Abstract
In hepatocytes, aging-associated decline in GSH has been linked to activation of neutral SMase (nSMase), accumulation of bioactive ceramide, and inflammation. In this study, we seek to test whether dietary supplementation with the cysteine precursor, L-2-oxothiazolidine-4-carboxylic acid (OTC), would correct the aging-associated differences in hepatic GSH, nSMase, and ceramide. Young and aged mice were placed on a diet that either lacked sulfur-containing amino acids (SAAs) or had 0.5% OTC for 4 weeks. Mice fed standard chow were used as an additional control. SAA-deficient mice exhibited significant aging-associated differences in hepatic GSH, GSH/GSSG, ceramide, and nSMase. C24:1 ceramide, the major ceramide species in liver, was affected the most by aging, followed by the less abundant C16:0 ceramide. OTC supplementation eliminated the aging-associated differences in hepatic GSH and GSH/GSSG ratio. Surprisingly, however, instead of decreasing, the nSMase activity and ceramide increased in the OTC-fed mice irrespective of their age. These effects were due to elevated nSMase-2 mRNA and protein and appeared to be direct. Similar increases were seen in HepG2 cells following treatment with OTC. The OTC-fed aged mice also exhibited hepatic steatosis and triacylglyceride accumulation. These results suggest that OTC is a potent stimulant of nSMase-2 expression and that there may be unanticipated complications of OTC supplementation.
Keywords: sphingomyelinase, ceramide, reduced glutathione supplementation, aging, diet, oxidative stress
GSH is the major antioxidant in the body responsible for maintaining the intracellular redox balance. Aging is accompanied by a progressive decline in the levels of GSH in humans (1–3) and rodents (4–7), as well as in senescent cells in culture (8), resulting in elevated reactive oxygen species (ROS) and a state of chronic oxidative stress. Dietary GSH supplementation has been considered as a potential treatment for various aging-related diseases that are linked to oxidative stress, including metabolic, cardiovascular, and Alzheimer’s diseases, as well as various types of cancers (9–11). The direct delivery of GSH, however, is inefficient and could even be toxic; therefore, various precursors of GSH synthesis have been widely used instead (12).
The availability of l-cysteine is the rate-limiting factor for GSH synthesis. Several cysteine pro-drugs, including L-2-oxothiazolidine-4-carboxylic acid (OTC), have been successfully used to elevate GSH levels (13). Administration of OTC has been shown to effectively elevate hepatic GSH concentration in healthy guinea pigs (14), as well as in rats and mice with experimentally induced GSH deficiency due to acetaminophen and alcohol consumption (7, 15, 16). In humans, OTC supplementation leads to a significant increase in blood GSH in healthy volunteers (17, 18), as well as in dialysis and HIV patients (19–21). Typically, dietary OTC supplementation has beneficial effects and protects against acetaminophen-, alcohol-, or thioacetamide-induced hepatic damage (15, 16, 22, 23).
The effects GSH has on cellular functions are mediated through changes in the cellular redox state, as well as through direct protein modifications causing allosteric activation or inhibition of function. Studies have suggested that GSH also targets a lipid-signaling enzyme, neutral SMase (nSMase)-2 (24–28). nSMase-2 is a SM-hydrolyzing enzyme that is activated during cellular stress by cytokines such as TNF-α and IL-1β to generate ceramide (29, 30). The latter is a bioactive metabolite that activates distinct sets of downstream target proteins, including Stress-activated protein kinase/c-Jun N terminal kinase (SAPK/JNK), ERK, Protein phosphatase 2A (PP2A), and Protein phosphatase 1 (PP1) (31–34), as well as p38 (35) and Protein Kinase R (PKR) (36), which in turn propagate inflammation or induce cell death and growth arrest. Several studies have suggested that in vitro GSH acts as an inhibitor of nSMase-2 activity, which helps maintain low basal nSMase activity in a healthy state. Addition of GSH to cell lysates, or to partially purified nSMase-2, inhibits nSMase enzymatic activity, suggesting that it might be a direct inhibitor of the enzyme (26–28). It has been suggested that depletion of cellular GSH in conditions associated with elevated oxidative stress (including aging) triggers the activation of nSMase-2 and the accumulation of ceramide, and thus causes cellular death/inflammation (28).
This model was previously studied in the context of aging using primary hepatocytes (28). Hepatocytes isolated from aged rats showed very low GSH levels, accounting for only 30% of the GSH content of hepatocytes from young rats. This was paralleled by a 30–40% increase in total cellular nSMase activity. Gene silencing approaches indicated that nSMase-2 was responsible for this aging-associated increase in nSMase activity. Treatment of hepatocytes from young animals with buthionine sulphoximine, an inhibitor of GSH synthase, decreased cellular GSH content and activated nSMase-2 to values similar to those observed in aged hepatocytes. Inversely, the addition of N-acetyl cysteine to hepatocytes from aged animals successfully restored GSH content and nSMase-2 activity. The upregulation of hepatic inflammatory response observed in the aged animals decreased after replenishment of cellular GSH or inhibition of nSMase-2. These experiments suggested that age-associated depletion of GSH in old animals might be important for the onset of a chronic inflammatory phenotype via upregulation of nSMase-2 activity. The link between GSH and nSMase-2, however, has never been studied in vivo. Thus, the goal of the present study was to test the relations between aging, hepatic GSH, and nSMase-2 activity in mice. The specific hypothesis was that restoration of GSH content in aged livers by dietary supplementation with a cysteine pro-drug, OTC, would inhibit nSMase-2 activity and would have beneficial effects on hepatic functions in the aged mice. The study, however, unexpectedly showed that OTC has novel GSH-independent effects on nSMase-2 mRNA levels. These data are the first evidence for transcriptional regulation of nSMase-2 mRNA in the liver, which is physiologically relevant and determines the overall hepatic nSMase activity and ceramide content.
MATERIALS AND METHODS
Materials
N-(6-((7-nitro-2-1,3-benzoxadiazol-4-yl)amino)hexanoyl)-sphingosine-1-phosphocholine (C6-NBD-SM) was from Molecular Probes Inc. (Eugene, OR) and N-heptadecanoyl-D-erythro-sphingosine (C17:0 ceramide) was purchased from Avanti Polar Lipids (Alabaster, AL). Palmitic acid and OTC were from Sigma-Aldrich (St. Louis, MO). The Bioxytech® GSH/GSSG-412™ assay kit was from OXIS International (Foster City, CA). DiscretePak™ alkaline phosphatase (ALP) and DiscretePak™ alanine aminotransferase (ALT) reagent kits were from Catachem (Bridgeport, CN). TRIZOL® reagent was from Invitrogen (Carlsbad, CA). The forward and reverse primers for RT-PCR were purchased from Integrated DNA Technologies Inc. (Coralville, IA). Rabbit anti-nSMase-2 and nSMase-1 antibodies were custom generated by Invitrogen and verified in our lab. Other antibodies were from the following manufacturers: anti cyclophilin-A was from Cell Signaling (Beverly, MA); anti-β-actin and ALP-conjugated secondary antibodies were from Sigma-Aldrich. The Lowry total protein determination kit (Dc protein assay) was from BioRad (Hercules, CA). All other reagents were from Fisher Scientific (Pittsburgh, PA).
Animals and diets
Young (4 months) and aged (22 months) male C57Bl6 mice were purchased from the National Institute of Aging (Bethesda, MD) and housed in the Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facility at the University of Kentucky Medical Center. The animals were maintained at 12 h light/dark cycle in micro-isolation and had unlimited access to water and standard (Std) chow diet (2918, Teklad-Global, 18% protein rodent diet; Harlan-Teklad, Indianapolis, IN). After a 1 week acclimatization period, mice were randomly placed on three different diets: sulfur-containing amino acid deficient (SAA def.) control diet (TD.08437, Harlan-Teklad); SAA def. diet supplemented with 5 g/kg OTC (0.5% OTC diet) (TD.99366, Harlan-Teklad); or continued on the Std chow diet for a period of 4 weeks. At the end of the 4 week period, mice were deeply anesthetized, blood was collected by heart puncture, and the serum was obtained in serum separator tubes. Liver and other tissues were dissected, weighed, flash-frozen in liquid nitrogen, and kept at −80°C for later processing. All experiments with animals were included in our animal protocol, which was approved by the Institutional Animal Care and Use Committee and carried out accordance with the recommendations of American Veterinary Medical Association.
Analysis of ceramide species by HPLC-ESI/tandem mass spectrometry
Total lipid extracts were prepared from 10 mg of liver tissue, using acidified organic solvents (21) with the addition of C17 ceramide (Avanti Polar Lipids) as an internal standard. The organic phase was collected, evaporated under N2, and then the dried lipids were reconstituted in methanol. Ceramide species were quantitated by HPLC-ESI/tandem mass spectrometry using a 4000 Q-Trap hybrid linear ion trap triple-quadrupole mass spectrometer as described earlier (21). Recovery was determined by reference to the internal standards and quantification was accomplished using calibration curves generated using synthetic standards for each ceramide species (Avanti Polar Lipids). Total ceramide mass was calculated as the sum of all detected ceramide species.
