Abstract
Accurate knowledge of greater palatine foramen (GPF) anatomy is necessary when performing a variety of anaesthesiological, dental or surgical procedures. The first aim of this study was to localize the GPF in relation to multiple anatomical landmarks. The second aim was to perform a systematic review of literature, and to conduct a meta-analysis on the subject of GPF position to aid clinicians in their practice. One-hundred and fifty dry, adult, human skulls and 1200 archived head computed tomography scans were assessed and measured in terms of GPF relation to other anatomical reference points. A systematic literature search was performed using the PubMed, Embase and Web of Science databases, and a meta-analysis on the subject of GPF relation to the maxillary molars was conducted. On average, in the Polish population, the GPF was positioned 15.9 ± 1.5 mm from the midline maxillary suture (MMS), 3.0 ± 1.2 mm from the alveolar ridge (AR) and 17.0 ± 1.5 mm from the posterior nasal spine (PNS); 74.7% of GPF were positioned opposite the third maxillary molar (M3). Twenty-seven studies were included in the systematic review and 23 in the meta-analysis (n = 6927 GPF). The pooled prevalence of the GPF being positioned opposite the M3 was 63.9% (95% confidence interval = 56.6–70.9%). Concluding, the GPF is most often located opposite the M3 in the majority of the world's populations. The maxillary molars are the best landmarks for locating the GPF. In edentulous patients the most useful points for approximating the position of the GPF are the AR, MMS and PNS. This study introduces an easy and repeatable classification to reference the GPF to the maxillary molars.
Keywords: anatomy, greater palatine foramen, meta-analysis, position, systematic review
Introduction
The maxillary nerve and its tributaries provide sensory innervation to the maxillary teeth, the palate, the nasal cavity, the sinuses and subsequently the skin of the midface (Howard-Swirzinski et al. 2010). The maxillary nerve originates from the trigeminal ganglion, passes through the cavernous sinus, proceeds to exit through the foramen rotundum, and finally enters the pterygopalatine fossa where it gives rise to multiple branches (Rodella et al. 2012). The anterior (greater) palatine nerve supplies the main sensory innervation to the palate. It branches off the maxillary nerve and passes through the greater palatine canal (GPC) to surface on the hard palate from the greater palatine foramen (GPF), and continues anteriorly, ending just short of the front incisors (Sharma & Garud, 2013).
The anterior palatine nerve block was first described in 1927 (Ikuta et al. 2013). This procedure can be performed using two intra-oral approaches – the high tuberosity approach or the GPC approach (Piagkou et al. 2012). The latter is associated with a higher success rate and a lower incidence of complications (Wong & Sved, 1991). However, the major clinical difficulty of this method is to accurately locate the position of the GPF, which is the palatal exit point of the GPC (Wong & Sved, 1991). The GPC approach is of additional clinical significance as it allows to reduce bleeding during nasal septum surgical repair (Mercuri, 1979), endoscopic sinus surgery and septorhinoplasty (Das et al. 2006; Douglas & Wormald, 2006). What is more, accurate GPF localization is needed when aiming to mobilize the greater palatine artery during oroantral fistulae closure using mucoperiosteal pedicled palatal flaps (Bell, 2011; Piagkou et al. 2012) or during palatal mucosa graft harvesting for periodontal proposes (Klosek & Rungruang, 2009). All of the above underline the essential need to thoroughly understand the anatomy and anatomical variability of the GPF and its associated landmarks.
Matsuda (1927) was the first to report on the localization of the GPF. The majority of textbooks still locate the GPF in a very general way, for example near the lateral or posterolateral palatal border, medial or opposite the third maxillary molar (M3; Romanes, 1981). Anaesthesia textbooks seem to be a little more specific in that matter, loosely positioning the GPF in relation to the maxillary molars (Shane, 1975).
Up-to-date, anatomical studies on the placement of the GPF have been conducted in numerous populations, such as African (Langenegger et al. 1983; Hassanali & Mwaniki, 1984; Ajmani, 1994; Osunwoke et al. 2011), American (Malamed & Trieger, 1983; Fu et al. 2011), Brazilian (Chrcanovic BR & Custódio, 2010; Teixeira et al. 2010; Urbano et al. 2010; Lopes et al. 2011; Ikuta et al. 2013), Chinese (Wang et al. 1988), European (Malamed & Trieger, 1983; Piagkou et al. 2012; Nimigean et al. 2013), Indian (Westmoreland & Blanton, 1982; Ajmani, 1994; Sujatha et al. 2005; Saralaya & Nayak, 2007; Kumar et al. 2011; D'Souza et al. 2012; Dave et al. 2013; Jotania et al. 2013; Sharma & Garud, 2013), Korean (Lee et al. 2001; Hwang et al. 2011) and Thai (Methathrathip et al. 2005; Klosek & Rungruang, 2009). However, the main limitation of all of the previously mentioned studies is that they were conducted on a very limited number of subjects/samples, not exceeding 300 skulls or 50 computed tomography (CT) scans or cone-beam CT scans. Even the most recent studies (Ikuta et al. 2013) see the need for further investigation to establish an exact reference point or position of the GPF. The growing number of publications on the subject of GPF position makes it difficult for a clinician to draw practical conclusions, especially when some of the studies present conflicting results.
Thus, the first aim of the present study was to localize the GPF relative to multiple anatomical landmarks in a large sample of Polish adult skulls and head CTs. The second aim of the study was to perform a comprehensive review of literature on the subject of GPF position, and to conduct a meta-analysis on the prevalence of GPF location in regard to the maxillary molars to aid clinicians in their practice.
Materials and methods
Study materials
The present study was conducted on 150 dry, adult, sexed, human skulls obtained from the Museum of the Department of Anatomy cranial collection (Jagiellonian University Medical College – http://www.katedra-anatomii.cm-uj.krakow.pl/?q=muzeum-katedry) and 1200 archived adult head CT scans (Department of Radiology, Jagiellonian University Medical College and Department of Radiology and J. Dietl's Specialistic Hospital, Krakow, Poland). Data regarding sex and age of the analysed dry skulls were obtained from the records of the Museum of the Department of Anatomy.
The CT images were acquired using a Siemens Somatom Sensation 16 and a Toshiba Aquilion 64. The following study parameters were applied: exposure 120 kV, 74 mA, 60 mAs; rotation time: 0.5 s; slice thickness: 0.5 mm. Patient's sex and age data were acquired from patient files.
Study inclusion criteria were full eruption of third molars on both sides of the maxilla, presence of all maxillary teeth, participant/specimen age over 21 years, and absence of any pathological (including developmental and traumatic) changes in the region of the maxilla.
Measurements
The measurements were performed using a digital caliper (Mitutoyo, Japan; dry skulls) or the eFilm Workstation 3.4 (Merge Healthcare; CT scans). For the CT scans, maximum intensity projections, multi-planar reconstructions and volume rendering reconstructions were examined in three planes: coronal; sagittal; and transverse. All measurements were recorded to the nearest 0.01 mm, and after statistical analysis were rounded up to the nearest 0.1 mm for data presentation. All bilateral measurements were performed symmetrically. Each measurement was taken twice by the same observer and, in cases of any discrepancies, the mean of the two values was recorded. After all of the samples were measured, 20% of randomly chosen samples were re-measured by an observer who did not partake in assessing the samples the first time. Inter-class correlations (ICCs) were calculated. The level of agreement between the assessments was very high (ICC = 0.92–0.96).
The centre of the GPF was established while measuring its anterior–posterior (AP) and lateral–medial (LM) dimensions. GPF centre was set at the point of the intersection of two straight lines representing the longest AP and LM GPF dimensions. If necessary this was corrected visually, using the GPF form factor. The form factor was obtained by dividing the AP by LM dimensions. If the GPF was circular in shape, the obtained value was equal to 1. Values > 1 indicated an AP elongated GPF, and values < 1 indicated a LM elongated foramen (Jaffar & Hamadah, 2003).
The following assessments were performed (Fig. 1).
Fig. 1.
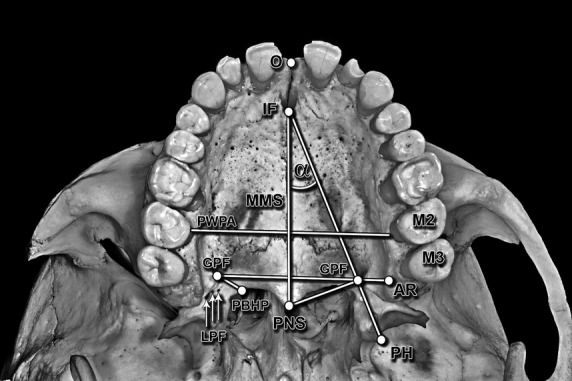
Ventral photograph of the hard palate illustrating performed measurements. O, orale; IF, incisive foramen; MMS, midline maxillary suture; PWPA, posterior width of the palatal arch; GPF, greater palatine foramen; LPF, lesser palatine foramina; PBHP, posterior border of the hard palate; PNS, posterior nasal spine; AR, alveolar ridge; PH, tip of the pterygoid hamulus; M2, second maxillary molar; M3, third maxillary molar; α, the PNS–IF–GPF angle.
