Abstract
Microsomal epoxide hydrolase (EPHX1, EC 3.3.2.9) is a highly abundant α/β-hydrolase enzyme that is known for its catalytical epoxide hydrolase activity. A wide range of EPHX1 functions have been demonstrated including xenobiotic metabolism; however, characterization of its endogenous substrates is limited. In this study, we present evidence that EPHX1 metabolizes the abundant endocannabinoid 2-arachidonoylglycerol (2-AG) to free arachidonic acid (AA) and glycerol. The EPHX1 metabolism of 2-AG was demonstrated using commercially available EPHX1 microsomes as well as PC-3 cells overexpressing EPHX1. Conversely, EPHX1 siRNA markedly reduced the EPHX1 expression and 2-AG metabolism in HepG2 cells and LNCaP cells. A selective EPHX1 inhibitor, 10-hydroxystearamide, inhibited 2-AG metabolism and hydrolysis of a well-known EPHX1 substrate, cis-stilbene oxide. Among the inhibitors studied, a serine hydrolase inhibitor, methoxy-arachidonyl fluorophosphate, was the most potent inhibitor of 2-AG metabolism by EPHX1 microsomes. These results demonstrate that 2-AG is an endogenous substrate for EPHX1, a potential role of EPHX1 in the endocannabinoid signaling and a new AA biosynthetic pathway.
Keywords: arachidonic acid, prostate carcinoma cells, anandamide
Microsomal epoxide hydrolase (EPHX1, EC 3.3.2.9) is a xenobiotic metabolizing enzyme that is functionally associated with the cytochrome P450 family. The commonly known function of EPHX1 is to metabolize xenobiotics including environmental chemicals and many therapeutic drugs. This enzymatic action converts lipophilic and sometimes reactive epoxides to more polar 1,2-diols. It also activates (pro)toxins and (pro)carcinogens (1, 2). In addition to xenobiotic metabolism, EPHX1 regulates endogenous steroid metabolism (3), bile acid transportation (4), and the vitamin K1 reductase complex that is responsible for vitamin K1 oxide reduction activity (5). While a number of diverse functions of EPHX1 have been demonstrated, only a few naturally endogenous substrates for EPHX1 have been characterized (3, 6–8).
2-Arachidonoylglycerol (2-AG) is a highly abundant endogenous ligand for the cannabinoid receptor type 1 (CB1) (9–11). Through the activation of CB1, 2-AG plays a major role in a variety of physiological processes. The actions of 2-AG are tightly regulated by enzymatic hydrolysis, a major deactivation pathway. Monoacylglycerol lipase (MGL) (12, 13) and possibly FA amide hydrolase (FAAH) (14) are responsible for hydrolysis of 2-AG to free arachidonic acid (AA) and glycerol. Non-FAAH or non-MGL enzymes in porcine membranes (15) and mouse microglial cells (16) have been demonstrated to hydrolyze 2-AG. Recent studies discovered two integral membrane enzymes, α/β-hydrolase fold 6 and 12, that contributed to ∼4% and 9% of total 2-AG hydrolysis, respectively, in mouse brain membrane (17, 18).
The amino acid sequence of human EPHX1 indicates an epoxide hydrolase N terminus at the amino acids 50–161 and an α/β-hydrolase domain (ABHD) at the amino acids 178–395. The ABHD is commonly associated with esterase and lipase activities (19–22). Interestingly, previous structure-function studies in EPHX1 inhibition demonstrated that a number of long-chain FA derivatives or valproic acid derivatives are potent, partially competitive inhibitors of EPHX1-mediated hydrolysis of cis-stilbene oxide (cSO) or (S)-(+) styrene oxide, well-known EPHX1 substrates (23–25). The results suggest that these compounds can bind to EPHX1 and may modify and/or interrupt the catalytic sites for the substrates. These results also suggest that ester compounds containing a long-chain unsaturated FA backbone may fit in a catalytic binding pocket and be metabolized by EPHX1. In this study, we demonstrate that the long-chain unsaturated FA glycerol ester, the endocannabinoid 2-AG, is metabolized by EPHX1 to free AA and glycerol. It is not known whether EPHX1 contributes to 2-AG hydrolysis in vivo. The results suggest potential roles of EPHX1 in regulating endocannabinoid signaling and free AA biosynthesis.
MATERIALS AND METHODS
Materials
Microsomes of control, vector-transfected, and EPHX1-transfected human lymphoblasts were obtained from BD Biosciences (San Jose, CA) and Sigma-Aldrich (St. Louis, MO). Human prostate carcinoma cells (PC-3 and LNCaP) and hepatocellular carcinoma cells (HepG2) were obtained from ATCC (Rockville, MD). 2-AG, [2H5]2-AG, [2H8]2-AG, [2H8]anandamide ([2H8]AEA), N-arachidonoyl dopamine (AA-dopamine), arachidonoyl serinol (AA-serinol), 4-nitrophenyl-4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184), methoxy-arachidonyl fluorophosphate (MAFP), (Z)-octa-9-decenamide (oleamide), (3′-(aminocarbonyl) [1,1’-biphenyl]-3-yl)-cyclohexylcarbamate (URB597), cyclohexyl [1,1’-biphenyl]-3-ylcarbamate (URB602), MGL primary antibody, and FAAH primary antibody were obtained from Cayman Chemical Co. (Ann Arbor, MI). 2-Arachidonoyl-[3H]glycerol (2-AG*, 3H-labeled on the glycerol moiety) and [3H]AEA (AEA*, 3H-labeled on the ethanolamine moiety) were obtained from American Radiolabeled Chemical (St. Louis, MO). [14C]2-AG (labeled at C1 position) was synthesized by Dr. J. R. Falck. 10-Hydroxystearamide (10-HSA), 3-(dodecylthio)-1,1,1-trifluoropropan-2-one (DDTFP), 3-(decylthio)-1,1,1-trifluoropropan-2-one (DETFP), 3-(octylthio)-1,1,1-trifluoropropan-2-one (OTFP), and recombinant human soluble epoxide hydrolase (EPHX2; EC 3.3.2.10) were generously provided by Dr. Bruce Hammock. Primary antibody against human EPHX1 was obtained from BD Biosciences and Santa Cruz Biotechnologies (Santa Cruz, CA). Goat anti-rabbit and rabbit anti-mouse IgG-HRP secondary antibodies were obtained from Zymed Laboratories Inc. (South San Francisco, CA). Goat anti-mouse IgG-HRP secondary antibody and Pluronic F-127, the trade name of a nonionic surfactant polyol, were obtained from Invitrogen (Eugene, OR). pCMV6-XL4 vector and pCMV6-XL4 containing EPHX1 cDNA were obtained from Origene Technologies (Rockford, MD). EPHX1 siRNAs, a functional nontargeting control (siControl), and DharmaFECT1 transfection reagent were obtained from Dharmacon Inc. (Lafayette, CO). NADPH regenerating system was obtained from BD Biosciences. Primary antibodies against human β-actin, cSO, FA-free BSA, SDS, and CHAPS were obtained from Sigma-Aldrich. Primary monoclonal antibody against human pan-cadherin was obtained from Abcam (Cambridge, MA). ECL for Western blotting detection and BCA protein determination assay kits were obtained from Pierce (Rockville, IL). SDS-PAGE ReadyGels™ and Mini-PROTEAN TGX gels were obtained from BioRad (Hercules, CA). Distilled, deionized water was used in all experiments.
