crit. Care Med. 33: 2521–2526. [DOI] [PubMed] [Google Scholar]
- 105.Roselaar S. E., Daugherty A. 1998. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J. Lipid Res. 39: 1740–1743. [PubMed] [Google Scholar]
- 106.Levine D. M., Parker T. S., Donnelly T. M., Walsh A., Rubin A. L. 1993. In vivo protection against endotoxin by plasma high density lipoprotein. Proc. Natl. Acad. Sci. USA. 90: 12040–12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hasty A. H., Linton M. F., Brandt S. J., Babaev E. R., Gleaves L. A., Fazio S. 1999. Retroviral gene therapy in apoE-deficient mice: apoE expression in the artery wall reduces early foam cell lesion formation. Circulation. 99: 2571–2576. [DOI] [PubMed] [Google Scholar]
- 108.Curtiss L. K., Boisvert W. A. 2000. Apolipoprotein E and atherosclerosis. Curr. Opin. Lipidol. 11: 243–251. [DOI] [PubMed] [Google Scholar]
- 109.Shimano H., Ohsuga J., Shimada M., Namba Y., Gotoda T., Harada K., Katsuki M., Yazaki Y., Yamada N. 1995. Inhibition of diet-induced atheroma formation in transgenic mice expressing apolipoprotein E in the arterial wall. J. Clin. Invest. 95: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bellosta S., Mahley R. W., Sanan D. A., Murata J., Newland D. L., Taylor J. M., Pitas R. E. 1995. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. J. Clin. Invest. 96: 2170–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baitsch D., Bock H. H., Engel T., Telgmann R., Muller-Tidow C., Varga G., Bot M., Herz J., Robenek H., von Eckardstein A., et al. 2011. Apolipoprotein E induces anti-inflammatory phenotype in macrophages. Arterioscler. Thromb. Vasc. Biol. 31: 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fazio S., Babaev V. R., Murray A. B., Hasty A. H., Carter K. J., Gleaves L. A., Atkinson J. B., Linton M. F. 1997. Increased atherosclerosis in C57BL/6 mice reconstituted with apolipoprotein E null macrophages. Proc. Natl. Acad. Sci. USA. 94: 4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Potteaux S., Gautier E. L., Hutchison S. B., van Rooijen N., Rader D. J., Thomas M. J., Sorci-Thomas M. G., Randolph G. J. 2011. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of ApoE−/− mice during disease regression. J. Clin. Invest. 121: 2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoekstra M., Frodermann V., van den Aardweg T., van der Sluis R. J., Kuiper J. 2013. Leukocytosis and enhanced susceptibility to endotoxemia but not atherosclerosis in adrenalectomized apoE knockout mice. PLoS ONE. 8: e80441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murphy A. J., Akhtari M., Tolani S., Pagler T., Bijl N., Kuo C. L., Wang M., Sanson M., Abramowicz S., Welch C., et al. 2011. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 121: 4138–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tolani S., Pagler T. A., Murphy A. J., Bochem A. E., Abramowicz S., Welch C., Nagareddy P. R., Holleran S., Hovingh G. K., Kuivenhoven J. A., et al. 2013. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: studies in mice and FH children. Atherosclerosis. 229: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tall A. R., Yvan-Charvet L., Westerterp M., Murphy A. J. 2012. Cholesterol efflux a novel regulator of myelopoiesis and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 32: 2547–2552. [DOI] [PubMed] [Google Scholar]
- 118.Murphy A. J., Westerterp M., Yvan-Charvet L., Tall A. R. 2012. Anti-atherogenic mechanisms of high density lipoprotein: effects on myeloid cells. Biochim. Biophys. Acta. 1821: 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brown M. S., Goldstein J. L. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232: 34–47. [DOI] [PubMed] [Google Scholar]
- 120.Mahley R. W., Ji Z. S. 1999. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J. Lipid Res. 40: 1–16. [PubMed] [Google Scholar]
- 121.Sharifov O. F., Nayyar G., Garber D. W., Handattu S. P., Mishra V. K., Goldberg D., Anantharamaiah G. M., Gupta H. 2011. Apolipoprotein E mimetics and cholesterol-lowering properties. Am. J. Cardiovasc. Drugs. 11: 371–381. [DOI] [PubMed] [Google Scholar]
- 122.Datta G., Garber D. W., Chung B. H., Chaddha M., Dashti N., Bradley W. A., Gianturco S. H., Anantharamaiah G. M. 2001. Cationic domain 141-150 of apoE covalently linked to a class A amphipathic helix enhances atherogenic lipoprotein metabolism in vitro and in vivo. J. Lipid Res. 42: 959–966. [PubMed] [Google Scholar]