Measurements from the centre of the GPF: shortest perpendicular distance to the midline maxillary suture (MMS); shortest distance to the posterior border of the hard palate (PBHP), to the centre of the incisive foramen (IF), to the alveolar ridge (AR), to the tip of the pterygoid hamulus (PH), to the posterior nasal spine (PNS), to the centres of the second maxillary molar (M2) and the M3, and to the centre of the opposite GPF.
Measurement of the MMS–IF–GPF angle, maximal palatal vault height (at the level of the second molar a perpendicular line was drawn from the AR level to the MMS), palatal vault breadth (at the level of the second molars) and palatal vault length (distance between the orale and staphylion points).
Evaluation of the presence of the GPF posterior palatine crest and the number of lesser palatine foramen (LPFs), as well as palatal vault shape.
Evaluation of GPF opening direction, and GPF position in relation to the maxillary molars (a perpendicular line from the GPF to the MMS was drawn, and later extended to the maxillary molars).
Calculation of the palatine index (palatine breadth to palatine length ratio expressed as a percentage) and the palatal height index (palatine height to palatine breadth ratio expressed as a percentage).
Literature search
This study strictly adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. The whole process is depicted in Fig. 2. Embase, PubMed and Web of Science databases were searched, by two independent reviewers, for appropriate studies published up to 27 February 2014, without a lower date limit. Search keywords included ‘greater’, ‘palatine’ and ‘foramen’ in different combinations. Review of full-text articles was limited to the ones published in English. References of the identified articles were searched manually. Study inclusion criteria were: studies conducted on human skulls/head CT scans; participants age ≥ 21 years; full-text original articles only (excluding conference abstracts and review papers); ≥ 3 relevant GPF measurements. Inclusion or exclusion of studies was performed hierarchically based on the title of the report first, followed by the abstract, and finally by the full text.
Fig. 2.
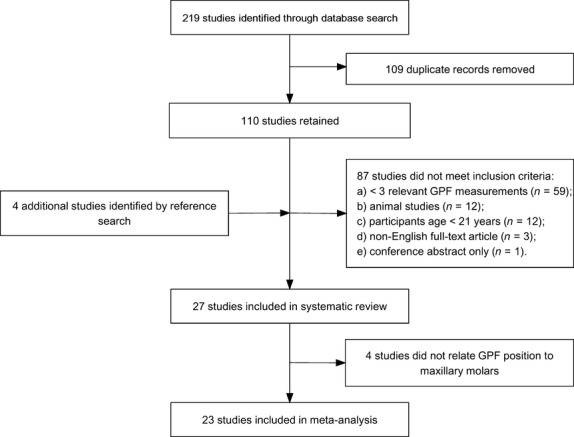
Flow-chart depicting literature search and study selection. GPF, greater palatine foramen.
One study was not included in this review as neither the author nor the Journal supplied an abstract or a full copy of the manuscript (Aterkar et al. 1995).
Data extraction and quality assessment were performed independently by two reviewers. The following data were extracted from the relevant studies: citation details; sample size and sample characteristics; as well as relevant measurements performed. In the present meta-analysis the relation of the GPF to the maxillary molars was analysed.
Statistical analysis
Statistical analysis was conducted using Statistica 10 PL (Statistica, Poland) and the MIX 2.0 PRO (BiostatXL) meta-analysis plugin for Excel. Elements of descriptive statistics were used (mean, standard deviation, percentage distribution). Side-related differences were evaluated using the Mann–Whitney U-test or the Student's t-test as appropriate. ICC was used to evaluate the level of agreement between measurement and re-measurement of the same sample.
Study heterogeneity was assessed using the Cochran Q-test and the I2 index. Values of about 25%, 50% and 75% were interpreted as low, medium and high heterogeneity, respectively. If substantial heterogeneity was observed the random-effects model was used (Huedo-Medina et al. 2006). In case significant heterogeneity was observed, a study subgroup analysis was planned (studies grouped by geographical region). Funnel plot was used to graphically evaluate publication bias (Egger et al. 1997), while statistical assessment of publication bias was conducted through the Egger's and Begg's tests. For each study included in the meta-analysis, the 95% confidence interval for prevalence of the GPF being located opposite the M3 was calculated using the binomial distribution.
A P-value of < 0.05 was considered statistically significant.
Ethics
This study has been approved by the Jagiellonian University Medical College Bioethics Committee (registry no. KBET/161/B/2013), and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Results
The studied group comprised 150 dry human skulls and 1200 head CTs – yielding a total of 1350 samples (695 female; 51.5%). The mean age of the group was 44.9 ± 17.1 years – females (45.4 ± 16.6 years) vs. males (44.4 ± 17.7 years; P = 0.28). As the measurements performed on dry skulls closely corresponded with those made on the CT scans (ICC = 0.93–97), it was decided that both groups will be treated as one during statistical analysis.
The main measurements performed are presented in Table 1. Overall, the table shows that palate measurements differ significantly between sexes. On the other hand, the right and left sides seem to be symmetrical, taking into account most of the measurements. Table 2 presents the GPF position in relation to the maxillary molars across different studies. Tables 3 and 4 present additional data regarding the position of the GPF and the anatomy of the hard palate obtained from the present study, and at the same time contrast those data with the results of other studies.
Table 1.
Main measurements performed on the studied group
| Females (n = 695) Mean (SD) (mm) | Males (n = 655) Mean (SD) (mm) | Total (n = 1350) Mean (SD) (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | L | T | R | L | T | P-value* | R | L | T | P-value† | |
| Age (years) | 45.4 (16.6)‡ | 44.4 (17.7)‡ | 0.28 | 44.9 (17.1)‡ | – | ||||||
| GPF–MMS | 15.7 (1.5) | 15.4 (1.4) | 15.5 (1.5) | 16.4 (1.5) | 16.0 (1.5) | 16.2 (1.5) | P < 0.0001 | 16.1 (1.5) | 15.6 (1.5) | 15.9 (1.5) | P < 0.0001 |
| GPF–PBHP | 4.8 (1.0) | 4.7 (1.1) | 4.7 (1.0) | 4.9 (1.0) | 4.9 (1.0) | 4.9 (1.0) | P = 0.0002 | 4.9 (1.0) | 4.8 (1.0) | 4.8 (1.0) | P = 0.25 |
| GPF–IF | 33.2 (2.5) | 33.4 (2.7) | 33.3 (2.6) | 34.8 (3.3) | 35.3 (3.3) | 35.0 (3.3) | P < 0.0001 | 34.0 (3.0) | 34.3 (3.1) | 34.2 (3.0) | P = 0.004 |
| MMS–IF–GPF angle (°) | 26.8 (2.9) | 26.3 (2.9) | 26.5 (2.9) | 25.5 (2.8) | 25.7 (3.0) | 25.6 (2.9) | P < 0.0001 | 26.0 (2.9) | 26.3 (3.0) | 26.2 (2.9) | P = 0.002 |
| GPF–AR | 2.9 (1.4) | 2.9 (1.5) | 2.9 (1.4) | 3.1 (1.2) | 2.9 (1.1) | 3.0 (1.1) | P = 0.11 | 3.0 (1.3) | 2.9 (1.3) | 3.0 (1.2) | P = 0.22 |
| GPF–PH tip | 11.6 (0.8) | 11.6 (0.8) | 11.6 (0.8) | 12.2 (1.2) | 12.3 (1.4) | 12.2 (1.3) | P < 0.0001 | 11.9 (1.0) | 12.0 (1.1) | 11.9 (1.1) | P = 0.19 |
| GPF–PNS | 16.5 (1.2) | 16.5 (1.3) | 16.5 (1.2) | 17.4 (1.5) | 17.5 (1.5) | 17.4 (1.5) | P < 0.0001 | 17.0 (1.4) | 17.0 (1.5) | 17.0 (1.5) | P = 0.48 |
| GPF–M2 | 11.3 (2.2) | 11.4 (1.9) | 11.4 (2.0) | 12.2 (2.4) | 12.2 (2.3) | 12.2 (2.3) | P < 0.0001 | 11.8 (2.1) | 11.8 (2.1) | 11.8 (2.1) | P = 0.72 |
| GPF–M3 | 11.0 (2.1) | 11.0 (1.7) | 11.0 (1.9) | 11.4 (2.1) | 11.7 (2.4) | 11.5 (2.2) | P < 0.0001 | 11.3 (2.1) | 11.4 (2.1) | 11.3 (2.1) | P = 0.16 |
| GPF–GPF | 28.7 (2.5)‡ | 29.5 (2.6)‡ | P < 0.0001 | 29.1 (2.6)‡ | – | ||||||
| Palatal vault height | 12.1 (2.5)‡ | 14.2 (2.5)‡ | P < 0.0001 | 13.1 (2.7)‡ | – | ||||||
| Palatal vault breadth | 45.7 (3.0)‡ | 48.1 (3.1)‡ | P < 0.0001 | 46.9 (3.3)‡ | – | ||||||
| Palatal vault length | 45.0 (3.9)‡ | 49.1 (4.2)‡ | P < 0.0001 | 47.0 (4.5)‡ | – | ||||||
| PI | 101.6 (4.7) | 98.0 (6.0) | P < 0.0001 | 99.8 (5.4)‡ | – | ||||||
| PHI | 26.5 (2.9) | 29.5 (3.7) | P < 0.0001 | 30.1 (3.1)‡ | – | ||||||
AR, alveolar ridge; GPF, greater palatine foramen; IF, incisive foramen; L, left side; M2, second maxillary molar; M3, third maxillary molar; MMS, midline maxillary suture; PBHP, posterior border of the hard palate; PH, pterygoid hamulus; PHI, palatine height index; PI, palatine index; PNS, posterior nasal spine; R, right side; SD, standard deviation; T, value for both sides.
