Abstract
Past studies documented the presence of epididymal/testicular fusion anomalies and persistence of a patent processus vaginalis in a small case-series of cryptorchid and/or hydrocele patients. The primary aim of this study was to determine the prevalence of the epididymal/testicular anomalies in a series of more than 1000 cryptorchid patients compared with controls. Secondary aims were: (i) to investigate the association between the cryptorchidism and the patency of p. vaginalis; and (ii) to correlate the epididymal/testicular fusion anomalies with the position of the testis and with the patency of the p. vaginalis. The clinical and surgical data of 1002 cryptorchid patients and 230 controls were retrospectively retrieved and analysed. Epididymal/testicular fusion anomalies were classified as: (i) normal anatomy; (ii) minor anomalies; and (iii) major anomalies. Statistical analysis was performed using the Student's t-test and Chi-square tests. The prevalence of the epididymal/testicular fusion anomalies was higher in the cryptorchid group compared with that of the control group (minor and major anomalies in cryptorchids vs. controls, respectively: 42.2 vs. 5.6% and 9.3 vs. 1.6%, P < 0.0001). Moreover, we documented a correlation of these anomalies with a more proximal localization of the testis (minor and major anomalies in proximal vs. distal location of the testis, respectively: 62.5 vs. 34.8% and 19.1 vs. 6.3%, P < 0.0001) and with the persistence of a widely patent p. vaginalis (minor and major anomalies in widely patent p. vaginalis vs. narrow duct, respectively: 51.7 vs. 42.2 and 11.9% vs. 7.8%, P < 0.001). In conclusion, the epididymal/testicular fusion anomalies were strongly associated with cryptorchidism and the persistence of a widely patent peritoneal vaginal duct. Although it remains unclear whether these anomalies cause non-descent of the testis or, conversely, result from the cryptorchidism or from the persistence of a widely patent duct, our data re-enforce this association.
Keywords: cryptorchidism, epididymal and testis anomaly, epididymal/testicular fusion, epididymis, orchidopexy
Introduction
Cryptorchidism is a common paediatric pathology with an incidence of 6.9% in live males at birth, ranging from 3.4% in term births and 30.1% in pre-terms (Ghirri et al. 2002). The pathogenesis of this condition and its aetiology have been extensively studied, and multiple factors (including anatomical and hormonal variations; Rajfer & Walsh, 1977) have been related to this disease, including a putative causal role for testicular/epididymal fusion anomalies (Mininberg & Schlossberg, 1983). Of note, in the vast majority of boys with cryptorchidism, it has been also documented a patent processus vaginalis (Mininberg & Schlossberg, 1983).
Epididymal anomalies associated with the undescended testis were firstly reported in 1851 according to Davis (Davis et al. 1974). Since then, few studies have investigated the prevalence of these anomalies in cryptorchid patients (reported ranging from 32 to 72%) and their role in testicular migration (Marshall & Shermeta, 1979; Mininberg & Schlossberg, 1983; Heath et al. 1984; Gill et al. 1989; Koff & Scaletscky, 1990; Elder, 1992; Mollaeian et al. 1994).
The majority of these studies were small case-series, performed without control groups and conducted 20–30 years ago. However, a large case-series recently investigated the histological findings associated with epididymal/testicular non-fusion (Kraft et al. 2011).
Moreover, the presence of epididymal anomalies has also been documented in boys who have undergone surgery for hydrocele (Han & Kang, 2002).
Several classifications were proposed for these anomalies, the first one in 1971 (Scorer & Farrington, 1971); later, Gill et al. (1989) proposed a schema for differentiating anomalies of ductal fusion and ductal suspension.
The primary aim of this large retrospective case–control study was to determine the prevalence of epididymal/testicular fusion anomalies in a population of more than 1000 cryptorchid patients compared with a control group of 230 patients. Secondary aims were to correlate: (i) cryptorchidism and patency of the p. vaginalis; and (ii) epididymal/testicular fusion anomalies and the position of the testis and the persistence of a widely patent p. vaginalis. Moreover, we propose a simple and clinically useful classification of these anomalies that might replace the previous ones in this field.
Materials and methods
Clinical, surgical and anatomical findings of consecutive patients who underwent a surgical procedure for cryptorchidism from 1984 to 1994 were recorded in a database; the retrieved data were reviewed and analysed.
