Abstract
Background:
Plastic and reconstructive surgeons are commonly faced with chronic ulcerations and consecutive wound infections of the feet as complications in patients with diabetes and/or peripheral arterial occlusive disease (PAOD). Microcirculatory changes seem to play an important role. However, the evaluation of functional changes in the soft tissue microcirculation at the plantar foot using combined Laser-Doppler and Photospectrometry System has not yet been performed in patients with DM or PAOD.
Methods:
A prospective, controlled cohort study was designed consisting of a total of 107 subjects allocated to 1 of 3 groups—group A: healthy subjects (57% males, 63.3 y); group B: patients with diabetes mellitus (DM) (53% males, 59.4 y); and group C: patients with PAOD (81% males, 66.1 y). Microcirculatory data were assessed using a combined Laser-Doppler and Photospectrometry System.
Results:
Global cutaneous oxygen saturation microcirculation at the plantar foot of healthy individuals was 8.4% higher than in patients with DM and 8.1% higher than in patients with PAOD (both P = 0.033). Patients with diabetes did not show significant differences in global cutaneous blood flow when compared with either healthy subjects or patients suffering from PAOD.
Conclusions:
Functional microcirculation at the plantar foot differs between healthy subjects and patients suffering from diabetes or PAOD of the same age. Patients with either diabetes or PAOD demonstrate deteriorated cutaneous oxygen saturation with equivalent blood perfusion at the plantar foot. More clinical studies have to be conducted to evaluate therapeutical methods that might ameliorate cutaneous oxygen saturation within diabetic foot disease and PAOD.
Lower limb ischemia with poor wound healing is found in patients with diabetic foot disease and peripheral arterial occlusive disease (PAOD) and remains a major reason for lower limb amputations. Specifically, more than 60% of nontraumatic lower limb amputations in the United States are performed in patients with diabetes.1 In addition, elderly patients with diabetes have a higher prevalence of PAOD (up to 6 times more) than patients without diabetes.2 Functional changes in the microcirculation are found in patients with diabetes. These changes are considered to play an important role in the pathogenesis of diabetic foot syndrome.3,4 Even though the existence of a “small vessels disease”5,6 has been disproved,7 structural and functional changes within the microvasculature of the skin still persist and are thought to be causative of diabetic foot syndrome.8,9 Several theories regarding the pathogenesis are still in discussion.4 The hemodynamic hypothesis suggests that the onset of hyperglycemia in early stages of diabetes mediates blood flow dysregulations which result in an increased flow, capillary filtration capacity,10 and microvascular pressure.11 It is thought that these changes lead to structural alterations of diabetic microangiopathy, for example, a thickened basement membrane with microvascular sclerosis. Transport processes through the capillary membrane and even the migration of leukocytes may also be impaired.3 In addition, the reduced elasticity of microvascular vessels due to the aforementioned changes impairs vasodilatation and may in combination with lost secretory functions of the endothelium result in a diminished hyperemic response in diabetic patients. This could limit nutrient supply to the tissue under conditions of stress.12–14 Another hypothesis, the “capillary steal syndrome,” suggests that the influence of autonomic neuropathy in patients with diabetes impedes sympathetic innervation of the microvasculature, causing a loss of vasoconstriction, thus directing blood away from the capillaries through arteriovenous shunts.15,16 Studies supporting this theory have shown an increased venous oxygenation in patients with diabetic foot disease and peripheral polyneuropathy.17
As PAOD also plays an important role in the development of chronic ulcerations and consecutive wound infections, investigations have been conducted to elucidate possible microcirculatory changes in patients with impeded macrovascular circulation. The pathogenesis of PAOD can be described with the development of atherosclerosis which involves changes in both the macro- and microcirculation.18 Even though Laser Doppler studies showed a normal baseline leg skin perfusion in stage II PAOD patients,19,20 impaired postischemic hyperemic reaction,19 an abnormal flowmotion pattern,20 and impeded vascular reactivity21,22 have been shown.
