Abstract
Background and objective
Our objective was to investigate the efficacy and safety of dexa methasone (DEX) implant for the treatment of pseudophakic cystoid macular edema (PCME) in diabetic patients.
Study design
This was a prospective, non-randomized, interventional case series of 43 participants. Eighteen patients were enrolled in the DEX implant group and 25 were enrolled in an intravitreal triamcinolone acetonide (IVTA) group.
Main outcome measures
The primary efficacy measurement was the percentage of patients who gained improvements of more than ten letters in best corrected visual acuity (BCVA) during 6 months of follow-up. Other efficacy measurements included change in BCVA, change in central macular thickness (CMT), and number of retreatments. The primary safety evaluation was the percentage of patients with intraocular hypertension and variation in intraocular pressure (IOP) during 6 months of follow-up. Other adverse events, such as conjunctival hemorrhage, eye pain, secondary infection, endophthalmitis, noninfectious inflammation, retinal detachment, and implant migration, were also recorded during follow-up.
Results
At month 1, we observed that the percentage of patients gaining improvement of more than ten letters was similar in both groups (P=0.625). As patients in the IVTA group were retreated several times, this effect persisted throughout the study (P=0.941 at month 2, P=0.553 at month 3, P=0.856 at month 6). Variations in CMT were noticed at week 1 and reached their maximum at month 1. No significant difference was found between the two groups (P=0.831 at week 1, P=0.783 at month 1). At month 1, the variation in IOP reached its maximum in the DEX implant group and then decreased slightly. However, in the IVTA group, it increased continuously throughout the study. Conjunctival hemorrhage and eye pain were found in both groups, but both were rated as mild in severity, and no significant difference was found (P=0.184, P=0.766, respectively).
Conclusion
Both IVTA and DEX implants could effectively restore visual function and recover morphological change in diabetic patients with PCME for at least 6 months, but repeated intravitreal injection was required in the IVTA group. DEX implant is well tolerated. We suggest that intravitreal injection of DEX implant is a promising new therapeutic option for diabetic patients with PCME.
Keywords: dexamethasone implant, intravitreal triamcinolone acetonide, pseudophakic cystoid macular edema, diabetic retinopathy, corticosteroid, inflammation
Introduction
Pseudophakic cystoid macular edema (PCME) is one of the most common causes of visual impairment after uneventful cataract surgery.1 With the development of surgical technique, instrumentation, and lens design in recent years, the incidence of clinical PCME is very low in the normal population (0.2%–2%).2 However, it is still high in diabetic patients, even in the absence of diabetic retinopathy.3 The general prognosis for PCME is good; however, in diabetic patients, PCME is unlikely to spontaneously resolve, and visual acuity is frequently poor.4
The pathogenesis of PCME is likely multifactorial, but inflammation appears to be the major reason. It is widely accepted that the purpose of anti-inflammatory treatment is to not only treat but also prevent the development of PCME. Topical nonsteroidal anti-inflammatory drugs are commonly used in the perioperative period of cataract surgery, but not all patients are sensitive to them. Corticosteroids have excellent anti-inflammatory properties and a synergistic effect when combined with nonsteroidal anti-inflammatory drugs. Triamcinolone acetonide (TA) is an anti-inflammatory drug and immunomodulator used in a variety of ocular diseases. Previous studies have suggested that intravitreal TA (IVTA) is efficacious in the treatment of refractory PCME.5–7 However, IVTA has a high rate of side effects, such as glucocorticoid-induced glaucoma, posterior subcapsular cataract formation, and endophthalmitis, which restrict its application.8
Dexamethasone (DEX) has an outstanding anti-inflammatory activity that is about six-fold that of TA. Recently, a biocompatible intravitreal DEX implant (Ozurdex®; Allergan Inc., Irvine, CA, USA) has been developed that shows a persistent release of 0.7 mg DEX in two phases: a high concentration in the first 6 weeks, and a lower concentration during the next 6 months. Until now, many studies have suggested that DEX implant intravitreal injection was a promising treatment of cystoid macular edema (CME) arising from a variety of disorders.9–11 However, no study has yet reported the efficacy and safety of DEX implant on PCME in diabetic patients. Currently, a phase II study is enrolling patients to investigate Ozurdex in managing combined CME and diabetes mellitus after cataract surgery, but no outcome is yet available.12
Consequently, the purpose of this study is to 1) investigate the efficacy of DEX implant for the treatment of PCME in diabetic patients; and 2) compare the efficacy and safety profile between DEX implant and IVTA in diabetic patients with PCME.
Materials and methods
Study design and patient recruitment
This project was a prospective, non-randomized, interventional study in diabetic patients with PCME between December 2011 and October 2013, following the tenets of the Declaration of Helsinki. The study received approval from the Institutional Review Board of Yellow River Hospital. All potential risks and possible benefits of either treatment were clearly explained to patients. Written informed consent was obtained from all patients.