Cell culture
HepG2 cells were maintained in essential modified Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine serum, sodium pyruvate (100 mM), l-glutamine (2 mM), streptomycin (100 µg/ml), and penicillin (100 U/ml). PC-3 and LNCaP cells were maintained in RPMI 1640 medium as previously described (26). Cells were grown in 75 cm2 polystyrene tissue culture flasks at 37°C in 5% CO2 in air.
Determination of hydrolysis of [14C]2-AG to [14C]AA by EPHX1 microsomes
[14C]2-AG (10 μM) was incubated with EPHX1 microsomes (30 μg) from BD Biosciences or Sigma-Aldrich in PBS buffer containing essentially FA-free BSA (1 mg/ml) at 37°C for 15 min at pH 7.4 or 9.0. Samples were extracted by solid phase extraction as previously described (27). Samples were separated by HPLC using a C18 reverse phase column (4.6 × 250 mm2, Nucleosil, Phenomenex) and water-acetonitrile containing 0.1% acetic acid as a mobile phase at a flow rate of 1.0 ml/min. The mobile phase started at 50% acetonitrile and linearly increased to 100% acetonitrile in 35 min. The eluent was collected at 5 fractions/min and counted for radioactivity. The retention times of the radioactive peaks of [14C]2-AG and [14C]AA in the samples were compared with the retention times of 2-AG and AA standards.
Determination of hydrolysis activity of EPHX1
2-AG* was used as enzyme substrate for determination of 2-AG hydrolysis activity, and AEA* (3H-labeled on the ethanolamine moiety) was used as a substrate for determination of AEA hydrolysis activity. The percent hydrolysis of 2-AG* and AEA* was determined as previously described (26) and normalized to the hydrolysis of the control (as the relative hydrolysis activity).
Determination of 2-AG hydrolysis by EPHX1 using LC-ESI/MS
In this series of experiments, 2-AG was used as a substrate. In some cases, [2H5]2-AG was used as a substrate to make certain that there is no contribution from endogenous 2-AG in the samples. After the hydrolysis reaction, samples were added with [2H8]2-AG or [2H8]AEA as an internal standard, extracted by solid phase extraction, and analyzed by using LC-ESI/MS (Agilent 1100 LC-MSD, SL model) as previously described (27, 28). The detection was made in the positive ion mode. For quantitative measurement, m/z 379, 384, 387, and 356 were used for 2-AG, [2H5]2-AG, [2H8]2-AG, and [2H8]AEA, respectively. The concentrations of 2-AG (or [2H5]2-AG) were calculated by comparing their ratios of peak areas to the standard curves. The results were normalized to the protein content and compared with the control.
Determination of cSO metabolism by GC-MS
After the incubation of cSO, a known substrate for EPHX1, with the membrane fractions of PC-3 cells overexpressing EPHX1, the amount of unhydrolyzed cSO was determined by GC-MS (H/P 5890 GC coupled to the H/P 5971 MS, Hewlett-Packard, Palo Alto, CA). The peak area of the selected m/z 167 was used to quantify cSO as compared with the standard curve.
Overexpression of EPHX1 in PC-3 cells
PC-3 cells were chosen for EPHX1 overexpression because of their low level of endogenous EPHX1 expression. PC-3 cells, at ∼70% confluence, were transfected with 5 μg of purified pCMV6-XL4 vector or purified pCMV6-XL4 containing EPHX1 cDNA using Lipofectamine (0.1%, v/v) in Opti-MEM reduced serum media. Concentrations of cDNA and transfection times were initially optimized. After 5 h of transfection, RPMI 1640 feed medium was replaced with 10% serum feed medium and incubated for an additional incubation of 24 h posttransfection. Cells were lysed, and samples were separated for membrane fractions by centrifugation at 100,000 g at 4°C for 60 min. Then, EPHX1 enzyme activity for 2-AG hydrolysis in the membrane fractions was determined using 2-AG or [2H5]2-AG as a substrate.
In another set of experiments, the intact PC-3 cells were used after transfection for [2H5]2-AG incubation. In this case, control and transfected PC-3 cells were pretreated with JZL184 (100 nM), a known selective MGL inhibitor, to block the 2-AG hydrolysis by the highly abundant MGL for 10 min at 37°C. Then, [2H5]2-AG was added as a substrate and incubated for 30 min. Then, cells were lysed, and samples were analyzed for EPHX1 protein expression. Samples were extracted by solid phase extraction (27), and the remaining (unmetabolized) [2H5]2-AG was determined by LC/MS as described previously.
EPHX1 gene silencing in HepG2 and LNCaP cells
HepG2 and LNCaP cells contain high EPHX1 expression and 2-AG hydrolysis activity. EPHX1 expression in HepG2 and LNCaP cells was knocked down using specific EPHX1 siRNA of four separate sequences. A functional nontargeting siRNA that was bioinformatically designed by Dharmacon Inc. to have ≥4 mismatches with known human genes was included as a control (siControl). Different siRNA concentrations and transfection times were optimized for the maximal suppression of EPHX1 expression. Cells were transfected at 37°C in antibiotic-free medium with DharmaFECT1 alone, siControl, or EPHX1 siRNA. At 5 h posttransfection, the transfection medium was replaced with RPMI 1640 feed medium for 24 h, and cells were harvested for Western immunoblot analysis and enzyme activity.
Western blot analysis of EPHX1, MGL, and FAAH
Proteins in samples were electrophoretically separated by SDS-PAGE (Ready Gels) or Mini-PROTEAN TGX gels and transferred to a nitrocellulose membrane (BioRad). Blots were incubated with EPHX1 primary antibody (1:250) (BD Biosciences or Santa Cruz Biotechnology) or MGL primary antibody (1:200) or FAAH primary antibody (1:200), followed by HRP-conjugated secondary antibody. Protein concentrations and β-actin or pan-cadherin were used as loading controls. Detection was made by using ECL Western Blotting Substrate (Pierce) and captured by Fuji film X-ray (Tokyo, Japan). Band densities were analyzed using Image J software from the National Institutes of Health.
