Abstract
The thoracolumbar fascia (TLF) consists of aponeurotic and fascial layers that interweave the paraspinal and abdominal muscles into a complex matrix stabilizing the lumbosacral spine. To better understand low back pain, it is essential to appreciate how these muscles cooperate to influence lumbopelvic stability. This study tested the following hypotheses: (i) pressure within the TLF's paraspinal muscular compartment (PMC) alters load transfer between the TLF's posterior and middle layers (PLF and MLF); and (ii) with increased tension of the common tendon of the transversus abdominis (CTrA) and internal oblique muscles and incremental PMC pressure, fascial tension is primarily transferred to the PLF. In cadaveric axial sections, paraspinal muscles were replaced with inflatable tubes to simulate paraspinal muscle contraction. At each inflation increment, tension was created in the CTrA to simulate contraction of the deep abdominal muscles. Fluoroscopic images and load cells captured changes in the size, shape and tension of the PMC due to inflation, with and without tension to the CTrA. In the absence of PMC pressure, increasing tension on the CTrA resulted in anterior and lateral movement of the PMC. PMC inflation in the absence of tension to the CTrA resulted in a small increase in the PMC perimeter and a larger posterior displacement. Combining PMC inflation and tension to the CTrA resulted in an incremental increase in PLF tension without significantly altering tension in the MLF. Paraspinal muscle contraction leads to posterior displacement of the PLF. When expansion is combined with abdominal muscle contraction, the CTrA and internal oblique transfers tension almost exclusively to the PLF, thereby girdling the paraspinal muscles. The lateral border of the PMC is restrained from displacement to maintain integrity. Posterior movement of the PMC represents an increase of the PLF extension moment arm. Dysfunctional paraspinal muscles would reduce the posterior displacement of the PLF and increase the compliance of the lateral border. The resulting change in PMC geometry could diminish any effects of increased tension of the CTrA. This study reveals a co-dependent mechanism involving balanced tension between deep abdominal and lumbar spinal muscles, which are linked through the aponeurotic components of the TLF. This implies the existence of a point of equal tension between the paraspinal muscles and the transversus abdominis and internal oblique muscles, acting through the CTrA.
Keywords: abdominal muscles, erector spinae, middle lamina, multifidus, posterior lamina, spine, thoracolumbar fascia, transversus abdominis
Introduction
Stabilization and movement of the lumbosacral spine is contingent on the complex interaction between muscles, ligaments and fascia surrounding the torso. The thoracolumbar fascia (TLF) represents a girdling structure consisting of several aponeurotic and fascial layers that separates the paraspinal muscles from the muscles of the posterior abdominal wall. Understanding the complex function of the TLF and its associated fascial compartments is critical to anatomical and biomechanical analysis, and implementation of effective treatment in patients with lumbopelvic pain.
The TLF envelops the back muscles from the sacral region, through the thoracic region, and plays an important role in posture, load transfer and respiration (Gracovetsky et al. 1981, 1985; Bogduk & MacIntosh, 1984; Carr et al. 1985; Tesh et al. 1987; Vleeming et al. 1995; Barker & Briggs, 1999, 2007; Hodges et al. 2003; Barker et al. 2004, 2006; Schuenke et al. 2012; Willard et al. 2012). The TLF is comprised of three layers of which both the fibrous posterior layer (PLF) and the middle layer (MLF) have a significant biomechanical function (Gracovetsky et al. 1981, 1985; Bogduk & MacIntosh, 1984; Carr et al. 1985; Tesh et al. 1987; Hukins et al. 1990; Vleeming et al. 1995; Barker & Briggs, 1999; Barker et al. 2004, 2006; Urquhart & Hodges, 2007; Gatton et al. 2010; Schuenke et al. 2012; Willard et al. 2012). The delicate anterior layer merely represents the thin transversalis fascia lining the deep surface of transversus abdominus and the quadratus lumborum muscles (Willard et al. 2012).
Superficial lamina of the PLF
The PLF is a composite of superficial and deep laminae of connective tissue. The superficial lamina derives from the aponeurosis of the latissimus dorsi (LD; Bogduk & MacIntosh, 1984; Tesh et al. 1987; Vleeming et al. 1995; Barker & Briggs, 1999; Gatton et al. 2010; Willard et al. 2012) and is part of a collective sheath of fascia, bridging from the first rib down to the xiphoid process anteriorly, and from the cranial base to the sacrum posteriorly (Stecco et al. 2009; Willard et al. 2012). This fascial sheath contains muscles such as the pectoralis major and minor, rhomboid major and minor, trapezius, serratus anterior (Barker et al. 2004; Willard et al. 2012) and the expansive LD, reaching far caudally and forming the superficial lamina of the PLF. In addition, the aponeurosis of this muscle partially crosses the midline to connect to the fascia of the contralateral gluteus maximus muscle (GM; Bogduk & MacIntosh, 1984; Vleeming et al. 1995; Barker et al. 2004).
Deep lamina of the PLF
The deep lamina of the PLF extends from the spinous processes to the transverse processes, and is distinct from both the superficial lamina of the PLF and the middle layer of the MLF. Cranially, the deep lamina most likely begins on the occipital bone and extends caudally to its fusion with the superficial lamina over the sacrum. The lateral margin of the deep lamina of the TLF is located at the common intersection of the hypaxial (e.g. ventral trunk muscles) and epaxial (paraspinal) muscles (Willard et al. 2012). Several authors have studied the deep lamina of the PLF (Bogduk & MacIntosh, 1984; Tesh et al. 1987; Vleeming et al. 1995; Barker & Briggs, 1999). Bogduk & MacIntosh (1984) described the deep lamina as having alternating bands of dense fibers, which they termed accessory ligaments and proposed that the deep lamina stems most likely from the crossed fibers of the aponeurosis tendon of the LD. Vleeming et al. (1995) and Barker & Briggs (1999) describe the same fascial bands; however, typically characterizing the deep lamina of the PLF as being mainly formed by the aponeurosis tendon of the serratus posterior inferior muscle.
The arrangement of a fascial compartment in the lumbar spine, created by a fascial sheath encapsulating the paraspinal muscles, has been noted or illustrated by numerous authors (Spalteholz, 1923; Schaeffer, 1953; Hollinshead, 1969; Grant, 1972; Farfan, 1973; Gracovetsky et al. 1977; Bogduk & MacIntosh, 1984; Clemente, 1985; Tesh et al. 1987; Vleeming et al. 1995; Barker et al. 2004; Gatton et al. 2010). Standring (2008) described a designated osteofascial compartment for the paraspinal muscles. Many authors cited above utilize the deep lamina of the PLF to describe the inner posterior wall of this encapsulating sheath and the MLF to describe the anterior wall. However, most of these descriptions are based on the assumption that the deep lamina of the PLF is a longitudinally oriented, flat fascial sheath (Willard et al. 2012).
The lateral border of the deep lamina contributes to the lateral raphe (Schaeffer, 1953; Bogduk & MacIntosh, 1984; Tesh et al. 1987; Vleeming et al. 1995; Barker et al. 2004). Spalteholz (1923) describes the lateral border as curving around the paraspinal muscles to join anteriorly to the MLF. Tesh et al. (1987) describe the deep lamina as encircling the paraspinal muscles. Likewise, Carr et al. (1985) measured intra-compartmental TLF pressure and concluded that sustained pressure within the TLF is only possible if the paraspinal muscles are enclosed by a continuous fascial sheath.
A recent study has examined the extent of the deep lamina and confirmed that it forms a sheath surrounding the paraspinal muscles, which has been termed the paraspinal retinacular sheath (PRS). This sheath represents the innermost part of the deep lamina of the PLF (Schuenke et al. 2012), and is attached to the spinous process posteriorly and the transverse process anteriorly. Laterally, the PRS forms a junction with the common transversus tendon (CTrA), deriving from the inner oblique muscle below the transverse process of L3 (Bogduk & MacIntosh, 1984; Barker et al. 2004; Urquhart & Hodges, 2005) but mainly from the transversus abdominus. The CTrA forms a strong anchor or seam for the transmission of force between the abdominal muscles anteriorly and the paraspinal muscles posteriorly (Fig. 1).
Fig. 1.
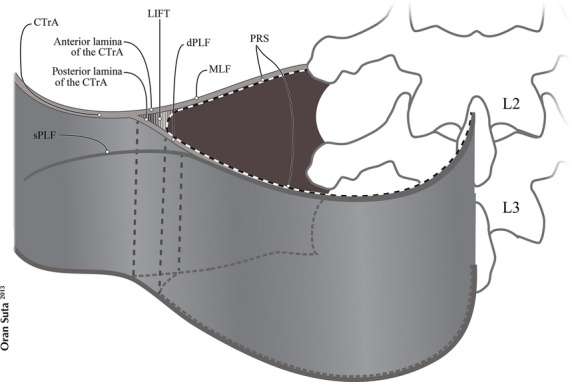
Modified with permission from fig. 4, Schuenke et al. (2012). A schematic and simplified view of the bifurcation of the common transversus tendon (CTrA) and the paraspinal retinacular sheath (PRS), creating the lumbar interfascial triangle (LIFT). The CTrA bifurcates into anterior and posterior laminae. The anterior lamina contributes to the middle layer of the thoracolumbar fascia (MLF). The posterior lamina contributes to the deep lamina of the posterior layer of the thoracolumbar fascia (PLF). The junction of the CTrA with the PRS creates the LIFT. dPLF, deep lamina of PLF; sPLF, superficial lamina of PLF.
The CTrA and the PRS enclose a triangular-shaped, fat-filled space, the lateral interfascial triangle (LIFT), which derives from the bifurcation of the anterior and posterior lamina of the CTrA, and the portion of PRS that spans between them. This LIFT may act as a fulcrum distributing laterally mediated tension, to balance different viscoelastic moduli, along either the MLF or PLF. The presence of the LIFT explains why an externally ridged-union of dense connective tissue is formed, called the lateral raphe (Bogduk & MacIntosh, 1984; Schuenke et al. 2012).