Inclusion criteria
Inclusion criteria were as follows: 1) a diagnosis of diabetes mellitus (any subtype) with no or mild diabetic retinopathy; 2) persistent PCME after administration of topical 0.1% diclofenac sodium eye drops (Bausch and Lomb, Tampa, FL, USA) in combination with TobraDex eye drops (Alcon, Beijing, the People’s Republic of China) during the first month after cataract surgery; 3) best corrected electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) visual acuity letter score ≥35 (approximately 20/200 or better); 4) glycated hemoglobin (HbA1c) <8% as per investigation less than 3 months prior to study enrolment; and 5) no complications during surgery, especially rupture of the posterior capsule and vitreous loss.
Exclusion criteria
Exclusion criteria were as follows: 1) systemic use of corticosteroids within 90 days; 2) intraocular hypertension (intraocular pressure [IOP] ≥21 mmHg); 3) area of foveal avascular zone in arteriovenous phase of fluorescence fundus angiography (FFA) >1,000 μm in diameter; 4) history of periocular/intraocular antivascular endothelial growth factor or corticosteroid injection; 5) history of vitrectomy; or 6) history or evidence of proliferative diabetic retinopathy or diabetic macular edema in any eye before surgery.
Baseline evaluation
On initial examination, best corrected visual acuity (BCVA), central macular thickness (CMT), IOP, FFA, and slit-lamp biomicroscopy were performed. Detailed clinical history was likewise recorded.
Trained optometrists masked to both groups measured the BCVA using the E-ETDRS method as reported by Beck et al.13 IOP was recorded using Goldmann applanation tonometry. IOP ≥21 mmHg was defined as intraocular hypertension. A single vitreoretinal fellow (Yalong Dang) performed the slit lamp, fundus, and IOP evaluations.
Baseline FFA examinations were obtained. Each FFA was used to rule out the presence of retinal neovascularization and to measure the diameter of the foveal avascular zone in the arteriovenous phase.
Spectral domain optical coherence tomography (SD-OCT; 3D OCT-2000, Topcon Corporation, Japan) was used to detect the CMT as previously described by Adhi et al.14 Briefly, after dilating the pupil with Tropicamide eye drops (Santen, Osaka, Japan), a three-dimensional macula protocol was used to measure the CMT. CMT was defined as the macular thickness of the innermost 1 mm ring. Mean macular thickness was defined as the average macular thickness from all nine regions of the ETDRS map. All scans were taken by two independent observers.
Follow-up evaluation
Patients underwent BCVA, CMT measurements, and slit lamp and fundus evaluations on the first postoperative day and thereafter at the end of the 1st week, and the 1st, 2nd, 3rd, and 6th months.
Postoperative adverse events, such as intraocular hypertension, secondary ocular infections, endophthalmitis, noninfectious inflammation, retinal detachments, conjunctival hemorrhage, and implant migration were monitored at each follow-up.
Patients with IOP over 30 mmHg received IOP-lowering medications.
Retreatment criteria
Need for retreatment was assessed at month 2, 3, 4, 5, and 6 after the first injection. To be eligible for retreatment, patients had to meet all three of the following criteria: 1) CMT increase of 100 μm or more compared with the previous result; 2) a minimum interval of at least 2 months between treatments had passed for IVTA retreatment and at least 6 months for DEX implant retreatment; 3) the patient was not at significant risk from retreatment and may benefit from retreatment.
Outcome measures
The primary efficacy measurement was the percentage of patients who gained more than ten letter improvements in BCVA during 6 months of follow-up. Other efficacy measurements included changes in BCVA, changes in CMT, and number of retreatments.
The primary safety evaluation was the percentage of patients with intraocular hypertension and the variation in IOP during the 6-month follow-up. Intravitreal injection-related effects, such as endophthalmitis, noninfectious inflammation, retinal detachment, conjunctival hemorrhage, and implant migration were also recorded.
Statistical analyses
All data were analyzed by Predictive Analytics Software (PASW statistics, version 18.0; SPSS Inc., Chicago, IL, USA). Categorical data were analyzed using Fisher’s exact test. Continuous data were analyzed using the Student’s independent t-test or one-way analysis of variance (ANOVA). Results were considered significant at P-values <0.05.
Results
Baseline characteristics
A total of 43 patients (18 patients in the DEX implant group, 25 in the IVTA group) were enrolled in the study. As shown in Table 1, patients in both groups were similar regarding age, sex, BCVA, CMT, IOP, type of diabetes mellitus, duration of macular edema, and stage of diabetic retinopathy at baseline.
Table 1.