Statistical analysis
The means of the measured values of each treatment group were compared using Student’s t-test. Means were considered statistically different from one another if P < 0.05.
RESULTS
Presence of EPHX1, MGL, and FAAH in microsomes
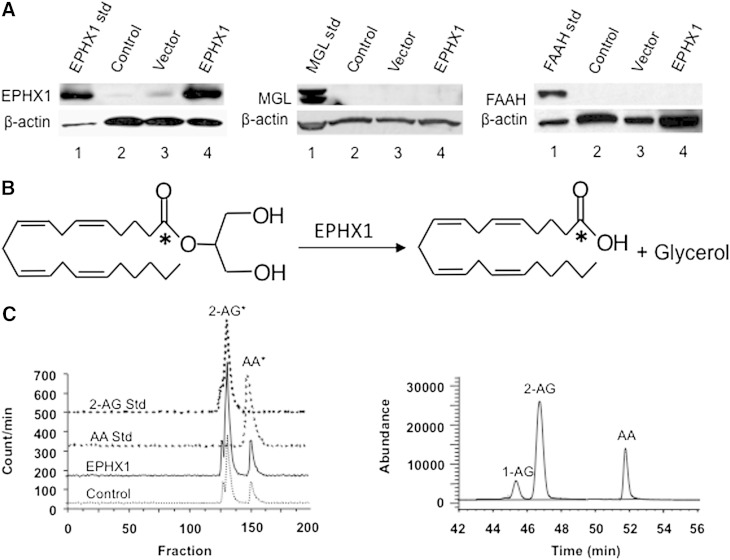
The presence of EPHX1 and other major enzymes metabolizing 2-AG, MGL, and FAAH, in control microsomes, vector microsomes, and EPHX1 microsomes from BD Biosciences, were determined by Western immunoblotting. EPHX1 immunoreactive bands were detected at very low levels in the control and vector microsomes, while an intense immunoreactive band was detected in the EPHX1 microsomes (Fig. 1A, left panel). Immunoreactive bands for MGL and FAAH were not detected in these microsomes at 30 μg protein (Fig. 1A, middle and right panels). These results indicate that EPHX1 microsomes contained high EPHX1 protein but contained MGL and FAAH at levels below detection by Western blot analysis at 30 μg of microsomes.
Fig. 1.
EPHX1 microsomes hydrolyze 2-AG to AA and glycerol. A: Examples of Western immunoblots for EPHX1 (left panel), MGL (middle panel), and FAAH (right panel) in control (lane 2), vector (lane 3), and EPHX1 (lane 4) microsomes (BD Gentest microsomes). Also shown are standard EPHX1, MGL, and FAAH (lane 1). B: Diagram depicting the conversion of 2-AG to free AA and glycerol by EPHX1. C: HPLC chromatograms of the conversion of [14C]2-AG to [14C]AA by control and EPHX1 microsomes as detected by radioactivity (left panel). Also shown are HPLC chromatograms of [14C]2-AG and [14C]AA standards. The conversion of 2-AG to free AA by EPHX1 microsomes as detected by LC/MS (right panel). 2-AG can rearrange to 1/3-AG isomers (as detected in a small amount).
Hydrolysis of 2-AG to AA by EPHX1 microsomes
To test that EPHX1 microsomes metabolize 2-AG to free AA and glycerol (shown in Fig. 1B), two sets of experiments were performed. First, control and EPHX1 microsomes were incubated with [14C]2-AG in PBS buffer containing essentially FA-free BSA (1 mg/ml) pH 7.4 at 37°C for 15 min, and radioactive products separated on a reverse phase LC. Fractions were collected and counted for radioactivity. In the second set of experiments, microsomes were incubated with 2-AG under the same conditions, and 2-AG and its metabolite (AA) were analyzed by LC/MS. The left chromatogram of Fig. 1C indicates the radioactive peaks comigrated with 2-AG standard and a small peak comigrated with 1/3-AG, rearranged regioisomers of 2-AG. The chromatogram also shows the radioactive reaction product that comigrated with the AA standard. LC/MS analysis of the incubation of 2-AG with EPHX1 microsomes also indicates the production of AA (Fig. 1C, right panel). These results indicate that EPHX1 microsomes convert 2-AG to free AA and glycerol.
Factors affecting 2-AG hydrolysis by EPHX1
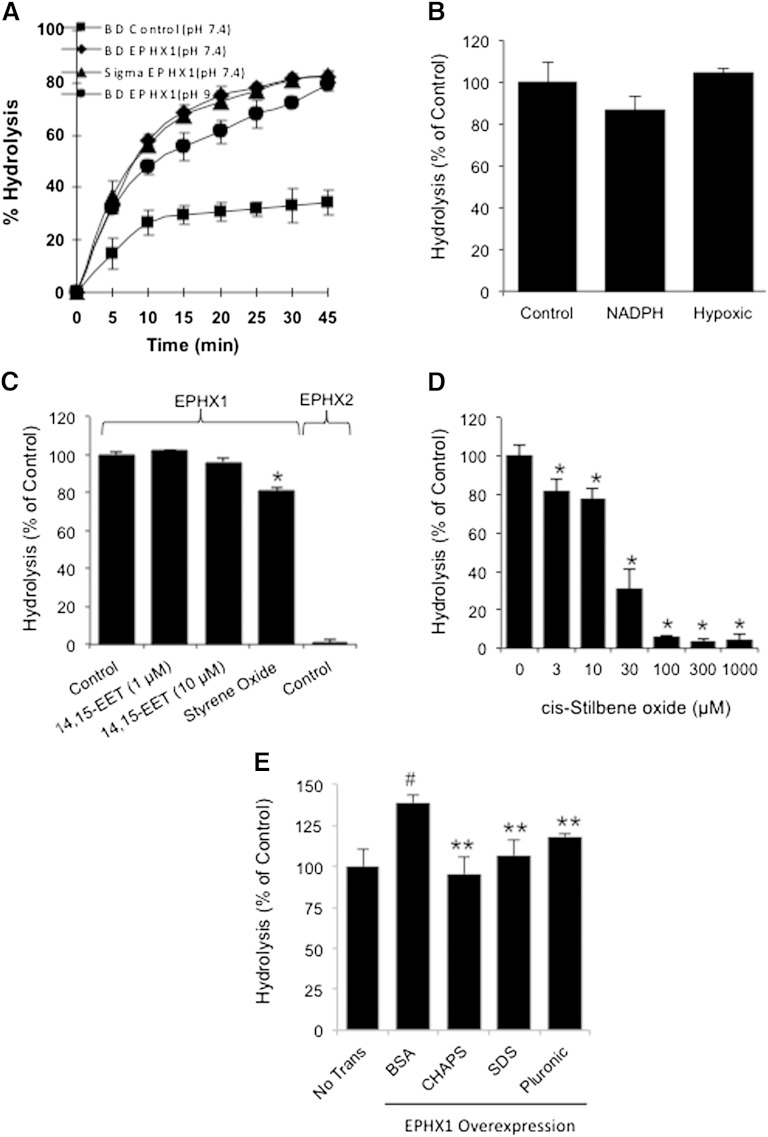
2-AG hydrolysis by EPHX1 microsomes was determined in various reaction conditions. EPHX1 microsomes from two suppliers were incubated with 2-AG* (3H-labeled on the glycerol moiety) as a substrate for 2-AG hydrolysis or AEA* (3H-labeled on the ethanolamine moiety) as a substrate for AEA hydrolysis. 2-AG hydrolysis by control microsomes from BD Biosciences was also determined. The reaction was performed at 37°C in PBS buffer containing essentially FA-free BSA (1 mg/ml) at pH 7.4 for various reaction times. The percent hydrolysis of these substrates was determined as previously described (29). At these conditions, the hydrolysis of 2-AG increased with incubation time and reached the maximum after 30 min (Fig. 2A). The rate and extent of hydrolysis was similar with microsomes from the two suppliers (Fig. 2A). The hydrolysis of 2-AG also took place in the basic solutions, at pH 9.0 (Fig. 2A). In the presence of JZL184 (1–100 nM), a potent MGL-selective inhibitor (30), the 2-AG hydrolysis was not altered when compared with the control without JZL184 (data not shown), suggesting that the 2-AG hydrolysis in the EPHX1 microsomes was not from the activity of MGL (a major known enzyme hydrolyzing 2-AG). AEA was not hydrolyzed by the EPHX1 microsomes, further indicating that the 2-AG hydrolysis in EPHX1 microsomes was not from the activity of FAAH.