Data from patients who underwent surgical procedures requiring exploration of the tunica vaginalis (including elective procedures for congenital hernia, emergencies procedures for testicular torsion and torsion of testicular appendices or orchitis/epididymitis) during the same time period were recorded, retrieved for this study and used as control group.
All the records, including the patient's demographics (side of cryptorchidism, age at the time of surgery, co-morbidities), surgical procedures and anatomical findings were reviewed.
Anatomical records included: the position of the testis (abdominal, inguinal canal, ectopic) and the insertion of the gubernaculums testis (at the pubis region, scrotal, other, absent); moreover, a description of the p. vaginalis was provided when detected as: narrow (obliterated or threadlike) or wide (if it could admit the tips of a surgical forceps/scissor). Special attention was given reviewing the anatomical relationship between the epididymis and testis (described in the surgical procedure reports).
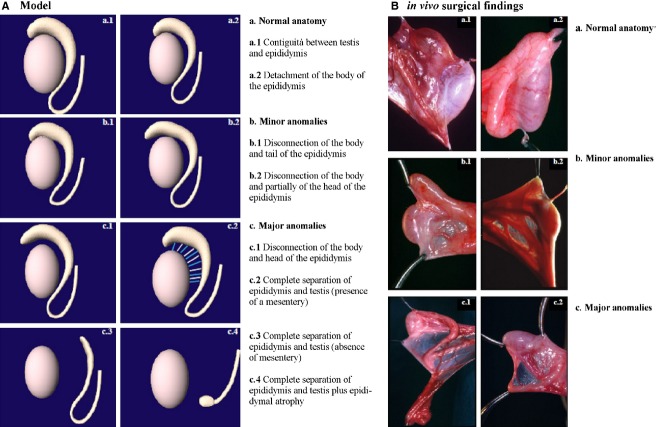
We used our own classification based on the degree of dissociation between the epididymis and testis that was documented at the surgical procedure, as follows (Fig. 1): (i) normal anatomy (contiguity between the testis and epididymis or detachment of the body of the epididymis); (ii) minor anomalies (disconnection of the body and tail, or disconnection of the body and partially of the head of the epididymis); and (iii) major anomalies (disconnection of the body and head, or complete separation between the epididymis and testis with or without a mesentery and with epididymal atrophy). This classification has been used for analysis and interpretation of results.
Fig. 1.
(A) Proposed classification of epididymal/testicular fusion anomalies: [(a) normal anatomy (a.1) contiguity between the testis and epididymis or (a.2) detachment of the body of the epididymis]; [(b) minor anomalies (b.1) disconnection of the body and tail, or (b.2) disconnection of the body and partially of the head of the epididymis] and [(c) major anomalies (c.1) disconnection of the body and head, or complete separation between the epididymis and the testis with (c.2) or without (c.3) a mesentery or with epididymal atrophy (c.4)]. (B) Examples of epididymal/testicular fusion anomalies associated with cryptorchidism found in this study: in vivo [(a) normal anatomy (a.1) contiguity between the testis and epididymis or (a.2) detachment of the body of the epididymis]; [(b) minor anomalies (b.1) disconnection of the body and tail, or (b.2) disconnection of the body and partially of the head of the epididymis] and [(c) major anomalies complete separation between the epididymis and testis with (c.1) or without (c.2) a mesentery].
Statistical analysis
Patients and controls were compared using the Student's t-test and Chi-square's tests. A two-tailed P-value < 0.05 was considered statistically significant. All analyses were performed using the MedCalc software version 11.4.4.0. A post hoc analysis was performed for evaluating the power of the tests using G*Power software version 3.1.2.
Results
The study population consisted of 1002 patients who underwent 1002 surgical procedures for cryptorchidism from 1984 to 1994, and 230 patients who underwent 250 scrotal explorations for other pathologies during the same period (control group). Table 1 shows the patients’ demographics and surgical procedures in the study population and controls. Nine-hundred and seventy-two patients underwent elective procedures for primary cryptorchidism, 22 emergency procedures (e.g. due to a hernia associated to the cryptorchidism) and eight first-stage orchidopexies. Of note, 231 patients (23%) had a bilateral cryptorchidism, but we focused the analysis exclusively on the undescended testis surgically treated. All orchidopexies were performed by the conventional technique of placing the testis in the sub-dartos space.
Table 1.