- 123.Dyer C. A., Cistola D. P., Parry G. C., Curtiss L. K. 1995. Structural features of synthetic peptides of apolipoprotein E that bind the LDL receptor. J. Lipid Res. 36: 80–88. [PubMed] [Google Scholar]
- 124.Clay M. A., Anantharamaiah G. M., Mistry M. J., Balasubramaniam A., Harmony J. A. 1995. Localization of a domain in apolipoprotein E with both cytostatic and cytotoxic activity. Biochemistry. 34: 11142–11151. [DOI] [PubMed] [Google Scholar]
- 125.Nikoulin I. R., Curtiss L. K. 1998. An apolipoprotein E synthetic peptide targets to lipoproteins in plasma and mediates both cellular lipoprotein interactions in vitro and acute clearance of cholesterol-rich lipoproteins in vivo. J. Clin. Invest. 101: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vedhachalam C., Narayanaswami V., Neto N., Forte T. M., Phillips M. C., Lund-Katz S., Bielicki J. K. 2007. The C-terminal lipid-binding domain of apolipoprotein E is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry. 46: 2583–2593. [DOI] [PubMed] [Google Scholar]
- 127.Zheng Y., Patel A. B., Narayanaswami V., Hura G. L., Hang B., Bielicki J. K. 2011. HDL mimetic peptide ATI-5261 forms an oligomeric assembly in solution that dissociates to monomers upon dilution. Biochemistry. 50: 4068–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li F. Q., Sempowski G. D., McKenna S. E., Laskowitz D. T., Colton C. A., Vitek M. P. 2006. Apolipoprotein E-derived peptides ameliorate clinical disability and inflammatory infiltrates into the spinal cord in a murine model of multiple sclerosis. J. Pharmacol. Exp. Ther. 318: 956–965. [DOI] [PubMed] [Google Scholar]
- 129.Laskowitz D. T., Mckenna S. E., Song P., Wang H., Durham L., Yeung N., Christensen D., Vitek M. P. 2007. COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J. Neurotrauma. 24: 1093–1107. [DOI] [PubMed] [Google Scholar]
- 130.Sheng Z., Prorok M., Brown B. E., Castellino F. J. 2008. N-methyl-D-aspartate receptor inhibition by an apolipoprotein E-derived peptide relies on low-density lipoprotein receptor-associated protein. Neuropharmacology. 55: 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Datta G., White C. R., Dashti N., Chaddha M., Palgunachari M. N., Gupta H., Handattu S. P., Garber D. W., Anantharamaiah G. M. 2010. Anti-inflammatory properties and recycling of Ac-hE18a-NH2: implications in the inhibition of atherosclerosis. Atherosclerosis. 208: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gupta H., White C. R., Handattu S., Garber D. W., Datta G., Chaddha M., Dai L., Gianturco S. H., Bradley W. A., Anantharamaiah G. M. 2005. An apolipoprotein E mimetic peptide dramatically lowers atherogenic lipoproteins and restores endothelial function in Watanabe heritable hyperlipidemic rabbits. Circulation. 111: 3112–3118. [DOI] [PubMed] [Google Scholar]
- 133.Handattu S. P., Nayyar G., Garber D. W., Palgunachari M. N., Monroe C. E., Keenum T. D., Mishra V. K., Datta G., Anantharamaiah G. M. 2013. Two apolipoprotein E mimetic peptides with similar cholesterol reducing properties exhibit differential atheroprotective effects in LDL-R null mice. Atherosclerosis. 227: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garber D. W., Handattu S., Aslan I., Datta G., Chaddha M., Anantharamaiah G. M. 2003. Effect of an arginine-rich amphipathic helical peptide on plasma cholesterol in dyslipidemic mice. Atherosclerosis. 168: 229–237. [DOI] [PubMed] [Google Scholar]
- 135.Goldberg D. I., Nayyar G., Garber D. W., Handattu S. P., Anatharamaiah G. M. 2013. Sustained effects of apolipoprotein E mimetic peptides on established atherosclerotic lesions in apo E null mice. Circulation. 128: A10759. [Google Scholar]
- 136.Datta G., Chaddha M., Handattu S. P., Palgunachari M. N., Nayyar G., Garber D. W., Gupta H., White C. R., Anantharamaiah G. M. 2010. ApoE mimetic peptide reduces plasma lipid hydroperoxide content with a concomitant increase in HDL paraoxonase activity. Adv. Exp. Med. Biol. 660: 1–4. [DOI] [PubMed] [Google Scholar]
- 137.Handattu S. P., Monroe C. E., Nayyar G., Palgunachari M. N., Kadish I., van Groen T., Anantharamaiah G. M., Garber D. W. 2013. In vivo and in vitro effects of an apolipoprotein E mimetic peptide on amyloid-β pathology. J. Alzheimers Dis. 36: 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raffai R. L., Loeb S. M., Weisgraber K. H. 2005. Apolipoprotein E promotes the regression of atherosclerosis independently of lowering plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 25: 436–441. [DOI] [PubMed] [Google Scholar]