Fig. 2.
Factors affecting 2-AG hydrolysis by EPHX1. A: Time course of 2-AG hydrolysis by control microsomes and EPHX1 microsomes from BD Biosciences at pH 7.4 (also EPHX1 microsomes from Sigma Chemical Co.) and 9.0; AEA hydrolysis in BD microsomes at pH 7.4. AEA was not hydrolyzed by EPHX1 microsomes. B: Effects of NADPH regenerating system and hypoxia on the relative 2-AG hydrolysis in EPHX1 microsomes. C: Effects of epoxide hydrolase substrates and EPHX2 on the relative 2-AG hydrolysis. 14,15-EET (a known substrate for EPHX2) at 1 µM and 10 µM did not alter the relative 2-AG hydrolysis by EPHX1 microsomes. Styrene oxide at 100 µM inhibited 2-AG hydrolysis by 18%. EPHX2 (5 μg, 37°C for 30 min) did not hydrolyze 2-AG. D: Effects of cSO on the reduction of the relative 2-AG hydrolysis by EPHX1 microsomes. E: In the presence of BSA, the relative 2-AG hydrolysis in membrane fractions of PC-3 cells overexpressing EPHX1 increased as compared with the nontransfected (control) PC-3 cells. Detergents, CHAPS, SDS, and Pluronic F-127 diminished the relative 2-AG hydrolysis by the membrane fractions of PC-3 cells overexpressing EPHX1. The 2-AG hydrolysis in the presence of detergents was lower than in the presence of BSA and similar to the nontransfected (control) cells. The “% of control” represents the 2-AG hydrolysis in EPHX1-transfected microsomes under treatment and was normalized to the hydrolysis of 2-AG in EPHX1-transfected microsomes in the absence of treatment. Values are mean ± SEM (n = 3–6). *, significantly lower than control with P < 0.01; #, significantly higher than nontransfected (control) cells with P < 0.01; and **, significantly lower than the relative 2-AG hydrolysis in the membrane fractions of PC-3 cells overexpressing EPHX1 the presence of BSA with P < 0.01.
2-AG hydrolysis by EPHX1 microsomes was also determined in the presence of a NADPH regenerating system or under hypoxic conditions (<0.5% oxygen hypoxic chamber). The 2-AG hydrolysis by EPHX1 microsomes with the NADPH regenerating system was not significantly different from the control reaction of EPHX1 microsomes alone (Fig. 2B). The 2-AG hydrolysis by EPHX1 microsomes under the hypoxic conditions was not significantly different from the normoxic (control) conditions (Fig. 2B). These results suggest that the 2-AG hydrolysis by EPHX1 microsomes is not from other enzymes such as flavin-containing monooxygenases, cytochrome c reductase, cytochrome b5 reductase, or cytochrome P450 enzymes that might be present in the microsomes.
Next, the effects of other substrates of EPHX1 or EPHX2 were tested on the 2-AG hydrolysis. 14,15-Epoxyexicosatrienoic acid (14,15-EET, a known substrate for EPHX2) at 1 and 10 μM was not hydrolyzed, and it did not significantly alter the 2-AG hydrolysis by EPHX1 microsomes (Fig. 2C). Styrene oxide (100 μM) reduced the 2-AG hydrolysis by ∼18%. 2-AG was not hydrolyzed by the recombinant human EPHX2 in the same reaction conditions (at 37°C for 30 min) used for EPHX1 microsomes (Fig. 2C). Interestingly, cSO, a known substrate for EPHX1, reduced the relative 2-AG hydrolysis in a concentration-dependent manner (Fig. 2D).
In another set of experiments, the relative 2-AG hydrolysis in membrane fractions of the nontransfected (control) PC-3 cells or PC-3 cells overexpressing EPHX1 was carried out in the presence of BSA or detergents such as CHAPS (4 mM), SDS (7 mM), and Pluronic F-127 (1%). The hydrolysis by membrane fractions of PC-3 cells overexpressing EPHX1 in the presence of BSA was significantly higher than the nontransfected (control) cells. However, in the presence of detergents, the hydrolysis was significantly lower than the reaction in the presence of BSA and similar to the nontransfected (control) cells (Fig. 2E). These results suggest that detergents interfere with the 2-AG hydrolysis activity by EPHX1.