Three studies specifically analyzed lateral force transfer through the CTrA to the TLF. Tesh et al. (1987) were the first to simulate intra-compartmental pressure (ICP) within the paraspinal compartment. They replaced the paraspinal muscles with foam dowels to create a static tension within the TLF. Their study focused primarily on tension in the PLF. Barker et al. (2004) studied lumbar neutral zone movement, and applied both static and cyclical loading to lumbar segments. They analyzed the effect of CTrA pull to the TLF, to examine the significance of the transverse abdominus and internal oblique muscles to segmental stability of the spine. The authors simulated tension through the CTrA, and found tension increasing primarily through the MLF as the mechanical pull to the CTrA was directed transversely (parallel to the MLF), without simulation of paraspinal contraction of the paraspinal muscle compartment (PMC). Hodges et al. (2003), in a porcine study, analyzed pull through the transverse abdominus muscle, exclusively focusing on the force transfer through the MLF as the PLF was cut. The study concluded that tensing the fascia (MLF) produces an extension moment.
To better understand low back and pelvic girdle pain, it is essential to develop a detailed understanding of how abdominal and spinal muscles cooperate to influence lumbopelvic motion and postural stability. Specifically, how activation of the middle parts of the transverse abdominus and internal oblique muscles influence force transfer to the PLF and MLF. The aim of the present study is to analyze the effect of incrementally raising inflation within the PMC (simulating paraspinal contraction), without and combined with simultaneous CTrA tension (simulating transverse abdominus/internal oblique contraction), on force transfer through the PLF and MLF.
To the authors' knowledge, this is the first study in which incremental ICP of the PMC is examined. The results could provide a better understanding of the relationship between dysfunction of the paraspinal muscles in patients with low back pain (LBP), in combination with force transfer from the deep abdominal muscles via the CTrA to the outer perimeter of the PMC, influencing lumbar stability.
Materials and methods
Specimen characteristics and preparation
Seven embalmed (70% isopropyl alcohol, 2% phenol, 1% formaldehyde) human specimens (three male, four female; 69.9 ± 17.3 years) were studied. On one specimen, the skin and superficial fascia had been dissected before axial sectioning. In comparison to the other six specimens, there were no statistically significant differences, and the data of seven specimens were pooled.
A total of 14 axial slabs were sectioned (approximately 2 cm thickness) using an industrial band saw (Hobart 5801; Troy, OH, USA). Prior to sectioning, the lumbar region of each cadaver was assessed with a C-arm fluoroscope (Exposcope 7000; Ziehm Imaging, Orlando, FL, USA) to identify planes that contain transverse processes bilaterally. The transverse processes were marked by the transverse placement of a needle, and axial sections made between the needle positions. Left and right PMCs of all seven specimens were analyzed individually, resulting in N = 14. None of the samples revealed evidence of lumbosacral pathology or surgical procedures in the lumbar region. Conducting the measurements at the level of the transverse processes is essential, because the MLF loses its insertion at inter-transverse levels in order to create a passageway for the dorsal neurovasculature. Only axial sections through levels L2 and L3 were used in this study, because sections including L1 contained rib fragments. Similarly, sections through the L4 level were not included, because they contained portions of the iliac crest.
Objectives
-
To test the hypothesis that changes of ICP within the PMC (mimicking incremental contraction of paraspinal muscles) alters the load transfer between the PLF and MLF. In order to test this, the following took place.
The perimeter of the left and right PMC (from transverse process to spinous process) was measured at three stages of ICP ‘without’ tension to the CTrA.
Using the same pressure increments (as in 1A), the perpendicular straight-line distance ‘without’ CTrA tension was measured from the lateral tip of the transverse process to the posterior border of the PLF, to analyze posterior displacement of the PLF (Fig. 2).
-
To test the hypothesis that ‘with’ tension of the CTrA and incremental PMC pressure, the fascial tension is primarily transferred to the PLF, rather than the MLF. In order to test this, measurements similar to those described in 1A and 1B were repeated with 8.5 N tension being exerted bilaterally through the CTrA.
Load cells were used to measure unidirectional tension along anterior and posterior CTrA laminae ‘with’ CTrA tension (Fig. 3a). This was repeated under three incremental stages of TLF compartmental pressure.
Only one load cell was used in experiment 2A, to measure unidirectional tension for each anterior lamina and posterior lamina of the CTrA, analyzing load transfer, respectively, to the MLF and PLF, ‘during CTrA tension’ while incrementally inflating the PMCs (Fig. 3b). After finalizing the experiments (2A), it became obvious that the PLF became significantly distended. It is reasonable to expect that during each incremental inflation, the rubber tubes (simulating paraspinal contraction) impose a posteriorly-directed force on the PLF. However, for most experiments, the load cells were oriented to quantify force in the anterolateral direction ‘during CTrA tension’, to differentiate force transfer between the MLF and PLF. Subsequently, the load cell would not directly measure inflation-induced tension in the posterior direction because inflation has the biggest effect on the PLF, partially minimizing the force in the unidirectional load cell with each incremental inflation.
Fig. 2.
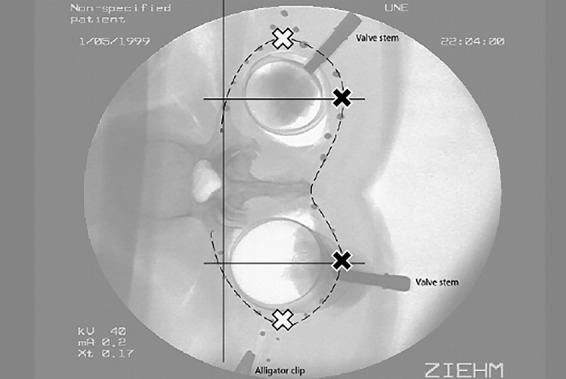
Analyzing posterior and lateral displacement of the borders of the TLF compartment ‘with incremental inflation’. Beads (black circles) were affixed to the PMC in order to track movement of individual points. Posterior displacement of the posterior border was measured on a perpendicular straight line from the lateral-most point of the transverse process to the posterior border of the PLF (Method 4; indicated by black crosses). This line was then used as a reference line for measuring medial-to-lateral displacement of the PMC (SLDlat). This was measured from the perpendicular straight line to the lateral-most point of the PMC (indicated by white crosses). These measurements were done with (Method 1a) and without (as shown) CTrA tension.
Fig. 3.
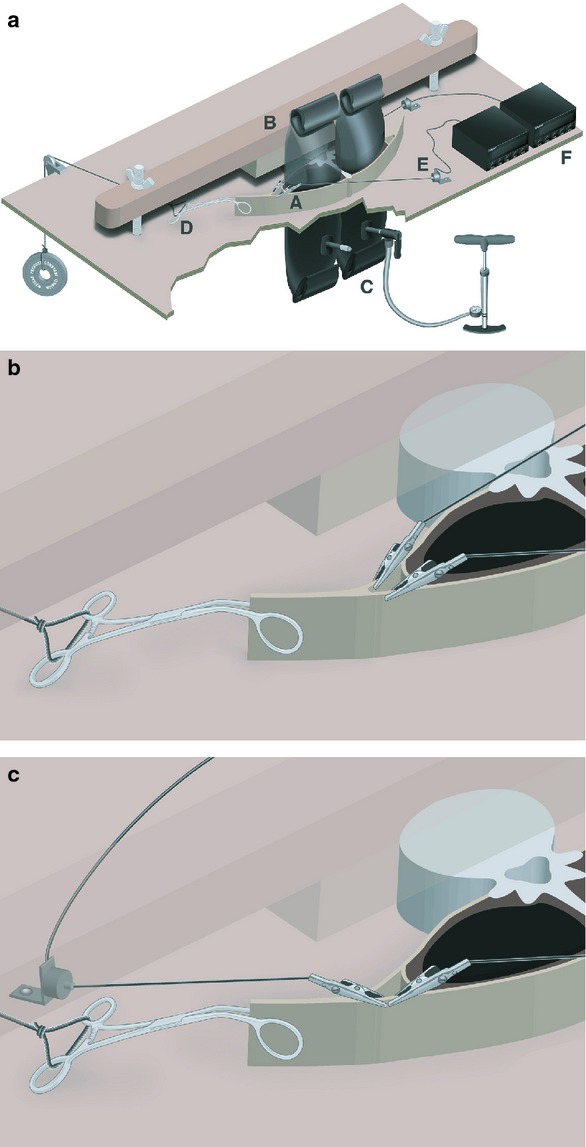
(a) Experimental apparatus design. Cadaveric slab (A) is placed on a wooden platform with holes to accommodate inflatable tubes (C) attached to a positive displacement pump (note: jagged cut-out is to demonstrate spatial context). To prevent vertebral rotation, a cross-bar (B) is placed across the vertebral body and clamped down using wingnuts on threaded bolts. A hemostatic clamp (D) attaches the common aponeurosis of the transversus abdominis and internal oblique muscles the CTrA to a constant load. Alligator clips attach the anterior and posterior laminae of the common aponeurosis to load cells (E) that connect to load cell meters with digital display (F). (b) Experimental apparatus design. Magnified view of alligator clip placement for differentiating MLF/PLF force transfer (Method 5). Alligator clips are attaching the anterior and posterior laminae of the common aponeurosis to load cells (not shown). Hemostatic clamp is attaching the common aponeurosis to a known load (not shown). For simplicity, the inflatable tubes are not shown in this image. (c) Experimental apparatus design. Magnified view of alligator clip placement for analyzing the effect of inflation on PLF force transfer (Method 6). Alligator clips are attached along the posterior laminae of the common aponeurosis in opposing directions. Hemostatic clamp is attaching the common aponeurosis to a known load (not shown). For simplicity, the inflatable tubes are not shown in this image.
Therefore, to quantify this inflation-induced, posteriorly-oriented force, an additional set of experiments (bidirectional tension measurements along the posterior CTrA lamina with CTrA tension) was conducted bilaterally on two axial slabs (measuring in total four compartments per inflation condition). One load cell was positioned as before along the PLF in the posteromedial direction, additionally a second load cell was positioned along the PLF in an anterolateral direction (Fig. 3c). The ‘CTrA was tensed’ with the same load of 8.5 N along the CTrA and the same incremental inflation stages as in experiment 2A.
Testing sequence
The following methods were used (details outlined below).
Simulate ‘tensioning of CTrA’ with paraspinal muscles intact followed by analysis of perimeter changes of the PMC.
Remove paraspinal muscles and insert a custom-made butyl inflation device with valves into the right and left PMCs (see Fig. 3a).
Inflate incrementally the PMC to simulate contraction of paraspinal muscles and concurrently simulate contraction of transverse abdominus/internal oblique by ‘tensing the CTrA’.
Analyze both the perimeter changes of the PMC, and measure the force differential between MLF and PLF with load cells.