Palatine index: palatine breadth to palatine length ratio (%); palatine height index: palatine height to palatine breadth ratio (%).
Palatine breadth: measured at the level of maxillary second molars. Palatine length: distance between the orale and staphylion points.
P-value for the comparison between female and male total values.
P-value for the comparison between total right and left values.
Total value only.
Table 2.
Data comparison between studies regarding GPF position in relation to the maxillary molars
| GPF position (%) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior to M2 | Opposite M2 | Between M2 and M3 | Medial to M3 | Opposite M3 | Distal to M3 | ||||||||||||||
| Study | Population (number of GPF in the sample) | R | L | Total | R | L | Total | R | L | Total | R | L | Total | R | L | Total | R | L | Total |
| European studies (total GPF n = 3007) | |||||||||||||||||||
| Tomaszewska et al. 2014 (this study) | Polish (n = 2700) | – | 15.5 | 17.1 | 16.3 | 6.8 | 6.8 | 6.8 | – | 75.4 | 74.0 | 74.7 | 1.1 | 1.1 | 2.2 | ||||
| Nimigean et al. 2013 | Romanian (n = 200) | – | 9.0* | 15.0* | – | 73.0* | 3.0* | ||||||||||||
| Piagkou et al. 2012 | Greek (n = 107) | – | 16.6 | 17.0 | 16.8 | – | – | 76.9 | 75.5 | 75.7 | 7.4 | 7.5 | 7.5 | ||||||
| African studies (total GPF n = 880) | |||||||||||||||||||
| Osunwoke et al. 2011 | Nigerian (n = 300) | – | 1.3 | 0.7 | 1.0 | 23.3 | 22.0 | 22.7 | – | 74.0 | 75.3 | 74.7 | 1.3 | 2.0 | 1.7 | ||||
| Ajmani, 1994 | Nigerian (n = 130) | – | 10.8 | 15.4 | 13.1 | 36.9 | 40.0 | 38.5 | 43.1 | 36.9 | 40.0 | 9.2 | 7.7 | 8.5 | – | ||||
| Hassanali & Mwaniki, 1984 | Kenyan (n = 250) | – | 10.4* | 13.6* | – | 76.0* | – | ||||||||||||
| Langenegger et al. 1983 | South African (n = 200) | – | – | 1.0 | 0.5 | – | 1.0 | 0.5 | – | 2.0 | 1.0 | 61.0 | 62.0 | 61.5 | 39.0 | 34.0 | 36.5 | ||
| Asian studies (total GPF n = 530) | |||||||||||||||||||
| Klosek & Rungruang, 2009 | Thai (n = 82) | (F): 14.3* (M): 0 | (F): 35.7*, † (M): 65.0*, † | (F): 35.7%* (M): 10.0* | – | (F): 14.3* (M): 25.0* | – | ||||||||||||
| Methathrathip et al. 2005 | Thai (n = 160) | 0.0* | 5.6*, † | 23.1* | – | 64.4*, † | 6.9* | ||||||||||||
| Wang et al. 1988 | Chinese (Taiwan) (n = 200) | 0.0‡ | 2.0‡ | 1.0 | 14.0 | 20.0 | 17 | 46.0 | 51.0 | 48.5 | – | 40.0 | 27.0 | 44.5 | – | ||||
| Brazilian studies (total GPF n = 260) | |||||||||||||||||||
| Ikuta et al. 2013 | Brazilian (n = 100) | – | – | – | 3.0* | 92* | 5* | ||||||||||||
| Chrcanovic and Custodio, 2010 | Brazilian (n = 160) | – | – | 6.2* | – | 54.9* | 38.9* | ||||||||||||
| Indian studies (total GPF n = 1813) | |||||||||||||||||||
| Dave et al. 2013 | Indian (W) (n = 200) | – | – | 6.0 | 1.0 | 3.5 | – | 88.0¶ | 94.0¶ | 87.5¶ | 12.0 | 4.0 | 8.0 | ||||||
| Jotania et al. 2013 | Indian (W) (n = 120) | – | 5.0 | 3.3 | 4.2 | 23.3 | 11.7 | 17.5 | – | 71.7 | 85.0 | 78.3 | – | ||||||
| Sharma & Garud, 2013 | Indian (W) (n = 139) | – | 8.7§ | 8.7§ | 8.6 | – | – | 69.6§ | 77.1§ | 73.4 | 21.7 | 14.3 | 18.0 | ||||||
| D'Souza et al. 2012 | Indian (SW) (n = 80) | – | 2.5 | 2.5 | 2.5 | 22.5 | 25 | 23.8 | – | 75.0 | 72.5 | 73.8 | – | ||||||
| Kumar et al. 2011 | Indian (N) (n = 200) | – | 5.0 | 5.0 | 5.0 | 9.0 | 9.0 | 9.0 | – | 85.0 | 85.0 | 85.0 | 1.0 | 1.0 | 1.0 | ||||
| Saralaya & Nayak, 2007 | Indian (SW) (n = 264) | – | 0.8 | 0 | 0.4 | 25 | 23.5 | 24.2 | – | 73.5 | 75.8 | 74.6 | 0.8 | 0.8 | 0.8 | ||||
| Sujatha et al. 2005 | Indian (S) (n = 142) | – | 0.9* | 13.1* | – | 86.0 | – | ||||||||||||
| Ajmani, 1994 | Indian (N) (n = 68) | – | – | 29.4 | 35.3 | 32.4 | 26.5 | 17.6 | 22.1 | 41.2 | 44.1 | 42.7 | 2.9 | 2.9 | 2.9 | ||||
| Westmoreland & Blanton, 1982 | Indian (E) (n = 600) | – | 8.7 | 10.6 | 9.7 | 34.7 | 32.7 | 33.7 | – | 50.7 | 50.7 | 50.7 | 6.0 | 6.0 | 6.0 | ||||
| Studies from other regions | |||||||||||||||||||
| Fu et al. 2011 | American (n = 21) | – | 19.1*, † | 66.6* | – | 14.3*, † | – | ||||||||||||
| Jaffar & Hamadah, 2003 | Caucasian (Iraqi) (n = 100) | – | 12.0* | 19.0* | – | 55.0% | 14.0% | ||||||||||||
| Malamed & Trieger, 1983 | Mixed (n = 316) | – | 39.9* | – | – | 60.1* | – | ||||||||||||
GPF, greater palatine foramen; M2, second maxillary molar; M3, third maxillary molar; F, female; M, male; (N), north; (S), south; (E), east; (W), west – referring to the geographical region from which the study samples were collected.
The authors report total values only.
The authors describe this location as palatal instead of opposite.
The authors report it is ‘interproximal to the M1 and M2’.
This percentage is the sum of what the authors measure to be located between ‘the posterior half of the M2 and the anterior half of the M3’.
The authors report that the GPF is located ‘medial to the M3’, however according to the definition given in the manuscript this was reclassified to ‘opposite to the M3’.
‘R%’ and ‘L%’ – the ratio of the number of GPF in a particular relation to the molar teeth on the right or left sides to all GPF on that side.
‘total’ – ratio of the total number of GPF on both sides in a particular relation to the molar teeth to the total number of GPF in the examined group.
This table presents the data from 23 relevant studies. The study population from the work of Ajmani [17] has been divided in two, as the study analyses two different populations (African and Indian). Hence the 24 positions in the table.
Table 3.