To further evaluate the underlying mechanisms of impaired microcirculation in patients with diabetes and PAOD, a variety of techniques for in vivo assessment of microcirculatory effects have been developed. Combined Laser-Doppler and Photospectrometry has been shown to be a useful tool for quantitative, noninvasive tissue measurements,23–30 and studies published in the literature have investigated microcirculatory parameters in diabetic patients and patients with PAOD using this technique.23,30–33
To our knowledge, no study has been published as of yet, mapping functional microcirculatory parameters at the plantar foot in healthy subjects and in patients with diabetes mellitus and PAOD using combined Laser-Doppler and Photospectrometry systems. Therefore, we hypothesized that the functional microcirculation at the plantar foot significantly differs between healthy subjects and patients with diabetes mellitus and/or PAOD.
SUBJECTS AND METHODS
Clinical Trial Registration
The trial was registered at ClinicalTrials.gov with the identifier number NCT01235312. The trial was performed in accordance with the Declaration of Helsinki (2000) of the World Medical Association. Institutional review board approval was granted through the local institutional review board committee. Written consent was obtained from each study subject before the start of the investigation.
Reporting Standards
Data are reported according to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.
Study Design and Setting
The study was designed as a prospective, controlled, and blinded cohort study. Data collection was performed between August 2011 and May 2012 using combined Laser-Doppler and Photospectrometry. For mapping of the microcirculatory parameters, 10 standardized measurement points were chosen equally distributed over the plantar foot (Fig. 1).
Fig. 1.
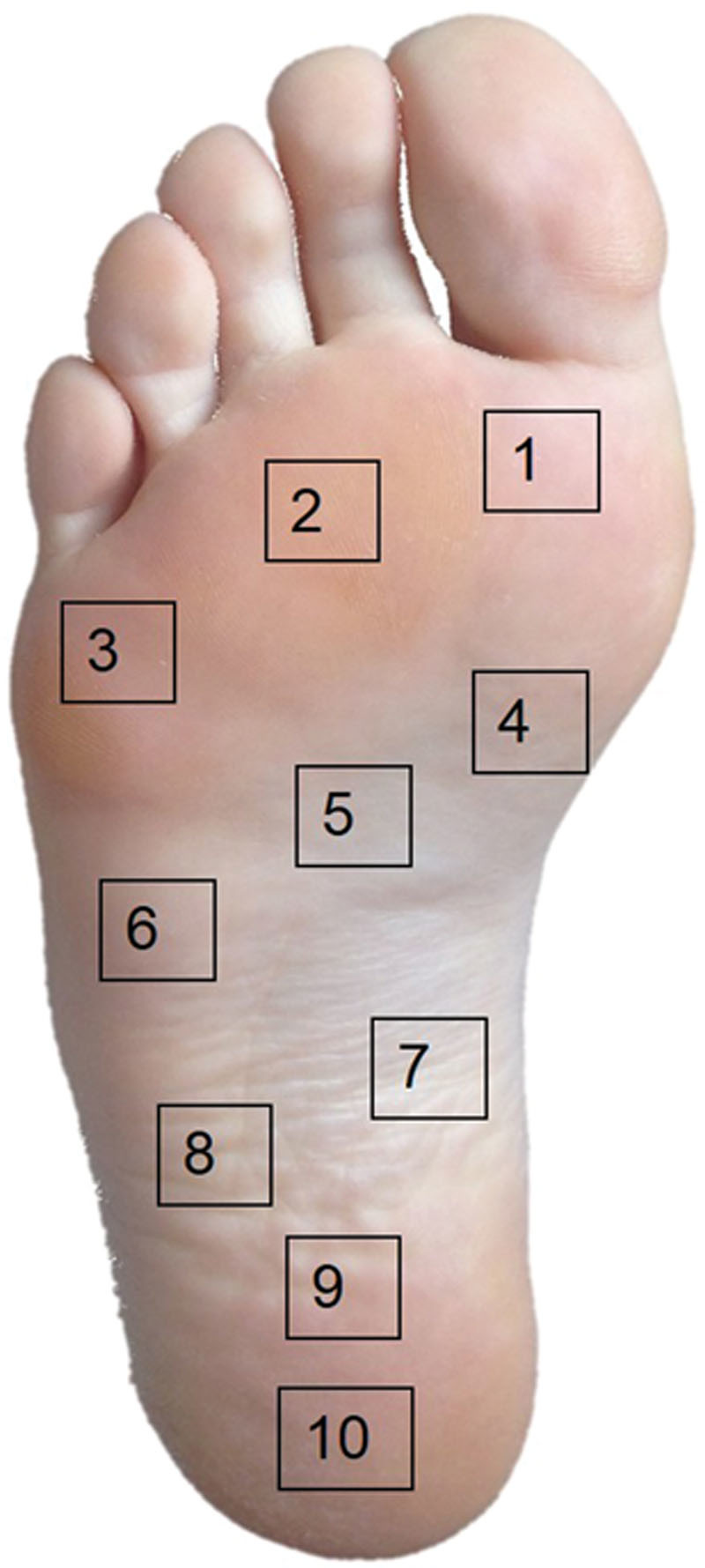
Location of 10 measurement points at the plantar foot.