Clinical, surgical and anatomical features in the study population and in the control group
| Study population – 1002 patients/1002 procedures | Control group – 230 patients/250 procedures | P-value | ||
|---|---|---|---|---|
| Age – years (years), months | ||||
| Mean (SD) | 5 years, 6 months (3 years, 3 months) | Mean (SD) | 6 years, 2 months (4 years, 1 month) | 0.1* |
| Median | 5 years | Median | 6 years | |
| Range | 1 month – 16 years | Range | 3 months – 13 years | |
| Side – total 1002, n (%) | Side – total 250, n (%) | |||
| Right | 568 (56.7%) | Right | 145 (58.0%) | 0.73† |
| Left | 434 (43.3%) | Left | 105 (42.0%) | |
| Surgical procedure – total 1002, n (%) | Surgical procedure – total 250, n (%) | |||
| Primary orchidopexy | 972 (97.0%) | Torsion of testicular appendices/testis | 166 (66.4%) | n/a |
| Emergency procedure | 22 (2.2%) | Orchitis/epididymitis and others | 45 (18.0%) | |
| First stage orchidopexy | 8 (0.8%) | Incarcerated congenital inguinal hernia | 39 (15.6%) | |
| Testis localization – total 1002, n (%) | ||||
| Intra-abdominal | 116 (11.6%) | |||
| Inguinal canal- proximal | 157 (15.7%) | |||
| Inguinal canal-distal | 243 (24.3%) | |||
| Superficial inguinal ring | 287 (28.6%) | |||
| Ectopic testis | 165 (16.5%) | |||
| Atresia/not found | 34 (3.4%) | |||
| Insertion of the gubernaculum – total 594, n (%) | ||||
| Sopra-pubic | 288 (48.5%) | |||
| Scrotal | 131 (22.1%) | |||
| Other | 76 (12.8%) | |||
| Not detected | 99 (16.7%) | |||
| Total | 594 (100%) | |||
| Processus vaginalis – total 900, n (%) | ||||
| Detected | 698 (77.6%) | |||
| Not detected | 202 (22.4%) | |||
t-test.
Chi-square test.
Moreover, an inguinal hernia associated with cryptorchidism was noted and surgically corrected in 97 patients (9.7% of the study population). The study population and the control group were homogeneous regarding age and side of the surgical procedures (p ns, Table 1).
Table 1 shows the anatomical findings associated with cryptorchidism. In 165 patients (16.5%) an ectopic testis was documented: it was an interstitial ectopy (testis located at the distal inguinal ring, above the external oblique muscle's fascia) in 113 patients (68.5%), an inguino-crural ectopy (testis located at the Scarpa's Triangle) in 24 patients (14.5%) and suprapubic in 28 cases (17.0%).
The size of the testis was reported in 628 patients (62.8%) ranging from 4.0 to 30.0 mm (calculated as the longest diameter; mean 13.6 mm, median 13.0 mm, SD 3.6 mm). The insertion of the gubernaculum testis is also shown in Table 1 (594 patients, 59.3% of the case-series), along with the presence/absence of the p. vaginalis: it was not detected in 202 patients, otherwise in 698 the duct was detected and classified by the surgeon at operation as: narrow in 388 patients; wide in 304 patients; and detected but not categorized in the remaining six patients. We documented a strong association between the proximal level position of the testis and the persistence of a wide p. vaginalis [Chi-square test: P < 0.0001, post hoc analysis: effect size w 0.2, power (1 − β) 0.99; Table 2].
Table 2.
Level of cryptorchidism vs. patency of the processus vaginalis
| Narrow n (%) | Wide n (%) | P-value | |
|---|---|---|---|
| Position of the testis | |||
| Intra-abdominal | 34 (8.8) | 42 (13.8) | < 0.0001† |
| Inguinal canal-proximal | 54 (14.0) | 82 (27.0) | |
| Inguinal canal-distal | 112 (29.0) | 74 (24.3) | |
| Superficial inguinal ring | 132 (34.2) | 67 (22.0) | |
| Ectopic testis | 54 (14.0) | 39 (12.8) | |
| Total* | 386 (100) | 304 (100) | |
In two patients with a narrow processus vaginalis the testis was not documented.
Chi-square P < 0.0001.
Data regarding the epididymal and testicular anatomical relationship were available in 895 patients (89.3% of the study group) and in all the controls.
Table 3 shows the prevalence of epididymal and testicular anomalies in the study and control groups according to the classification shown in Fig. 1, and documenting a prevalence of statistical value of the fusion anomalies in cryptorchid patients.
Table 3.