Baseline characteristics
| DEX implant group (n=18) |
IVTA group (n=25) |
P-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | 65.6±12.0 | 68.9±11.8 | 0.561 |
| Sex (male:female) | 10:8 | 16:9 | 0.576 |
| Duration of diabetes mellitus (years) | |||
| Mean ± SD | 18.5±8.3 | 15.5±10.6 | 0.432 |
| Range | 9~37 | 7~38 | |
| Type of diabetes mellitus | |||
| Insulin independent | 15 | 21 | 0.953 |
| Insulin dependent | 3 | 4 | |
| Stage of diabetic retinopathy | |||
| No diabetic retinopathy | 11 | 12 | 0.395 |
| NPDR | 7 | 13 | |
| Duration of macular edema (months) | |||
| Mean ± SD | 3.7±1.8 | 4.2±1.5 | 0.674 |
| Range | 0.75~6 | 1~7.5 | |
| BCVA (letters) | |||
| Mean ± SD | 66.3±8.2 | 64.5±9.6 | 0.936 |
| Range | 36~75 | 38~76 | |
| CMT (μm) | |||
| Mean ± SD | 387.5±96.4 | 405.2±120.6 | 0.891 |
| Range | 284~598 | 278~649 | |
| IOP (mmHg) | |||
| Mean ± SD | 15.4±3.4 | 16.2±1.9 | 0.742 |
| Range | 7.8~21.7 | 9.4~20.6 | |
Notes: Among-group comparison using Fisher’s exact test or Student’s independent t-test, P-values <0.05 were considered to be statistically significant.
Abbreviations: BCVA, best corrected visual acuity; CMT, central macular thickness; DEX, dexamethasone; IOP, intraocular pressure; IVTA, intravitreal triamcinolone acetonide; NPDR, nonproliferative diabetic retinopathy; SD, standard deviation.
Number of retreatments
As shown in Table 2, all patients in the DEX implant group received a single intravitreal injection during the study. However, four patients at month 2, three patients at month 3, two patients at month 4, and one patient at month 5 received a second intravitreal injection of TA. Moreover, one patient received a third intravitreal injection at month 5 in the IVTA group.
Table 2.
Number of retreatments
| Week 1 | Month
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| DEX implant group | 0 | 0 | 0 | 0 | 0 | 0 |
| IVTA group | 0 | 0 | 4 | 3 | 2 | 1, 1a |
Note:
A patient in the IVTA group received a third injection of IVTA.
Abbreviations: DEX, dexamethasone; IVTA, intravitreal triamcinolone acetonide.
Efficacy analysis
BCVA
At baseline, the mean BCVA in the DEX implant group and in the IVTA group was (66.3±8.2) letters and (64.5±9.6) letters, respectively (among-group P=0.936, Table 1).
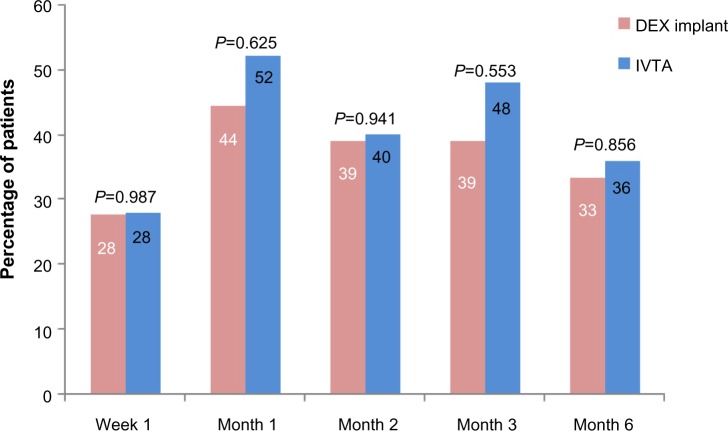
Figure 1 shows the percentage of patients who gained improvements of more than ten letters in BCVA from baseline at each follow-up. At week 1, the same percentage of patients achieved improvement of at least ten letters in both groups (28% in both groups; P=0.987). Both groups reached their peak at month 1 (52% in the IVTA group, 44% in the DEX implant group; P=0.625). We also noticed a higher percentage in the IVTA group at months 1, 2, 3, and 6, but the difference was not significant (52% vs 44%, P=0.625; 40% vs 39%, P=0.941; 48% vs 39%, P=0.553; and 36% vs 33%, P=0.856, respectively).
Figure 1.
Percentage of patients achieving improvement of more than ten letters in best corrected visual acuity from baseline.
Notes: P-values represent the comparison between the dexamethasone implant group and the intravitreal triamcinolone acetonide group. A P-value of <0.05 was considered to be statistically significant.
Abbreviations: DEX, dexamethasone; IVTA, intravitreal triamcinolone acetonide.