Testing methods
Preventing vertebral displacement
Performed in all experiments
The vertebral body of the cadaveric slab was clamped to a customized baseboard, to prevent movement of the vertebra, but not to impede any soft tissue movement. Also, the board was designed in such a way to permit insertion of inflatable tubes through the PMC (Fig. 3a). A customized compression bar was tightened in place over the vertebral body thereby eliminating unwanted motion of the bony structures (Fig. 3a).
Marking the perimeter of the PMC
In order to track perimeter changes of the fascial compartments, copper beads (2–8 mm; Sigma-Aldrich, USA) were affixed (methyl 2-cyanoacrylate Loctite; Henkel, USA) at approximately 1.5-cm intervals along the anterior and posterior lamina of the CTrA and the PRS (Fig. 2). Care was taken to ensure that beads adhered only to fascia and not to adjacent muscles.
Method 1. Loading the CTrA
Method 1a
The left and right CTrA were pulled anterolaterally (mimicking the curved shape and direction of the transverse abdominus and internal oblique muscles of each individual specimen) to generate bilateral forces of 8.5 N. Two load cells (LCMFD-10N; Omega Engineering, Stamford, CT, USA) measured the generated tension. A tension of 8.5 N was selected, based on the work of Barker et al. (2004) who demonstrated that the CTrA in cadavers could strain at 10 N.
Method 1b
The CTrA was loaded with 8.5 N of tension anterolaterally, via self-locking hemostats, simulating the normal function of the CTrA. Tension load cells were either attached, respectively, to the anterior and posterior lamina of the CTrA (Fig. 3b), or both attached to the posterior lamina in opposite directions (described in objective 2B; Fig. 3c).
Method 2. Simulation of paraspinal muscle contraction
Paraspinal muscles were carefully removed so as not to damage the surrounding fascia. Custom-made inflatable butyl rubber tubes (uninflated diameter 2.54 cm, length 10 cm) were placed inside the right and left PMC. To simulate contraction of the paraspinal muscles, the tubes were inflated. Due to inter- and intra-specimen variation of paraspinal muscle size, a standard initial pressure could not be used. Instead, the first inflation (Inf1) was the minimum pressure required to hold the tube in the compartment. Subsequent inflations were set at 1.5-cm increments above the tube circumference of the initial inflation. All circumferential measurements were recorded using a soft, tailor's tape measure immediately above the superior surface of the axial section. The average intra-tube circumferences for Inf1, Inf2 and Inf3 were 12.04 cm, 13.54 cm, 15.04 cm, respectively. Intra-tube pressure was also measured using Vernier LabPro (Vernier Software and Technology, Beaverton, OR, USA). The average intra-tube pressures for Inf1, Inf2 and Inf3 were 79.1 mm Hg, 99.8 mm Hg and 106.5 mm Hg, respectively. A study of healthy young individuals showed that the mean submaximal muscle contraction pressure was 175 mm Hg, during isometric and concentric extension exercises (Styf, 1987). Compared with the present study, the average inflation pressure is 50% less.
Method 3. Measuring the perimeter of the TLF
The pre-tensed (neutral) position of the copper beads was imaged using a C-arm fluoroscope. During ‘tensioning the CTrA’ with 8.5 N, a second image was captured. In order to compare the positioning of the beads between the neutral and tensed CTrA, the opacity of the tensed image was reduced to 40%. The tensed image was superimposed to the neutral image using Adobe Illustrator (Adobe Systems, San Jose, CA, USA). For every incremental inflation, new pre-tensed and tensed images were captured and compared.
During superimposition, vertebral processes from each image were aligned. Subsequently, a curved line was drawn, connecting all of the beads for a given inflation condition to measure perimeter changes (NIH ImageJ software) around the PMC. There was no statistical difference between the right and left MLF, nor for the right and left PLF, for a given inflation condition. Therefore, lengths of the left and right MLF and PLF were pooled for each inflation condition.
Method 4. Analyzing posterior and lateral displacement of the PMC
The straight-line perpendicular distance from the lateral-most tip of the transverse process to the posterior part of the PLF in the x-plane (Fig. 2) was measured using ImageJ software, with MTrackJ plug-in (Meijering et al. 2012). The distance from the aforementioned straight line to the lateral-most point of the border of the PMC was also measured. These measurements were taken under the same inflation increments as described in Method 3, and performed both ‘without CTrA tension’ and with 8.5 N CTrA tension.
Method 5. Differentiating MLF/PLF force transfer resulting from CTrA tension
Tension load cells were attached to the anterior and posterior laminae of the CTrA, using alligator clips (Fig. 3b). The anterior lamina is continuous with the MLF, and the posterior lamina is continuous with the PLF. A reading of each load cell was recorded without CTrA tension (neutral). A load (8.5 N, after accounting for frictional and drag components) was then applied anterolaterally on to the CTrA, using self-locking hemostats, and was suspended on a pulley. Each step of incrementally pressurizing the PMC, with the inflatable tubes, was recorded with load cells, analyzing the relative force transfer between MLF and PLF. The differential in load transfer through the MLF and PLF of the CTrA is calculated by:
Method 6. Analyzing the effect of inflation on PLF force transfer
To quantify the subtractive effect of incremental inflation on specifically PLF tension, two axial slabs (measuring four compartments per inflation condition) were tested. One alligator clip connected to a load cell was positioned as before along the PLF in the posteromedial direction. Another alligator clip connected to a second load cell was positioned along the PLF in an anterolateral direction. The same CTrA load (8.5 N) and incremental inflations (as described in Method 2) were used (see Fig. 3c).
Results
Fascial perimeter ‘without’ CtrA tension
The perimeter of each MLF and PLF was measured (Method 3) with muscles intact and at each inflation increment (Method 2). There were no statistical differences in perimeter length between the right and left MLF, or the right and left PLF for any given inflation condition. Consequently, measurements of the right and left MLF were pooled and likewise for the PLF (Fig. 4). The perimeter length of the MLF did not change significantly with inflation (P = 0.78). In contrast, the length of PLF increased significantly with inflation (P = 0.046). Post hoc analyses revealed that the length of the PLF increases significantly between the muscles intact condition (before inflation) and Inf3 (P = 0.012). There was a trend towards significance between muscles intact and Inf2 (P = 0.051), and between Inf1 and Inf3 (P = 0.092). In the muscles intact condition and all three inflation increments, the perimeter of the PLF is significantly longer than the perimeter of the MLF (P = 0.001).
Fig. 4.
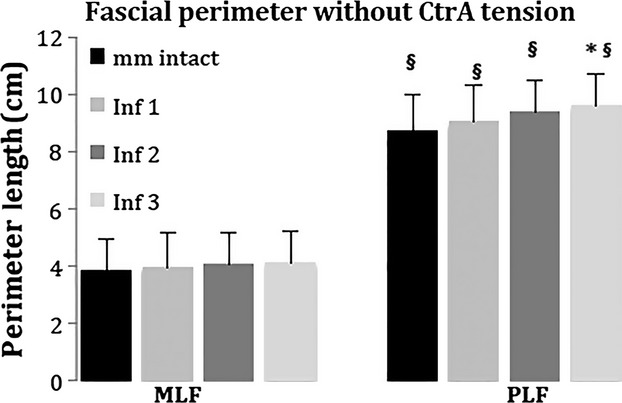
The length of fibrous middle layer (MLF) and fibrous posterior layer (PLF) at each increment of ICP ‘without CTrA tension’. There is statistical significance (*) between the muscles intact and Inf3 conditions, and (§) between MLF and PLF for a given ICP.
Analyzing posterior and lateral displacement of the PLF
Without CTrA tension
The perpendicular straight-line distance in the posterior direction (SLDpost) was measured from the lateral tip of the transverse process to the PLF (Method 4; see Fig. 2). The SLDpost increases with inflation (P = 0.0005). Post hoc analyses indicate that the SLDpost in the muscles intact condition is significantly shorter than the SLDpost in Inf1 (P = 0.0086), Inf2 (P = 0.0011) and Inf3 (P = 0.0001). The SLDpost does not significantly differ between the three inflation conditions (Inf1 vs. Inf2, P = 0.52; Inf1 vs. Inf3, P = 0.18; Inf2 vs. Inf3, P = 0.49).
The aforementioned line drawn perpendicular to the lateral tip of the transverse process (described above; see Fig. 2) was then used as a reference line from which to measure the straight-line lateral displacement (SLDlat) to the lateral-most point of the PMC. The SLDlat did not significantly differ between the muscle intact condition and any of the inflation increments (P = 0.68).
With CTrA tension
To determine the net effect of CTrA tension (Method 1a) and inflation (Method 2) of the PMC (i.e. co-contraction of transverse abdominus/internal oblique and paraspinal muscles), the perpendicular SLDpost of the baseline condition (Method 4; no CTrA tension, no PMC pressure) was compared with 8.5 N CTrA tension with incremental inflation. For example:
Predictably, when the CTrA is tensed with no pressure in the PMC, the PLF moves anteriorly (Fig. 5). However, with each incremental inflation the amount of anterior movement due to CTrA tension is significantly reduced (P = 0.0001). In fact, the anterior movement due to CTrA tension is negated by the inflation-induced posterior movement such that the net displacement of the PLF is in the posterior direction. The amount of CTrA-induced anterior movement of the PLF is also significantly reduced in inflations Inf2 (P = 0.024) and Inf3 (P = 0.0008), relative to Inf1. There was no significant difference between Inf2 and Inf3 (P = 0.32).
Fig. 5.
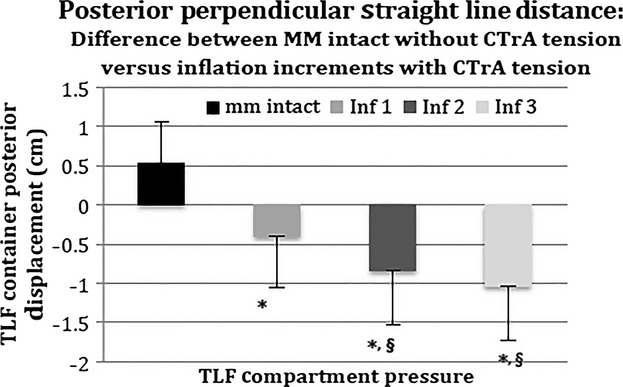
Straight-line distance from the tip of the transverse process to the PLF under different inflation increments (see also Fig. 2): comparison of baseline condition (mm intact, no CTrA tension) with 8.5 N CTrA tension and incremental inflation. A positive value indicates anterior displacement. A negative value indicates posterior displacement. *Significant difference from mm intact. §Significant difference from inf1. TLF, thoracolumbar fascia.