Determination of hepatic glutathione content
Whole liver homogenates (20%) were prepared in a buffer containing 50 mM Tris-HCl (pH, 7.4), 1 mM EDTA, and protease and phosphatase inhibitors. GSH+GSSG and GSSG contents were determined in 50 μl aliquots using the Bioxytech® GSH/GSSG-412™ assay kit according to the manufacturer’s instructions. GSH was calculated as GSSG values were subtracted from the total glutathione (GSH+GSSG).
Cell culture
HepG2 cells (ATCC, Manassas, VA) were maintained in MEM (Gibco, Invitrogen) supplemented with 10% FBS (Atlanta-Biologicals, Atlanta, GA). Cells were seeded in 6-well plates (100,000 cells/well) and allowed 18 h to attach to the bottom. Cell culture medium was replaced with fresh medium supplemented with the respective concentrations of OTC in the beginning of the treatment and every third day after that. Cells were harvested 1, 3, or 6 days after treatment, as indicated.
SMase activity assays
Liver homogenates were prepared in a buffer containing 50 mM Tris-HCl (pH, 7.4), 1 mM β-mercaptoethanol (β-Me), 0.1% Triton X-100, phosphatase inhibitors (1 mM Na2VO4, 1 mM NaF), and 1:100 (v/v) protease inhibitor cocktail (Sigma-Aldrich). nSMase and acidic SMase (ASMase) activities were measured using C6-NBD-SM from Molecular Probes Inc. as a substrate. Liver lysates (80 μg protein/assay) or HepG2 cell lysates in PBS (20 μg protein/assay) were incubated in a reaction buffer containing 50 mM Tris-HCl (pH 7.4), 7.5 mM MgCl2, and 10 μM C6-NBD-SM, for nSMase activity. Because the inclusion of β-Me in the homogenates/assays might influence the OTC effects (in case these were mediated via redox-sensitive mechanisms), preliminary assays were done where the concentration of β-Me in the lysis or assay buffers was varied (from 0 to 10 mM). The results from these studies indicated that albeit β-Me inclusion affected the basal nSMase activity to some extent, it did not alter the elevation seen in the OTC-treated groups. For ASMase activity, liver lysates were incubated in 420 mM acetate buffer (pH 4.5) containing 10 μM C6-NBD-SM. Reactions were allowed to proceed for 1 h at 37°C and stopped by the addition of 1 ml of methanol. After another incubation at 37°C for 30 min, the samples were centrifuged at 1,000 g for 5 min and the generation of the fluorescent product, C6-NBD-ceramide ((6-((N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)amino)hexanoyl)Sphingosine), was monitored by a reverse phase HPLC using methanol:water:phosphoric acid (850:150:0.15, by volume) as a mobile phase (30).
SDS-PAGE and Western blotting
Whole liver lysates were prepared in a buffer containing 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1.0% Triton X-100, 1:100 (v/v) protease inhibitor cocktail, and phosphatase inhibitors (1 mM Na2VO4, 1 mM NaF). HepG2 cells were pelleted by centrifugation at 500 g for 4 min at 4°C, rinsed, and lysed in 50 μl of lysis buffer [1 mM EDTA, 1.0% Triton X-100, 1 mM Na2VO4, 1 mM NaF, 1:100 (v/v) protease inhibitor cocktail, and 50 mM Tris-HCl (pH 7.4)] on ice for 30 min. Cell and tissue lysates were centrifuged at 16,000 g for 10 min at 4°C and protein concentration was measured. Proteins (usually 80–100 μg/lane) were resolved by 10% SDS-PAGE and transferred to Immobilon®-P polyvinylidene difluoride membrane (Millipore Corporation, Billerica, MA). nSMase-1 and nSMase-2 proteins were detected using the antibodies described in the Materials section. Protein-antibody interactions were visualized using ECF substrate and a Storm860 Phospho-imager scanning instrument. Data were analyzed and quantified using ImageQuant5.0 software (Molecular Dynamics, Sunnyvale, CA).
Liver histology
Immediately upon dissection, part of the liver tissue was embedded in OCT compound from Tissue-Tek® (Sakura Finetek USA, Inc., Torrance, CA) and frozen for future processing. Frozen liver tissue samples were sectioned (8–10 μm), stained with either hematoxylin and eosin (H and E) or Oil Red-O, and observed under a light microscope.
Triacylglycerol determination
The mass of triacylglycerols (TAGs) in the liver was measured in total lipid extracts using an L-type Triglyceride M kit from Wako Chemicals USA, Inc. (Richmond, VA) following the manufacturer’s instructions.
Total RNA isolation, quantitative reverse transcription, and real-time PCR
Total RNA was extracted from 80 mg liver or 1 × 106 HepG2 cells using TRIZOL® reagent (Invitrogen) following the manufacturer’s instructions. Reverse transcription was done with 2 μg total RNA using random hexamers (Roche, Indianapolis, IN) and Superscript II™ reverse transcriptase (Invitrogen). Quantitative RT-PCR analysis was performed with the following primers: nSMase-2, – forward 5′-gcaggaggtgtttgacaag-3′, reverse 5′-tctttggtcctgaggtgtg-3′ β-actin, forward 5′-tatggagaagatttggcacc-3′, reverse 5′-gtccagacgcaggatggcat-3′ using JumpStart Taq DNA polymerase (Sigma-Aldrich). PCR products were separated by electrophoresis on 1.8% agarose gel containing 0.02% ethidium bromide and visualized on a Scion Image gel imaging system. Subsequent quantification and analyses were performed using ImageQuant5.0 software (Molecular Dynamics).
Quantitative real-time PCR analyses were performed using TaqMan® gene expression assays (Applied Biosystems) specific for mouse (Mm00491359_m1) and human (Hs00920354_m1) nSMase-2 gene. Reactions were done in 96-well plates according to the manufacturer’s instructions and analyzed in absolute quantitation experiments using Applied Biosystems’s 7500 real-time PCR system equipped with SDSv1.2x software. Expression assays for β-actin (Hs99999903_m1, human; Mm00607939_s1, mouse) were used as endogenous normalization controls. Levels of nSMase-2 mRNA were normalized to β-actin mRNA.
Serum ALP and ALT activity assays
In vitro quantitative determination of ALP and ALT activities was carried out in mouse serum using DiscretePak™ ALP and DiscretePak™ ALT reagent kits (Catachem, Bridgeport, CT) following the manufacturer’s recommendations in 10 μl (for ALT) or 6 μl (for ALP) of serum. Absorbance was monitored at 340 nm (for ALT) and 405 nm (for ALP) for up to 10 min. Final values were calculated according to the instructions and normalized per gram of liver.
RESULTS
Impact of diets on body and liver weight
Studies in hepatocytes isolated from young and aged animals consistently show a 50–60% decrease in GSH content during aging. The goal of this study was first to test whether similar differences are seen in vivo and to determine if dietary supplementation with a GSH precursor, OTC, could alleviate the aging-associated differences. Assessment of hepatic GSH content in animals, however, is difficult. Fluctuations in endogenous GSH content due to the different nutritional status of each animal, as well as differences in metabolism, has led to inconsistency in the results of several major studies (37–44) with a similar goal. In an attempt to alleviate these inherent limitations, three different dietary regimens were used with young (4 months) and aged (22 months) C57Bl6 mice. In the first group, mice were fed regular chow diet and served as a Std control. To diminish the effects of the GSH and GSH precursors present in the regular rodent chow, a second group of mice was placed on a diet of defined amino acid composition that was devoid of the two GSH precursors, cysteine and methionine (SAA def. diet). A third group, consisting of age- and gender-matched animals, was fed the same SAA def. diet supplemented with OTC (see Table 1 for the exact composition of the diets). Mice were placed on these diets for 4 weeks. Significant diet- and aging-associated differences in the body and liver weight (supplementary Table I) were observed. As compared with mice on a Std diet, mice fed the SAA def. diet exhibited lower body weight (17.96 ± 0.43 g for young and 21.00 ± 0.99 g for aged vs. 32.26 ± 0.63 g for young and 31.78 ± 1.87 g for aged, respectively). Adding OTC to the diet partially reversed the changes in body weight. The average weights of the OTC-fed animal were 20.23 ± 1.50 g and 26.72 ± 1.72 g, for young and aged respectively. Similar differences were observed in liver weight, with the livers of aged mice being statistically heavier than the livers of young animals across all diets, and mice on the SAA def. diet having significantly lower liver weight as compared with those fed a Std or OTC-supplemented diet. No significant liver toxicity was observed; although varying between the groups (from 50 to 200 units/l), the activity of ALP and ALT (supplementary Fig. I) remained well below the range typically associated with toxicity (i.e., between 2,000 and 10,000 units/l).
TABLE 1.