Participants
Study Population and Recruitment
Eligibility criteria were male and female subjects aged 18–75 years old either healthy or suffering from diabetes mellitus or PAOD.
After inclusion in the trial, participants were assigned to 1 of 3 groups according to their medical history. Group A was the control group consisting only of healthy subjects. Group B included patients with nonischemic diabetes mellitus type I or II. Group C consisted of patients with a medical history of PAOD.
Eligibility criteria for the control group were healthy subjects excluding diabetes mellitus, Raynaud’s disease, evident plantar malperfusion, soft tissue inflammation or osteomyelitis, vasculitis, PAOD, chronic kidney or liver disease, cardiac dysfunction, arterial hypotension, and any type of vasoactive medication, that is, β-blockers, calcium channel blockers, nitroglycerin, or equivalents.
Exclusion criteria for groups B and C were soft tissue inflammation or osteomyelitis, vasculitis, and liver disease. The study population was a consecutive series of participants defined by the aforementioned selection criteria.
Variables
Determination of Vital Parameters of M icrocirculation
Hemoglobin and blood flow are measured concurrently in the Oxygen to see (O2C) system. The standardization for measuring both parameters separately and together (blood flow by Laser-Doppler technique and hemoglobin oxygenation and hemoglobin concentration by photospectrometry) has been described in detail elsewhere.23–30 Specifically, the O2C system measures these microcirculatory parameters using an optical fiber probe. The fiber probe incorporates both the laser Doppler method and broadband light spectrometry. The probe used in this study assessed the following parameters at a depth of 8 mm:
Cutaneous tissue oxygen saturation (%)
Cutaneous relative postcapillary venous filling pressure (arbitrary units, AU)
Cutaneous capillary blood flow (AU)
Microcirculatory data assessment using the O2C system was performed by the same experienced examiner. Data were blinded for statistical analysis.
Bias
Structural measurement bias was avoided by standardization of environmental factors during assessment of microcirculatory data, that is, assessment by the same examiner, uniform positioning of the subject during measurement, and standardization of ambient light and temperature. Artifacts from microcirculatory measurements were avoided by fixation of each measurement probe on every position for at least 90 seconds.
Retrieval biases were prevented by an independent literature search through the National Library of Medicine. Funding bias was eliminated by the exclusion of any financial disclosure of any contributing author.
Statistical Analysis
One-way analysis of variance was performed for statistical analysis between groups for microcirculatory data, biometrical data, and patient’s characteristics. To complete the multivariate hypothesis testing, significance of the diabetes group B and the PAOD group C was tested vs healthy group A by using Fisher’s least significant difference and Bonferroni post hoc test. P-values less than 0.05 were considered to be statistically significant. The SPSS statistical software package 20 for Windows (IBM, NY) was used for statistical analysis.
RESULTS
Participants and Descriptive Data
A total of 107 patients met the inclusion and exclusion criteria and were included in the study after written informed consent was obtained.
Detailed patients’ characteristics such as age, height, weight, body mass index (BMI), concomitant diseases, and medication are reported in Table 1. Subjects were allocated to groups A/B/C according to their medical history. Group A was the control group consisting of healthy subjects (57% male; 63.3 ± 12.2 y; BMI, 27.1 ± 4.7). Group B included patients with nonischemic diabetes mellitus with an average diabetes duration of 12.5 years (53% male; 59.4 ± 17.5 y; BMI, 29.6 ± 6.2). Group C consisted of patients with a medical history of PAOD (81% male; 66.1 ± 11.3 y; BMI, 27.4 ± 4.6). The average duration of PAOD of these patients is 4.75 years. Statistical analysis showed an equal distribution of patients between groups regarding gender, age, height, weight, and BMI.