Similarly, to determine the net effect of CTrA tension and inflation of the PMC (i.e. co-contraction of transverse abdominus/internal oblique and paraspinal muscles), the SLDlat of the baseline condition (no CTrA tension, no PMC pressure) was compared with 8.5 N CTrA tension at each inflation increment (Fig. 6). With muscles intact (no PMC pressure), 8.5 N CTrA tension resulted in lateral movement of the SLDlat relative to muscles intact without CTrA tension. With inflation (pressure in PMC), the amount of lateral movement due to CTrA tension is significantly reduced (P = 0.047). In fact, the lateral movement due to CTrA tension is negated by the inflation-induced medial movement such that the net displacement of the TLF is in the medial and posterior directions. This supports the concept that co-contraction of the transverse abdominus/internal oblique and the paraspinal muscles transfer forces mainly through the PLF, hence posteriorly girdling the lumbar spine. Post hoc analyses indicated that the amount of CTrA-induced medial movement of the PLF is significantly reduced during inflations Inf2 (P = 0.03) and Inf3 (P = 0.0063), relative to mm intact. There was no significant difference between Inf1 and mm intact (P = 0.14). There were no significant differences in SLDlat between the three inflation increments.
Fig. 6.
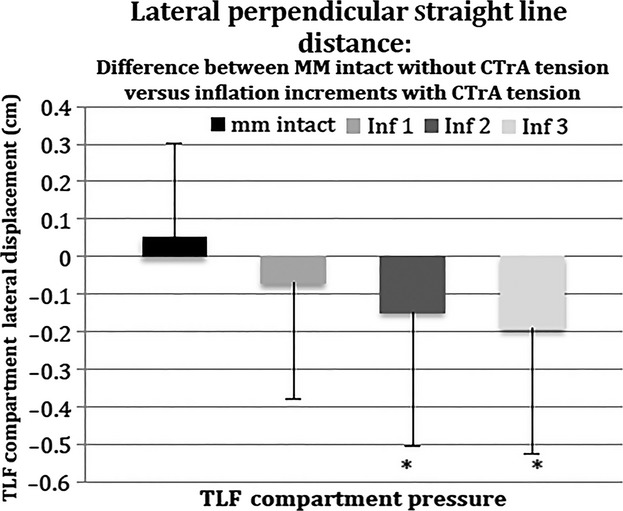
Perpendicular straight-line distance from the most lateral point of the PMC, projected to the line running from the tip of the transverse process to the PLF under different inflation increments (see also Fig. 2): comparison of baseline condition (mm intact, no CTrA tension) with 8.5 N CTrA tension and incremental inflation. A positive value indicates lateral displacement. A negative value indicates medial displacement. *Significantly different from mm intact. TLF, thoracolumbar fascia.
To determine whether a relationship exists between the inflation-induced posterior displacement vs. medial displacement of the walls of the PMC, a medial displacement-to-posterior displacement ratio was calculated for each inflation increment and CTrA tension. The ratios were 0.18, 0.181 and 0.185 for Inf1, Inf2 and Inf3, respectively. This indicates a consistent medial displacement of the lateral wall of the PMC of approximately 0.18 cm for each 1 cm of posterior wall displacement.
Common transversus tendon tension in the absence of a pressurized PMC (mm intact) results in an anterolateral displacement of the PMC (black bars in Figs 5 and 6; gray line in Fig. 7b). However, this CTrA tension-dependent anterolateral displacement is counteracted by incremental pressure of the PMC (e.g. simulating paraspinal muscle contraction), resulting in an overall posteromedial movement of the PMC (gray bars in Figs 5 and 6; dashed line in Fig. 7b). In this case, a theoretical point of equal tension between the paraspinal muscles and transverse abdominus and internal obliques pull through the CTrA is attained.
Fig. 7.
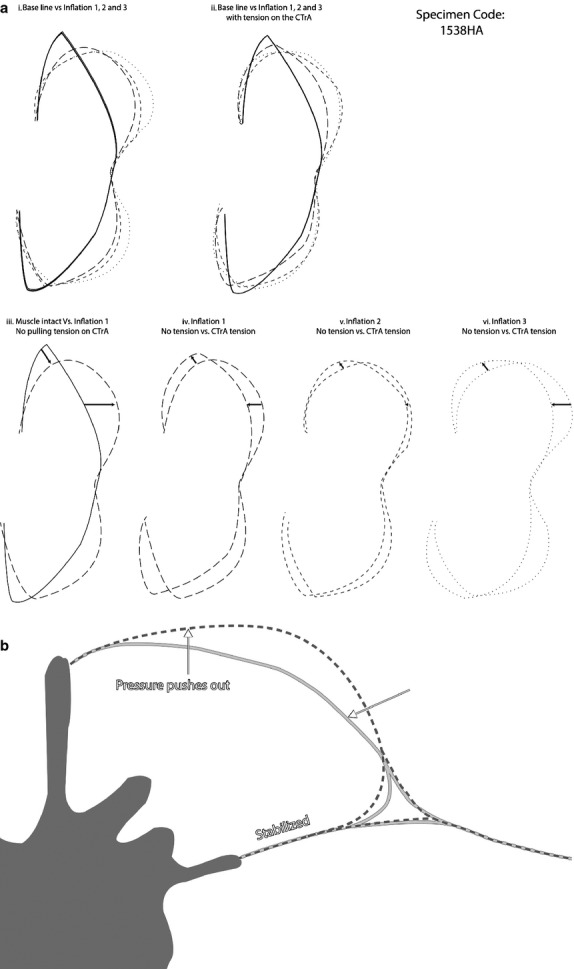
(a) Effect of inflation and tension on the PMC. Sample-specific example with comparisons of: (i) muscles intact (solid line) compared with inflation 1 (large dashed line), inflation 2 (small dashed line) and inflation 3 (dotted line) without tension in the common tendon of the transversus abdominis and internal oblique muscles (CTrA); (ii) muscles intact (solid line) compared with inflation 1 (large dashed line), inflation 2 (small dashed line) and inflation 3 (dotted line) with CTrA tension; (iii) muscles intact (solid line) to inflation 1 (large dashed line) without CTrA tension, arrows indicate the direction of movement that results from inflation; (iv) inflation 1 without and with CTrA tension, arrows indicate the direction of movement that results from CTrA tension; (v) inflation 2 without and with CTrA tension, arrows indicate the direction of movement that results from CTrA tension; (vi) inflation 3 without and with CTrA tension, arrows indicate the direction of movement that results from CTrA tension. (b) Effect of inflation and tension on the PMC. Composite drawing of the generalized effects of inflation on the PMC based on the mean (across all samples) displacement of beads from the muscles intact (gray line) to inflation 3 (dashed line). The PMC is pushed posteriorly and medially, as indicated by the arrows.
Differentiating MLF/PLF force transfer: unidirectional tension measurement along anterior and posterior CTrA laminae in the presence of tension of the CTrA and inflation of the PMC
The amount of force transmitted through MLF and PLF (Method 5) was measured while applying 8.5 N force through the CTrA (Method 1b) at each inflation increment (Method 2). At each level of inflation, a significantly greater proportion of the force was transmitted through the PLF, relative to the MLF (P = 0.0001; Fig. 8). The force transmission through the MLF did not differ significantly between each increment of inflation (P = 0.74). Conversely, force transmission through the PLF is elevated at Inf1 and then gradually declines with further incremental inflation (P = 0.013). Post hoc analyses indicate that force transmission through the PLF in Inf3 is significantly lower than Inf1 (P = 0.0068), which will be further elucidated in Fig. 9. There was also a trend toward significance between Inf1 and Inf2 (P = 0.087). There was no significance between Inf2 and Inf3 (P = 0.2). It should be noted that it was not possible to obtain this measurement in the muscles intact condition, because there was no space to place the alligator clips.
Fig. 8.
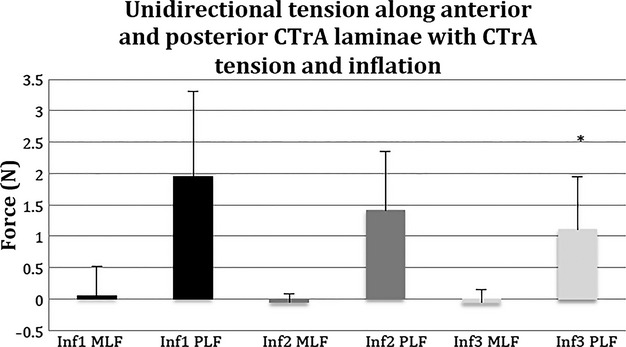
Unidirectional tension measurements along anterior and posterior CTrA laminae with CTrA tension and inflation. The amount of CTrA-mediated force directed through the fibrous posterior layer (PLF) appears to decrease with incremental PMC pressure, yet the amount of CTrA-mediated force directed through the fibrous middle layer (MLF) appears to be minimal and basically unaffected by incremental PMC pressure. *Indicated statistical significance between Inf1 and Inf3.
Fig. 9.
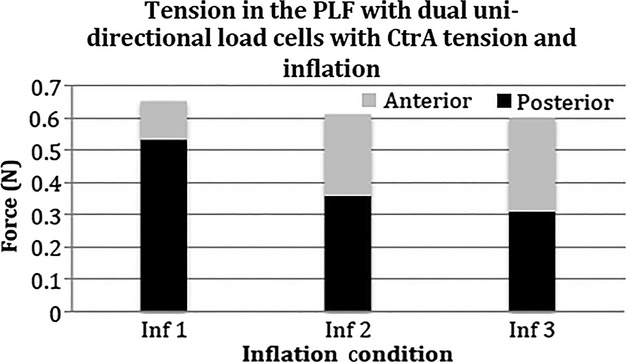
Effects of inflation and tension in the common tendon of the transversus abdominis and internal oblique (CTrA) on the PLF. With incremental inflation, posteriorly directed forces (black bars) decline and anteriorly directed forces (gray bars) increase. The sum of dual load cells measurements of anteriorly and posteriorly directed forces through the PLF does not differ across the three inflation increments.
The data presented in Fig. 8 (Method 5) correspond with the data in Fig. 5 (Method 4). Because the load cells are unidirectional, they only measured tension in the direction of the CTrA. Inflation moved the posterior part of the PLF in the opposite direction from the load cell measuring CTrA tension (Fig. 5), thereby having a subtractive effect on force measured through the PLF (Fig. 8).