Comparison of studies on the subject of GPF – part I
| Study | Population (number of samples –skulls/CT scans) | Type of investigation and sample characteristics | GPF dimensions (SD)(mm) | GPF–MMS (SD)(mm) | GPF–PBHP (SD)(mm) | GPF–IF (SD)(mm) | MMS–IF–GPF angle (SD)(°) | Mucosa thickness over GPF (SD)(mm)¶ | GPF–AR (SD)(mm) | GPF–PH tip (SD)(mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AP | LM | R | L | R | L | R | L | R | L | R | L | R | L | ||||
| European studies (total sample n = 1749) | |||||||||||||||||
| Tomaszewska et al. 2014 (this study) | Polish (n = 1350) | Sinus CT scans (n = 1200) Dry human skulls; sexed (n = 150) mean age 44.9 ± 17.1 years; Total: 655M, 695F | 5.1 (0.5) | 3.0 (0.8) | 16.1 (1.5) | 15.6 (1.5) | 4.9 (1.0) | 4.8 (1.0) | 34.0 (3.0) | 34.3 (3.1) | 26.0 (2.9) | 26.3 (3.0) | – | 3.0 (1.3) | 2.9 (1.3) | 11.9 (1.0) | 12.0 (1.1) |
| 15.9 (1.5)* | 4.8 (1.0)* | 34.2 (3.0)* | 26.2 (2.9)* | 3.0 (1.2) | 11.9 (1.1) | ||||||||||||
| Nimigean et al. 2013 | Romanian (n = 100) | Dry human skulls; age range 25–40 years; sexed | 4.9 (0.9) | 3.0 (0.9) | 14.5 (0.7) | 4.4 (1.1) | – | – | 6.0 | – | 12.0 (1.8)* | ||||||
| Piagkou et al. 2012 | Greek (n = 71) | Dry human skulls; adult; unsexed | 5.3 (0.9) | 2.7 (0.5) | 15.3 (1.3) | 4.6 (1.0) | 4.7 (1.1) | – | – | – | 3.1 (1.7) | 2.9 (1.6) | – | ||||
| African studies (total sample n = 440) | |||||||||||||||||
| Osunwoke et al. 2011 | Nigerian (n = 150) | Dry human skulls; adult; sexed (100%M) | – | 15.0 (2.1) | 15.0 (2.1) | 14.3 (1.5) | 5.0 (1.2) | 5.1 (5.1) | 41.1 (5.0) | – | – | – | – | ||||
| Ajmani, 1994 | Nigerian (n = 65) | Dry human skulls; adult, unsexed | – | 15.4 (0.2) | 15.4 (0.2) | 3.5 (0.2) | 3.5 (0.2) | – | – | – | – | – | |||||
| Hassanali & Mwaniki, 1984 | Kenyan (n = 125) | Dry human skulls; adult; sexed (60M, 22F, 43U) | – | – | – | – | – | – | – | – | |||||||
| Langenegger et al. 1983 | South African (n = 100) | Dry human skulls; mean age 42.7 years; sexed (50M, 50F) | – | 2.5 (0.5) | 15.1 (2.6) | 14.9 (1.8) | – | – | – | – | – | 12.3 (1.8) | 12.6 (1.9) | ||||
| Asian studies (total sample n = 351) | |||||||||||||||||
| Hwang et al. 2011 | Korean (n = 50) | Head HRCT scans; mean age 51.0 years; sexed (22M, 28F) | 4.5 (0.7) | 2.2 (0.4) | 16.2 (1.3) * | – | – | – | 10.7 (1.8) | – | – | ||||||
| Klosek & Rungruang, 2009 | Thai (n = 41) | Human cadavers; mean age 71.2 years; sexed (24M, 17F) | F: 5.1 (1.0) M: 4.9 (8.3) | F: 2.8 (1.0) M: 2.6 (8.3) | 14.7 (3.3) | – | 34.0 (7.3) | – | – | – | – | ||||||
| 3.25 (0.5) † | |||||||||||||||||
| Methathrathip et al. 2005 | Thai (n = 160) | Dry human skulls (n = 105) – mean age 48.1 years; sexed (68M, 37F); Human cadavers (n = 55) | 4.9 (0.9) | 2.7 (0.5) | 16.2 (1.3) | 2.1 (1.3) | – | – | 6.7 (2.3) | – | – | ||||||
| Wang et al. 1988 | Chinese (n = 100) | Dry human skulls; adult; sexed | – | 16.0 (0.2) | 16.0 (0.1) | 3.9 (0.1) | 4.3 (0.1) | – | – | – | – | – | |||||
| 16.0 (0.1) | 4.1 (0.1) | ||||||||||||||||
| Brazilian studies (total sample n = 408) | |||||||||||||||||
| Ikuta et al. 2013 | Brazilian (n = 50) | CBCT scans; mean age 35.8 years; sexed (27M, 23F) | 3.1† (0.5) | 15.2 (1.45) | – | – | – | – | 7.9 (2.0) | – | |||||||
| Lopes et al. 2011 | Brazilian (n = 94) | Dry human skulls; adult; sexed (65M, 29F) | – | 15.6 (1.3) | 15.4 (1.4) | 3.4 (1.2) | 3.5 (1.1) | – | – | – | – | – | |||||
| Chrcanovic & Custódio, 2010 | Brazilian (n = 80) | Dry human skulls; age unknown; unsexed | – | 14.7 (1.6) | 14.4 (1.4) | 3.4 (1.1)* | 36.2 (3.2) | 36.5 (3.3) | 22.1 (2.7) | 23.3(2.5) | – | – | – | ||||
| Teixeira et al. 2010 | Brazilian (n = 141) | Dry human skulls; mean age 31.6 ± 13.1 years; sexed (82M, 59F) | – | 15.7 (1.6) | 16.2 (1.6) | – | 39.3 (3.4) | 39.1 (3.8) | – | – | – | – | |||||
| Urbano et al. 2010 | Brazilian (n = 43) | Dry human skulls; adult; unsexed | – | 16.6 | 16.4 | 4.5 | 4.6 | – | – | – | – | – | |||||
| Indian studies (total sample n = 937) | |||||||||||||||||
| Dave et al. 2013 | Indian (W) (n = 100) | Dry human skulls; adult; sexed (60M, 39F, 1U) | – | F: 16.2 (1.6) M: 16.7 (1.1) | F: 16.4 (1.1) M: 16.6 (1.1) | F: 6.7 (1.2) M: 6.9 (1.3) | F: 7.0 (1.3) M: 6.9 (1.2) | – | – | – | – | – | |||||
| Sharma & Garud, 2013 | Indian (W) (n = 100) | Dry human skulls; adult; unsexed | 4.7 (1.1) | 3.25 (0.5) † | 14.7 (1.4) | 14.7 (1.4) | 3.4 (1.5) | 3.4 (1.5) | 35.4 (2.8) | 35.7 (2.6) | – | – | – | 12.1 (2.5) | 11.5 (1.9) | ||
| Jotania et al. 2013 | Indian (W) (n = 60) | Dry human skulls; adult; unsexed | – | 14.8 (1.2) | 14.8 (1.5) | – | – | – | – | – | – | ||||||
| D'Souza et al. 2012 | Indian (SW) (n = 40) | Dry human skulls; adult; unsexed | – | 14.6 (1.5) | 14.4 (1.4) | – | – | – | – | – | – | ||||||
| Kumar et al. 2011 | Indian (N) (n = 100) | Dry human skulls; adult; unsexed | – | 14.3 (1.4) | 14.4 (1.3) | 3.6 (0.9) | 3.6 (0.9) | 36.6 (2.2) | 35.7 (3.9) | – | – | – | – | ||||
| 14.3 (1.3) * | 3.6 (0.9) * | 36.2 (3.21) * | |||||||||||||||
| Saralaya & Nayak, 2007 | Indian (SW) (n = 132) | Dry human skulls; adult; unsexed | – | 14.7 (0.2) | 14.7 (0.2) | 4.2 (0.1) | 4.2 (0.1) | 37.2 (0.3) | 37.4 (0.3) | 21.1 (4.2) | 21.1 (4.2) | – | – | – | |||
| 14.7 (0.3) * | 4.2 (0.2) * | 37.3 (0.7) * | 21.1 (2.0) * | ||||||||||||||
| Sujatha et al. 2005 | Indian (S) (n = 71) | Dry human skulls; adult; unsexed | – | – | – | – | – | – | – | – | |||||||
| Ajmani, 1994 | Indian (N) (n = 34) | Dry human skulls; adult, unsexed | – | 14.7 (1.0) | 14.6 (1.1) | 3.7 (1.2) | 3.7 (1.4) | – | – | – | – | – | |||||
| Westmoreland & Blanton, 1982 | Indian (E) (n = 300) | Dry human skulls; adult; unsexed | – | 14.8 (0.1) | 15.0 (0.1) | 1.9 (0.04) | 1.9 (0.04) | – | – | – | – | – | |||||
| Studies from other regions | |||||||||||||||||
| Fu et al. 2011 | American (n = 11) | Fresh cadaver heads; mean age 75.7 years; 100% M | – | – | – | – | – | – | – | – | |||||||
| Jaffar & Hamadah, 2003 | Caucasian (Iraqi) (n = 50) | Dry human skulls; adult; unsexed | 4.6 (1.0) | 2.8 (0.6) | 15.7 (1.4) * | 4.9, (0.1) *,‡ | – | – | – | – | – | ||||||
| Malamed & Trieger, 1983 | Mixed (61.8% European, 27.9% American, 10.3% African) (n = 204) | Dry human skulls; adult; unsexed | – | – | 7.0*,§ | – | – | – | – | 12.2*,§ | |||||||
AP, anterior–posterior GPF dimension; AR, alveolar ridge; CBCT, cone-beam computed tomography; F, female; GPF, greater palatine foramen; HRCT, high-resolution computed tomography; IF, incisive foramen; LM, lateral–medial GPF dimension; M, male; MMS, midline maxillary suture; n, number; NA, not available; PBHP, posterior border of the hard palate; PH, pterygoid hamulus; SD, standard deviation; U, unknown. (E), east; (N), north; (S), south; (W), west – referring to the geographical region from which the study samples were collected.
This table presents the data from 27 studies containing relevant measurements. The study population from the work of Ajmani (17) has been divided in two, as the study analyses two different populations (African and Indian). Hence the 28 positions in the table.
Mean value for both sides.
GPF diameter.