Epididymal and testicular anomalies associated with cryptorchidism, comparison with the control group
| Study population 895 patients/895 surgical procedures n (%) | Control group 230 patients/250 surgical procedures n (%) | P-value | |
|---|---|---|---|
| Normal anatomy | P < 0.0001* | ||
| Contiguity between testis and epididymis | 36 (4.0) | 18 (7.2) | |
| Detachment of the body of the epididymis | 398 (44.5) | 214 (85.6) | |
| Minor anomalies | |||
| Disconnection of the body and tail of the epididymis | 230 (25.7) | 10 (4.0) | |
| Disconnection of the body and partially of the head of the epididymis | 148 (16.5) | 4 (1.6) | |
| Major anomalies | |||
| Disconnection of the body and of the head of the epididymis | 16 (1.8) | 2 (0.8) | |
| Complete separation between epididymis and testis (presence of a mesentery) | 54 (6.0) | 0 | |
| Complete separation between epididymis and testis (absence of a mesentery) ± epididymal atrophy | 13 (1.5) | 2 (0.8) | |
Chi-square test.
There was no significant association between the side of the cryptorchidism and the presence of fusion anomalies [Chi-square test: P 0.44, post hoc analysis: effect size w 0.2, power (1– β) 0.99], conversely we reported a significant association between the presence of the anomalies and a proximal localization of the retained testis [Chi-square test: P < 0.0001, post hoc analysis: effect size w 0.2, power (1 − β) 0.99]. Moreover, the normal anatomy was associated with the presence of a narrow p. vaginalis, whereas the anomalies were more common if the duct presented as wide [Chi-square test: P 0.001, post hoc analysis: effect size w 0.2, power (1 − β) 0.99; Table 4].
Table 4.
Epididymal and testicular anomalies: association with the cryptorchidism features
| Side of cryptorchidism, 895 patients n (%) P 0.44* | Position of undescended testis, 895 patients n (%) P < 0.0001* | Patent processus vaginalis, 664 patients n (%) P 0.001* | |||||
|---|---|---|---|---|---|---|---|
| Right | Left | Proximal** | Distal*** | Ectopic | Narrow | Wide | |
| Normal anatomy | 262 (50.3) | 172 (46.0) | 47 (18.4) | 283 (59.0) | 104 (65.4) | 185 (50.0) | 107 (36.4) |
| Minor anomalies | 213 (40.9) | 165 (44.1) | 160 (62.5) | 167 (34.8) | 51 (32.1) | 156 (42.2 | 152 (51.7 |
| Major anomalies | 46 (8.8) | 37 (9.9) | 49 (19.1) | 30 (6.3) | 4 (2.5) | 29 (7.8) | 35 (11.9) |
| Total | 521 (100 | 374 (100) | 256 (100) | 480 (100) | 159 (100) | 370 (100) | 294 (100) |
Chi-square test.
Proximal testis included testis located intra-abdominally and at the proximal edge of the inguinal canal.
Distal testis included testis located at the distal edge of the inguinal canal/superficial inguinal ring.
Discussion
The embryogenesis of the male reproductive system is a complex process. The testis and the head of the epididymis arise from the genital ridge, whereas the body of the epididymis and the vas deferens arise from the wolfian duct; the union by canalization of the rete testis and mesonephric tubules begins at 12 weeks and it is probably completed at puberty (Cromie, 1978; Gill et al. 1989; Mollaeian et al. 1994).
The regulation of normal testicular descent involves numerous mechanical components (including the gubernaculum, the epididymis and intra-abdominal pressure) and hormones; moreover, animal studies provided evidences regarding the role of a complex network of androgens, genitofemoral nerve and calcitonin gene-related peptide implied in the descent (Hutson et al. 2013).
The anatomic pathway for testicular descent is provided by the gubernaculum and by the epididymis. A possible role for the epididymis in testicular descent has been proposed by Mininberg & Schlossberg (1983); on the same extent, Bedford (in the animal model) hypothesized that testicular migration into the scrotum is primarily to preserve normal epididymal function (Bedford, 1978).
Nevertheless, according to the studies conducted by Hadziselimovic & Herzog (1983), the gubernaculum testis should be renamed as gubernaculum epididymis, as it inserts in the epididymis and not in the testis, and thus drives the epididymal descent (ruling the testicular descent indirectly). Intriguingly, because no mammals present descended testes and undescended epididymis, the primary role of the epididymis in the testicular descent should be emphasized.
Because of all these close relationships, it is not surprising that epididymal abnormalities may also be associated with an undescended testis (Marshall & Shermeta, 1979).