Table 1.
Patient Characteristics: Group A (Healthy Subjects) vs Group B (Diabetes Mellitus) vs Group C (PAOD)
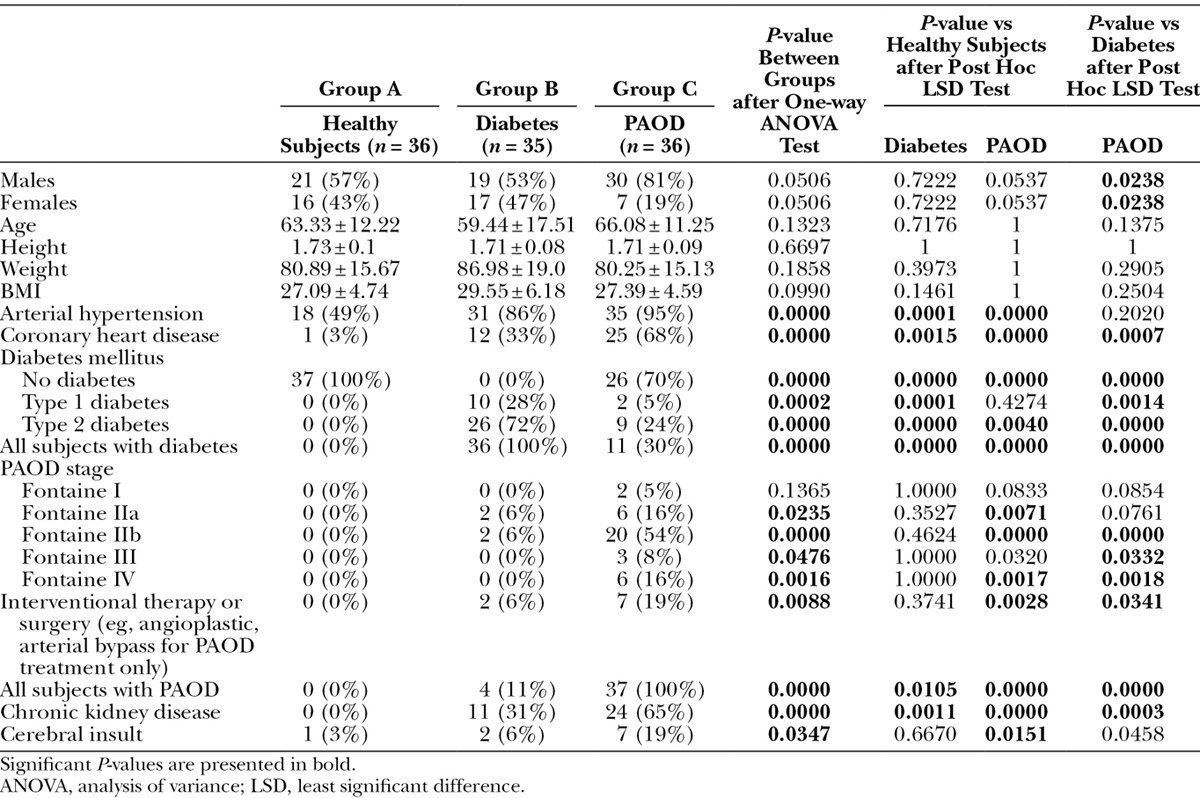
The number of patients suffering from arterial hypertension, cardiac diseases, diabetes mellitus, PAOD, and chronic kidney diseases were significantly higher in groups B and C compared to group A (all parameters P < 0.05). A significantly higher prevalence of patients with cardiac diseases, PAOD, and chronic kidney disease were apparent in group C (PAOD) relative to group B (diabetes mellitus) and a significantly lower number of patients suffering from diabetes mellitus in group C vs group B (all P < 0.05)
Microcirculatory Analysis
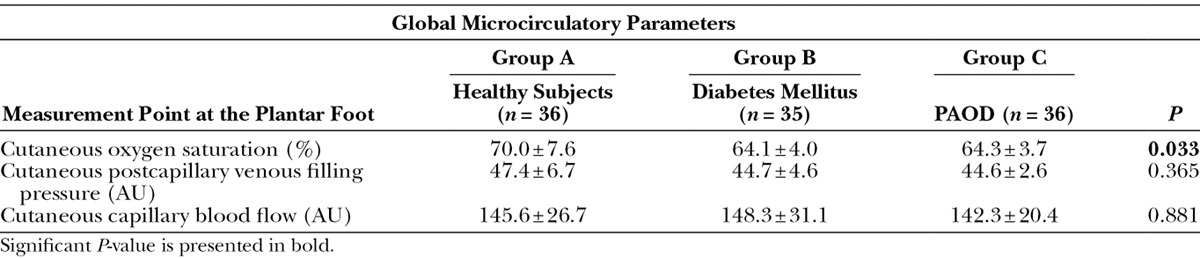
The microcirculatory data of the plantar side of the foot were averaged and displayed as mean and standard deviation. Detailed microcirculatory data of all assessed measurement points are reported in Table 2.