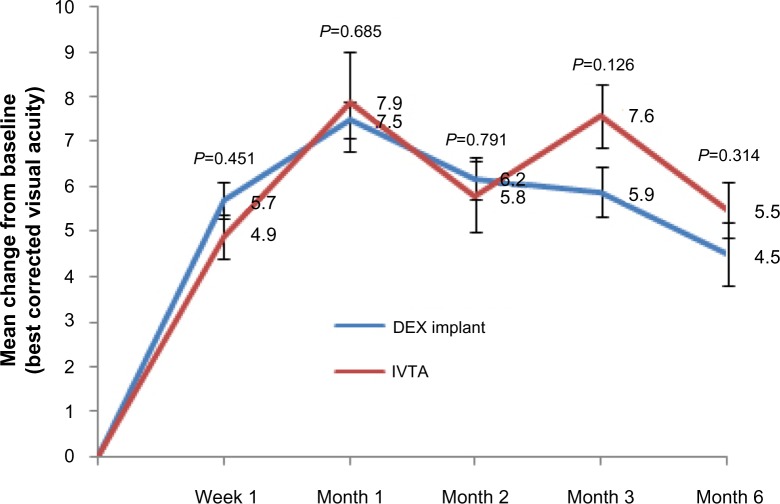
Figure 2 shows the variation of BCVA from baseline in the two groups. Parallel with the results shown in Figure 1, both groups achieved improvement of several letters in BCVA at week 1. The maximum improvement in BCVA was also noticed at month 1. In the DEX implant group, a slight but sustained decrease in the variation was observed at months 2, 3, and 5. However, in the IVTA group, the variation in BCVA reached its second crest at month 3 and then decreased to 5.5±0.63 letters at month 6. No significant difference was observed between the two groups at each follow-up (P=0.451, 0.685, 0.791, 0.126, 0.314, respectively).
Figure 2.
Mean variation in best corrected visual acuity from baseline in either group.
Notes: The mean ± standard deviation best corrected visual acuity at baseline was 66.3±8.2 letters in the dexamethasone implant group and 64.5±9.6 letters in the intravitreal triamcinolone acetonide group (P=0.936). Error bars are the standard errors of the mean. P-values represent the comparison between the dexamethasone implant group and the intravitreal triamcinolone acetonide group. A P-value of <0.05 was considered to be statistically significant.
Abbreviations: DEX, dexamethasone; IVTA, intravitreal triamcinolone acetonide.
CMT
At baseline, mean CMT in the DEX implant group and in the IVTA group was 387.5±96.4 μm and 405.2±120.6 μm, respectively (among-group P=0.891, Table 1).
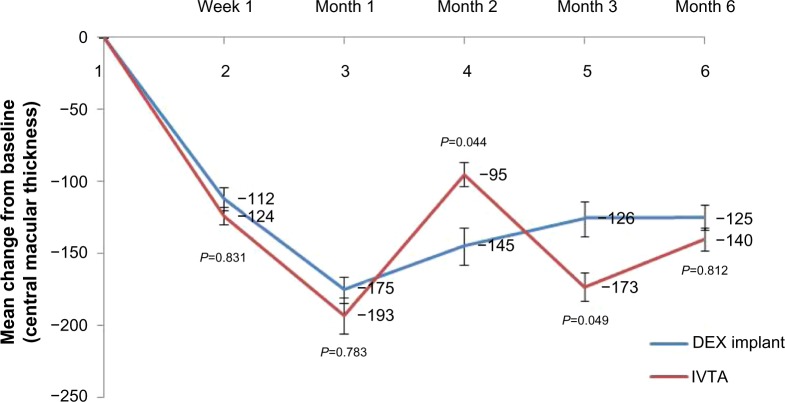
As shown in Figure 3, the variation of CMT was noticed at week 1 (112±8 μm in the DEX implant group, 124±6 μm in the IVTA group) and reached its maximum at month 1 (175±9 μm in the DEX implant group, 193±12.5 μm in the IVTA group). No significant difference was found between the two groups (P=0.831, P=0.783, respectively). At month 2, the variation in CMT was sharply reduced in the IVTA group. After retreatment in four patients, it reached another crest at month 3 and then reduced to 140±8 μm at month 6. In the DEX implant group, a slight but sustained decrease in the variation of CMT was found at months 2, 3, and 6. We also noticed significant differences between the two groups in months 2 and 3 (P=0.044, P=0.049, respectively). An interesting case was also reported (Figure 6).
Figure 3.
Mean variation of central macular thickness from baseline in either group.
Notes: The mean ± standard deviation central macular thickness at baseline was 387.5±96.4 μm in the dexamethasone implant group and 405.2±120.6 μm in the intravitreal triamcinolone acetonide group (P=0.891). Error bars are standard errors of the mean. P-values represent the comparison between the dexamethasone implant group and the intravitreal triamcinolone acetonide group. A P-value of <0.05 was considered to be statistically significant.
Abbreviations: DEX, dexamethasone; IVTA, intravitreal triamcinolone acetonide.
Figure 6.