Distance between GPF centre and the most lateral point of the PBHP (in all other studies distance between GPF and the nearest medial aspect of the PBHP).
Authors only measure with an accuracy of 0.5 mm. Range for GPF–PBHP distance 3–12 mm. Range for GPF–PH tip 5.0–20.5 mm.
Measured only on CT scans.
Table 4.
Comparison of studies on the subject of GPF – part II
| Study | Population (number of samples) | GPF opening direction (%) | Palatal vault shape | Mean palatal vault height (SD) (mm) | Mean palatal breadth (SD) (mm) | Mean palate length (SD) (mm) | PI (%) | PHI (%) | GPF posterior palatine crest (%) | Mean LPF number (SD) (range) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-A-M | I-A-L | Anterior | Vertical | R | L | R | L | ||||||||
| European studies (total sample n = 1521) | |||||||||||||||
| Tomaszewska et al. 2014 (this study) | Polish (n = 1350) | 82.6 | 3.8 | 7.4 | 5.2 | U-shaped 100% Flat: 22.0% Arched: 58.7% High-arched: 19.3% | 13.1 (2.7) | 46.9 (3.3) | 47.0 (4.5) | 99.8 (5.4) | 30.1 (3.1) | 32.4* | 30.7* | 1.6* | 1.5* |
| (0–5)* | |||||||||||||||
| Nimigean et al. 2013 | Romanian (n = 100) | 82.0 | – | 13.0 | 5.0 | – | – | 46.9 | – | – | – | – | – | ||
| Piagkou et al. 2012 | Greek (n = 71) | – | – | – | – | – | – | – | 57.8 | 56.3 | 1.8 | 1.6 | |||
| (1–5) | |||||||||||||||
| African studies (total sample n = 440) | |||||||||||||||
| Osunwoke et al. 2011 | Nigerian (n = 150) | – | – | – | – | – | – | – | – | 1.3 (0.5) | 1.2 (0.4) | ||||
| Ajmani, 1994 | Nigerian (n = 65) | 58.7 | 38.7 | – | – | U-shaped: 100% | 30–80 mm | – | – | – | – | 24.6† | – | ||
| Hassanali & Mwaniki, 1984 | Kenyan (n = 125) | 74.0 | – | – | 26 | – | 12.2 (1.6) | 40.2 (3.6) | 49.2 (3.6) | 82.0 (7.8) | 30.3 (6.3) | 43.7† | 1.6 | 1.7 | |
| (0–5) | |||||||||||||||
| Langenegger et al. 1983 | South African (n = 100) | – | – | – | – | – | – | – | 90.0 | 91.0 | – | ||||
| Asian studies (total sample n = 301) | |||||||||||||||
| Klosek & Rungruang, 2009 | Thai (n = 41) | – | – | 14.8 (3.6) | 31.1 (5.2) | 51.4 (5.8) | 61.0 (13.0) | – | – | – | |||||
| Methathrathip et al. 2005 | Thai (n = 160) | – | – | – | 97.6 | – | – | – | – | – | – | – | – | ||
| Wang et al. 1988 | Chinese (Taiwan) (n = 100) | – | – | 90.0 | 10.0 | – | – | – | – | – | – | – | – | ||
| Brazilian studies (total sample n = 80) | |||||||||||||||
| Chrcanovic & Custódio, 2010 | Brazilian (n = 80) | 18.8 | 0.0 | 69.4 | 11.9 | – | – | – | 52.4 (4.6) | – | – | – | – | ||
| Indian studies (total sample n = 866) | |||||||||||||||
| Dave et al. 2013 | Indian (W) (n = 100) | – | – | 4.0 | 96.0 | – | – | – | – | – | – | – | – | ||
| Jotania et al. 2013 | Indian (W) (n = 60) | – | – | – | 37.8 | 49.7 | 76.1 | – | – | 1.7 | 1.7 | ||||
| (0–4) | |||||||||||||||
| Sharma & Garud, 2013 | Indian (W) (n = 100) | 49.5 | 3.5 | 2.0 | 45.0 | – | – | – | – | – | – | 32.3 | 23.2 | 1.4 | 1.4 |
| 27.8† | (0–5) | ||||||||||||||
| D'Souza et al. 2012 | Indian (SW) (n =40) | – | – | – | – | – | – | – | – | Single LPF: 62.5% 2 LPF: 30% 3 LPF: 7.5% | |||||
| Kumar et al. 2011 | Indian (N) (n = 100) | 19.0 | 73.0 | 1.0 | 7.0 | Flat: 20% Arched: 69% High-arched: 11% | – | – | – | – | – | 1.0† | 1.2 (0.5) | 1.3 (0.5) | |
| (0–3) | |||||||||||||||
| Saralaya & Nayak, 2007 | Indian (SW) (n = 132) | 46.2 | 12.5 | 41.3 | – | Flat: 37.1% Arched: 46.2% High-arched: 16.7% | – | – | – | – | – | – | (1–4) | ||
| Ajmani, 1994 | Indian (N) (n = 34) | 91.4 | – | – | – | U-shaped: 100% | 30–80 mm | – | – | – | – | 25.3† | – | ||
| Westmoreland & Blanton, 1982 | Indian (E) (n = 300) | – | – | 18.0 | 82.0 | U-shaped: 100% | 40–80 mm | – | – | – | – | 16.0† | – | ||
| Studies from other regions | |||||||||||||||
| Fu et al. 2011 | American (n = 11) | – | – | 14.1 (2.5) | – | – | – | – | – | – | |||||
| Jaffar & Hamadah, 2003 | Caucasian (Iraqi) (n = 50) | 60.0 | – | 36.0 | 4.0 | – | – | 39.3 (3.4) | 50.8 (3.6) | 77.6 (6.0) | – | 67.0%† | Single LPF: 41%multiple LPF: 55%absent LPF: 4% | ||
| Malamed & Trieger, 1983 | Mixed (n = 204) | – | – | 38.7 | 61.3 | – | – | – | – | – | – | – | – | ||
F, females; GPF, greater palatine foramen; LPF, lesser palatine foramen/foramina; M, males; SD, standard deviation.GPF opening direction: I-A-M: inferiorly antero-medially; I-A-L: inferiorly antero-laterally.
PI (palatine index): palatine breadth to palatine length ratio (%); PHI (palatine height index): palatine height to palatine breadth ratio (%).
Palatine breadth: measured at the level of maxillary second molars. Palatine length: distance between the orale and staphylion points.
This table presents the data from 21 studies containing relevant measurements. The study population from the work of Ajmani (17) has been divided in two, as the study analyses two different populations (African and Indian). Hence the 22 positions in the table.
Measured only in 150 dry, adult skulls.
Mean value for both sides.
Figure 2 presents a flowchart depicting the results of the literature search. Out of the 27 studies included in the systematic review (Tables 4), only 23 (n = 6927 GPF) were included in the meta-analysis, as four studies did not relate the GPF to the maxillary molars.
Considering the most common GPF position – opposite the M3 – the Q-test showed high heterogeneity (Q = 744.7; P < 0.0001), as confirmed by the I2-test (96.9%; 95% CI = 96.2–97.5%) for the 23 studies included in the meta-analysis (Fig. 3). However, both Egger's (P = 0.16) and Begg's tests (P = 0.21) did not indicate the presence of study bias. Thus, the pooled prevalence of the GPF being positioned opposite the M3 was calculated to be 63.9% (95% CI = 56.6–70.9%; Fig. 4).
Fig. 3.
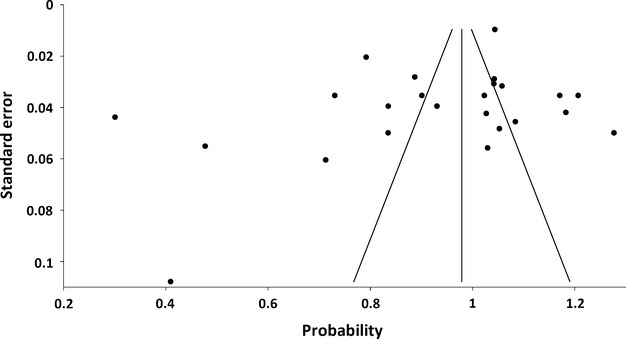
Funnel plot presenting study heterogeneity and potential publication bias. Each dot depicts an included study. Both Egger's test (P = 0.16) and Begg's test (P = 0.21) do not indicate the presence of study bias.
Fig. 4.
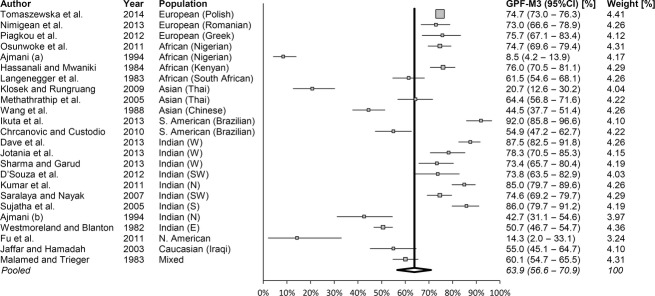
Forest plot showing studies (n = 23) that report on the prevalence of the greater palatine foramen (GPF) being positioned opposite to the third maxillary molar (M3; Q = 744.7; P < 0.0001 and I2 = 96.91%). Note: weights were calculated using random-effects analysis. Total number of GPF in the analysis n = 6927. GPF–M3, the GPF being opposite to the M3. N, north; S, south; E, east; W, west; CI, confidence interval.