EPHX1 gene manipulation and 2-AG hydrolysis
Two approaches were used to alter the EPHX1 expression and the 2-AG hydrolysis. First, the EPHX1 siRNA was used to knockdown EPHX1 in HepG2 and LNCaP cells, cells that contain high basal EPHX1 expression. The expression of EPHX1 in HepG2 and LNCaP cells is shown in Fig. 3. For EPHX1 siRNA knockdown in HePG2 and LNCaP cells, the siRNA sense sequence -GAGGAAACUUUGCCACUUGUU and antisense sequence -CAAGUGGCAAAGUUUCCUCUU yielded the best knockdown, and the sense sequence -UGAAAGGCCUGCACUUGAACC and antisense sequence -UUCAAGUGCAGGCCUUUCAUU yielded the second-best result. Thus, the first duplex was subsequently used for all other experiments. EPHX1 siRNA markedly decreased EPHX1 protein expression in the membrane fractions of HepG2 cells (Fig. 3A, left panel) and LNCaP cells (Fig. 3B, left panel) as compared with the control and siControl. The 2-AG hydrolysis decreased in the membrane fractions of siEPHX1 HepG2 cells (Fig. 3A, right panel) and the membrane fractions of siEPHX1 LNCaP cells (Fig. 3B, right panel) as compared with the control and siControl membrane fractions.
Fig. 3.
Effects of EPHX1 gene manipulation on enzyme expression and 2-AG hydrolysis. A: Gene silencing by EPHX1 siRNA in HepG2 cells. Western blot depicting immunoreactive bands corresponding to EPHX1 and β-actin in membrane fractions of control (nontransfected), siControl, and EPHX1 siRNA-transfected HepG2 cells (left panel). The 2-AG hydrolysis in the membrane fractions of EPHX1 siRNA-transfected HepG2 cells (right panel). B: Gene silencing by EPHX1 siRNA in LNCaP cells. Western blot depicting immunoreactive bands corresponding to EPHX1 and β-actin in membrane fractions of control (nontransfected), siControl, and EPHX1 siRNA-transfected LNCaP cells (left panel). The 2-AG hydrolysis in the membrane fractions of EPHX1 siRNA-transfected LNCaP cells (right panel). C: Overexpression of EPHX1 in PC-3 cells. Western blots depicting immunoreactive bands corresponding to EPHX1 and β-actin in PC-3 cell membrane fractions of control, vector control, and EPHX1 overexpression (left panel). The 2-AG hydrolysis in the membrane fractions of EPHX1-transfected PC-3 cells (middle panel) and the cSO hydrolysis in the membrane fractions of EPHX1-transfected PC-3 cells (right panel). D: Overexpression of EPHX1 in PC-3 cells. Intact, transfected PC-3 cells were preincubated with JZL184 (100 nM) to block 2-AG hydrolysis by MGL and then incubated with [2H5]2-AG as a substrate. Cells were lysed, and samples were analyzed for the remaining [2H5]2-AG concentrations by LC/MS. Western blots depicting immunoreactive bands corresponding to EPHX1 and pan-cadherin in cell membrane fractions of control, vector control, and EPHX1 overexpression (left panel). The 2-AG hydrolysis in the intact transfected PC-3 cells as compared with the nontransfected and vector-transfected cells (right panel). Western blots are representative of three to four separate experiments. The 2-AG hydrolysis values are mean ± SEM (n = 9–12). *, significantly lower than control with P < 0.001; and #, significantly higher than control with P < 0.001.
Second, PC-3 cells were transfected with the EPHX1 cDNA to increase EPHX1 expression. EPHX1 expression in the membrane fraction of PC-3 cells significantly increased after transfection with EPHX1 cDNA as compared with control and vector-transfected cells (Fig. 3C, left panel). The 2-AG hydrolysis in the membrane fractions of PC-3 cells overexpressing EPHX1 increased as compared with the membrane fractions of the control and vector-transfected cells (Fig. 3C, middle panel). Furthermore, the cSO hydrolysis by the membrane fractions was determined. The cSO hydrolysis in the overexpressed EPHX1 markedly increased above the control and vector-transfected membranes (Fig. 3C, right panel). These results indicated that transfection with EPHX1 cDNA markedly increased the EPHX1 protein and cSO hydrolysis activity. Despite a very low endogenous expression of EPHX1 protein in PC-3 cells, these cells have a relatively high 2-AG hydrolysis (28) in the control cells. Other enzymes metabolizing 2-AG contributed to 2-AG hydrolysis as reflected by the smaller increase of 2-AG hydrolysis than the increase of cSO hydrolysis (Fig. 3C).
The 2-AG hydrolysis in intact PC-3 cells overexpressing EPHX1 was also determined. These intact cells were pretreated with JZL184 to block MGL activity. EPHX1 expression was markedly increased in PC-3 cells overexpressing EPHX1 as compared with the control or vector-transfected cells (Fig. 3D, left panel). The 2-AG hydrolysis in intact PC-3 cells overexpressing EPHX1 (as normalized to protein content in samples) increased as compared with the intact control and vector-transfected cells (Fig. 3D, right panel).
Kinetics of 2-AG hydrolysis by EPHX1 microsomes
The 2-AG hydrolysis was determined at various 2-AG concentrations at 37°C in pH 7.4 PBS buffer at 10 min of reaction time. The specific activity of 2-AG hydrolysis (nmol/min/mg of protein) in EPHX1 microsomes increased as a function of 2-AG concentrations (Fig. 4A). The Hanes-Woolf plot was used to determine the Michaelis-Menten constant (Km) of 2-AG hydrolysis by EPHX1 microsomes. The plot of ratio [2-AG]/V0 versus [2-AG] yielded a linear relationship with the x-intercept of −Km (Fig. 4B). The apparent Km for 2-AG hydrolysis by EPHX1 microsomes was 42 µM.
Fig. 4.
Enzyme kinetics of 2-AG hydrolysis by EPHX1 microsomes. A: Apparent specific activity of 2-AG hydrolysis by EPHX1 microsomes (nmol/min/mg protein) as a function of 2-AG concentrations. Reaction time was 10 min, except reaction time for 2.5 and 5.0 µM was 5 min. B: The Hanes-Woolf plot was used to determine the apparent Michaelis-Menten constant (Km) of 2-AG hydrolysis by EPHX1 microsomes. Values are mean ± SEM (n = 6).