- 139.Kothapalli D., Liu S. L., Bae Y. H., Monslow J., Xu T., Hawthome E. A., Byfield F. J., Castagnini P., Rao S., Radar D. J., et al. 2012. Cardiovascular protection by ApoE and ApoE-HDL linked to suppression of ECM gene expression and arterial stiffening. Cell Reports. 2: 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Swertfeger D. K., Hui D. Y. 2001. Apolipoprotein E: A cholesterol transport protein with lipid transport-independent cell signaling properties. Front. Biosci. 6: D526–D535. [DOI] [PubMed] [Google Scholar]
- 141.Nayyar G., Garber D. W., Palgunachari M. N., Monroe C. E., Keenum T. D., Handattu S. P., Mishra V. K., Anantharamaiah G. M. 2012. Apolipoprotein E mimetic is more effective than apolipoprotein A-I mimetic in reducing lesion formation in older female apo E null mice. Atherosclerosis. 224: 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharifov O. F., Nayyar G., Ternovoy V. V., Palgunachari M. N., Garber D. W., Anantharamaiah G., Gupta H. Comparison of anti-endotoxin activity of apoE and apoA mimetic derivatives of a model amphipathic peptide 18A. Innate Immun. Epub ahead of print. Dec 9, 2013; 10.1177/1753425913514621. [DOI] [PubMed] [Google Scholar]
- 143.Tytler E. M., Segrest J. P., Epand R. M., Nie S. Q., Epand R. F., Mishra V. K., Venkatachalapathi Y. V., Anantharamaiah G. M. 1993. Reciprocal effects of apolipoprotein and lytic peptide analogs on membranes. Cross- sectional molecular shapes of amphipathic alpha-helixes control membrane stability. J. Biol. Chem. 268: 22112–22118. [PubMed] [Google Scholar]
- 144.Handattu S. P., Datta G., Epand R. M., Epand R. F., Palgunachari M. N., Mishra V. K., Monroe C. E., Keenum T. D., Chaddha M., Anantharamaiah G. M., et al. 2010. Oral administration of L-mR18L, a single domain cationic amphipathic helical peptide, inhibits lesion formation in ApoE null mice. J. Lipid Res. 51: 3491–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wool G., Reardon C. A., Getz G. S. Mimetic peptides of human apoA-I helix 10 get together to lower lipids and ameliorate atherosclerosis: is the action in the gut? J. Lipid Res. Epub ahead of print. August 1, 2014; 10.1194/jlr.E053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dong B., Wu M., Li H., Kraemer F. B., Adeli K., Seidah N. G., Wook Park S., Liu J. 2010. Strong induction of PCSK9 gene expression through HNF1α and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J. Lipid Res. 51: 1486–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Antonopoulos A. S., Margaritis M., Lee R., Channon K., Antoniades C. 2012. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr. Pharm. Des. 18: 1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stein E. A., Mellis S., Yancopoulos G. D., Stahl N., Logan D., Smith W. B., Lisbon E., Gutierrez M., Webb C., Wu R., et al. 2012. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N. Engl. J. Med. 366: 1108–1118. [DOI] [PubMed] [Google Scholar]
- 149.Farnier M. 2013. PCSK9 inhibitors. Curr. Opin. Lipidol. 24: 251–258. [DOI] [PubMed] [Google Scholar]
- 150.Raal F. J., Giugliano R. P., Sabatine M. S., Koren M. J., Langslet G., Bays H., Blom D., Eriksson M., Dent R., Wasserman S. M., et al. 2014. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J. Am. Coll. Cardiol. 63: 1278–1288. [DOI] [PubMed] [Google Scholar]
- 151.Stein E. A., Honarpour N., Wasserman S. M., Xu F., Scott R., Raal F. J. 2013. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 128: 2113–2120. [DOI] [PubMed] [Google Scholar]
- 152.Hemphill L. C. 2010. Familial hypercholesterolemia: Current treatment options and patient selection for low-density lipoprotein apheresis. J. Clin. Lipidol. 4: 346–349. [DOI] [PubMed] [Google Scholar]
- 153.Raal F. J., Santos R. D. 2012. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis. 223: 262–268. [DOI] [PubMed] [Google Scholar]
- 154.Fotakis P., Vezeridis A., Dafnis I., Chroni A., Kardassis D., Zannis V. I. 2014. apoE3[K146N/R147W] acts as a dominant negative apoE form that prevents remnant clearance and inhibits the biogenesis of HDL. J. Lipid Res. 55: 1310–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]