To explore the source of the heterogeneity, the studies were subdivided into groups based on geographical location. For European studies, the Q-test showed almost no heterogeneity (Q = 0.36; P = 0.84), confirmed by the I2-test (0.0%). For the rest of the geographical regions, the heterogeneity was still medium to high: for African studies Q = 236.7 (P < 0.0001), I2 = 98.72% (95% CI = 98.2–99.1%); for Asian studies Q = 45.7 (P < 0.0001), I2 = 95.6% (95% CI = 91.6–97.7%); and for Indian studies Q = 208.5 (P < 0.0001), I2 = 96.2% (95% CI = 94.4–97.4%). Figure 5 depicts a forest plot for studies pooled in groups according to the geographical region they originated from. However, due to manual choosing of the studies, selectivity publication bias appeared: Egger's test (P = 0.005) and Begg's test (P = 0.09).
Fig. 5.
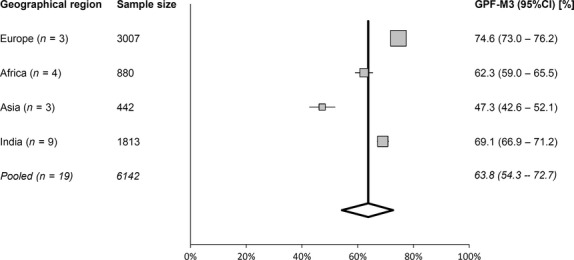
Forest plot showing studies pooled into the geographical region they originated from, and reporting on the prevalence of the greater palatine foramen (GPF) being positioned opposite to the third molar (M3; Q = 151.5; P < 0.0001 and I2 = 98.02%). Note: weights were calculated using random-effects analysis. Sample size: number of GPF in the sample. Studies not included in this analysis are Malamed & Trieger (1983), Jaffar & Hamadah (2003), Chrcanovic & Custudio (2010), Fu et al. (2011) and Ikuta et al. (2013). CI, confidence interval.
Discussion
As mentioned earlier, classic anatomical or surgical textbooks localize the GPF in a very general manner, leading to inconsistencies in physician training. The majority of studies regarding GPF position have been conducted on dry adult skull, thus delivering limited information on the gender and age of the samples (Table 3). Though our study has shown that measurements conducted on dry skulls are equal to those performed on CT scans, the latter method has the added value of knowing the participants exact age, gender, ethnicity and very often other important medical parameters. It also allows to gather a substantially larger patient sample for statistical analysis.
Though many studies (Table 3) have been conducted on the subject of GPF location, new clinical reports are constantly published stating the difficulties with locating this anatomical reference point (Piagkou et al. 2012; Ikuta et al. 2013). The anatomy of the GPF is bound to gain even more attention, as through the GPF it is possible to stimulate the pterygopalatine ganglion (Piagkou et al. 2012). This can be used in stroke patients to reduce the stroke's effect, but also to intervene in patients with cluster and migraine headaches, as well as cerebral vasospasm conditions (Oluigbo et al. 2011). From the clinical point of view, it should be stressed that in-depth knowledge of GPF anatomy is mandatory, as this is the most important site in the palate, having the greatest and most precise representation in the somatosensory cortex (Bessho et al. 2007).
According to the literature search, this study is by far the largest to date: both in terms of the number of samples and measurements. It has shown that the GPF is most often located opposite the M3 – on average in two-thirds to three-quarters of all patients, both in Europe and worldwide. The prepared systematic review and meta-analysis will be of great value to clinicians, allowing to adequately prepare oneself before performing procedures using the GPC approach or in the vicinity of the GPF. Even if one of the molar teeth is missing, the other reference points will supply enough data to easily localize the GPF. Additionally, this study has brought to light the fact that the position of the GPF, as well as its distance from certain anatomical reference points, might be used to distinguish between male and female skulls during forensic examination.
Location of the GPF in relation to the maxillary molars
The first attempt to reference the GPF to other anatomical points was undertaken by Matsuda (1927) on a mixed ethnical sample of 380 skulls. Slavkin et al. (1966) noted that the GPF moves posteriorly with the eruption of new teeth in children. This was the reason why we chose not to include specimens/scans from patients younger than 21 years. Although numerous studies have been conducted, there is still no agreement as to whether the position of the GPF is prone to ethnical variability, with Wang et al. (1988) and Chrcanovic & Custudio (2010) supporting this claim with their studies, and Jaffar & Hamadah (2003) refuting this theory. Though the heterogeneity of the studies included in our meta-analysis might suggest that the diversity may be due to genetic factors, we should look to the subgroup analysis for the more probable answer. European studies show no heterogeneity at all in terms of the position of the GPF, even though they originate from different regions, whereas Indian studies (Saralaya & Nayak, 2007; D'Souza et al. 2012; Dave et al. 2013; Jotania et al. 2013; Sharma & Garud, 2013) conducted on the same population from one western state demonstrate significant heterogeneity. After analysing all of the studies, and the measurements they performed, we think that the causes of GPF position diversity are differences in the quality of measurements performed, as well as the way the GPF was related to the maxillary molars. That is why we would like to introduce a unified classification that would help to position the GPF in relation to the maxillary molars. The position of the GPF in relation to the maxillary molars should be assessed as described in the Materials and methods, and the possible GPF position would be one of the five listed below (Fig. 6).
Fig. 6.
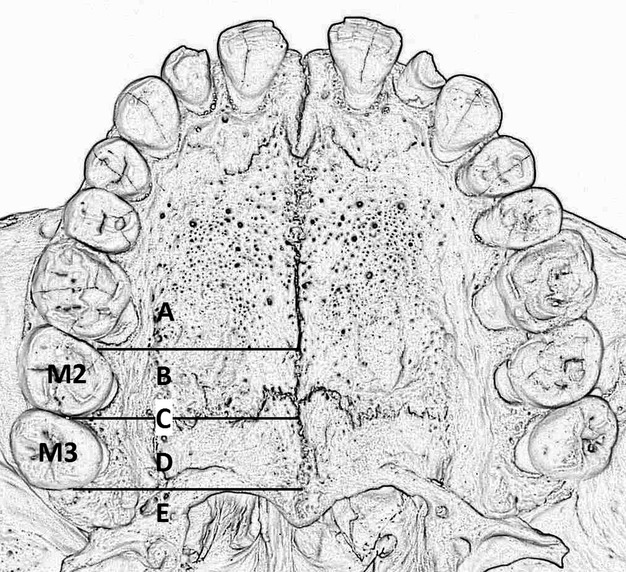
Classification of the GPF position in relation to the maxillary molars. (A) Mesial to the second maxillary molar (M2; mesial/anterior to the mesial surface of the M2); (B) opposite (medial) the M2 (from the mesial surface of the M2 to the distal surface of the M2); (C) between (interproximal) the M2 and the third maxillary molar (M3; at the level of the contact surface between the M2 and the M3); (D) opposite (medial) the M3 (from the mesial surface of the M3 to the distal surface of the M3); (E) distal to the M3 (distal/posterior to the distal surface of the M3).
(A) Mesial to the M2 (mesial/anterior to the mesial surface of the M2).
(B) Opposite (medial) the M2 (from the mesial surface of the M2 to the distal surface of the M2).
(C) Between (interproximal) the M2 and the M3 (at the level of the contact surface between the M2 and the M3).
(D) Opposite (medial) the M3 (from the mesial surface of the M3 to the distal surface of the M3).
(E) Distal to the M3 (distal/posterior to the distal surface of the M3).
An additional aspect to consider when referencing the GPF to the maxillary molars is the shape of the GPF. In most populations the GPF is elongated (Langenegger et al. 1983; Ajmani, 1994; Klosek & Rungruang, 2009) rather than oval in shape. It is important to measure the relation of the GPF centre to the maxillary molars, rather than one of its borders. To assist researchers in designing future studies, a detailed description of how to determine the centre of the GPF has been given in the Materials and methods.
GPF–MMS, GPF–PBHP, GPF–PH and MMS–IF–GPF angle measurements
The GPF–MMS and GPF–PBHP measurement values found in this study demonstrate rather significant inter-population variability (Table 3). Slavkin et al. (1966) stated that GPF location variability may occur due to sutural growth between the maxilla and the palatine bone. We also have to bear in mind that the antero-posterior dimension of the palate increases with the eruption of the posterior teeth.
Hawkins & Isen (1998) have described the location of the GPF to be along a straight line from the tip of the PH to the ipsilateral cingulum of the lateral incisor (Fig. 1). The PH is palpable on the postero-lateral side of the soft palate, about 2 mm posteriorly from the pterygomaxillary suture (Nimigean et al. 2013). Thus, we consider the tip of the PH an accurate reference point, located consistently throughout different studies about 12 mm in a straight line from the GPF (Table 3).