Composition of the diets
| Ingredientsa | Diet | ||
| SAA Def. | 0.5% OTC | Std Chow | |
| l-Alanine | 5.2 | 4.2 | 11.0 |
| l-Arginine HCl | 12.1 | 12.1 | 10.0 |
| l-Asparagine | 6.0 | 5.0 | 0.0 |
| l-Aspartic acid | 5.2 | 4.2 | 14.0 |
| l-Glutamic acid | 40.0 | 40.0 | 34.0 |
| Glycine | 26.0 | 26.0 | 8.0 |
| l-Histidine HCl | 4.5 | 4.5 | 4.0 |
| l-Isoleucine | 8.2 | 8.2 | 8.0 |
| l-Leucine | 11.1 | 11.1 | 18.0 |
| l-Lysine HCl | 18.0 | 18.0 | 9.0 |
| l-Phenylalanine | 7.5 | 7.5 | 10.0 |
| l-Proline | 3.5 | 3.5 | 16.0 |
| l-Serine | 5.2 | 5.2 | 11.0 |
| l-Threonine | 8.2 | 8.2 | 7.0 |
| l-Tryptophan | 1.8 | 1.8 | 2.0 |
| l-Tyrosine | 5.0 | 5.0 | 6.0 |
| l-Valine | 8.2 | 8.2 | 9.0 |
| l-Cysteine | 0.0 | 0.0 | 3.0 |
| l-Methionine | 0.0 | 0.0 | 4.0 |
| OTC | 0.0 | 5.0 | 0.0 |
| Carbohydrates | 652.0 | 650.0 | 442.0 |
| Fats | 80.0 | 80.0 | 62.0 |
| Fibers | 34.0 | 34.0 | 35.0 |
| Minerals | 43.2 | 43.2 | 35.4 |
| Vitamins | 13.0 | 13.0 | 12.5 |
Diets were purchased from Harlan-Teklad. SAA def. and 0.5% OTC diets were custom-made amino acid- defined diets. Std chow was an 18% protein rodent diet from natural sources.
Amino acid profile and other ingredients in the diets are presented as g/kg of dry weight diet.
Aging-associated changes in hepatic GSH/GSSG levels and nSMase activity in vivo
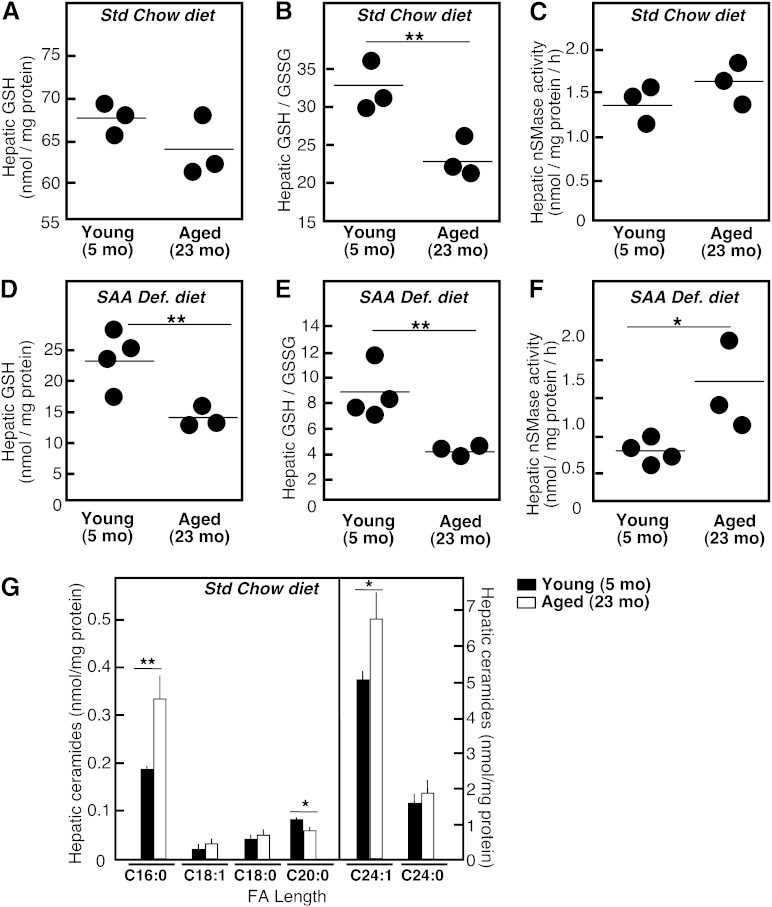
In mice fed the Std diet, no statistically significant aging-associated changes in hepatic GSH content or GSH/GSSG ratio were measurable (Fig. 1A, B). The aging-associated differences in nSMase activity also did not reach statistical significance (Fig. 1C). These data are in contrast to our earlier observations for primary hepatocytes, where a 70% drop in the total GSH content and almost 2-fold elevation in nSMase activity were observed in hepatocytes isolated from aged rats as compared with those from young rats (28).
Fig. 1.
Aging-associated changes in GSH/GSSG levels, nSMase activity, and ceramide species in liver. Young (5 months) and aged (23 months) C57Bl6 mice were randomly placed on either a Std chow or a SAA def. diet for 4 weeks. Twenty percent homogenates from livers were prepared and used for analyses. Hepatic GSH and GSSG (A, B, D, E) were measured in individual animals (n = 3) using a Bioxytech® GSH/GSSG-412™ assay kit as outlined in the Materials and Methods. nSMase activity (C, F) was measured in whole liver homogenates (40 μg of protein/assay) using a fluorescently labeled analog NBD-SM as a substrate. The mean values for each individual mouse are shown. Quantification of the different ceramide species in the liver of young and aged animals was done by HPLC-ESI/tandem mass spectrometry (G). Mean values ± SD are shown (n = 3), *P < 0.05, **P < 0.01 according to Student’s t-test.
Aging-associated differences in GSH levels and in nSMase activity were readily apparent in mice on the SAA def. diet. As expected, there was an overall decline in total hepatic GSH content in all SAA def. diet-fed mice. However, the hepatic GSH content (Fig. 1D) and GSH/GSSG ratio (Fig. 1E) in aged animals were lower than those in young animals by 35 and 200%, respectively. nSMase activity was 2-fold higher in the aged animals than that measured in the young animals (Fig. 1F). Thus, the changes observed in mice on a SAA def. diet reflect the aging-associated changes in GSH and nSMase activity observed in isolated hepatocytes.
Aging-associated accumulation of C16:0 and C24:1 ceramides
Measurement of hepatic levels of ceramide, the product of nSMase activity, in the liver of mice fed the regular chow diet showed a slight tendency to increase with aging, but the differences did not reach statistical significance. However, statistically significant changes were seen when mass spectrometry was used to individually quantify ceramides with different FA chain length. Notably, the two major ceramide species in the liver, C24:1 and C16:0 ceramide, were elevated with aging. The levels of C20:1 ceramide seemed to decrease with aging, while levels of C18:1, C18:0, C20:0, and C24:0 ceramide were not affected (Fig. 1G). The same ceramide species were elevated in aged SAA def. diet-fed mice as compared with young mice on the same diet. These data are consistent with an aging-associated stimulation of the activity of nSMase-2 specifically, rather than that of nSMase-1, because C24:1-SM is the preferred substrate of the former (45).
Alleviation of aging-associated differences in hepatic GSH by dietary OTC supplementation
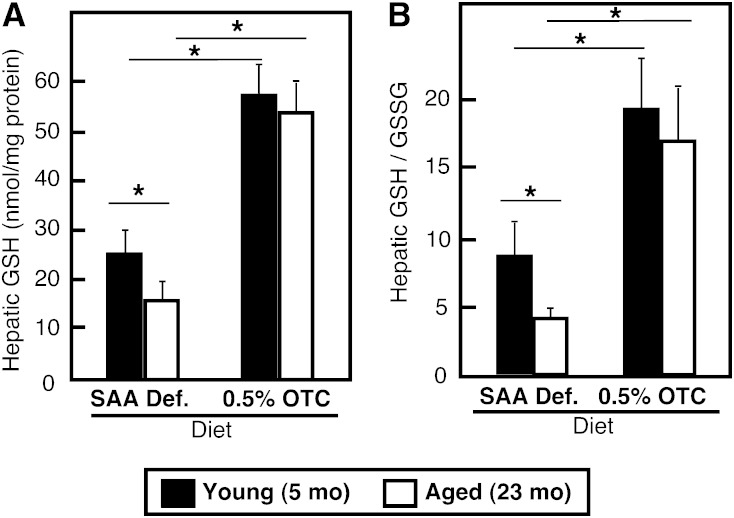
Substantial elevation in hepatic GSH content was achieved in mice fed with the OTC-supplemented diet. The total GSH content increased up to 3-fold (Fig. 2A) in animals of all ages, while the GSH/GSSG ratio was elevated between 2.5- and 3-fold (Fig. 2B). These changes were well within the physiological range of GSH, as they closely resembled the values typically observed in mice on a chow diet. More importantly, mice on an OTC diet exhibited no aging-associated differences in hepatic GSH levels and GSH/GSSG ratio. Together these results show that OTC provided in the diet can effectively modulate hepatic GSH content in an age-specific manner.
Fig. 2.
Effect of dietary OTC supplementation on hepatic GSH content. Young and aged C57Bl6 mice were placed on either a 0.5% OTC or a SAA def. control diet for 4 weeks. Hepatic GSH (A) and GSH/GSSG (B) contents were measured in whole liver homogenates prepared from individual animals (n = 3–4 per group) using a Bioxytech® GSH/GSSG-412™ assay kit. Data are shown as mean values ± SD. Statistical analyses were done by two-way ANOVA. The main effects of the diet and age are indicated with an asterisk (*P < 0.05). No interaction between both factors was present.