Bi-directional tension measurements analyzing the effect of inflation on PLF force transfer with CTrA tension
To better understand the subtractive effect that PMC inflation had on the unidirectional tension measured in the CTrA (described above, observed in Fig. 8), two uni-directional load cells were placed on the PLF: (i) one measured tension in the anterolateral direction (i.e. in the direction of pull for the transverse abdominus and internal obliques through the CTrA); (ii) the other measured tension in the posteromedial direction (i.e. along the PLF toward the spinous process; Method 6). An 8.5 N load was then suspended from the CTrA, as described previously (Method 1b). As inflation increased, the amount of measured posteromedially oriented tension in the PLF decreased (black bars, Fig. 9). Conversely, the measured amount of anterolaterally oriented tension in the PLF increased as a result of the tension of the CTrA, but only with increased inflation (gray bars, Fig. 9). The sum of anterolateral and posteromedial forces did not differ between levels of inflation. These data confirm that with increased inflation of the PMC, the tension progressively increases on the CTrA.
Summary of results
Incremental inflation of the PMC, in the absence of tension on the CTrA, produced a minimal but significant increase in length of the PLF accompanied by its posterior displacement (Fig. 4). However, incremental inflation within the PMC does not alter MLF length (Fig. 4) or generate any displacement of the lateral wall of the PMC.
In the absence of inflation within the PMC (mm intact), increasing CTrA tension results in anterior and lateral movement of the borders of the compartment (black bars in Figs 5 and 6; gray line in Fig. 7b). However, when CTrA tension is coupled with inflation, the net displacement of the borders of the compartment is posterior and slightly medial (gray bars in Figs 5 and 6; dashed line in Fig. 7b). Similarly, as pressure within the PMC increases, tension through the CTrA is significantly counteracted (Figs 8 and 9). Tension of the CTrA is predominately passed through the PLF, with very little impact on the MLF, regardless of the level of pressure in the PMC (Fig. 8). Collectively, this implies that an adequate paraspinal muscle contraction can counter the tension created by transverse abdominus and internal oblique contraction and vice versa. Further, it implies the existence of a point of equal tension between the paraspinal muscles and the transverse abdominus and internal oblique muscles acting through the CTrA producing increased force closure and self-bracing of the spine (Vleeming et al. 1990a,b).
Discussion
To the best of the authors' knowledge, this is the first study in which simulated incremental contraction of the paraspinal muscles within the TLF compartment is combined with applied tension to the CTrA of the transverse abdominus and internal oblique muscles. This study was undertaken to examine the relative force transfer through the MLF and PLF of the PMC.
The analysis of perimeter changes in the PMC in the setting of increasing compartment pressures, combined with measurements of load transfer from the CTrA through the PLF and MLF lamina, produced the following results: (i) in the absence of PMC inflation (mimicking the lack of muscle contraction), CTrA tension results in anterior and lateral movement of the PLF (black bar in Figs 5 and 6); (ii) the combination of PMC inflation (mimicking muscle contraction via increased pressure) with increased CTrA tension substantially displaces the PLF in a posterior and slightly medial direction.
With each incremental inflation, the displacement triggered by force transfer from the CTrA to the PLF leveled off, indicating that a point of equal tension between paraspinal muscles and CTrA is reached. At this point of equilibrium, pressure within the PMC counters the pull created by the CTrA and further displacement ceases. This indicates that with increasing PMC inflation, the angle of load transfer between the anterolateral pull of CTrA to the PLF is further optimized, creating an increasingly linear pull to the PLF.
This is in contrast to the MLF, where the angle of pull from the CTrA to the MLF becomes less optimal with increased PMC inflation.
In this study, incrementally increased pressure in the PMC was used to mimic the volume growing effect of muscle contraction. The pressure-generated displacement of the PLF was coupled on the transverse and sagittal planes. For each 1 cm of expansion in the posterior direction (sagittal axis) there was a 0.18 cm movement inward (medial direction) on the transverse axis. This indicates that the lateral border of the TLF is not expanding and is even slightly displaced medially, compared with a much larger posterior displacement of the PLF. This tension effect could be explained by the fact that submaximal inflation (Inf3) of the TLF compartment is counteracting the strain of pulling the CTrA. In this case, a theoretical point of equalized tension between paraspinal muscles and the transverse abdominus and internal oblique muscles has been attained. These results show that increasing tension on the CTrA (mimicking deep abdominal muscle contraction), combined with PMC inflation, transfers significantly more load to the PLF in comparison to the MLF thereby girdling the spine posteriorly. On average, the maximal posterior expansion of the PLF obtained when comparing a neutral position (muscles intact in the PMC and no tension on the CTrA) with that of maximal stress on the PLF (level 3 inflation of the PMC with 8.5 N tension on the CTrA) was an increase of 1.56 cm. From a biomechanical prospective, the shift of the load to the PLF places the strain on the thick lumbar spinous processes and supraspinous ligaments rather than passing this stress through the MLF to the much thinner lumbar transverse process. In addition, this strain is partially transmitted to the contralateral site of the PLF (Vleeming et al. 1995; Barker & Briggs, 1999). The results of this study could improve our understanding of LBP patients, showing that in the setting of paraspinal and deep abdominal muscle weakness or dysfunction, tension to the PLF potentially can unbalance the girdling relationship between the PLF and the abdominal muscles.
Comparisons to previous studies
Tesh et al. (1987) created an innovative in vitro study to analyze whether an extension moment could be generated in the lumbar spine by simulating lateral pull to the PLF and indirectly to the supraspinous ligaments. To simulate compression within the PMC, two foam dowels were inserted bilaterally into the emptied PMC of two sequential lumbar segments. An incremental loading sequence was applied to the CTrA to create tension up to a maximum of 98 N. The results of the Tesh et al. (1987) study confirmed that by applying tension to the PLF, the lumbar spine is stabilized; however, to a lesser degree than the higher values reported in a related study by Gracovetsky and co-workers (Gracovetsky et al. 1981; Gracovetsky, 1985). The insertion of foam dowels, as applied in Tesh's study, created an unknown static tension, keeping the dowels in place but did not mimic various levels of paraspinal contractions. In the present study, custom-made butyl inflatable tubes were inserted, in order to simulate three stages of contraction/inflation (inf), with and without CTrA tension.
Barker et al. (2006) investigated the effects of placing tension on the CTrA–PLF/MLF couple on segmental lumbar stiffness during flexion and extension movements. In their study, the tension placed on the CTrA at vertebral level L3 was transmitted almost twice as effectively to the MLF as compared with the PLF. Barker et al. (2006) cautiously comments ‘while segmental studies indicate that the PLF resists flexion and it has a greater moment arm than the MLF, its midline attachments are relatively unthickened and mobile, so its efficiency in influencing segmental motion may be dependent on the activation of the paraspinal muscles'. The present study confirms that by mimicking activation of the paraspinal muscles, combined with CTrA pull, the PLF becomes preferentially tensed while the MLF is barely influenced (Fig. 8).
To effectively analyze the transfer of load between MLF and PLF subsequent to tension placed on the CTrA, it is critical to replicate the normal compartmental pressure in the PMC as closely as possible, thus some form of compartmental inflation has to be employed. In addition, it is necessary to deliver the load from the CTrA in a manner that best mimics its in vivo angle. For this reason, the current study used the angle of the CTrA normally found in the individual specimen under study; this angle created by the anterolateral trajectory of the CTrA resembled a hoop surrounding the abdomen. Tesh et al. (1987) and Barker et al. (2006) used a synthetic angle of 90 ° from the midline. A transverse or horizontal pull to the CTrA, as used in these two studies, creates a non-physiological linear strain, predominantly in line with the fiber direction of the MLF.
Barker et al. (2006) applied a 20 N strain to the CTrA tendon to simulate moderate transverse abdominus tension. The present study applies on average 8.5 N load to the CTrA. Although this is a lower force than used in the study by Barker et al. (2006), this force was chosen to match the fact that the current study used only one vertebral body slab, whereas Barker et al. (2006) used a thicker slab containing two vertebral bodies. More than 8.5 N force could rupture the relatively small CTrA associated with a singular vertebral level.
Analyzing the extension moment of the PLF
It has been postulated by Gracovetsky and co-workers (Gracovetsky et al. 1977, 1981; Gracovetsky, 1985) that, if lateral force through the CTrA is applied to the TLF when its lateral border is restrained, such as by inflation of the PMC, a longitudinal tension will develop in the PLF. Assuming that the PLF behaves like an ideal net structure, the potential of the PLF to transform horizontal CTrA tension into a longitudinal tension at the level of the spinous process will enable an extension moment in the lumbar spine (Gracovetsky et al. 1977, 1981; Gracovetsky, 1985; Macintosh & Bogduk, 1987; Tesh et al. 1987; Hukins et al. 1990; Dolan et al. 1994; Adams & Dolan, 2007). In this manner, a mechanical conversion (gain) takes place between forces acting on the PLF. This was coined ‘the hydraulic amplifier mechanism’ (Gracovetsky et al. 1977). This gain is the ratio between longitudinal tension of the PLF and horizontal pull through the CTrA, and was found to be dependent on the inclination angle of the collagen fibers of the PLF between its lateral border and the midline (Tesh et al. 1987).
Although several values have been calculated for the optimal angle (Bogduk & MacIntosh, 1984; Vleeming et al. 1995; Barker & Briggs, 1999), a comparison between various anatomical studies of the superficial lamina of the PLF (Willard et al. 2012) confirmed an average fiber direction of up to 40 ° from horizontal and a cross-hatched fiber appearance below T12. In this sense, the PLF resembles the annulus fibrosis in which tension in the crossed fibers acts to contain the pressure of the intervertebral disc (Macintosh & Bogduk, 1987; Tesh et al. 1987). Restraining lateral expansion of the TLF is both an effect of the cross-hatched orientation of the collagen fibers in the PLF, alternating between the superficial and deep lamina (Bogduk & MacIntosh, 1984; Tesh et al. 1987; Hukins et al. 1990; Vleeming et al. 1995; Barker & Briggs, 1999), and the lateral tension from the CTrA (Tesh et al. 1987; Hodges et al. 2003; Barker et al. 2004, 2006). In the present study, CTrA tension combined with PMC inflation restrains the lateral border and even creates a small medial displacement. This restraint of the lateral border matches well with a reported four times stronger lateral strength of the PLF, compared with longitudinal strength (Tesh et al. 1987).