The MMS–IF–GPF angle values found in our study differ from those found by Chrcanovic & Custudio (2010) (right = 22.12 °; left = 23.30 °) and Saralaya & Nayak (2007) (right = 21.1 °; left = 21.2 °). Though statistically significant differences were found between the right and left angles, these discrepancies still remain clinically insignificant. Taking into account the ease with which the MMS can be located, the knowledge of the mean value of the MMS–IF–GPF angle will help physicians determine the angle at which the needle should be introduced into the GPF.
Locating the GPF in edentulous patients
When palpating for the GPF in edentulous patients, we would suggest triangulating the position of the GPF using the AR, MMS and the PNS, as they are constant and easily identifiable. The tip of the PH can also be added as an extra reference point. Though the posterior margin of the hard palate is clinically visible due to a narrow mucous membranous band of lighter colour (Nimigean et al. 2013) corresponding to it, we do not recommend using it as a reference point, as it is not clinically practical. The differences in the GPF–PBHP distance can be explained by growth at the level of the transverse palatine suture, and by the fact that palate length increases anteriorly from this suture after lateral teeth eruption. At the same time, growth is significantly reduced posteriorly from this line (Nimigean et al. 2013). Another explanation lies in the fact that some authors refer the GPF to the lateral-most aspect of the PBHP (Jaffar & Hamadah, 2003), while others to the greatest concavity of the PBHP (Westmoreland & Blanton, 1982; Ajmani, 1994).
When clinically utilizing all of the mentioned reference points and measurements given, the needle traverses the shortest possible route before administering the anaesthetic. The physician also avoids the risk of creating a haematoma resulting from pterygoid plexus vein puncture, as well as the possibility of anaesthetic deposition into the fat pad (Yamamoto et al. 2004).
Opening direction of the GPF
The inter-study comparison of the opening direction of the GPF (Table 4) proved to be difficult, as authors used different estimation methods. That is why we cannot agree with the statement of Wang et al. (1988) that the discrepancies are due to racial variations. The only sure way to determine the opening direction of the GPF is to base on horizontal and sagittal CT scans. Assessments utilizing dry skulls and needles only provide a very rough estimate of the GPF opening direction. Further CT-based studies are needed to accurately assess the opening direction of the GPF in different populations. In our study, vertical GPF openings were rather rare; however, their existence may explain the occasional clinical difficulty encountered while attempting to insert the needle point into the GPF and the pterygopalatine canal. Additionally, according to Slavkin et al. (1966), the frequency of anatomical obstruction of the needle in the GPC increases with age.
Posterior palatine crest of the GPF and the LPF
The presence of the posterior palatine crest is highly variable between studies (Table 4). This is where palatal tendons and muscles attach (Hassanali & Mwaniki, 1984). The posterior palatine crest, if present, may act as a natural barrier preventing the needle from venturing into the nasopharynx (Malamed & Trieger, 1983). Jaffar & Hamadah (2003) consider the presence of the posterior palatine crest important in prosthetic dentistry, but taking into account the thickness of the mucosa covering the GPF and the resulting minimal risk of developing pressure lesions in patients with removable dental prostheses, we think this finding is of no clinical significance.
We did not find any literature data suggesting that the number of LPFs may have clinical meaning. However, the absence of LPF, as it was found in this and several other studies (Table 4), may cause the lesser palatine nerve to exit through the GPF, and thus be prone to anaesthesia when blocking the greater palatine nerve. On the other hand, a high number of LPFs may lead to the formation of a single large LPF, as Jaffar & Hamadah (2003) have found. Such an anatomical variation may lead to mistaking the LPF for the GPF, and thus anaesthetizing the lesser palatine nerve, leading to a gagging sensation in the soft palate (Hassanali & Mwaniki, 1984).
Gender- and side-related differences
Male skulls are generally larger and more massive than female ones (Teixeira et al. 2010). Analysis of the obtained results has shown that most of the GPF-related measurements significantly differ between sexes (Table 1). This shows that the position of the GPF in relation to other anatomical landmarks could be used in forensic examination to identify a person's sex. Our study has shown that the GPF–MMS and the GPF–IF distances differ between sides. This stands in agreement with the study of Teixeira et al. (2010), but disagrees with some of the other studies included in the systematic review (Jaffar & Hamadah, 2003; Methathrathip et al. 2005; Saralaya & Nayak, 2007). These differences might be the result of palatal development, which is dependent on the function of several ossification centres (Slavkin et al. 1966).
Strong sides, limitations and novelty of the current study
The strong sides of the present project included the largest study sample size and number of individual measurements performed of all the studies published up-to-date on the subject of GPF position. The CT scan vs. traditional dry skull measurements comparison, as well as random measurement verification, were further factors guaranteeing high precision of the results. The use of CT scans allowed to gather data on the patients’ sex and age. Finally, the systematic review was performed according to PRISMA guidelines, and the subsequent meta-analysis of observational studies was the very first regarding GPF position, and created a unified, clinically useful review of all pertinent studies.
Several limitations of the present study should also be mentioned. Most of the included studies had small study groups. Heterogeneity among studies may be another limitation of our meta-analysis; however, we applied a random-effects model that takes possible heterogeneity into consideration (Xia et al. 2014). Exclusion of conference abstracts and non-English language studies may have led to publication bias; however, the performed statistical tests showed no bias was present in the performed meta-analysis. Despite these limitations, meta-analysis is a cost-effective and reasonable approach to evaluating sporadic, inconsistent and small sample size studies (Xu et al. 2014).
The novelty of this study stems from its design – it is the first systematic review and meta-analysis on the subject of GPF position, with added original data. Utilizing such an approach, the authors were able to present, for the first time, the pooled prevalence of GPF position in relation to the maxillary molars – both in the general world population, as well as divided by geographical regions. Following, the authors developed a clear, concise, easy to use and most importantly unambiguous classification system of positioning the GPF in relation to the maxillary molars. This study is also the first to present a straightforward methodology of determining the centre of the GPF, thus allowing to conduct reliable and repetitive measurements in future studies regarding GPF anatomy. The pooled data presented in this manuscript will allow clinicians to adequately prepare before performing procedures using the GPC approach or in the vicinity of the GPF, regardless of the geographical region they are working in. Finally, this study presents evidence that GPF position may not be so prone to anatomical variability. The large discrepancies between certain studies originate rather from differences in measurement methodology. By implementing the above-mentioned classification and methodology, this study aims to prevent such discrepancies from occurring in future studies regarding GPF anatomy.
Conclusions
Concluding, a clear understanding of the location and anatomy of the GPF is needed in order to properly administer anaesthetic through this foramen during maxillofacial procedures. Using a systematic review and meta-analysis, we demonstrated that the GPF is most often located opposite the M3 in the majority of the world's populations. In Poles, the GPF is located approximately 11 mm in a straight line from the M3. The maxillary molars are the best landmarks for locating the GPF. In edentulous patients the most useful points for approximating the position of the GPF are the AR, MMS and the PNS. Taking into account the estimated location of the GPF, clinicians should remember that when harvesting a palatal mucosa graft, they should avoid crossing the distal surface of the M2. Additionally, this study introduces an easy and repeatable classification to reference the GPF to the maxillary molars. Further CT-based studies are needed to estimate the opening direction of the GPF in different populations.
Acknowledgments
The authors wish to thank Mr Jacenty Urbaniak for his excellent photographs.
Conflict of interest statement
All authors declare that they have no conflict of interest or financial relationship to disclose.
Funding
This study has been funded using Jagiellonian University statutory funds. Krzysztof A. Tomaszewski received a scholarship to prepare his PhD thesis from the National Science Center, Poland under award number DEC-2013/08/T/NZ5/00020.
Author contributions
Design and planning of the study: IMT, MN, JAW. Data collection (measuring the samples): IMT, KAT, EKK, IZP, MN. Data interpretation: IMT, KAT (statistics and radiological interpretation); AU (radiological interpretation). Bibliographic search: IMT, JAW. Drafting and revising the manuscript: IMT. Obtaining funding and critical revision of the manuscript: MN, JAW. All authors have read and approved the final version of the manuscript. All co-authors confirm the above-mentioned contributions and consent to the fact that this study is a part of Iwona M. Tomaszewska's PhD thesis. The co-authors confirm that Iwona M. Tomaszewska has contributed significantly (70% in total) to every part of this study, as stated above.