Table 2.
Global Cutaneous Oxygen Saturation, Global Cutaneous Postcapillary Venous Filling Pressure, and Global Cutaneous Capillary Blood Flow of Groups A (Healthy Subjects) vs B (Diabetes Mellitus) vs C (PAOD) at all 10 Measurement Points at the Plantar Side of the Foot
Cutaneous Oxygen Saturation
Global cutaneous oxygen saturation microcirculation at the plantar foot of healthy individuals was 8.4% higher than in patients with diabetes mellitus and 8.1% higher than in patients with PAOD (P = 0.033) (group A vs group B: 70.0% ± 7.6% vs 64.1% ± 4.0%; P = 0.061; group A vs group C: 70.0% ± 10.9% vs 64.3% ± 3.7%; P = 0.076).
There was no statistically significant difference between subjects with diabetes mellitus and PAOD (64.1% ± 4.0% vs 64.3% ± 3.7%; P = 1.0).
Postcapillary Venous Filling Pressure
Global cutaneous postcapillary venous filling pressure at the plantar side of the foot of healthy subjects was not significantly different compared to patients with diabetes mellitus (47.4 ± 6.7 AU vs 44.7 ± 4.6 AU; P = 0.692) and compared to patients with PAOD (47.4 ± 6.7 AU vs 44.6 ± 2.6 AU, respectively; P = 0.638). Cutaneous postcapillary venous filling pressure at the plantar side of the foot was not statistically different between patients with diabetes mellitus and PAOD (44.7 ± 4.6 AU vs 44.6 ± 2.6 AU, respectively; P = 1.0).
Capillary Blood Flow
Global cutaneous capillary blood flow at the plantar side of the foot of healthy subjects was not different in patients with diabetes mellitus (group A: 145.6 ± 26.7 AU vs group B: 148.3 ± 31.1 AU; P = 1.0) and patients suffering from PAOD (group A: 145.6 ± 26.7 AU vs group C: 142.3 ± 20.4 AU; P = 1.0). Similar to postcapillary venous pressure, there was no difference between groups B and C (148.3 ± 31.1 AU vs 142.3 ± 20.4 AU, respectively; P = 1.0).
DISCUSSION
The aim of this investigation was to evaluate the functional cutaneous microcirculatory perfusion of the plantar foot in both males and females, to determine functional foot microcirculation relevant to altered wound healing processes. We therefore hypothesized that functional microcirculation at the plantar side of the foot differs between healthy patients and patients with diabetes or PAOD.
Our study assessed different parameters of functional microcirculation at the plantar foot between healthy subjects and patients with diabetes or PAOD. Patients with either diabetes or PAOD showed a global deterioration in cutaneous oxygen saturation, despite maintaining global blood perfusion and global postcapillary venous filling pressure at the plantar foot relative to healthy subjects. However, local sampling at various points of the plantar foot revealed no changes between healthy controls and patients with either diabetes or PAOD.