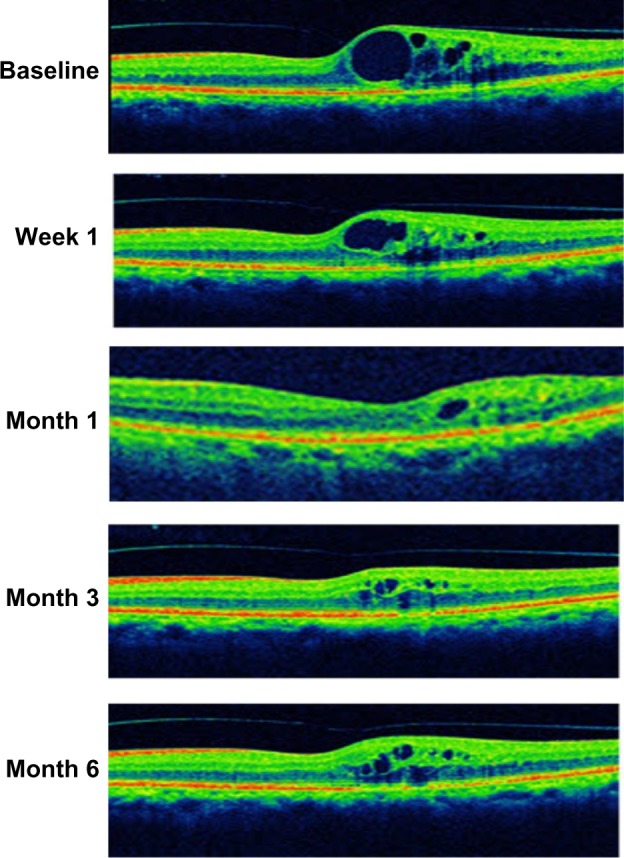
Case report: a 73-year-old male was diagnosed with pseudophakic cystoid macular edema 11 months prior to treatment.
Notes: At baseline, the central macular thickness was 413 μm and the best corrected visual acuity was 66 ETDRS letters. After intravitreal injection of dexamethasone implant, central macular thickness sharply decreased to 387 μm at week 1 and reached the minimum at month 1 (268 μm). At that time, the best correct visual acuity improved to 79 ETDRS letters. However, a slight but sustained increase in central macular thickness was observed at month 3 and month 6. The best corrected visual acuity also reduced to 74 ETDRS letters at the end of the study.
Abbreviation: ETDRS, Early Treatment Diabetic Retinopathy Study.
Safety analysis
IOP
Table 1 shows that the mean IOP was 15.4±3.4 mmHg in the DEX implant group and 16.2±1.9 mmHg in the IVTA group at baseline. No significant difference was noticed (P=0.742).
Figure 4 displays the variation in IOP. At the first week after treatment, the changes were obscure (P=0.283). At month 1, the variation in IOP reached the maximum in the DEX implant group and then slightly decreased. However, the variation in IOP continuously increased throughout the study in the IVTA group. Moreover, we noticed significant differences between the two groups at months 3 and 6 (P=0.011, P=0.006, respectively).
Figure 4.
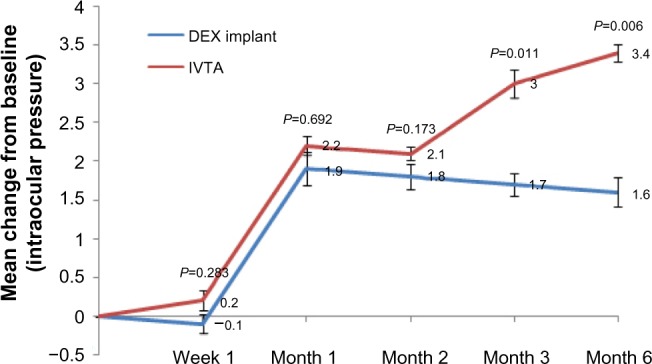
Mean variation in intraocular pressure from baseline in each group.
Notes: The mean ± standard deviation intraocular pressure at baseline was 15.4±3.4 mmHg in the dexamethasone implant group and 16.2±1.9 mmHg in the intravitreal triamcinolone acetonide group (P=0.742). Error bars are standard errors of the mean. P-values represent the comparison between the dexamethasone implant group and the intravitreal triamcinolone acetonide group. A P-value of <0.05 was considered to be statistically significant.
Abbreviations: DEX, dexamethasone; IVTA, intravitreal triamcinolone acetonide.
As shown in Figure 5, intraocular hypertension occurred in 6% of patients in the DEX implant group at months 1, 2, and 3. However, more than 12% of patients had intraocular hypertension in the IVTA group after month 1, and a significant difference occurred at month 6 (P=0.044).
Figure 5.
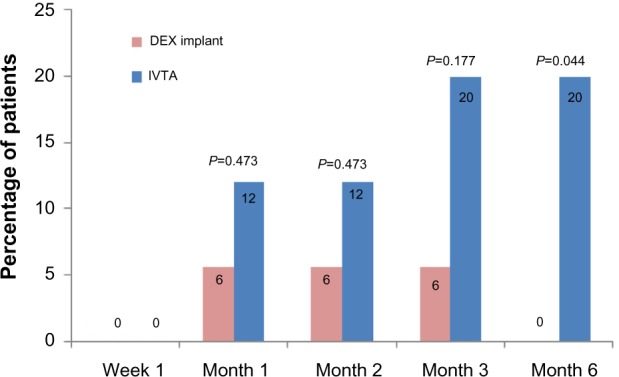
Percentage of patients with intraocular hypertension in each group.