In vivo, a circumferential hoop tension occurs during lifting, by raising intra-abdominal pressure (IAP) and/or contracting the transverse abdominus and internal oblique muscles (Hodges & Richardson, 1996, 1999; Urquhart & Hodges, 2005), and thereby creating a horizontal force to the PLF. Using a biomechanical model, Hukins et al. (1990) showed that limiting radial expansion of the PMC due to contraction of the deep abdominal muscles could increase the tension within the TLF compartment by 30%, leading to a proportional increase in the extensor moment exerted by paraspinal muscles, as suggested by Gracovetsky and co-workers (Gracovetsky et al. 1977; Gracovetsky, 1985). The extension moment of the PLF depends on the distance to the instantaneous center of rotation of the vertebral body, which is calculated on average as 6–8 cm, possessing an excellent moment arm to resist flexion (Gracovetsky et al. 1981, 1985; McGill & Norman, 1985; Tesh et al. 1987; Hukins et al. 1990; Dolan et al. 1994; Barker et al. 2006; Adams & Dolan, 2007; Gatton et al. 2010). The present study shows an average posterior displacement of the PLF of 1.56 cm, when the PMC is submaximally inflated (Inf3); this represents a substantial increase of the moment arm of the PLF. In addition, this extensor moment becomes significantly larger in flexed postures (Gracovetsky et al. 1981; Tesh et al. 1987; Dolan et al. 1994; Adams & Dolan, 2007) and will also increase tension over the lumbosacral area, affecting stability of the pelvis (Vleeming et al. 2012). It has been calculated that the PLF could resist longitudinal forces exceeding 1 kN (Adams & Dolan, 2007). However, it is reasonable to expect expansion of the PMC to be substantially less in healthy trained individuals, with stronger paraspinal muscles, and hence stronger aponeuroses of the paraspinal muscles and PLF, resulting in an earlier and larger increase of pressure within the PMC.
Dolan & Adams (1993) reported that in lifting weights the total extensor moment is unrelated to the electromyogram (EMG) activity of the paraspinal muscles. Less than 25% of this ‘passive’ extensor moment comes from intervertebral discs and ligaments. The rest of this passive force appears to arise from non-contractile tissues, such as the PLF, the supraspinous ligaments and the surrounding fascial tissues within the erectors as well as raised IAP. Bogduk & MacIntosh (1984) report a significant flexion resistant role of the PLF; nonetheless, they argue that the hydraulic amplifier mechanism does not occur, because of the thinness and inconsistency of the deep lamina of the PLF. However, a recent study revealed that the paraspinal muscles are enclosed by an intact fascial sheath, the PRS (Schuenke et al. 2012). The PRS is a circular extension of the deep layer of the PLF, extending between the spinous process and the transverse process. The PRS contributes to the LIFT, formed by the anterior and posterior laminae of the CtrA (Fig. 1; Schuenke et al. 2012). This triangle becomes the intermediary point of force transfer, between deep abdominal muscles and the paraspinal muscles within the TLF. Hence, the LIFT equalizes tension between adjacent pressure vessels like the hypaxial abdominal and epaxial TLF fascial compartments. Therefore, encapsulation of the paraspinal muscles by this retinaculum makes it possible to create pressure within the PMC. The results of the present study suggest that a mechanical conversion (gain) is achieved in which horizontal tension is transformed into longitudinal tension, as proposed by Gracovetsky (1985) and Hukins et al. (1990).
Impairment of muscles and fascia contributing to the TLF
Various studies of abdominal and lumbar paraspinal muscles in LBP patients have reported significant pathological modifications in structure and function (Parkkola et al. 1993; Hides et al. 1994, 1995; Mooney et al. 1997; Danneels et al. 2000; Kader et al. 2000; Mengiardi et al. 2006; Dickx et al. 2008, 2010; Chan et al. 2012). Deconditioning, fatty involution, size and shape changes and alterations in motor control for the abdominal and paraspinal muscles, particularly the deep lumbar multifidus, have been defined in patients with LBP. All of these conditions have the potential to alter the effective load transfer characteristics of the TLF. Even in well-conditioned athletes such as ballet dancers, examination of those with low back and hip pain revealed alterations in cross-sectional area (CSA) of the multifidus muscle correlating with the side of the pain (Gildea et al. 2013).
Besides changes in size of the paraspinal muscles, the stiffness of these muscles is altered in patients with LBP. Ultrasound elastography studies confirmed that the stiffness of the multifidus muscle in certain postures increases in male LBP patients, compared with asymptomatic male controls, indicating adaptation of muscle contractile function (Chan et al. 2012). Another study, using the same methodology, quantified shear plane motion within the deep and superficial lamina of the PLF, demonstrating that the shear strain between these layers was on average reduced by 20% in female and male patients with LBP. A sexual dimorphism was also found in this study, indicating that males had significantly lower shear strain values compared with females (Langevin et al. 2011). The authors suggest that this reduction of strain could be attributed to intrinsic connective tissue pathology and chronic inflammation of the PLF (Langevin et al. 2011). Moreover, mechanical testing of isolated PLF samples of humans and animals shows an increase in stiffness with deformation, when stretched for long periods (Tesh et al. 1987; Yahia et al. 1992; Vleeming et al. 1995; Schleip et al. 2012). In addition, patients with LBP have demonstrable changes in the histological characteristics of the PLF (Bednar et al. 1995).
The present study demonstrates that robust paraspinal muscle contractions are required within the PMC to enable the pressure increase necessary to alter the geometric shape of this fascial structure. Compartment inflation leads especially to a posterior displacement of the erector spinae aponeurosis and PLF, thus enhancing the moment arm for spinal extension. However, when this expansion is combined with deep abdominal muscle contraction, the CTrA will transfer tension to the PLF and vice versa, thereby bracing and girdling the paraspinal muscles. Simultaneously, the lateral border of the PMC is restrained from lateral displacement and, in fact, is displaced slightly medially serving to maintain compartmental integrity around the paraspinal muscles. It could be assumed that changes in paraspinal muscle structural (Danneels et al. 2000) and functional (Dickx et al. 2010) properties in patients with chronic LBP could reduce the posterior displacement of the PLF and increase the compliance of the lateral border, thereby diminishing an effect of increased abdominal muscle tension.
Maintaining ICP is critical to normal function of the muscles (Kjellmer, 1964; Garfin et al. 1981; Carr et al. 1985; Styf, 1987; Styf & Lysell, 1987). There appears to be an optimal pressure either above or below which there is loss of efficacy in function. Compartmental pressures vary depending on activity, increasing significantly with exercise (Kjellmer, 1964). In addition, compartmental pressure is not constant throughout the full range of motion, but may vary considerably with specific postures and activities (Carr et al. 1985). Specifically, from a neutral pressure on vertical, relaxed standing, PMC pressure in normal volunteers raises slightly on extension, drops slightly on flexion but returns to normal on full flexion (90 °) of the spine (Carr et al. 1985). Opening a fascial compartment, thereby reducing its ICP, decreases muscle contraction force (Garfin et al. 1981). It has also been reported that increased pressure can be recorded in lumbar fascial compartments of a cohort of patients with LBP (Styf & Lysell, 1987). The present study shows that depending on the level of activation of the paraspinal and deep abdominal muscles, intra-abdominal and intra-compartmental PMC pressures affect each other through the tightening of the CTrA. This phenomenon was also indirectly studied by Carr et al. (1985), showing a strong increase of ICP within the PMC when the IAP was increased by applying the Valsalva maneuver.
The studies of Carr et al. (1985) and Schuenke et al. (2012) have demonstrated that lumbar paraspinal muscles are contained within the TLF compartment that will support increased pressure. It is noteworthy that patients with LBP consistently show higher ICP values within this compartment, associated with increased flexion and loading as compared with healthy controls (Konno et al. 1994). These findings in patients could be an indication that a diminished volume filling function of the multifidus requires more work from its residual fibers and from associated muscles such as the longissimus and the iliocostalis. Less inflation of the PMC reduces posterior PLF displacement and alters the tension transfer between the PLF and CTrA.
Conversely, continuous activation of paraspinal muscles in patients with LBP increases pressure within the PMC. It could be hypothesized that either diminished or too much tension of the paraspinal muscles, converted to the CTrA, could lead to an inhibitory response of the deep abdominal muscles, resulting in dysfunctional deep abdominal muscles as frequently reported in patients with chronic LBP (Hodges, 2008).
The flexion relaxation response (FRR)
During increased spinal flexion, diminution of EMG activity of the paraspinal muscles frequently occurs in healthy persons (Fick, 1911; Floyd & Silver, 1951). This phenomenon is termed the FRR (Andersson et al. 1977). The ability to diminish lumbar spinal muscle activity during increased flexion could be a consequence of tone in healthy robust spinal muscles sufficiently inflating the TLF compartment.
Kaigle et al. (1998) studied patients with chronic LBP and healthy volunteers during dynamic spinal flexion and extension exercises. The results showed that both intervertebral motion and trunk mobility were significantly restricted in patients compared with healthy individuals. The FRR in full flexion of the controls showed a 78% decrease in myoelectric activity, compared with patients with LBP showing an average reduction of 13% and most patients showing no reduction. The authors conclude that the FRR occurs exclusively in subjects reaching a complete segmental intervertebral flexion, considerable before full trunk flexion was achieved. The authors comment that ongoing spinal muscle activation in the patient group during flexion precludes full intervertebral motion, tensing the ligaments, thereby preventing the FRR (Kaigle et al. 1998).
The present study shows that in the absence of sufficient PMC inflation, CTrA tension results in anterior and lateral movement of especially the PLF. This suggests that decreased CSA of the paraspinal muscles, with less volume load to fill the PMC, combined with or without CTrA tension, will displace the TLF anteriorly and laterally, thereby reducing the extension moment of the PLF. The diminished efficacy of the extensor moment could be another reason why persistent muscle activation, compensating for less tension within the TLF compartment, precludes full intervertebral motion during deep flexion.