Despite confirming our hypothesis, the pathogenesis of the diabetic foot still remains controversial.34 In addition to vasculopathy, there are additional pathways and risk factors that can lead to foot ulcerations including peripheral neuropathy, foot trauma, foot deformity, lower limb ischemia, foot edema, and callus deformation. The combination of these findings, especially neuropathy, minor foot trauma, and foot deformity, is considered to be the leading cause of diabetic foot ulcers.35 Structural changes seen within the microvasculature of patients with diabetes, for example thickened capillary basement membranes, reduced capillary size, and pericyte degeneration have also been documented in the current scientific literature.13,36–40 However, data and theorized mechanisms regarding the role of a functional microcirculation are still controversial and require new approaches for the assessment of microcirculatory alterations in patients with diabetes.4 Combined Laser-Doppler and Photospectrometry allows quantitative measurements of soft tissue microcirculation measuring simultaneously cutaneous oxygen saturation, cutaneous postcapillary venous filling pressure, and cutaneous capillary blood flow. We thus initiated the reassessment of these microcirculatory parameters in patients with diabetes to compare them with healthy volunteers and patients with PAOD.
Recent studies support the hemodynamic hypothesis in the pathogenesis of diabetic microangiopathy, demonstrating increased capillary blood pressure in diabetic patients.10,11,41 Beckert et al23 could demonstrate a significant increase in capillary blood flow at the forefoot in diabetic foot ulcer patients compared to healthy controls, without demonstrating significant changes in cutaneous oxygen saturation using the same combined Laser-Doppler and Photospectrometry as we did in our study (O2C). However, their data are not fully comparable to our study because they had a different focus of investigation, by trying to distinguish between healing and nonhealing diabetic foot ulcers. Microcirculatory parameters might be compounded due to inflammatory processes. Several other studies contradict the hemodynamic hypothesis by reporting increased capillary blood flow and/or increased capillary flow pressure in patients with diabetes.4,42,43 In concordance to these results, we also could not detect significant changes in capillary blood flow in either diabetic patients or in PAOD patients. Iwase et al31 showed that transcutaneous oxygen pressure (TcPO2) at the dorsal foot in patients with diabetes was reduced compared to healthy controls, whereas skin blood flow did not differ significantly. This is also in agreement with our results as we reported deteriorated cutaneous oxygen saturation with equivalent blood perfusion in patients with diabetes mellitus. Iwase et al suggested that these findings might be explained with sympathetic vascular dysfunction shunting the microcirculating blood away from nutritive capillaries through anastomotic arteriovenous shunts, which would in generally confirm the “capillary steal syndrome” hypothesis.
One of the major advantages of the combined Laser-Doppler and Photospectrometry System that we used in our study is that it is capable of assessing the tissue oxygen saturation of nutritive capillaries by measuring the venular (postcapillary) oxygen saturation in the capillary bed.44 As our findings demonstrated deteriorated cutaneous oxygen saturation in these nourishing capillaries of the skin with equivalent blood perfusion and equivalent postcapillary venous filling pressure in patients with diabetes compared to healthy controls, our results support the theory of the capillary steal syndrome.
Nagase et al45 tried another approach to assess vascular alteration in nonulcer diabetic patients by taking images of the plantar foot using Infrared Thermographic Imaging in 129 diabetic patients and 32 healthy controls. They found a much higher variation in the plantar thermographic patterns in the diabetes group than in healthy controls. This established a new conceptual classification of the plantar patterns according to the plantar angiosomes and their own findings. The higher variations in plantar thermographic patterns of patients with diabetes may be one reason why current studies are not consistent with respect to a functional microcirculation in patients with diabetes. Nevertheless, a potential limitation of this study by Nagase et al may be related to age unmatched groups, a parameter controlled for in our study which may make our data more conclusive.
As most subjects in our PAOD group were stage II patients, capillary blood flow should have remained normal at rest, due to microcirculatory compensation, observations shown elsewhere.19,20 It therefore seems that the total amount of blood flow alone is not crucial for impeded microcirculation in patients with PAOD. In this regard, Bongard and Fagrell46 reported that resting blood flow in the skin microcirculation in patients with PAOD was even increased, but maldistributed. Moreover, through TcPO2 measurements, reduced O2 levels could be demonstrated in patients with PAOD,20,47 which is in line with our results as we found deteriorated cutaneous oxygen saturation with equivalent global blood perfusion in patients suffering from PAOD. In addition, studies have indicated that vascular reactivity is impeded in patients with PAOD21,22 and skin postischemic hyperemic reaction is also impaired.19 Furthermore, in patients with critical limb ischemia, a reduced local vasoconstrictor response during limb lowering, in addition to pain while at rest, showed an increase in blood flow,48 implying a capillary hypertension with more filtration, which suggests a further compromise of the nutritive capillaries while increasing the oxygen diffusion distance.20 These results and findings shown by vital capillaroscopy, for example, dilated papillary capillaries, indistinct capillaries, capillary hemorrhage with extravasation, and a reduced number of blood filled capillaries,49 may be possible reasons for changes in functional microcirculation in patients with PAOD.