Notes: P-values represent the comparison between the dexamethasone implant group and the intravitreal triamcinolone acetonide group. A P-value of <0.05 was considered to be statistically significant.
Abbreviations: DEX, dexamethasone; IVTA, intravitreal triamcinolone acetonide.
Other adverse events
Other adverse events are summarized in Table 3. Conjunctival hemorrhage occurred in 22.2% of patients in the DEX implant group and 8% of patients in the IVTA group (P=0.184). Eye pain occurred in 27.8% of patients in the DEX implant group, and 32% of patients in the IVTA group (P=0.766). Secondary infection, endophthalmitis, noninfectious inflammation, retinal detachment, and implant migration were not observed in the study. All adverse events were rated as mild in severity and considered by the investigator to be linked to the surgical procedure.
Table 3.
Other adverse events observed during 6 months of follow-up
| DEX implant group (n=18) |
IVTA group (n=25) |
P-value | |
|---|---|---|---|
| Secondary infection | 0 | 0 | – |
| Endophthalmitis | 0 | 0 | – |
| Noninfectious inflammation | 0 | 0 | – |
| Retinal detachment | 0 | 0 | – |
| Conjunctival hemorrhage | 4 (22.2) | 2 (8) | 0.184 |
| Implant migration | 0 | 0 | – |
| Eye pain | 5 (27.8) | 8 (32) | 0.766 |
Notes: Among-group comparison using Fisher’s exact t-test, P-values <0.05 were considered to be statistically significant. Data are presented as n (%) unless otherwise indicated.
Abbreviations: DEX, dexamethasone; IVTA, intravitreal triamcinolone acetonide.
Discussion
Diabetes mellitus is one of the most important risk factors in PCME. The incidence of PCME in diabetic patients is significantly greater than in those without the disease,3 especially for diabetic retinopathy.15 As inflammation plays a crucial role in PCME, inhibitors of inflammatory mediators are well recognized as a core therapeutic choice. Our results suggest that both the IVTA and the DEX implant effectively improved BCVA and decreased CMT in diabetic patients with PCME during 6 months of follow-up, but these effects required more intravitreal injections in the IVTA group. In addition, we also reported a relatively high percentage of patients with intraocular hypertension in the IVTA group. To our knowledge, this is the first study to compare the efficacy and safety of IVTA and DEX implant for the treatment of PCME in diabetic patients. This study will help us to fully understand the therapeutic effect of corticosteroids and make an advisable decision in clinical practice.
DEX implant is a novel biodegradable drug-delivery system that has been approved by the US Food and Drug Administration. Recent studies have suggested that intravitreal injection of DEX implant could effectively restore visual function and recover morphological changes in various types of CME.16–19 In a 12-month, randomized, multicenter trial reported by Callanan et al,18 a greater improvement in BCVA was observed in eyes treated with DEX implant plus laser than in eyes treated with laser alone. Similarly, the decrease in CMT from baseline was significantly greater in the DEX implant plus laser group than in the laser alone group at four of the eight follow-up visits. Another phase II study also reported that more than one-third of patients achieved improvements of at least ten letters in the DEX implant group at 3 months after intravitreal injection, compared with only 12.3% of patients in the observation group.19 Nowadays, most investigators mainly focus on diabetic macular edema,18,19 uveitis-related macular edema,17 retinal vein occlusion-related macular edema,20 or PCME in healthy populations.17 However, PCME in diabetic patients is more complex than in healthy populations or other types of CME. It might partly be attributable to surgery-related trauma or diabetes itself. In our study, we found that 44% of patients achieved ten letters or more in BCVA at the end of the first month after intravitreal injection. Distinct from the variation of BCVA in the IVTA group, this effect persisted to 6 months without additional injections. This is the only study to report that DEX implant efficiently restored visual function and morphological change in diabetic patients with PCME.