Pharmacological inhibition of 2-AG hydrolysis
A number of hydrolase inhibitors and compounds that are known to inhibit 2-AG hydrolysis were tested for their ability to inhibit the activity of EPHX1 microsomes. 10-HSA, a selective, partially competitive EPHX1 inhibitor (24) inhibited the 2-AG hydrolysis in a concentration-dependent manner (Fig. 5A). 10-HSA also inhibited the 2-AG hydrolysis in the membrane fractions of PC-3 cells overexpressing EPHX1 (Fig. 5B). It is apparent that 10-HSA inhibition of the 2-AG hydrolysis by EPHX1 microsomes or membrane fractions of PC-3 cells overexpressing EPHX1 is less potent than its inhibition of cSO hydrolysis by rat recombinant EPHX1 (24).
Fig. 5.
Effect of inhibitors on 2-AG hydrolysis by EPHX1. A: Inhibition of 2-AG hydrolysis in EPHX1 microsomes by a known EPHX1 inhibitor, 10-HSA. B: Inhibition of 2-AG hydrolysis in membrane fractions of PC-3 cells overexpressing EPHX1 by 10-HSA. C: Inhibition of 2-AG hydrolysis by EPHX1 microsomes by inhibitors of serine hydrolases, carboxyesterases and other compounds that known to potentially inhibit 2-AG hydrolysis. Values are mean ± SEM (n = 6–9). *, significantly lower than control with P < 0.001.
Among the inhibitors/compounds tested on EPHX1 microsomes, MAFP, an irreversible inhibitor for serine hydrolases, exhibited a very potent inhibition of 2-AG hydrolysis with IC50 at ∼7.9 nM (Fig. 5C). A series of compounds containing a trifluoromethyl ketone moiety that inhibit carboxyesterases, OTFP, DDTFP, and DETFP, moderately inhibited 2-AG hydrolysis. MGL inhibitor URB602 (31) and FAAH inhibitor URB597 (32) only inhibited 2-AG hydrolysis by EPHX1 microsomes at concentrations >10–100 µM. Other AA-derived MGL inhibitors such as AA-serinol (33) and AA-dopamine (34) also had low potency for inhibition of 2-AG hydrolysis by EPHX1 microsomes. Oleamide, a sleep-inducing factor that binds and is hydrolyzed by FAAH (35), minimally inhibited 2-AG hydrolysis by EPHX1 microsomes. The inhibition of 2-AG hydrolysis by MGL and FAAH inhibitors at high concentrations suggests that these inhibitors exhibit a weak inhibition of 2-AG hydrolysis by EPHX1.
DISCUSSION
These results demonstrate that microsomes overexpressing EPHX1 metabolize 2-AG to free AA and glycerol. Two important enzymes hydrolyzing 2-AG, MGL and FAAH, were not detected by Western blot analysis in these microsomes. However, 2-AG hydrolysis occurred in the control and vector-transfected microsomes indicating that there is a contribution from an unknown 2-AG hydrolyzing enzyme(s) in these microsomes. Therefore, the 2-AG hydrolysis in the vector-transfected microsomes was subtracted from the 2-AG hydrolysis in the overexpressed EPHX1 microsomes. The 2-AG hydrolysis at pH 7.4 was similar to the hydrolysis at pH 9.0, with or without an NADPH regenerating system and was also identical in the normoxic and hypoxic conditions. These characteristics support the enzymatic function of EPHX1.
In HepG2 and LNCaP cells (cells with high abundance of EPHX1), EPHX1-specific siRNA markedly decreased EPHX1 protein levels as well as the relative 2-AG hydrolysis. These results strong support the activity of EPHX1 in metabolizing 2-AG.
On the other hand, overexpression of EPHX1 in PC-3 cells increased EPHX1 protein levels and the relative 2-AG hydrolysis as well as a known EPHX1 substrate, cSO, in the membrane fractions. The relative 2-AG hydrolysis was also increased in intact PC-3 cells overexpressing EPHX1 and pretreated with JZL184. The increase of 2-AG hydrolysis in EPHX1 microsomes and membrane fractions of PC-3 cells overexpressing EPHX1 was abolished by a pretreatment with the selective EPHX1 inhibitor 10-HSA. These findings further indicate the role of EPHX1 in 2-AG hydrolysis. The increase of 2-AG hydrolysis in the membrane fractions of PC-3 cells overexpressing EPHX1 was smaller than the increase of cSO hydrolysis. This smaller increase in the relative 2-AG hydrolysis is the result of a higher basal 2-AG hydrolysis (from other enzymes metabolizing 2-AG) than cSO hydrolysis in PC-3 cells.
Interestingly, the well-known EPHX1 substrate cSO blocked 2-AG hydrolysis by EPHX1 microsomes in a concentration-dependent manner. This suggests a competition and/or a modification of the substrate binding site of EPHX1 for 2-AG by cSO. Because EPHX1 contains an ABHD, its esterase activity may be responsible for conversion of 2-AG to free AA and glycerol (19, 21, 22). The catalytic residues of Asp107 and His275, located in a hydrophobic environment between the epoxide hydrolase and α/β hydrolase domains, may play an important role in 2-AG hydrolysis and the inhibition by cSO (19).
There are a number of serine hydrolase inhibitors that inhibit 2-AG hydrolysis. We previously demonstrated that MAFP potently inhibited 2-AG hydrolysis in prostate carcinoma cells (26). In this study, MAFP was a very potent inhibitor of 2-AG hydrolysis in EPHX1 microsomes. A series of carboxyesterase inhibitors such as OTFP, DDTFP, and DETFP were moderately potent inhibitors for EPHX1. These results suggest that the long-chain FA backbone of these compounds may represent a structural requirement for fitting into the hydrophobic pocket of the catalytic region of EPHX1 and inhibiting 2-AG hydrolysis. Consistent with the cellular localization of EPHX1 to microsomes, the increase of 2-AG hydrolysis in cells overexpressing EPHX1 or the decrease of 2-AG hydrolysis in cells that were treated with EPHX1 siRNA took place only in the membrane fractions. The cytosolic EPHX2 did not hydrolyze 2-AG.
The results from this study provide evidence that EPHX1 has a new enzymatic function to metabolize 2-AG to free AA. This has many biological implications that will require further exploration. EPHX1 hydrolysis of 2-AG has an important role in the regulation of endocannabinoid signaling. In the current study, EPHX1 was highly expressed in LNCaP prostate cancer cells and may therefore reduce 2-AG inhibition of cell migration/invasion and proliferation that we previously observed (26, 29, 36). However, the implications go beyond prostate cancer. EPHX1 is highly expressed in the brain and may regulate 2-AG activity in stress, depression, and pain (37–40). Furthermore, free AA is further metabolized by cyclooxygenases, lipoxygenases, and cytochrome P450s to biologically active eicosanoids that mediate inflammation, vasodilation, vasoconstriction, cell growth, and migration. EPHX1 may participate in an important pathway in these physiological and pathological processes by providing free AA. Interestingly, a recent study demonstrated multiple forms of expression of EPHX1, and the results implicated the multiple roles of EPHX1 in various cells (41). In the current study, an EPHX1 form(s) responsible for 2-AG hydrolysis was not characterized, and this possibility deserves future detailed investigation. In conclusion, the highly abundant EPHX1 may possess a broader role in various organs and regulate a larger number of pathophysiological processes than previously recognized.