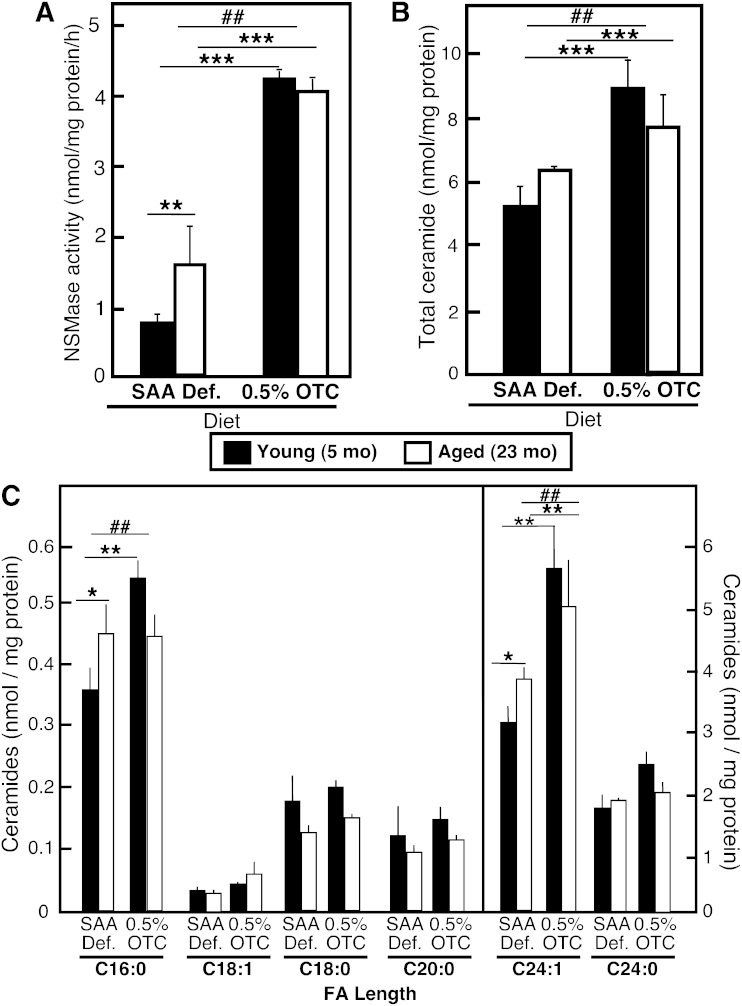
Effects of the OTC diet on hepatic ceramide levels and nSMase activity
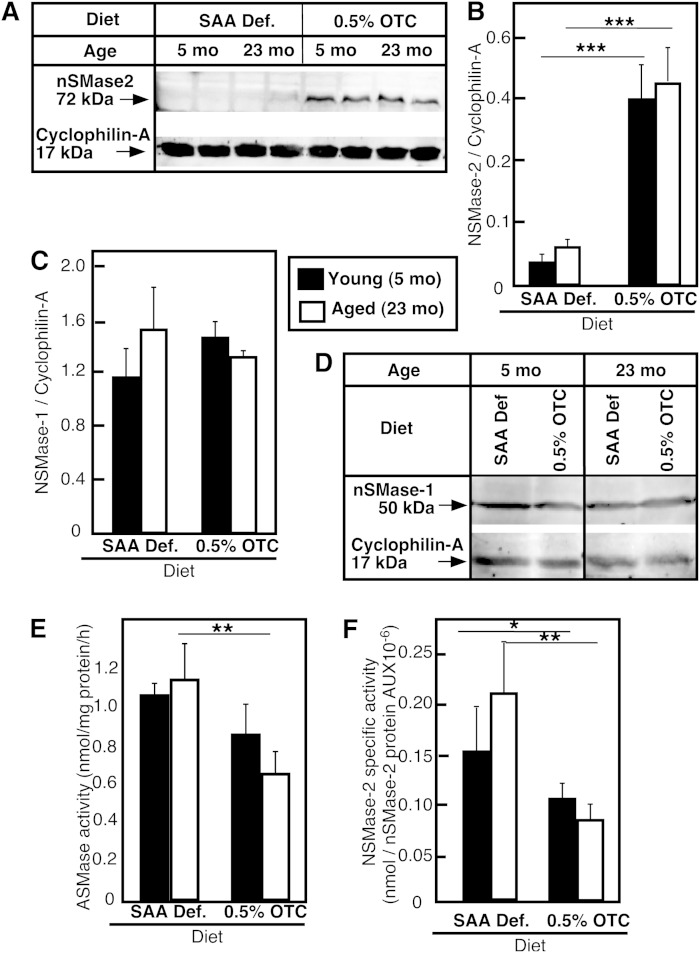
Our earlier studies in isolated hepatocytes showed that restoration of normal GSH levels in hepatocytes of aged rats also restored normal nSMase activity and ceramide content (28), consistent with the proposed role of GSH as an inhibitor of nSMase; therefore, we anticipated that restoration of GSH content in aged animals should inhibit nSMase activity and respectively decrease ceramide content. Surprisingly, however, in spite the significant elevation in hepatic GSH content, all mice on the OTC diet exhibited 5- to 6-fold higher hepatic nSMase activity as compared with the activity observed in mice fed either the Std chow or the SAA def. control diet (Fig. 3A). Accordingly, the levels of the SMase product, ceramide, also increased (Fig. 3B), with C24:1 ceramide being affected the most (Fig. 3C). The assessment of nSMase-2 protein content in the liver revealed that the increases in activity were likely caused by substantial elevation of nSMase-2 protein in young and aged mice fed the OTC diet (Fig. 4A, B). These effects of OTC appear to be specific for nSMase-2, as neither nSMase-1 nor ASMase were affected, based on the lack of change in nSMase-1 protein content (Fig. 4C, D) or ASMase activity (Fig. 4E). Furthermore, these effects were most likely GSH-independent, because a comparison of GSH levels and nSMase-2 expression in all groups of animals shows no correlation between GSH content, ceramide levels, or nSMase-2 expression.
Fig. 3.
Effect of dietary OTC supplementation on nSMase activity and ceramide levels in mouse livers. Young and aged C57Bl6 mice were placed on either a 0.5% OTC or a SAA def. control diet for 4 weeks. nSMase activity (A) was assessed in whole liver homogenates (40 μg of protein/assay) using a fluorescently labeled NBD-SM as a substrate. Total ceramide (B) and ceramide species with different FA length (C) were measured in total lipid extracts by HPLC-ESI/tandem mass spectrometry. Mean values ± SD are shown (n = 3–4 mice in each group). Statistical analysis was done by two-way ANOVA followed by Bonferroni posttest analyses. The statistical significance of the main effects (aging and diet) are shown with asterisks (*P < 0.05, **P < 0.01, ***P < 0.001). The statistical significance of the interaction effect is shown with number signs (##P < 0.001).
Fig. 4.
Effect of dietary OTC supplementation on nSMase-2 protein in mouse livers. Young and aged C57Bl6 mice were placed on either a 0.5% OTC or a SAA def. control diet for 4 weeks. nSMase-2 (A, B) and nSMase-1 (C, D) protein levels in whole liver lysates were determined by Western blot using specific rabbit polyclonal antibodies against each protein. Cyclophilin-A was used to control for equal loading and for normalization. Mean values ± SD (n = 3–4 animals per group) of the ratio between the respective nSMase protein and the loading control are shown. ASMase activity (E) was determined in whole liver homogenates as described in the Materials and Methods. nSMase-2 specific activity (F) was calculated as the total nSMase activity measured for each individual mouse divided by the nSMase-2 protein amount quantified by Western blotting. Data are shown as mean values ± SD (n = 3–4 animals per group). Statistical analyses were done according to two-way ANOVA and Bonferroni posttest analyses. The statistical significance of the main effect of diet and aging is shown by asterisks (*P < 0.05, **P < 0.01, and ***P < 0.001). No statistically significant interaction effects were present.
Interestingly, the fold increase in nSMase-2 protein levels observed in mice on the OTC diet was up to 5-fold, while the corresponding changes in activity were only 2.5-fold, indicating that change in activity did not follow exactly the change in protein content. Normalization of the nSMase activity measured in vitro to the amount of nSMase-2 protein, instead of the total protein content, indicated that in the OTC-fed mice, hepatic nSMase-2 had lower specific activity than the enzyme of SAA def.-fed mice (Fig. 4F). One possible reason for this lower specific activity might be that GSH, in which content increased substantially in the OTC-fed mice, effectively inhibited the intrinsic activity of nSMase-2, similar to the effects seen in vitro when GSH content was increased via N-acetyl cysteine supplementation. Whatever the explanation, these results provide compelling evidence that OTC regulates the expression of nSMase-2 mRNA in the liver. More importantly, this upregulation was physiologically important, as it resulted in increased nSMase-2 activity and accumulation of ceramide.