The most important findings of this study are the similar efficacy of IVTA and DEX implant in diabetic patients with PCME. At month 1, a similar percentage of patients were observed to have gained improvements of more than ten letters in the two groups (P=0.625). With several retreatments in the IVTA group, this effect persisted throughout the study (P=0.941 at month 2, P=0.553 at month 3, P=0.856 at month 6). CMT is another parameter with which to evaluate the efficacy of various treatments for patients with PCME. Consistent with the variation in BCVA, we noticed a remarkable reduction in CMT in both groups at each follow-up. However, an apparent mismatch between the variation of CMT and BCVA was evident during the study. At month 2, the mean variation of CMT sharply decreased in the IVTA group (by approximately 98 μm), and the difference between the two groups was statistically significant (P=0.044); however, no difference was observed in the variation in BCVA (P=0.791). The analogous phenomenon also occurred at month 3. These paradoxical results could be attributed to the weak correlation between decreases in CMT and improvement in BCVA, as previously described by several studies.21,22
Compared with IVTA, the DEX implant was well tolerated and had an acceptable safety profile. First, to sustain restored visual function within 6 months, an average of 1.44 injections were required in the IVTA group, but only a single injection in the DEX implant group. Second, the DEX implant is biocompatible. The copolymer degrades over time into carbon dioxide and water, and does not require surgical removal. Third, the most important feature of the DEX implant is its relatively low incidence of intraocular hypertension. Elevated IOP is a common steroid-related complication, but it seems more frequent and serious after IVTA. In a retrospective, consecutive case series23 in which a total of 528 eyes received a single IVTA, intraocular hypertension occurred in 53.2% of patients; 50.6% of them experienced an IOP elevation over 30% of baseline. Additionally, 28 of 43 (65.1%) eyes that received a second injection experienced an increase in IOP of at least 30% of baseline. Filtering surgery was required in several cases. Kocabora et al24 also analyzed IOP elevation secondary to IVTA and discussed its management. In their study, 27.7% of eyes received topical anti-glaucomatous therapy, and 4.8% of eyes required surgical intervention to lower IOP. The authors suggested that patients who received IVTA should be carefully monitored for elevated IOP. However, recent studies have suggested that the DEX implant had a favorable safety profile, even in a long-term follow-up or retreatment. In a 12-month, multicenter, prospective clinical trial,20 12.6% of patients after the first intravitreal injection of the DEX implant and 15.4% of patients after the second treatment had IOP increases of 10 mmHg or higher. In these patients, the IOP increases were transient and well controlled with topical medication. Similarly, in a randomized, multicenter, parallel-group, 12-month trial by the Ozurdex PLACID Study Group,18 a total of 15.2% (19/125) of patients had an increase in IOP from baseline of more than 10 mmHg, 16.8% (21/125) of patients had an IOP over 25 mmHg, and 4.0% (5/125) of patients had an IOP over 35 mmHg. All cases with intraocular hypertension were managed with IOP-lowering medication, and no surgery was required during the study. Consistent with the previous studies, as shown in Figures 4 and 5, the mean variation in IOP was significantly higher in the IVTA group than in the DEX implant group at months 3 and 6. The percentage of patients with intraocular hypertension was much greater in the IVTA group. The high rate of intraocular hypertension is probably attributable to the intrinsic characteristic of IVTA and high rate of retreatment.
Conjunctival hemorrhage was reported in 4 of 18 patients in the DEX implant group, and 2 of 25 patients in the IVTA group. Eye pain occurred in 5 of 18 patients in the DEX implant group, and 8 of 25 patients in the IVTA group. There was no significant difference between the two groups in the incidence of these adverse events in the overall patient population; therefore, this difference may be the result of chance or the effect of the underlying disease process.
This retrospective study has limitations. A relatively small number of patients enrolled in the study, and the study was not powered to show statistically significant between-group differences. In addition, we noticed a slight but sustained deterioration of BCVA and CMT in the DEX implant group at months 2, 3, and 6. The follow-up time is too short to obtain sufficient information to make an advisable decision on retreatment. While it is generally recognized that at least a 6-month interval is essential for DEX implant retreatment, a long-term observational study is also required to investigate the optimum interval.
Conclusion
Our findings suggest that 1) both IVTA and DEX implant could effectively restore visual function and recover morphological change in diabetic patients with PCME for at least 6 months, but repeated intravitreal injection is required in the IVTA group; 2) the DEX implant is well tolerated. Based on the results above, we conclude that intravitreal injection of DEX implant is a promising new therapeutic option for diabetic patients with PCME.
Acknowledgments
This study was also supported by the International Cooperation Project of Henan Province (2013GH11), the National Natural Science Foundation of China (No 81371017), and the Key Project of Science Research of Henan Province Education Bureau (No 13A320427).