Of note, the p. vaginalis develops within the gubernaculum, and its obliteration – along with regression of the gubernaculum – usually occurs immediately after testicular descent has been completed. Thus, both the descent of the testis and the obliteration of the p. vaginalis may occur as a result of the same stimuli (Heyns, 1987; Han & Kang, 2002).
Epididymal anomalies may occur due to abnormal involution of the mesonephric duct adjacent to the normal testis (Dickinson, 1973; Mollaeian et al. 1994). In this study, 48.5% of the cryptorchid patients had normal anatomy, whereas 51.5% had a degree of disconnection between the epididymis and testis, consistent with the findings of other studies conducted on smaller samples (range of fusion anomalies: 32–72.2%; Marshall & Shermeta, 1979; Mininberg & Schlossberg, 1983; Heath et al. 1984; Gill et al. 1989; Koff & Scaletscky, 1990; Elder, 1992; Mollaeian et al. 1994); indeed, Turek and associates reported 97% of normal epididymal anatomy in a series of 112 non-cryptorchid patients (Turek et al. 1994). On the other hand, Kraft et al. (2011) reported that 83.7% of the cryptorchid patients investigated in their study had complete fusion of the testis and epididymis, even though a different classification was used, and Sherma & Sen (2013) reported 8% of complete epididymal/testicular dissociation in a smaller case-series. Apart from this, our data are consistent with that of Kraft and others who documented an association between fusion anomalies and higher testes location of the undescended testis (Marshall & Shermeta, 1979; Gill et al. 1989; Kraft et al. 2011). However, Mininberg and Schlossberg reported that epididymal abnormalities were independent of the degree of the testicular descent, but no difference was noted comparing patients presenting unilateral and bilateral cryptorchidism (Mininberg & Schlossberg, 1983). Similarly to the results of Han and Kang, the epididymal anomalies in this study were strongly associated with a wide p. vaginalis (Han & Kang, 2002).
The limitations of this study include the retrospective nature of the analysis and the late age at orchidopexy (> 5 years); however, these data could be justified as patients were treated from 1984 to 1994, whereas current guidelines suggest performing orchidopexy earlier. Of note, one of the primary aims of the authors when collecting data was the evaluation of the impact of the fusion anomalies in the development of infertility in adulthood; later this aim has been dropped and we focused our analysis exclusively on the anatomical study, because the follow-up of patients was interrupted at an early stage due to organization and management difficulties. Nevertheless, in more recent years the correlation between cryptorchidism and infertility has been clearly demonstrated and, even though past studies suggested that such anomalies may affect future fertility (Mollaeian et al. 1994), recently Kraft reported no significant abnormalities in germ cells/tubule or adult dark spermatogonia/tubule counts in cryptorchids with testicular/epididymal non-fusion (Kraft et al. 2011).
It seems important to highlight, however, that the classification we herein propose is simple and of clinical use, and this study is one of the largest series ever published with the value of being a case–control study (although the ‘control group’ is composed by children with some kind of uro/andrological pathologies that required a surgical procedure).
Conclusions
Our study showed that epididymal/testicular fusion anomalies are much more common in cryptorchid patients than controls; moreover, these anomalies are strongly associated with a more proximal position of the testis. Although it is difficult to determine whether the presence of epididymal/testicular fusion anomalies impairs normal descent of the testis, or the non-descent of the testis impairs the normal conjunction of these structures, our data substantiate this association. This study showed an association between epididymal/testicular fusion anomalies and patency of the p. vaginalis, supporting the theory that the development of all these structures involved in the testicular descent are interrelated and may be influenced by a common stimulus.
Acknowledgments
None of the authors has any potential financial conflict of interest related to this manuscript.
Author contributions
Conception and design: SC; FF. Acquisition of data: SC; FF; MC. Analysis and interpretation of data: LL, SC; DC. Drafting the manuscript: LL; SC; DC. Critical revision: LL; SC; FF; MC; DC. Statistical analysis: SC; LL; MC. Supervision: SC; FF.