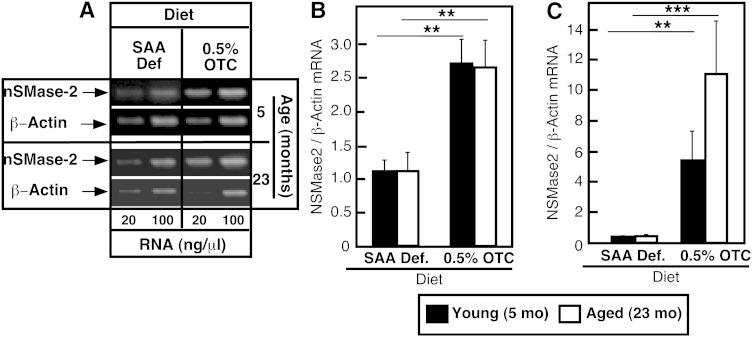
Quantitative RT-PCR (Fig. 5A, B), as well as real-time PCR (Fig. 5C), showed that hepatic nSMase-2 mRNA levels were substantially higher in the OTC-fed young or old mice, indicating that the effects of the diet on nSMase-2 protein were likely due to increased transcription/stability of nSMase-2 mRNA. The observed amplitude of change ranged between 2-fold (based on RT-PCR) and 15-fold (based on real-time PCR), which is not surprising having in mind the significantly better sensitivity of the latter method.
Fig. 5.
Effect of dietary OTC supplementation on nSMase-2 mRNA levels in liver. Young and aged C57Bl6 mice were placed on either a 0.5% OTC or a SAA def. control diet for 4 weeks. Levels of nSMase-2 mRNA in the liver were assessed by quantitative RT-PCR (A, B) and real-time PCR (C). The levels of β-actin mRNA were used for normalization. Data are shown as mean values ± SD (n = 3–4). Statistical analyses were done according to two-way ANOVA and Bonferroni posttest analyses. The statistical significance of the main effect of diet and aging is shown by asterisks (*P < 0.05, **P < 0.01, and ***P < 0.001). No statistically significant interaction effects were present.
nSMse-2 protein expression in HepG2 cells supplemented with OTC
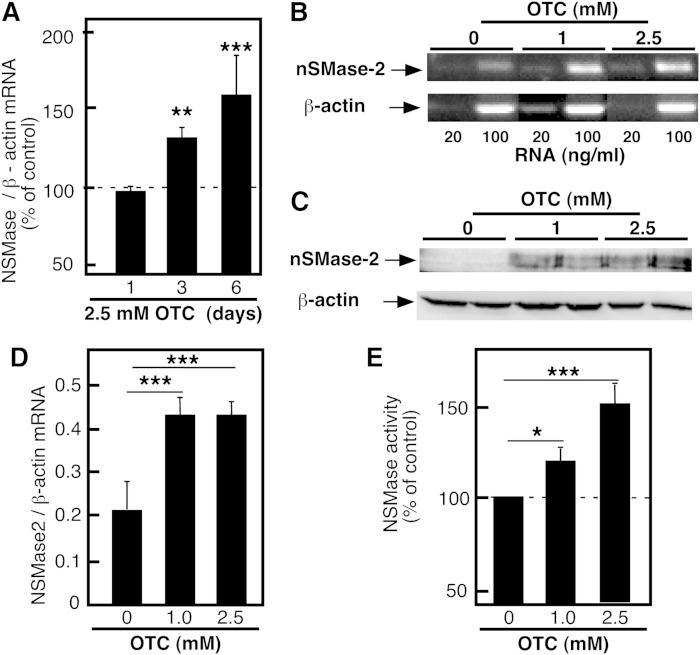
To begin investigating the mechanism of these effects of OTC on nSMase-2, HepG2 cells were used. Initially, OTC was added to HepG2 cells for up to 6 days at various concentrations ranging from 1 to 10 mM. No toxicity was observed (data not shown), however, concentrations at the higher end (7.5 and 10 nM) tended to acidify the medium. Therefore, OTC was added to HepG2 cells at a concentration of 2.5 mM and nSMase-2 mRNA expression was determined by real-time PCR after 1, 3, and 6 days of treatment (Fig. 6A). In agreement with the results seen in vivo, the OTC treatments led to a substantial increase in nSMase-2 mRNA after 3 and 6 days of treatment. The dose dependency of this effect was confirmed by RT-PCR of nSMase-2 mRNA (Fig. 6B, D) or by Western blotting to assess nSMase-2 protein (Fig. 6C), whose levels were similarly elevated in the OTC-treated cells. Finally, the OTC-treated cells also exhibited 2-fold higher nSMase activity (Fig. 6E). Together these data suggest that OTC is a novel inducer of nSMase-2 expression in the liver.
Fig. 6.
nSMase-2 protein and mRNA levels in HepG2 cells supplemented with OTC. HepG2 cells were cultured in complete growth medium supplemented with OTC (2.5 mM) for the indicated time (A) or for 6 days with the indicated concentrations (B–E). The levels of nSMase-2 mRNA were analyzed by real-time PCR (A) or quantitative RT-PCR (B, D). The levels of nSMase-2 protein were analyzed by Western blotting using nSMase-2 specific rabbit polyclonal antibody (C). β-Actin was used for normalization and as a control for equal loading. nSMase activity was measured in whole cell homogenates (D). Data are shown as a percent of the activity measured in vehicle-treated cells. Data are shown as mean values ± SD (n = 3). One-way ANOVA with Dunnett’s multiple comparison test are shown (*P < 0.05, ** P < 0.01, ***P < 0.001). Results are representative of at least three independent experiments.
Potential role of OTC-induced nSMase-2 protein expression in hepatic steatosis
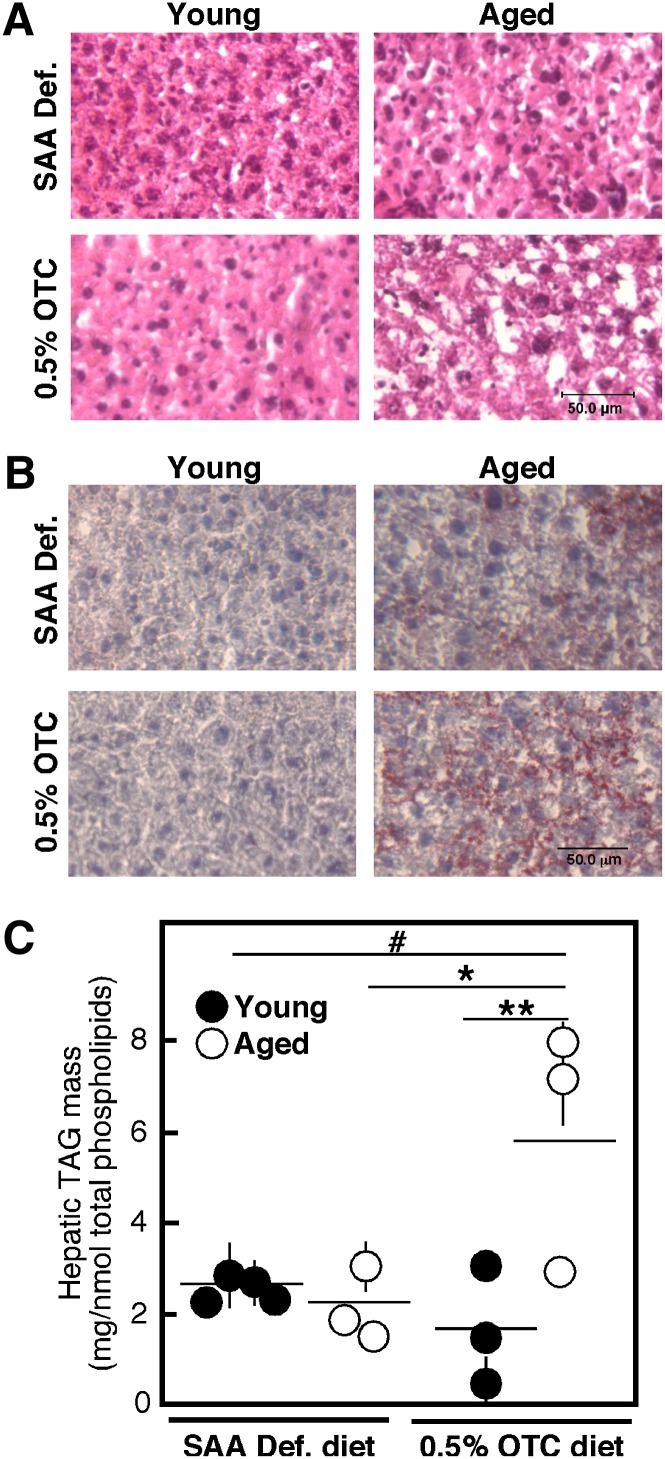
Close histological examination of the livers indicates that the OTC-fed mice begin to develop fatty liver or steatosis. H and E staining shows intact hepatic morphology (Fig. 7A); however, an increased proportion of white coloration, indicative for fatty deposit, was evident in the OTC-fed mice, especially at old age. The nature of these deposits was confirmed by Oil Red-O staining, indicating an increase in the accumulation of neutral fat (TAG and esterified cholesterol) in the liver of aged OTC-fed mice (Fig. 7B). These results suggested that at least in aged mice, OTC diet may cause increased TAG synthesis, and respectively, the development of fatty liver. The direct measurements of hepatic TAG mass confirmed that there is a statistically significant elevation of this lipid class in aged animals on the OTC diet (Fig. 7C). Based on earlier observations by ourselves and others, these effects could be linked to the observed upregulation of nSMase-2 protein. An elevation in hepatic ceramide has been observed and causatively linked to the development of the disease in various models of hepatic steatosis, including diet- and alcohol-induced TAG accumulation (46–48).