Trunk and extremity muscles contributing to PLF tension
In the present study, the biomechanical properties of the PMC were studied exclusively in the axial plane. However, the superficial and deep lamina of the PLF acts as an intermediary in transferring loads in three directions: between the upper and lower limbs; between left and right sides of the body; and between the abdominal wall and the lumbopelvic spine (Vleeming et al. 1995, 2012; Barker & Briggs, 1999). The superficial lamina of the PLF is formed mainly by the aponeurosis of the LD, additional connections to the deeper lamina of the PLF arise from the serratus posterior inferior muscles (Bogduk & MacIntosh, 1984; Vleeming et al. 1995; Barker & Briggs, 1999; Barker et al. 2004). This merging of abdominal and arm/trunk muscles into both laminae of the PLF creates a combined axial and frontal plane myofascial sling (Willard et al. 2012). Combinations of different trunk movements, like lumbar flexion/extension, lateral flexion and rotation, will generate specific directional tension to the PMC. For example, lumbopelvic flexion passively increases tension of the PLF (Dolan et al. 1994; Barker et al. 2004), which can be enhanced by combinations of trunk rotation and lateral flexion.
Transversus tendon muscle action is uniquely associated with increased postural demand, and contributes to general spine stabilization when the trunk is exposed to moderate flexion and extension moments (Crommert et al. 2011). Uni- or bilateral contractions of the deep abdominal muscles, with or without activation of the paraspinal muscles, cause different tensioning patterns to the PMC. As an example, during abdominal crunch exercises, the paraspinal and deep abdominal muscles will act antagonistically: flexing the pelvis and spine in sitting or standing, together with posterior trunk displacement, requires an eccentric contraction of the abdominal muscles combined with a relaxation of the paraspinal muscles. Progressively flexing the spine and pelvis with robust contraction of deep abdominal muscles will generate increased tension transfer from the CTrA to the PLF, girdling the spine in flexion. Flexing the spine is a typical example of opening up the kinematic chain and therefore diminishing ‘form’ closure of the spine, counteracted by increased ‘force’ closure (Vleeming et al. 1990a,b) by tensing the CTrA and PLF. In this scenario, deep abdominal muscles activity generates tension to the most superficial, posterior part of the PMC compartment.
Besides trunk muscles, several studies have clearly demonstrated that activity of the extremity muscles, like the LD, tense the PLF, both ipsi- and contralaterally, and reach as far as the contralateral GM muscle, even affecting resting tone of the contralateral hip (Bogduk & MacIntosh, 1984; Tesh et al. 1987; Vleeming et al. 1995; Mooney et al. 1997; Barker & Briggs, 1999; Barker et al. 2004; Willard et al. 2012; Carvalhais et al. 2013).
It is obvious that a multi-vector analysis will be needed to appreciate how paraspinal muscles activity in conjunction with deep abdominal muscles, and extremity muscles such as the LD and GM, effect the tension of the PLF and hence PMC pressure.
A flexible myofascial axial ring between the thorax and pelvis, replicating ribs
Vertebral stability in the thorax is greatly increased by interconnecting ribs to the spine and sternum; likewise in the pelvis, in which laterally flaring innominates could be regarded as fused ribs stemming from the sacrum and running anteriorly to the symphysis pubis (Tidwell & Carpenter, 2005; Gracovetsky, 2007). In humans, between the lower thorax and upper pelvis a greater space is present compared with the great apes. The latter have a restricted flexible zone between the rib cage and pelvis, because of their cranially extending pelvis and far caudally reaching lower ribs, restricting lumbar motion (Lovejoy, 2007). Humans, especially women, have a reduced height of the pelvis (Vleeming et al. 2012), creating more space between the thorax and pelvis, and this feature permits increased spinal mobility like flexion/extension and lateral flexion/rotation in the spine (Lovejoy, 2007).
Stability and mobility are opposing states in joints. To stabilize the lumbar spine, especially between L1 and L4 levels without ribs, the transverse abdominus and internal oblique muscles and their fascial aponeurosis resemble a flexible myofascial ring between the thorax and pelvis. This ring runs posteriorly from the transverse abdominus and internal oblique muscles, to the CTrA and via the MLF connecting to the transverse process, mimicking a dorsal rib construction. Anteriorly, the abdominal muscles fuse with the rectus abdominus fascia. The rectus muscle itself represents a contractible version of the inert bony sternum of the thorax or symphysis of the pelvis. Posteriorly, the PLF girdles the lumbar erector spinae and multifidus muscles. This axial abdominal–lumbar myofascial ring, between the thorax and pelvis, generates muscles contraction to compensate for the lack of rib stability. In essence, tension in this myofascial can be adjusted by altering PMC pressure. The present study has demonstrated that co-contraction of the paraspinal muscles and the deep abdominal muscles is capable of creating this girdling effect.
Methodological considerations
Sectioning the axial slabs results in diminished longitudinal tension, inherent in an intact TLF. This applies particularly to the PLF and the underlying common aponeurosis of the paraspinal muscles. This could be considered a limitation of the study. However, the present study is designed to inflate and pressurize the PMC and simultaneously tension the CTrA, which will increase both longitudinal and lateral tension. Another consideration is that studying elderly specimens generally is accompanied by a thinner fascia and aponeurosis. Therefore, expansion of the PMC as measured in the present study could be substantially larger in comparison to healthy individuals with robust paraspinal and deep abdominal muscles and a stronger PMC. The muscular strength difference in healthy persons could lead to an earlier PMC pressure, with less posterior displacement of the PLF compared with the specimen.
Concluding remarks
The present study demonstrates that robust paraspinal muscle contractions are required within the PMC to enable pressure increases sufficient to alter the geometric shape of this fascial structure. PMC inflation (mimicking paraspinal muscle contraction and generating pressure increase) results in a posterior displacement of the erector spinae aponeurosis and the closely associated PLF. However, when this expansion is combined with simulated deep abdominal muscle contraction, the CTrA will transfer tension almost exclusively to the PLF, thereby bracing and girdling the paraspinal muscles. Simultaneously, the lateral border of the PMC is moving slightly medially serving to maintain the PMC integrity. The present study shows an average posterior displacement of the PLF of 1.56 cm, when the PMC is submaximally inflated. This represents a substantial increase of the extensor moment arm of the PLF. Conversely, dysfunctional paraspinal muscles will reduce the pressure surge and hence the posterior displacement of the PLF. As a consequence, the compliance of the lateral border is increased, diminishing the effects of CTrA tension. When both the paraspinal and deep abdominal muscles are weak or dysfunctional, pressure increase and expansion of the PMC will be minimal.
This study shows a critical co-dependent mechanism between deep abdominal and lumbar spinal muscles linked to each other, especially through the PLF.
Acknowledgments
The authors would like to express appreciation to Mr Cole Southworth, Ms Dawn Whalen and Mr Innocent Ndzana for assisting in specimen preparation and data collection. The authors would also like to express appreciation to Mr Oran Suta for his artistic expertise and assistance on the figures. Lastly, the authors wish to express appreciation to Mr Hank Wheat for his knowledge and assistance in the design and construction of the research apparatuses.
References
- Adams MA, Dolan P. How to use the spine, pelvis, and legs effectively in lifting. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability & Lumbopelvic Pain: Integration of Research and Therapy. Edinburgh: Churchill Livingstone; 2007. pp. 167–184. [Google Scholar]
- Andersson GB, Ortengren R, Herberts P. Quantitative electromyographic studies of back muscle activity relatated to posture and loading. Orthop Clin North Am. 1977;8:85–96. [PubMed] [Google Scholar]
- Barker PJ, Briggs CA. Attachments of the posterior layer of lumbar fascia. Spine. 1999;24:1757–1764. doi: 10.1097/00007632-199909010-00002. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Briggs CA. Anatomy and biomechanics of the lumbar fasciae: implications for lumbopelvic control and clinical practice. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability & Lumbopelvic Pain: Integration of Research and Therapy. Edinburgh: Churchill Livingstone; 2007. pp. 63–73. [Google Scholar]
- Barker PJ, Briggs CA, Bogeski G. Tensile transmission across the lumbar fasciae in unembalmed cadavers: effects of tension to various muscular attachments. Spine. 2004;29:129–138. doi: 10.1097/01.BRS.0000107005.62513.32. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Guggenheimer KT, Grkovic I, et al. Effects of tensioning the lumbar fasciae on segmental stiffness during flexion and extension: Young Investigator Award winner. Spine. 2006;31:397–405. doi: 10.1097/01.brs.0000195869.18844.56. [DOI] [PubMed] [Google Scholar]
- Bednar DA, Orr FW, Simon GT. Observations on the pathomorphology of the thoracolumbar fascia in chronic mechanical back pain. A microscopic study. Spine. 1995;20:1161–1164. doi: 10.1097/00007632-199505150-00010. [DOI] [PubMed] [Google Scholar]
- Bogduk N, MacIntosh JE. The applied anatomy of the thoracolumbar fascia. Spine. 1984;9:164–170. doi: 10.1097/00007632-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Carr D, Gilbertson L, Frymoyer J, et al. Lumbar paraspinal compartment syndrome. A case report with physiologic and anatomic studies. Spine. 1985;10:816–820. [PubMed] [Google Scholar]
- Carvalhais VO, Ocarino Jde M, Araújo VL, et al. Myofascial force transmission between the latissimus dorsi and gluteus maximus muscles: an in vivo experiment. J Biomech. 2013;46:1003–1007. doi: 10.1016/j.jbiomech.2012.11.044. [DOI] [PubMed] [Google Scholar]
- Chan ST, Fung PK, Ng NY, et al. Dynamic changes of elasticity, cross-sectional area, and fat infiltration of multifidus at different postures in men with chronic low back pain. Spine J. 2012;12:381–388. doi: 10.1016/j.spinee.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Clemente CD. Gray's Anatomy of the Human Body. Philadelphia: Lea & Febiger; 1985. [Google Scholar]