Acknowledgments
The authors would like to thank Dr. Bruce Hammock for EPHX1 inhibitors and EPHX2 protein, and Frank E. Laib for his assistance in analysis of cSO by GC-MS.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- ABHD
- α/β-hydrolase domain
- AEA
- anandamide
- AEA*
- [3H]anandamide
- 2-AG
- 2-arachidonoylglycerol
- 2-AG*
- 2-arachidonoyl-[3H]glycerol
- cSO
- cis-stilbene oxide
- DDTFP
- 3-(dodecylthio)-1,1,1-trifluoropropan-2-one
- DETFP
- 3-(decylthio)-1,1,1-trifluoropropan-2-one
- EPHX1
- microsomal epoxide hydrolase
- EPHX2
- soluble epoxide hydrolase
- FAAH
- FA amide hydrolase
- 10-HSA
- 10-hydroxystearamide
- JZL184
- 4-nitrophenyl-4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate
- MAFP
- methoxy-arachidonyl fluorophosphate
- MGL
- monoacylglycerol lipase
- OTFP
- 3-(octylthio)-1,1,1-trifluoropropan-2-one
This work was supported by the Wisconsin Breast Cancer Showhouse; the Cancer Center of the Medical College of Wisconsin; National Institutes of Health Grants DA-09155 (W.B.C.), H-103673 (W.B.C.), HL-83279 (W.B.C.), and GM-31278 (J.R.F.); and Robert A. Welch Foundation Grant GL625910 (J.R.F.).
REFERENCES
- 1.Fretland A. J., Omiecinski C. J. 2000. Epoxide hydrolases: biochemistry and molecular biology. Chem. Biol. Interact. 129: 41–59. [DOI] [PubMed] [Google Scholar]
- 2.Graham M. A., Riley R. J., Kerr D. J. 1991. Drug metabolism in carcinogenesis and cancer chemotherapy. Pharmacol. Ther. 51: 275–289. [DOI] [PubMed] [Google Scholar]
- 3.Fändrich F., Degiuli B., Vogel-Bindel U., Arand M., Oesch F. 1995. Induction of rat liver microsomal epoxide hydrolase by its endogenous substrate 16 alpha, 17 alpha-epoxyestra-1,3,5-trien-3-ol. Xenobiotica. 25: 239–244. [DOI] [PubMed] [Google Scholar]
- 4.Alves C., von Dippe P., Amoui M., Levy D. 1993. Bile acid transport into hepatocyte smooth endoplasmic reticulum vesicles is mediated by microsomal epoxide hydrolase, a membrane protein exhibiting two distinct topological orientations. J. Biol. Chem. 268: 20148–20155. [PubMed] [Google Scholar]
- 5.Guenthner T. M., Cai D., Wallin R. 1998. Co-purification of microsomal epoxide hydrolase with the warfarin-sensitive vitamin K1 oxide reductase of the vitamin K cycle. Biochem. Pharmacol. 55: 169–175. [DOI] [PubMed] [Google Scholar]
- 6.Batt A. M., Siest G., Oesch F. 1984. Differential regulation of two microsomal epoxide hydrolases in hyperplastic nodules from rat liver. Carcinogenesis. 5: 1205–1206. [DOI] [PubMed] [Google Scholar]
- 7.Vogel-Bindel U., Bentley P., Oesch F. 1982. Endogenous role of microsomal epoxide hydrolase. Ontogenesis, induction inhibition, tissue distribution, immunological behaviour and purification of microsomal epoxide hydrolase with 16 alpha, 17 alpha-epoxyandrostene-3-one as substrate. Eur. J. Biochem. 126: 425–431. [PubMed] [Google Scholar]
- 8.Newman J. W., Morisseau C., Hammock B. D. 2005. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog. Lipid Res. 44: 1–51. [DOI] [PubMed] [Google Scholar]
- 9.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 1995. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215: 89–97. [DOI] [PubMed] [Google Scholar]
- 10.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N. E., Schatz A. R., Gopher A., Almog S., Martin B. R., Compton D. R., et al. 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50: 83–90. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura T., Kodaka T., Nakane S., Miyashita T., Kondo S., Suhara Y., Takayama H., Waku K., Seki C., Baba N., et al. 1999. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J. Biol. Chem. 274: 2794–2801. [DOI] [PubMed] [Google Scholar]
- 12.Dinh T. P., Carpenter D., Leslie F. M., Freund T. F., Katona I., Sensi S. L., Kathuria S., Piomelli D. 2002. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA. 99: 10819–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinh T. P., Freund T. F., Piomelli D. 2002. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem. Phys. Lipids. 121: 149–158. [DOI] [PubMed] [Google Scholar]
- 14.Goparaju S. K., Ueda N., Yamaguchi H., Yamamoto S. 1998. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 422: 69–73. [DOI] [PubMed] [Google Scholar]
- 15.Goparaju S. K., Ueda N., Taniguchi K., Yamamoto S. 1999. Enzymes of porcine brain hydrolyzing 2-arachidonoylglycerol, an endogenous ligand of cannabinoid receptors. Biochem. Pharmacol. 57: 417–423. [DOI] [PubMed] [Google Scholar]
- 16.Muccioli G. G., Xu C., Odah E., Cudaback E., Cisneros J. A., Lambert D. M., Lopez Rodriguez M. L., Bajjalieh S., Stella N. 2007. Identification of a novel endocannabinoid-hydrolyzing enzyme expressed by microglial cells. J. Neurosci. 27: 2883–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blankman J. L., Simon G. M., Cravatt B. F. 2007. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 14: 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrs W. R., Blankman J. L., Horne E. A., Thomazeau A., Lin Y. H., Coy J., Bodor A. L., Muccioli G. G., Hu S. S., Woodruff G., et al. 2010. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat. Neurosci. 13: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nardini M., Ridder I. S., Rozeboom H. J., Kalk K. H., Rink R., Janssen D. B., Dijkstra B. W. 1999. The x-ray structure of epoxide hydrolase from Agrobacterium radiobacter AD1. An enzyme to detoxify harmful epoxides. J. Biol. Chem. 274: 14579–14586. [PubMed] [Google Scholar]
- 20.Decker M., Arand M., Cronin A. 2009. Mammalian epoxide hydrolases in xenobiotic metabolism and signalling. Arch. Toxicol. 83: 297–318. [DOI] [PubMed] [Google Scholar]