Fig. 7.
Liver histology and TAG levels. Young and aged C57Bl6 mice were placed on either a 0.5% OTC or a SAA def. control diet for 4 weeks. Freshly dissected liver portions were flash-frozen, sliced, and stained with H and E (A) or Oil Red-O (B). Slides are representative of all animals in each group. The scale bar corresponds to 50 μm. Hepatic TAG mass was measured in total lipid extracts using an L-Type Triglyceride M kit and normalized to the total phospholipid content (C). Data from an individual mouse are shown. Statistical analysis was done by two-way ANOVA followed by Bonferroni posttest analyses. The statistical significance of the main effects (aging and diet) is shown by asterisks (*P < 0.05, **P < 0.01). The statistical significance of the interaction effect is shown by a number sign (#P < 0.01).
DISCUSSION
This study investigates the link between hepatic GSH, aging, and SMase activity. Excessive utilization of GSH, either for toxin conjugation or ROS scavenging, is characteristic of many pathophysiological conditions and may lead to chronic depletion of GSH and accumulation of GSSG in the tissue. Aging, in particular, is associated with a substantial increase in the GSSG/GSH ratio in the liver, the main site of GSH synthesis; however, whether this reflects depletion of GSH or only elevation in GSSG has not been clearly shown. Direct measurements of GSH in isolated hepatocytes indicated that hepatocytes from aged animals exhibit 2- to 3-fold lower levels of GSH as compared with young animals. Some in vivo studies confirm these results, reporting a net decrease in GSH content in the livers of 24-month-old rats as compared with 4-month-old rats (37–42). Other studies, however, found no change in hepatic GSH with age (43, 44). The results from our study indicate that the dietary supply of cysteine and cysteine precursors may be an important factor in determining whether or not hepatic GSH content will decrease with aging. Apparently the availability of dietary GSH precursors helps to maintain GSH content at any age, while feeding for 4 weeks with a diet depleted of cysteine precursors reveals significant aging-associated decline. It is likely that while on a SAA def. diet, the aged mice exhaust available GSH much faster than young mice due to increased demand of ROS scavenging and/or defects in their ability to synthesize GSH. Because only male mice were used in our experiments, it is unclear whether these observations reflect a trend common for both genders. One should not exclude the possibility that in females, the changes might be blunted, as male mice have been found to experience more dramatic age-associated change in GSH content than female mice (49).
An aging-associated elevation in ceramide content accompanies GSH depletion and is observed in liver, brain, plasma, and other tissues. While accumulation of ceramide can lead to increased inflammation and cell death, current evidence suggest that the FA make-up of ceramide is a key determinant of ceramide biological activity. It seems that ceramides with various chain lengths can be generated in a specific manner and have distinct bioactivities (50). The effects of aging on the FA composition of ceramide have been investigated in several models including mouse CD4+ T-cells (51), cardiomyocytes (52), and skeletal muscle (53). Our mass spectrometry-based analysis of ceramide species in the livers of aged and young mice reveals that aging leads to the accumulation of C16:0 and C24:1 ceramides and has no effect on the less abundant C18:0, C18:1, and C24:0 ceramides, while the level of C20:0 ceramide is modestly suppressed.
Studies in cell culture have also found that total nSMase activity is elevated with aging. The siRNAi approach indicated that nSMase-2, but not nSMase-1, accounted for these increases (54). Our analyses in mouse livers show similar differences that, however, reached statistical significance only in mice on the SAA def. diet but not in mice on Std chow. One possible explanation for this failure to observe strong aging-associated changes in nSMase activity in vivo could be the heterogeneity of the cell population making up the hepatic extracts. Furthermore, there is no specific assay to monitor the enzymatic activity of nSMase-2. In fact the assay reflects the sum of all hepatic nSMases, including the main housekeeping enzyme, nSMase-1, which is not affected by aging but is more abundant than nSMase-2. Whatever the case, the expected elevation in nSMase activity with aging was clearly seen when the mice were placed briefly on the SAA def. diet and the aging-associated differences in hepatic GSH levels were detected.
Our studies present evidence for transcriptional regulation of nSMase-2 by dietary OTC. The effects are specific and are paralleled by a production of functional protein and an elevation in ceramide. NSMase-2 is a signaling enzyme that typically is linked to the onset of apoptosis, cell growth arrest, and inflammation (55). Oxidative stress and pro-inflammatory cytokines, including IL-1β and TNF-α, are among the major inducers of nSMase activity; the effects are rapid (typically within 30 min of stimulation) and transient, involving posttranslational mechanisms such as change in phosphorylation status and translocation (56, 57). Our present study shows that the expression of nSMase-2 mRNA and protein is also regulated and could be affected by dietary means. The effects were clearly seen in vivo (in OTC-fed mice) as well as in vitro (in OTC-treated HepG2 cells), and were seemingly GSH-independent. Notably, nSMase-1 was not affected, thus confirming the specificity of the changes. More importantly, these changes in expression were functionally important, as they led to elevated hepatic ceramide content. Two very recent studies showed similar stimulation of nSMase-2 mRNA in response to all-trans retinoic acid (58) and cyclopamine (59) in cell culture. Together, these studies delineate a novel pathway for the regulation of nSMase activity and function, namely at mRNA level.
The exact mechanisms behind the OTC-induced upregulation of nSMase-2 mRNA are unclear. OTC-mediated activation of Nuclear factor erythroid 2-related transcription factor (NRF-2) has been observed in liver cells (23) and analysis of the 5′ proximal region of the nSMase-2 gene reveals the existence of an NRF-2 consensus binding site. Alternatively, an OTC metabolite could be involved. As discussed earlier, following its metabolic conversion by a cytosolic enzyme, 5-oxoprolinase, OTC serves as a precursor of cysteine and glutathione. Our studies, however, indicated that GSH is not the likely mediator because the changes in the GSH levels observed in vivo did not correlate with the changes in nSMase-2 mRNA or protein. Furthermore, in HepG2 cells, the OTC supplementation had very little effect on cellular glutathione levels (data not shown). Other OTC metabolites, however, could play a role. Hepatic cells efficiently convert OTC to taurine (60, 61), which is an LXR ligand and has been shown to affect hepatic lipid metabolism (62). OTC could also generate sulfate (60, 61) that, in turn, may affect hepatic functions such as drug detoxification and protein posttranslational modification.
In general, OTC is considered a beneficial supplement that may help in improving overall health in number of conditions associated with decreased GSH content (19, 22, 63–68). These could be specifically important for the aged population, where decreased ability to detoxify common drugs like acetaminophen has been linked to decline in GSH content (69). Therefore, our findings that OTC upregulates nSMase-2 expression and increases ceramide content (changes that are typically linked to propagation of inflammation and cell death) were unexpected. It is of note, however, that we also observed an elevation of hepatic TAG levels after the OTC diet, particularly in the aged mice. These changes in TAG synthesis might be causatively linked to the nSMase-2 upregulation, because in other systems, nSMase-2 and ceramide have been found to promote TAG synthesis and lipid droplet formation (70).
Supplementary Material
Footnotes
Abbreviations:
- ALP
- alkaline phosphatase
- ALT
- alanine aminotransferase
- ASMase
- acid SMase
- β-Me
- β-mercaptoethanol
- C6-NBD-SM
- N-(6-((7-nitro-2-1,3-benzoxadiazol-4-yl)amino)hexanoyl)-sphingosine-1-phosphocholine
- H and E
- hematoxylin and eosin
- nSMase
- neutral SMase
- OTC
- L-2-oxothiazolidine-4-carboxylic acid
- ROS
- reactive oxygen species
- SAA
- sulfur-containing amino acid
- SAA def.
- sulfur-containing amino acid deficient
- Std
- standard
- TAG
- triacylglycerol
This work was supported by National Institutes of Health Grants AG019223 and AG026711 (to M.N.K.), and GM50388, P20RR021954 for LC-MS/MS analyses (to A.J.M.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and one table.