- Crommert ME, Ekblom MM, Thorstensson A. Activation of transversus abdominis varies with postural demand in standing. Gait Posture. 2011;33:473–477. doi: 10.1016/j.gaitpost.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Danneels LA, Vanderstraeten GG, Cambier DC, et al. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9:266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickx N, Cagnie B, Achten E, et al. Changes in lumbar muscle activity because of induced muscle pain evaluated by muscle functional magnetic resonance imaging. Spine. 2008;33:E983–E989. doi: 10.1097/BRS.0b013e31818917d0. [DOI] [PubMed] [Google Scholar]
- Dickx N, Cagnie B, Parlevliet T, et al. The effect of unilateral muscle pain on recruitment of the lumbar multifidus during automatic contraction. An experimental pain study. Man Ther. 2010;15:364–369. doi: 10.1016/j.math.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Dolan P, Adams MA. The relationship between EMG activity and extensor moment generation in the erector spinae muscles during bending and lifting activities. J Biomech. 1993;26:513–522. doi: 10.1016/0021-9290(93)90013-5. [DOI] [PubMed] [Google Scholar]
- Dolan P, Mannion AF, Adams MA. Passive tissues help the back muscles to generate extensor movements during lifting. J Biomech. 1994;27:1077–1085. doi: 10.1016/0021-9290(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Farfan HF. Mechanical Disorders of the Low Back. Philadelphia: Lea and Febiger; 1973. [Google Scholar]
- Fick R. Handbuch der Anatomie und Mechanik der Gelenke. Jena: Gustav Fischer; 1911. [Google Scholar]
- Floyd WF, Silver PH. Function of erectores spinae in flexion of the trunk. Lancet. 1951;1:133–134. doi: 10.1016/s0140-6736(51)91212-3. [DOI] [PubMed] [Google Scholar]
- Garfin SR, Tipton CM, Mubarak SJ, et al. Role of fascia in maintenance of muscle tension and pressure. J Appl Physiol Respir Environ Exerc Physiol. 1981;51:317–320. doi: 10.1152/jappl.1981.51.2.317. [DOI] [PubMed] [Google Scholar]
- Gatton ML, Pearcy MJ, Pettet GJ, et al. A three-dimensional mathematical model of the thoracolumbar fascia and an estimate of its biomechanical effect. J Biomech. 2010;43:2792–2797. doi: 10.1016/j.jbiomech.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Gildea JE, Hides JA, Hodges PW. Size and symmetry of trunk muscles in ballet dancers with and without low back pain. J Orthop Sports Phys Ther. 2013;43:525–533. doi: 10.2519/jospt.2013.4523. [DOI] [PubMed] [Google Scholar]
- Gracovetsky S. An hypothesis for the role of the spine in human locomotion: a challenge to current thinking. J Biomed Eng. 1985;7:205–216. doi: 10.1016/0141-5425(85)90021-4. [DOI] [PubMed] [Google Scholar]
- Gracovetsky S. Is the sacroiliac joint an evolved costovertebral joint? In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 2007. pp. 185–198. [Google Scholar]
- Gracovetsky S, Farfan HF, Lamy C. A mathematical model of the lumbar spine using an optimized system to control muscles and ligaments. Orthop Clin North Am. 1977;8:135–153. [PubMed] [Google Scholar]
- Gracovetsky S, Farfan HF, Lamy C. The mechanism of the lumbar spine. Spine. 1981;6:249–262. doi: 10.1097/00007632-198105000-00007. [DOI] [PubMed] [Google Scholar]
- Gracovetsky S, Farfan H, Helleur C. The abdominal mechanism. Spine. 1985;10:317–324. doi: 10.1097/00007632-198505000-00005. [DOI] [PubMed] [Google Scholar]
- Grant JCB. An Atlas of Anatomy. Baltimore: Williams & Wilkins; 1972. [Google Scholar]
- Hides JA, Stokes MJ, Saide M, et al. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19:165–172. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- Hides JA, Richardson CA, Jull GA. Magnetic resonance imaging and ultrasonography of the lumbar multifidus muscle. Comparison of two different modalities. Spine. 1995;20:54–58. doi: 10.1097/00007632-199501000-00010. [DOI] [PubMed] [Google Scholar]
- Hodges PW. Transversus abdominis: a different view of the elephant. Br J Sports Med. 2008;42:941–944. doi: 10.1136/bjsm.2008.051037. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine. 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Transversus abdominis and the superficial abdominal muscles are controlled independently in a postural task. Neurosci Lett. 1999;265:91–94. doi: 10.1016/s0304-3940(99)00216-5. [DOI] [PubMed] [Google Scholar]
- Hodges P, Kaigle Holm A, Holm S, et al. Intervertebral stiffness of the spine is increased by evoked contraction of transversus abdominis and the diaphragm: in vivo porcine studies. Spine. 2003;28:2594–2601. doi: 10.1097/01.BRS.0000096676.14323.25. [DOI] [PubMed] [Google Scholar]
- Hollinshead WH. Anatomy for Surgeons: The Back and Limbs. New York: Hoeber-Harper; 1969. [Google Scholar]
- Hukins DW, Aspden RM, Hickey DS. Thoracolumbar fascia can increase the efficiency of the erector spinae muscles. Clin Biomech (Bristol, Avon) 1990;5:30–34. doi: 10.1016/0268-0033(90)90029-6. [DOI] [PubMed] [Google Scholar]
- Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55:145–149. doi: 10.1053/crad.1999.0340. [DOI] [PubMed] [Google Scholar]
- Kaigle AM, Wessberg P, Hansson TH. Muscular and kinematic behavior of the lumbar spine during flexion-extension. J Spinal Disord. 1998;11:163–174. [PubMed] [Google Scholar]
- Kjellmer I. An indirect method for estimating tissue pressure with special reference to tissue pressure in muscle during exercise. Acta Physiol Scand. 1964;62:31–40. doi: 10.1111/j.1748-1716.1964.tb03948.x. [DOI] [PubMed] [Google Scholar]
- Konno S, Kikuchi S, Nagaosa Y. The relationship between intramuscular pressure of the paraspinal muscles and low back pain. Spine. 1994;19:2186–2189. doi: 10.1097/00007632-199410000-00011. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Fox JR, Koptiuch C, et al. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet Disord. 2011;12:203–214. doi: 10.1186/1471-2474-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy CO. Evolution of the human lumbopelvic region and its relationship to some clinical deficits of the spine and pelvis. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 2007. pp. 141–158. [Google Scholar]
- Macintosh JE, Bogduk N. 1987 Volvo award in basic science. The morphology of the lumbar erector spinae. Spine. 1987;12:658–668. doi: 10.1097/00007632-198709000-00004. [DOI] [PubMed] [Google Scholar]
- McGill SM, Norman RW. Dynamically and statically determined low back moments during lifting. J Biomech. 1985;18:877–885. doi: 10.1016/0021-9290(85)90032-6. [DOI] [PubMed] [Google Scholar]
- Meijering E, Dzyubachyk O, Smal I. Methods for cell and particle tracking. Methods Enzymol. 2012;504:183–200. doi: 10.1016/B978-0-12-391857-4.00009-4. [DOI] [PubMed] [Google Scholar]
- Mengiardi B, Schmid MR, Boos N, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology. 2006;240:786–792. doi: 10.1148/radiol.2403050820. [DOI] [PubMed] [Google Scholar]
- Mooney V, Gulick J, Perlman M, et al. Relationships between myoelectric activity, strength, and MRI of lumbar extensor muscles in back pain patients and normal subjects. J Spinal Disord. 1997;10:348–356. [PubMed] [Google Scholar]
- Parkkola R, Rytökoski U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine. 1993;18:830–836. doi: 10.1097/00007632-199306000-00004. [DOI] [PubMed] [Google Scholar]
- Schaeffer JP. Morris's Human Anatomy. New York: McGraw-Hill; 1953. [Google Scholar]
- Schleip R, Duerselen L, Vleeming A, et al. Strain hardening of fascia: static stretching of dense fibrous connective tissues can induce a temporary stiffness increase accompanied by enhanced matrix hydration. J Bodyw Mov Ther. 2012;16:94–100. doi: 10.1016/j.jbmt.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Schuenke MD, Vleeming A, Von Hoof T, et al. Anatomical constituents of lumbar myofascial load transfer: an outline of the lateral raphe and the lumbar interlaminar triangle. J Anat. 2012;221:568–576. doi: 10.1111/j.1469-7580.2012.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalteholz W. Hand Atlas of Human Anatomy. Philadelphia: J.B. Lippincott; 1923. [Google Scholar]
- Standring S. Gray's Anatomy. 14th edn. Philadelphia: Churchill Livingstone; 2008. p. 1551. [Google Scholar]
- Stecco A, Masiero S, Macchi V, et al. The pectoral fascia: anatomical and histological study. J Bodyw Mov Ther. 2009;13:255–261. doi: 10.1016/j.jbmt.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Styf J. Pressure in the erector spinae muscle during exercise. Spine. 1987;12:675–679. doi: 10.1097/00007632-198709000-00006. [DOI] [PubMed] [Google Scholar]
- Styf J, Lysell E. Chronic compartment syndrome in the erector spinae muscle. Spine. 1987;12:680–682. doi: 10.1097/00007632-198709000-00007. [DOI] [PubMed] [Google Scholar]
- Tesh KM, Dunn JS, Evans JH. The abdominal muscles and vertebral stability. Spine. 1987;12:501–508. doi: 10.1097/00007632-198706000-00014. [DOI] [PubMed] [Google Scholar]
- Tidwell V, Carpenter K. Thunder-Lizards: The Sauropodomorph Dinosaurs (Life of the Past) Bloomington, IN: Indiana University Press; 2005. [Google Scholar]
- Urquhart DM, Hodges PW. Differential activity of regions of transversus abdominis during trunk rotation. Eur Spine J. 2005;14:393–400. doi: 10.1007/s00586-004-0799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart DM, Hodges PW. Clinical anatomy of the anterolateral abdominal muscles. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability & Lumbopelvic Pain: Integration of Research and Therapy. Edinburgh: Churchill Livingstone; 2007. pp. 75–84. [Google Scholar]
- Vleeming A, Stoeckart R, Volkers AC, et al. Relation between form and function in the sacroiliac joint. Part I: clinical anatomical aspects. Spine. 1990a;15:130–132. doi: 10.1097/00007632-199002000-00016. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Volkers AC, Snijders CJ, et al. Relation between form and function in the sacroiliac joint. Part II: biomechanical aspects. Spine. 1990b;15:133–136. doi: 10.1097/00007632-199002000-00017. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine. 1995;20:753–758. [PubMed] [Google Scholar]
- Vleeming A, Willard FH, Schuenke MD, et al. The sacroiliac joint: anatomy, function, and clinical implications. J Anat. 2012;221:537–567. doi: 10.1111/j.1469-7580.2012.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FH, Vleeming A, Schuenke MD, et al. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012;221:507–536. doi: 10.1111/j.1469-7580.2012.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahia L, Rhalmi S, Newman N, et al. Sensory innervation of human thoracolumbar fascia. An immunohistochemical study. Acta Orthop Scand. 1992;63:195–197. doi: 10.3109/17453679209154822. [DOI] [PubMed] [Google Scholar]