References
- Bedford JM. Anatomical evidence for the epididymis as the prime mover in the evolution of the scrotum. Am J Anat. 1978;152:483–507. doi: 10.1002/aja.1001520404. [DOI] [PubMed] [Google Scholar]
- Cromie WJ. Congenital anomalies of the testis, vas epididymis, and inguinal canal. Urol Clin North Am. 1978;5:237–252. [PubMed] [Google Scholar]
- Davis EL, Shpall RA, Goldstein AMB, et al. Congenitally uncoiled epididymis in a cryptorchid testis. J Urol. 1974;111:618–620. doi: 10.1016/s0022-5347(17)60030-2. [DOI] [PubMed] [Google Scholar]
- Dickinson SJ. Structural abnormalities in the undescended testis. J Pediatr Surg. 1973;8:523–527. doi: 10.1016/0022-3468(73)90215-7. [DOI] [PubMed] [Google Scholar]
- Elder JS. Epididymal anomalies associated with hydrocele/hernia and cryptorchidism: implications regarding testicular descent. J Urol. 1992;148:624–626. doi: 10.1016/s0022-5347(17)36672-7. [DOI] [PubMed] [Google Scholar]
- Ghirri P, Ciulli C, Vuerich M, et al. Incidence at birth and natural history of cryptorchidism: a study of 10,730 consecutive male infants. J Endocrinol Invest. 2002;25:709–715. doi: 10.1007/BF03345105. [DOI] [PubMed] [Google Scholar]
- Gill B, Kogan S, Starr S, et al. Significance of epididymal and ductal anomalies associated with testicular maldescent. J Urol. 1989;142:556–558. doi: 10.1016/s0022-5347(17)38814-6. [DOI] [PubMed] [Google Scholar]
- Hadziselimovic F, Herzog B. The development and descent of the epididymis. Eur J Paediatr. 1983;152(Suppl 2):S6–S9. doi: 10.1007/BF02125424. [DOI] [PubMed] [Google Scholar]
- Han CH, Kang SH. Epididymal anomalies associated with patent processus vaginalis in hydrocele and cryptorchidism. J Korean Med Sci. 2002;17:660–662. doi: 10.3346/jkms.2002.17.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AL, Man DW, Eckstein HB. Epididymal abnormalities associated with maldescent of testis. J Pediatr Surg. 1984;19:47–49. doi: 10.1016/s0022-3468(84)80014-7. [DOI] [PubMed] [Google Scholar]
- Heyns CF. The gubernaculum during testicular descent in the human fetus. J Anat. 1987;153:93–112. [PMC free article] [PubMed] [Google Scholar]
- Hutson JM, Southwell BR, Li R, et al. The regulation of testicular descent and effects of cryptorchidism. Endocr Rev. 2013;34:725–752. doi: 10.1210/er.2012-1089. [DOI] [PubMed] [Google Scholar]
- Koff WJ, Scaletscky R. Malformations of the epididymis in undescented testis. J Urol. 1990;143:340–343. doi: 10.1016/s0022-5347(17)39954-8. [DOI] [PubMed] [Google Scholar]
- Kraft KH, Mucksavage P, Canning DA, et al. Histological findings in patients with cryptorchidism and testis-epididymis nonfusion. J Urol. 2011;186:2045–2049. doi: 10.1016/j.juro.2011.07.037. [DOI] [PubMed] [Google Scholar]
- Marshall FF, Shermeta DW. Epididymal abnormalities associated with undescended testis. J Urol. 1979;121:341–343. doi: 10.1016/s0022-5347(17)56780-4. [DOI] [PubMed] [Google Scholar]
- Mininberg DT, Schlossberg S. The role of the epididymis in testicular descent. J Urol. 1983;129:1207–1208. doi: 10.1016/s0022-5347(17)52645-2. [DOI] [PubMed] [Google Scholar]
- Mollaeian M, Mehrabi V, Elahi B. Significance of epididymal and ductal anomalies associated with undescended testis: study in 652 cases. Urology. 1994;43:857–860. doi: 10.1016/0090-4295(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Rajfer J, Walsh PC. Testicular descent. Birth Defects Orig Artic Ser. 1977;13:107–122. [PubMed] [Google Scholar]
- Scorer CG, Farrington GH. Congenital Deformities of the Testis and Epididymis. New York: Appleton-Century-Crofts; 1971. pp. 136–144. [Google Scholar]
- Sherma S, Sen A. Complete testicular epididymal dissociation in the abdominal cryptorchid testis. J Ped Urol. 2013;9:1023–1027. doi: 10.1016/j.jpurol.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Turek PJ, Ewalt DH, Snyder HM, et al. Normal epididymal anatomy in boys. J Urol. 1994;151:726–727. doi: 10.1016/s0022-5347(17)35072-3. [DOI] [PubMed] [Google Scholar]