REFERENCES
- 1.Samiec P. S., Drews-Botsch C., Flagg E. W., Kurtz J. C., Sternberg P., Jr, Reed R. L., Jones D. P. 1998. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic. Biol. Med. 24: 699–704. [DOI] [PubMed] [Google Scholar]
- 2.Erden-İnal M., Sunal E., Kanbak G. 2002. Age-related changes in the glutathione redox system. Cell Biochem. Funct. 20: 61–66. [DOI] [PubMed] [Google Scholar]
- 3.Lang C. A., Naryshkin S., Schneider D. L., Mills B. J., Lindeman R. D. 1992. Low blood glutathione levels in healthy aging adults. J. Lab. Clin. Med. 120: 720–725. [PubMed] [Google Scholar]
- 4.Zhu Y., Carvey P. M., Ling Z. 2006. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 1090: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki T., Senda M., Kim S. N., Kojima S., Kubodera A. 2001. Age-related changes of glutathione content, glucose transport and metabolism, and mitochondrial electron transfer function in mouse brain. Nucl. Med. Biol. 28: 25–31. [DOI] [PubMed] [Google Scholar]
- 6.Paasche G., Huster D., Reichenbach A. 1998. The glutathione content of retinal Müller (glial) cells: the effects of aging and of application of free-radical scavengers. Ophthalmic Res. 30: 351–360. [DOI] [PubMed] [Google Scholar]
- 7.Nakata K., Kawase M., Ogino S., Kinoshita C., Murata H., Sakaue T., Ogata K., Ohmori S. 1996. Effects of age on levels of cysteine, glutathione and related enzyme activities in livers of mice and rats and an attempt to replenish hepatic glutathione level of mouse with cysteine derivatives. Mech. Ageing Dev. 90: 195–207. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi S., Zeydel M. 1982. gamma-Glutamyl transpeptidase and glutathione in aging IMR-90 fibroblasts and in differentiating 3T3 L1 preadipocytes. Arch. Biochem. Biophys. 214: 260–267. [DOI] [PubMed] [Google Scholar]
- 9.Chung H. Y., Lee E. K., Choi Y. J., Kim J. M., Kim D. H., Zou Y., Kim C. H., Lee J., Kim H. S., Kim N. D., et al. 2011. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J. Dent. Res. 90: 830–840. [DOI] [PubMed] [Google Scholar]
- 10.Ballatori N., Krance S. M., Notenboom S., Shi S., Tieu K., Hammond C. L. 2009. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 390: 191–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo T., Hirose M., Kageyama K. 2009. Roles of oxidative stress and redox regulation in atherosclerosis. J. Atheroscler. Thromb. 16: 532–538. [DOI] [PubMed] [Google Scholar]
- 12.Lu S. C. 1999. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 13: 1169–1183. [PubMed] [Google Scholar]
- 13.Cacciatore I., Cornacchia C., Pinnen F., Mollica A., Di Stefano A. 2010. Prodrug approach for increasing cellular glutathione levels. Molecules. 15: 1242–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishina H., Ohta J., Ubuka T. 1987. Effect of L-2-oxothiazolidine-4-carboxylate administration on glutathione and cysteine concentrations in guinea pig liver and kidney. Physiol. Chem. Phys. Med. NMR. 19: 9–13. [PubMed] [Google Scholar]
- 15.Williamson J. M., Boettcher B., Meister A. 1982. Intracellular cysteine delivery system that protects against toxicity by promoting glutathione synthesis. Proc. Natl. Acad. Sci. USA. 79: 6246–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iimuro Y., Bradford B. U., Yamashina S., Rusyn I., Nakagami M., Enomoto N., Kono H., Frey W., Forman D., Brenner D., et al. 2000. The glutathione precursor L-2-oxothiazolidine-4-carboxylic acid protects against liver injury due to chronic enteral ethanol exposure in the rat. Hepatology. 31: 391–398. [DOI] [PubMed] [Google Scholar]
- 17.Gwilt P. R., Radick L. E., Li X. Y., Whalen J. J., Leaf C. D. 1998. Pharmacokinetics of 2-oxothiazolidine-4-carboxylate, a cysteine prodrug, and cysteine. J. Clin. Pharmacol. 38: 945–950. [DOI] [PubMed] [Google Scholar]
- 18.Porta P., Aebi S., Summer K., Lauterburg B. H. 1991. L-2-oxothiazolidine-4-carboxylic acid, a cysteine prodrug: pharmacokinetics and effects on thiols in plasma and lymphocytes in human. J. Pharmacol. Exp. Ther. 257: 331–334. [PubMed] [Google Scholar]
- 19.Moberly J. B., Logan J., Borum P. R., Story K. O., Webb L. E., Jassal S. V., Mupas L., Rodela H., Alghamdi G. A., Moran J. E., et al. 1998. Elevation of whole-blood glutathione in peritoneal dialysis patients by L-2-oxothiazolidine-4-carboxylate, a cysteine prodrug (Procysteine). J. Am. Soc. Nephrol. 9: 1093–1099. [DOI] [PubMed] [Google Scholar]
- 20.Kalayjian R. C., Skowron G., Emgushov R. T., Chance M., Spell S. A., Borum P. R., Webb L. S., Mayer K. H., Jackson J. B., Yen-Lieberman B. 1994. A phase I/II trial of intravenous L-2-oxothiazolidine-4-carboxylic acid (procysteine) in asymptomatic HIV-infected subjects. J. Acquir. Immune Defic. Syndr. 7: 369–374. [PubMed] [Google Scholar]
- 21.Barditch-Crovo P., Noe D., Skowron G., Lederman M., Kalayjian R., Borum P., Buier R., Rowe W., Goldberg D., Lietman P. 1998. A phase I/II evaluation of oral L-2-oxothiazolidine-4-carboxylic acid in asymptomatic patients infected with human immunodeficiency virus. J. Clin. Pharmacol. 38: 357–363. [DOI] [PubMed] [Google Scholar]
- 22.Choi J., Park K. H., Kim S. Z., Shin J. H., Jang S. I. 2013. The ameliorative effects of L-2-oxothiazolidine-4-carboxylate on acetaminophen-induced hepatotoxicity in mice. Molecules. 18: 3467–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim I. H., Kim D. G., Hao P., Wang Y., Kim S. H., Kim S. W., Lee S. O., Lee S. T. 2012. Anti-fibrotic effects of L-2-oxothiazolidine-4-carboxylic acid via modulation of nuclear factor erythroid 2-related factor 2 in rats. BMB Rep. 45: 348–353. [DOI] [PubMed] [Google Scholar]
- 24.Adamy C., Mulder P., Khouzami L., Andrieu-abadie N., Defer N., Candiani G., Pavoine C., Caramelle P., Souktani R., Le Corvoisier P., et al. 2007. Neutral sphingomyelinase inhibition participates to the benefits of N-acetylcysteine treatment in post-myocardial infarction failing heart rats. J. Mol. Cell. Cardiol. 43: 344–353. [DOI] [PubMed] [Google Scholar]
- 25.Levy M., Castillo S. S., Goldkorn T. 2006. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem. Biophys. Res. Commun. 344: 900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B., Andrieu-Abadie N., Levade T., Zhang P., Obeid L. M., Hannun Y. A. 1998. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J. Biol. Chem. 273: 11313–11320. [DOI] [PubMed] [Google Scholar]
- 27.Liu B., Hannun Y. A. 1997. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J. Biol. Chem. 272: 16281–16287. [DOI] [PubMed] [Google Scholar]
- 28.Rutkute K., Asmis R. H., Nikolova-Karakashian M. N. 2007. Regulation of neutral sphingomyelinase-2 by GSH: a new insight to the role of oxidative stress in aging-associated inflammation. J. Lipid Res. 48: 2443–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatterjee S. 1994. Neutral sphingomyelinase action stimulates signal transduction of tumor necrosis factor-alpha in the synthesis of cholesteryl esters in human fibroblasts. J. Biol. Chem. 269: 879–882. [PubMed] [Google Scholar]
- 30.Nikolova-Karakashian M., Morgan E. T., Alexander C., Liotta D. C., Merrill A. H., Jr 1997. Bimodal regulation of ceramidase by interleukin-1beta. Implications for the regulation of cytochrome p450 2C11. J. Biol. Chem. 272: 18718–18724. [DOI] [PubMed] [Google Scholar]
- 31.Verheij M., Bose R., Lin X. H., Yao B., Jarvis W. D., Grant S., Birrer M. J., Szabo E., Zon L. I., Kyriakis J. M., et al. 1996. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 380: 75–79. [DOI] [PubMed] [Google Scholar]
- 32.Yan F., Polk D. B. 2001. Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 61: 963–969. [PubMed] [Google Scholar]
- 33.Chalfant C. E., Kishikawa K., Mumby M. C., Kamibayashi C., Bielawska A., Hannun Y. A. 1999. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A. Activation is stereospecific and regulated by phosphatidic acid. J. Biol. Chem. 274: 20313–20317. [DOI] [PubMed] [Google Scholar]
- 34.Dobrowsky R. T., Hannun Y. A. 1992. Ceramide stimulates a cytosolic protein phosphatase. J. Biol. Chem. 267: 5048–5051. [PubMed] [Google Scholar]
- 35.Scheid M. P., Foltz I. N., Young P. R., Schrader J. W., Duronio V. 1999. Ceramide and cyclic adenosine monophosphate (cAMP) induce cAMP response element binding protein phosphorylation via distinct signaling pathways while having opposite effects on myeloid cell survival. Blood. 93: 217–225. [PubMed] [Google Scholar]
- 36.Ruvolo P. P., Gao F., Blalock W. L., Deng X., May W. S. 2001. Ceramide regulates protein synthesis by a novel mechanism involving the cellular PKR activator RAX. J. Biol. Chem. 276: 11754–11758. [DOI] [PubMed] [Google Scholar]