- 21.Arand M., Muller F., Mecky A., Hinz W., Urban P., Pompon D., Kellner R., Oesch F. 1999. Catalytic triad of microsomal epoxide hydrolase: replacement of Glu404 with Asp leads to a strongly increased turnover rate. Biochem. J. 337: 37–43. [PMC free article] [PubMed] [Google Scholar]
- 22.Ollis D. L., Cheah E., Cygler M., Dijkstra B., Frolow F., Franken S. M., Harel M., Remington S. J., Silman I., Schrag J., et al. 1992. The alpha/beta hydrolase fold. Protein Eng. 5: 197–211. [DOI] [PubMed] [Google Scholar]
- 23.Morisseau C., Newman J. W., Dowdy D. L., Goodrow M. H., Hammock B. D. 2001. Inhibition of microsomal epoxide hydrolases by ureas, amides, and amines. Chem. Res. Toxicol. 14: 409–415. [DOI] [PubMed] [Google Scholar]
- 24.Morisseau C., Newman J. W., Wheelock C. E., Hill Iii T., Morin D., Buckpitt A. R., Hammock B. D. 2008. Development of metabolically stable inhibitors of Mammalian microsomal epoxide hydrolase. Chem. Res. Toxicol. 21: 951–957. [DOI] [PubMed] [Google Scholar]
- 25.Spiegelstein O., Kroetz D. L., Levy R. H., Yagen B., Hurst S. I., Levi M., Haj-Yehia A., Bialer M. 2000. Structure activity relationship of human microsomal epoxide hydrolase inhibition by amide and acid analogues of valproic acid. Pharm. Res. 17: 216–221. [DOI] [PubMed] [Google Scholar]
- 26.Nithipatikom K., Endsley M. P., Isbell M. A., Falck J. R., Iwamoto Y., Hillard C. J., Campbell W. B. 2004. 2-Arachidonoylglycerol: a novel inhibitor of androgen-independent prostate cancer cell invasion. Cancer Res. 64: 8826–8830. [DOI] [PubMed] [Google Scholar]
- 27.Nithipatikom K., Grall A. J., Holmes B. B., Harder D. R., Falck J. R., Campbell W. B. 2001. Liquid chromatographic-electrospray ionization-mass spectrometric analysis of cytochrome P450 metabolites of arachidonic acid. Anal. Biochem. 298: 327–336. [DOI] [PubMed] [Google Scholar]
- 28.Endsley M. P., Aggarwal N., Isbell M. A., Wheelock C. E., Hammock B. D., Falck J. R., Campbell W. B., Nithipatikom K. 2007. Diverse roles of 2-arachidonoylglycerol in invasion of prostate carcinoma cells: location, hydrolysis and 12-lipoxygenase metabolism. Int. J. Cancer. 121: 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endsley M. P., Thill R., Choudhry I., Williams C. L., Kajdacsy-Balla A., Campbell W. B., Nithipatikom K. 2008. Expression and function of fatty acid amide hydrolase in prostate cancer. Int. J. Cancer. 123: 1318–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long J. Z., Li W., Booker L., Burston J. J., Kinsey S. G., Schlosburg J. E., Pavon F. J., Serrano A. M., Selley D. E., Parsons L. H., et al. 2009. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 5: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander J. P., Cravatt B. F. 2005. Mechanism of carbamate inactivation of FAAH: implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chem. Biol. 12: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mor M., Rivara S., Lodola A., Plazzi P. V., Tarzia G., Duranti A., Tontini A., Piersanti G., Kathuria S., Piomelli D. 2004. Cyclohexylcarbamic acid 3′- or 4’-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure-activity relationships, and molecular modeling studies. J. Med. Chem. 47: 4998–5008. [DOI] [PubMed] [Google Scholar]
- 33.Ghafouri N., Tiger G., Razdan R. K., Mahadevan A., Pertwee R. G., Martin B. R., Fowler C. J. 2004. Inhibition of monoacylglycerol lipase and fatty acid amide hydrolase by analogues of 2-arachidonoylglycerol. Br. J. Pharmacol. 143: 774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Björklund E., Norén E., Nilsson J., Fowler C. J. 2010. Inhibition of monoacylglycerol lipase by troglitazone, N-arachidonoyl dopamine and the irreversible inhibitor JZL184: comparison of two different assays. Br. J. Pharmacol. 161: 1512–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cravatt B. F., Giang D. K., Mayfield S. P., Boger D. L., Lerner R. A., Gilula N. B. 1996. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 384: 83–87. [DOI] [PubMed] [Google Scholar]
- 36.Nithipatikom K., Isbell M. A., Endsley M. P., Woodliff J. E., Campbell W. B. 2011. Anti-proliferative effect of a putative endocannabinoid, 2-arachidonylglyceryl ether in prostate carcinoma cells. Prostaglandins Other Lipid Mediat. 94: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hohmann A. G., Suplita R. L., Bolton N. M., Neely M. H., Fegley D., Mangieri R., Krey J. F., Walker J. M., Holmes P. V., Crystal J. D., et al. 2005. An endocannabinoid mechanism for stress-induced analgesia. Nature. 435: 1108–1112. [DOI] [PubMed] [Google Scholar]
- 38.Petrovszki Z., Kovacs G., Tomboly C., Benedek G., Horvath G. 2012. The effects of peptide and lipid endocannabinoids on arthritic pain at the spinal level. Anesth. Analg. 114: 1346–1352. [DOI] [PubMed] [Google Scholar]
- 39.Rani Sagar D., Burston J. J., Woodhams S. G., Chapman V. 2012. Dynamic changes to the endocannabinoid system in models of chronic pain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367: 3300–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez-Jaimes L. J., Palmer J. A. 2011. The role of endocannabinoids in pain modulation and the therapeutic potential of inhibiting their enzymatic degradation. Curr. Pharm. Biotechnol. 12: 1644–1659. [DOI] [PubMed] [Google Scholar]
- 41.Duan H., Takagi A., Kayano H., Koyama I., Morisseau C., Hammock B. D., Akatsuka T. 2012. Monoclonal antibodies reveal multiple forms of expression of human microsomal epoxide hydrolase. Toxicol. Appl. Pharmacol. 260: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]