References
- Ajmani ML. Anatomical variation in position of the greater palatine foramen in the adult human skull. J Anat. 1994;184:635–637. [PMC free article] [PubMed] [Google Scholar]
- Aterkar S, Rawal PM, Kumar P. Position of greater palatine foramen in adults. J Anat Soc India. 1995;44:126–133. [Google Scholar]
- Bell G. Oro-antral fistulae and fractured tuberosities. Br Dent J. 2011;211:119–123. doi: 10.1038/sj.bdj.2011.620. [DOI] [PubMed] [Google Scholar]
- Bessho H, Shibukawa Y, Shintani M, et al. Localization of palatal area in human somatosensory cortex. J Dent Res. 2007;86:265–270. doi: 10.1177/154405910708600313. [DOI] [PubMed] [Google Scholar]
- Chrcanovic BR, Custódio AL. Anatomical variation in the position of the greater palatine foramen. J Oral Sci. 2010;52:109–113. doi: 10.2334/josnusd.52.109. [DOI] [PubMed] [Google Scholar]
- Das S, Kim D, Cannon TY, et al. High-resolution computed tomography analysis of the greater palatine canal. Am J Rhinol. 2006;20:603–608. doi: 10.2500/ajr.2006.20.2949. [DOI] [PubMed] [Google Scholar]
- Dave MR, Yagain VK, Anadkat S. A study of the anatomical variations in the position of the greater palatine foramen in adult human skulls and its clinical significance. Int J Morphol. 2013;31:578–583. [Google Scholar]
- Douglas R, Wormald PJ. Pterygopalatine fossa infiltration through the greater palatine foramen: where to bend the needle. Laryngoscope. 2006;116:1255–1257. doi: 10.1097/01.mlg.0000226005.43817.a2. [DOI] [PubMed] [Google Scholar]
- D'Souza AS, Mamatha H, Nayak J. Morphometric analysis of hard palate in south Indian skulls. Biomed Res. 2012;23:173–175. [Google Scholar]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JH, Hasso DG, Yeh CY, et al. The accuracy of identifying the greater palatine neurovascular bundle: a cadaver study. J Periodontol. 2011;82:1000–1006. doi: 10.1902/jop.2011.100619. [DOI] [PubMed] [Google Scholar]
- Hassanali J, Mwaniki D. Palatal analysis and osteology of the hard palate of the Kenyan African skulls. Anat Rec. 1984;209:273–280. doi: 10.1002/ar.1092090213. [DOI] [PubMed] [Google Scholar]
- Hawkins JM, Isen D. Maxillary nerve block: the pterygopalitine canal approach. J Calif Dent Assoc. 1998;26:658–664. [PubMed] [Google Scholar]
- Howard-Swirzinski K, Edwards PC, Saini TS, et al. Length and geometric patterns of the greater palatine canal observed in cone beam computed tomography. Int J Dent. 2010 doi: 10.1155/2010/292753. doi: 10.1155/2010/292753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Meth. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Hwang SH, Seo JH, Joo YH, et al. An anatomic study using three-dimensional reconstruction for pterygopalatine fossa infiltration via the greater palatine canal. Clin Anat. 2011;24:576–582. doi: 10.1002/ca.21134. [DOI] [PubMed] [Google Scholar]
- Ikuta CR, Cardoso CL, Ferreira-Júnior O, et al. Position of the greater palatine foramen: an anatomical study through cone beam computed tomography images. Surg Radiol Anat. 2013;35:837–842. doi: 10.1007/s00276-013-1151-z. [DOI] [PubMed] [Google Scholar]
- Jaffar AA, Hamadah HJ. An analysis of the position of the greater palatine foramen. J Basic Med Sci. 2003;3:24–32. [Google Scholar]
- Jotania B, Patel SV, Patel SM, et al. Morphometric analysis of hard palate. Int J Res Med. 2013;2:72–75. [Google Scholar]
- Klosek SK, Rungruang T. Anatomical study of the greater palatine artery and related structures of the palatal vault: considerations for palate as the subepithelial connective tissue graft donor site. Surg Radiol Anat. 2009;31:245–250. doi: 10.1007/s00276-008-0432-4. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sharma A, Singh P. Assessment of the relative location of greater palatine foramen in adult Indian skulls: consideration for maxillary nerve block. Eur J Anat. 2011;15:150–154. [Google Scholar]
- Langenegger JJ, Lownie JF, Cleaton-Jones PE. The relationship of the greater palatine foramen to the molar teeth and pterygoid hamulus in human skulls. J Dent. 1983;11:249–256. doi: 10.1016/0300-5712(83)90197-5. [DOI] [PubMed] [Google Scholar]
- Lee SP, Paik KS, Kim MK. Variations of the prominences of the bony palate and their relationship to complete dentures in Korean skulls. Clin Anat. 2001;14:324–329. doi: 10.1002/ca.1059. [DOI] [PubMed] [Google Scholar]
- Lopes PTC, Santos AMPV, Pereira GAM, et al. Morphometric analysis of the greater palatine foramen in dry Southern Brazilian adult skulls. Int J Morphol. 2011;29:420–423. [Google Scholar]
- Malamed SF, Trieger N. Intraoral maxillary nerve block: an anatomical and clinical study. Anesth Prog. 1983;30:44–48. [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y. Location of the dental foramina in human skulls from statistical observations. Int J Orthod Oral Surg Radiogr. 1927;13:299–305. [Google Scholar]
- Mercuri LG. Intraoral second division nerve block. Oral Surg Oral Med Oral Pathol. 1979;47:109–113. doi: 10.1016/0030-4220(79)90161-0. [DOI] [PubMed] [Google Scholar]
- Methathrathip D, Apinhasmit W, Chompoopong S, et al. Anatomy of greater palatine foramen and canal and pterygopalatine fossa in Thais: considerations for maxillary nerve block. Surg Radiol Anat. 2005;27:511–516. doi: 10.1007/s00276-005-0016-5. [DOI] [PubMed] [Google Scholar]
- Nimigean V, Nimigean VR, Buţincu L, et al. Anatomical and clinical considerations regarding the greater palatine foramen. Rom J Morphol Embryol. 2013;54:779–783. [PubMed] [Google Scholar]
- Oluigbo CO, Makonnen G, Narouze S, et al. Sphenopalatine ganglion interventions: technical aspects and application. Prog Neurol Surg. 2011;24:171–179. doi: 10.1159/000323049. [DOI] [PubMed] [Google Scholar]
- Osunwoke EA, Amah-Tariah FS, Bob-Manuel IF, et al. A study of the palatine formane in dry human skulls in south-south Nigeria. Scientia Africana. 2011;10:98–101. [Google Scholar]
- Piagkou M, Xanthos T, Anagnostopoulou S, et al. Anatomical variation and morphology in the position of the palatine foramina in adult human skulls from Greece. J Craniomaxillofac Surg. 2012;40:e206–e210. doi: 10.1016/j.jcms.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Rodella LF, Buffoli B, Labanca M, et al. A review of the mandibular and maxillary nerve supplies and their clinical relevance. Arch Oral Biol. 2012;57:323–334. doi: 10.1016/j.archoralbio.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Romanes GJ. Cunningham's Textbook of Anatomy. 12th edn. New York: Oxford University Press; 1981. p. 116. [Google Scholar]
- Saralaya V, Nayak SR. The relative position of the greater palatine foramen in dry Indian skulls. Singapore Med J. 2007;48:1143–1146. [PubMed] [Google Scholar]
- Shane SME. Principles of Sedation, Local and General Anesthesia in Dentistry. 1st edn. Illinois: The Charles C Thomas Company; 1975. p. 173. [Google Scholar]
- Sharma NA, Garud RS. Greater palatine foramen–key to successful hemimaxillary anaesthesia: a morphometric study and report of a rare aberration. Singapore Med J. 2013;54:152–159. doi: 10.11622/smedj.2013052. [DOI] [PubMed] [Google Scholar]
- Slavkin HC, Canter MR, Canter SR. An anatomic study of the pterygomaxillary region in the craniums of infants and children. Oral Surg. 1966;21:225–235. doi: 10.1016/0030-4220(66)90248-9. [DOI] [PubMed] [Google Scholar]
- Sujatha N, Manjunath KY, Balasubramanyam V. Variations of the location of the greater palatine foramina in dry human skulls. Indian J Dent Res. 2005;16:99–102. [PubMed] [Google Scholar]
- Teixeira CS, Souza VR, Maques CP, et al. Topography of the greater palatine foramen in macerated skulls. J Morphol Sci. 2010;27:88–92. [Google Scholar]
- Urbano ES, Melo KA, Costa ST. Morphologic study of the greater palatine canal. J Morphol Sci. 2010;27:102–104. [Google Scholar]
- Wang TM, Kuo KJ, Shih C, et al. Assessment of the relative locations of the greater palatine foramen in adult Chinese skulls. Acta Anat (Basel) 1988;132:182–186. doi: 10.1159/000146572. [DOI] [PubMed] [Google Scholar]
- Westmoreland EE, Blanton PL. An analysis of the variations in position of the greater palatine foramen in the adult human skull. Anat Rec. 1982;204:383–388. doi: 10.1002/ar.1092040412. [DOI] [PubMed] [Google Scholar]
- Wong JD, Sved AM. Maxillary nerve block anaesthesia via the greater palatine canal: a modified technique and case reports. Aust Dent J. 1991;36:15–21. doi: 10.1111/j.1834-7819.1991.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Xia H, Wang X-J, Zhous Q, et al. Efficacy and safety of talc pleurodesis for malignant pleural effusion: a meta-analysis. PLoS ONE. 2014;9:e87060. doi: 10.1371/journal.pone.0087060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Guan J, Yi H, et al. A systematic review and meta-analysis of the association between serotonergic gene polymorphisms and obstructive sleep apnea syndrome. PLoS ONE. 2014;9:e86460. doi: 10.1371/journal.pone.0086460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Curtin HD, Suwansa-ard P, et al. Identification of juxtaforaminal fat pads of the second division of the trigeminal pathway on MRI and CT. Am J Roentgenol. 2004;182:385–392. doi: 10.2214/ajr.182.2.1820385. [DOI] [PubMed] [Google Scholar]