- 37.Palomero J., Galan A. I., Munoz M. E., Tunon M. J., Gonzalez-Gallego J., Jimenez R. 2001. Effects of aging on the susceptibility to the toxic effects of cyclosporin A in rats. Changes in liver glutathione and antioxidant enzymes. Free Radic. Biol. Med. 30: 836–845. [DOI] [PubMed] [Google Scholar]
- 38.Jung K., Henke W. 1996. Developmental changes of antioxidant enzymes in kidney and liver from rats. Free Radic. Biol. Med. 20: 613–617. [DOI] [PubMed] [Google Scholar]
- 39.Farooqui M. Y., Day W. W., Zamorano D. M. 1987. Glutathione and lipid peroxidation in the aging rat. Comp. Biochem. Physiol. B. 88: 177–180. [DOI] [PubMed] [Google Scholar]
- 40.Hagen T. M., Vinarsky V., Wehr C. M., Ames B. N. 2000. (R)-alpha-lipoic acid reverses the age-associated increase in susceptibility of hepatocytes to tert-butylhydroperoxide both in vitro and in vivo. Antioxid. Redox Signal. 2: 473–483. [DOI] [PubMed] [Google Scholar]
- 41.Véricel E., Narce M., Ulmann L., Poisson J. P., Lagarde M. 1994. Age-related changes in antioxidant defence mechanisms and peroxidation in isolated hepatocytes from spontaneously hypertensive and normotensive rats. Mol. Cell. Biochem. 132: 25–29. [DOI] [PubMed] [Google Scholar]
- 42.Stio M., Iantomasi T., Favilli F., Marraccini P., Lunghi B., Vincenzini M. T., Treves C. 1994. Glutathione metabolism in heart and liver of the aging rat. Biochem. Cell Biol. 72: 58–61. [DOI] [PubMed] [Google Scholar]
- 43.Rebrin I., Kamzalov S., Sohal R. S. 2003. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 35: 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rikans L. E., Kosanke S. D. 1984. Effect of aging on liver glutathione levels and hepatocellular injury from carbon tetrachloride, allyl alcohol or galactosamine. Drug Chem. Toxicol. 7: 595–604. [DOI] [PubMed] [Google Scholar]
- 45.Marchesini N., Osta W., Bielawski J., Luberto C., Obeid L. M., Hannun Y. A. 2004. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J. Biol. Chem. 279: 25101–25111. [DOI] [PubMed] [Google Scholar]
- 46.Deevska G. M., Rozenova K. A., Giltiay N. V., Chambers M. A., White J., Boyanovsky B. B., Wei J., Daugherty A., Smart E. J., Reid M. B., et al. 2009. Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J. Biol. Chem. 284: 8359–8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deaciuc I. V., Nikolova-Karakashian M., Fortunato F., Lee E. Y., Hill D. B., McClain C. J. 2000. Apoptosis and dysregulated ceramide metabolism in a murine model of alcohol-enhanced lipopolysaccharide hepatotoxicity. Alcohol. Clin. Exp. Res. 24: 1557–1565. [PubMed] [Google Scholar]
- 48.Pagadala M., Kasumov T., McCullough A. J., Zein N. N., Kirwan J. P. 2012. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 23: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H., Liu H., Liu R. M. 2003. Gender difference in glutathione metabolism during aging in mice. Exp. Gerontol. 38: 507–517. [DOI] [PubMed] [Google Scholar]
- 50.Hannun Y. A., Obeid L. M. 2011. Many ceramides. J. Biol. Chem. 286: 27855–27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molano A., Huang Z., Marko M. G., Azzi A., Wu D., Wang E., Kelly S. L., Merrill A. H., Jr, Bunnell S. C., Meydani S. N. 2012. Age-dependent changes in the sphingolipid composition of mouse CD4+ T cell membranes and immune synapses implicate glucosylceramides in age-related T cell dysfunction. PLoS ONE. 7: e47650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monette J. S., Gomez L. A., Moreau R. F., Dunn K. C., Butler J. A., Finlay L. A., Michels A. J., Shay K. P., Smith E. J., Hagen T. M. 2011. (R)-alpha-lipoic acid treatment restores ceramide balance in aging rat cardiac mitochondria. Pharmacol. Res. 63: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rivas D. A., Morris E. P., Haran P. H., Pasha E. P., Morais Mda S., Dolnikowski G. G., Phillips E. M., Fielding R. A. 2012. Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J. Appl. Physiol. (1985). 113: 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutkute K., Karakashian A. A., Giltiay N. V., Dobierzewska A., Nikolova-Karakashian M. N. 2007. Aging in rat causes hepatic hyperresposiveness to interleukin-1beta which is mediated by neutral sphingomyelinase-2. Hepatology. 46: 1166–1176. [DOI] [PubMed] [Google Scholar]
- 55.Nikolova-Karakashian M., Karakashian A., Rutkute K. 2008. Role of neutral sphingomyelinases in aging and inflammation. Subcell. Biochem. 49: 469–486. [DOI] [PubMed] [Google Scholar]
- 56.Clarke C. J., Guthrie J. M., Hannun Y. A. 2008. Regulation of neutral sphingomyelinase-2 (nSMase2) by tumor necrosis factor-α involves protein kinase C-δ in lung epithelial cells. Mol. Pharmacol. 74: 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filosto S., Ashfaq M., Chung S., Fry W., Goldkorn T. 2012. Neutral sphingomyelinase 2 activity and protein stability are modulated by phosphorylation of five conserved serines. J. Biol. Chem. 287: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito H., Tanaka K., Hagiwara K., Kobayashi M., Hoshikawa A., Mizutani N., Takagi A., Kojima T., Sobue S., Ichihara M., et al. 2012. Transcriptional regulation of neutral sphingomyelinase 2 in all-trans retinoic acid-treated human breast cancer cell line, MCF-7. J. Biochem. 151: 599–610. [DOI] [PubMed] [Google Scholar]
- 59.Meyers-Needham M., Lewis J. A., Gencer S., Sentelle R. D., Saddoughi S. A., Clarke C. J., Hannun Y. A., Norell H., da Palma T. M., Nishimura M., et al. 2012. Off-target function of the sonic hedgehog inhibitor cyclopamine in mediating apoptosis via nitric oxide-dependent neutral sphingomyelinase 2/ceramide induction. Mol. Cancer Ther. 11: 1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banks M. F., Stipanuk M. H. 1994. The utilization of N-acetylcysteine and 2-oxothiazolidine-4-carboxylate by rat hepatocytes is limited by their rate of uptake and conversion to cysteine. J. Nutr. 124: 378–387. [DOI] [PubMed] [Google Scholar]
- 61.Yamada S., Abe T., Ohta J., Masuoka N., Ubuka T. 1995. Increase in tissue cysteine level and excretion of sulfate and taurine after intragastric administration ofL-2-oxothiazolidine-4-carboxylate in rats. Amino Acids. 8: 37–45. [DOI] [PubMed] [Google Scholar]
- 62.Hoang M. H., Jia Y., Jun H. J., Lee J. H., Hwang K. Y., Choi D. W., Um S. J., Lee B. Y., You S. G., Lee S. J. 2012. Taurine is a liver X receptor-alpha ligand and activates transcription of key genes in the reverse cholesterol transport without inducing hepatic lipogenesis. Mol. Nutr. Food Res. 56: 900–911. [DOI] [PubMed] [Google Scholar]
- 63.Jain A., Madsen D. C., Auld P. A. M., Frayer W. W., Schwartz M. K., Meister A., Mårtensson J. 1995. L-2-oxothiazolidine-4-carboxylate, a cysteine precursor, stimulates growth and normalizes tissue glutathione concentrations in rats fed a sulfur amino acid-deficient diet. J. Nutr. 125: 851-856. [DOI] [PubMed] [Google Scholar]
- 64.Bernard G. R., Wheeler A. P., Arons M. M., Morris P. E., Paz H. L., Russell J. A., Wright P. E. 1997. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 112: 164–172. [DOI] [PubMed] [Google Scholar]
- 65.Jurima-Romet M., Huang H. S. 1992. Enalapril hepatotoxicity in the rat. Effects of modulators of cytochrome P450 and glutathione. Biochem. Pharmacol. 44: 1803–1810. [DOI] [PubMed] [Google Scholar]
- 66.Crankshaw D. L., Berkeley L. I., Cohen J. F., Shirota F. N., Nagasawa H. T. 2002. Double-prodrugs of L-cysteine: differential protection against acetaminophen-induced hepatotoxicity in mice. J. Biochem. Mol. Toxicol. 16: 235–244. [DOI] [PubMed] [Google Scholar]
- 67.Gerard-Monnier D., Fougeat S., Gourvest J. F., Chaudiere J. 1993. Partial prevention of glutathione depletion in rats following acute intoxication with diethylmaleate. Clin. Physiol. Biochem. 10: 36–42. [PubMed] [Google Scholar]
- 68.Brodeur J., Goyal R. 1987. Effect of a cysteine prodrug, L-2-oxothiazolidine-4-carboxylic acid, on the metabolism and toxicity of bromobenzene: an acute study. Can. J. Physiol. Pharmacol. 65: 816–822. [DOI] [PubMed] [Google Scholar]
- 69.Maher P. 2005. The effects of stress and aging on glutathione metabolism. Ageing Res. Rev. 4: 288–314. [DOI] [PubMed] [Google Scholar]
- 70.Robciuc A., Hyotylainen T., Jauhiainen M., Holopainen J. M. 2012. Hyperosmolarity-induced lipid droplet formation depends on ceramide production by neutral sphingomyelinase 2. J. Lipid Res. 53: 2286–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.