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol. 2012;23(1):26–32. doi: 10.1097/ICU.0b013e32834cd5f8. [DOI] [PubMed] [Google Scholar]
- 2.Lobo C. Pseudophakic cystoid macular edema. Ophthalmologica. 2012;227(2):61–67. doi: 10.1159/000331277. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi K, Igarashi C, Hirata A, Hayashi H. Changes in diabetic macular oedema after phacoemulsification surgery. Eye (Lond) 2009;23(2):389–396. doi: 10.1038/sj.eye.6703022. [DOI] [PubMed] [Google Scholar]
- 4.Horozoglu F, Yanyali A, Aytug B, Nohutcu AF, Keskinbora KH. Macular thickness changes after phacoemulsification in previously vitrectomized eyes for diabetic macular edema. Retina. 2011;31(6):1095–1100. doi: 10.1097/IAE.0b013e3181f98cd5. [DOI] [PubMed] [Google Scholar]
- 5.Ahmadabadi HF, Mohammadi M, Beheshtnejad H, Mirshahi A. Effect of intravitreal triamcinolone acetonide injection on central macular thickness in diabetic patients having phacoemulsification. J Cataract Refract Surg. 2010;36(6):917–922. doi: 10.1016/j.jcrs.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Benhamou N, Massin P, Haouchine B, Audren F, Tadayoni R, Gaudric A. Intravitreal triamcinolone for refractory pseudophakic macular edema. Am J Ophthalmol. 2003;135(2):246–249. doi: 10.1016/s0002-9394(02)01938-4. [DOI] [PubMed] [Google Scholar]
- 7.Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide for pseudophakic cystoid macular edema. Am J Ophthalmol. 2003;136(2):384–386. doi: 10.1016/s0002-9394(03)00230-7. [DOI] [PubMed] [Google Scholar]
- 8.Jonas JB. Intravitreal triamcinolone acetonide: a change in a paradigm. Ophthalmic Res. 2006;38(4):218–245. doi: 10.1159/000093796. [DOI] [PubMed] [Google Scholar]
- 9.Zalewski D, Raczyńska D, Raczyńska K. Five-month observation of persistent diabetic macular edema after intravitreal injection of Ozurdex implant. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/364143. Article ID 364143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parravano M, Oddone F, Boccassini B, et al. Exploring the morphological and functional retinal changes after dexamethasone intravitreal implant (Ozurdex®) in macular edema due to retinal vein occlusion. Ophthalmic Res. 2014;51(3):153–160. doi: 10.1159/000357275. [DOI] [PubMed] [Google Scholar]
- 11.Pichi F, Specchia C, Vitale L, et al. Combination therapy with dexamethasone intravitreal implant and macular grid laser in patients with branch retinal vein occlusion. Am J Ophthalmol. 2014;157(3):607–615. e1. doi: 10.1016/j.ajo.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Northern California Retina Vitreous Associates Ozurdex for combined pseudophakic cystoid macular edema and diabetic macular edema after cataract surgery. [Accessed January 6, 2014]. Available from: http://clinicaltrials.gov/ct2/show/NCT01284478. NLM identifier: NCT01284478.
- 13.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 14.Adhi M, Aziz S, Muhammad K, Adhi MI. Macular thickness by age and gender in healthy eyes using spectral domain optical coherence tomography. PLoS One. 2012;7(5):e37638. doi: 10.1371/journal.pone.0037638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollack A, Leiba H, Bukelman A, Oliver M. Cystoid macular oedema following cataract extraction in patients with diabetes. Br J Ophthalmol. 1992;76(4):221–224. doi: 10.1136/bjo.76.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuppermann BD, Blumenkranz MS, Haller JA, et al. Dexamethasone DDS Phase II Study Group Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125(3):309–317. doi: 10.1001/archopht.125.3.309. [DOI] [PubMed] [Google Scholar]
- 17.Williams GA, Haller JA, Kuppermann BD, et al. Dexamethasone DDS Phase II Study Group Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol. 2009;147(6):1048–1054. doi: 10.1016/j.ajo.2008.12.033. e1–2. [DOI] [PubMed] [Google Scholar]
- 18.Callanan DG, Gupta S, Boyer DS, et al. Ozurdex PLACID Study Group Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120(9):1843–1851. doi: 10.1016/j.ophtha.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Haller JA, Kuppermann BD, Blumenkranz MS, et al. Dexamethasone DDS Phase II Study Group Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128(3):289–296. doi: 10.1001/archophthalmol.2010.21. [DOI] [PubMed] [Google Scholar]
- 20.Haller JA, Bandello F, Belfort R, Jr, et al. Ozurdex GENEVA Study Group Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118(12):2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Blumenkranz MS, Haller JA, Kuppermann BD, et al. Correlation of visual acuity and macular thickness measured by optical coherence tomography in patients with persistent macular edema. Retina. 2010;30(7):1090–1094. doi: 10.1097/IAE.0b013e3181dcfaf3. [DOI] [PubMed] [Google Scholar]
- 22.Diabetic Retinopathy Clinical Research Network. Browning DJ, Glassman AR, Aiello LP, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee DJ, Peck RE, Belmont J, et al. Intraocular pressure alterations following intravitreal triamcinolone acetonide. Br J Ophthalmol. 2006;90(8):999–1003. doi: 10.1136/bjo.2006.090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocabora MS, Yilmazli C, Taskapili M, Gulkilik G, Durmaz S. Development of ocular hypertension and persistent glaucoma after intravitreal injection of triamcinolone. Clin Ophthalmol. 2008;2(1):167–171. doi: 10.2147/opth.s2359. [DOI] [PMC free article] [PubMed] [Google Scholar]