There is no doubt that microcirculatory changes seem to play a major role in patients with diabetes and PAOD. In recent studies, it has been shown that among patients with critical limb ischemia, the microcirculatory assessment was a powerful indicator to predict amputation,32,50 whereas Fontaine staging, ankle blood pressure, or the presence of diabetes mellitus did not.50 Thus, it can be concluded that the microcirculation may have an even bigger impact on morbidity than an impeded macrovasculature. Moreover, Rajbhandari et al33 demonstrated a decreased microvascular oxygen saturation as an early indicator of diabetic foot ulcers occurring with healing.
Therefore, improvement of microcirculation should be considered as one of the primary goals of treatment. Glycemic control and metformin administration in patients with diabetes have been shown to have a positive influence on microcirculation.51–53 Similarly, we have demonstrated that remote ischemic preconditioning in healthy volunteers can improve the cutaneous microcirculation.27 Further studies are needed to elucidate whether this could also be useful for patients with diabetes mellitus or PAOD.
LIMITATIONS
There are 2 main limitations in our study. First, we did not match the microcirculatory findings in the diabetes group to either Hba1c values or polyneuropathy status, which may be factors affecting the microcirculatory parameters.11,33,54 However, our diabetes study population had an average diabetes duration of 12.5 years, so that a relevant microcirculatory damage should be expected. As far as we know, there are no studies suggesting these values above have any influence on microcirculatory parameters assessed using the O2C as a combined Laser-Doppler and Photospectrometry System.54
Another limitation are the intraindividual changes during a time period with respect to skin blood flow or other microcirculatory parameters, especially in patients with PAOD, as there are reports of intraindividual variabilities and fluctuations in patients with PAOD when measuring oxygen saturation using the transcutaneous oxygen tension (TcPO2) technique.55 However, combined Laser-Doppler and photospectrometry as a relatively new technique revealed a high reproducibility,56 even in patients with diabetes.30
CONCLUSIONS
According to our findings, we can conclude that the “capillary steal syndrome” hypothesis might be one possible pathway for reduced capillary oxygen saturation with equivalent blood perfusion and equivalent postcapillary venous filling pressure at the plantar foot in patients with diabetes. However, further investigations have to be conducted to elucidate the entire mechanisms leading to foot ulcers, including the assessment of microcirculatory effects and their implications. Impaired tissue oxygen saturation seems to play an essential role in foot ulcers and wound healing as both patient groups showed a significant deterioration of tissue oxygen saturation at the plantar foot. This is most interesting in patients with PAOD; these data suggest that impaired microcirculation may have an even bigger influence on morbidity than an impeded macrocirculation.
Interestingly, we could show that venous stasis and blood flow did not show any significant alterations in patients suffering from diabetes mellitus or PAOD, which may imply a benefit of therapeutic options that ameliorate cutaneous tissue oxygen saturation at the feet of these patients.
Footnotes
Disclosure: All authors disclose receiving funding for this work from National Institutes of Health (NIH), Wellcome Trust, or Howard Hughes Medical Institute (HHMI). The Article Processing Charge was paid for by the German Research Foundation (DFG).
Drs. Kabbani and Rotter contributed equally to this work.
REFERENCES
- 1.American Diabetes Association. Diabetes Statistics. Available at: http://www.diabetes.org/diabetes-statistics.jsp. Accessed January 27, 2013.
- 2.Meijer WT, Hoes AW, Rutgers D, et al. Peripheral arterial disease in the elderly: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–192. doi: 10.1161/01.atv.18.2.185. [DOI] [PubMed] [Google Scholar]
- 3.Flynn MD, Tooke JE. Aetiology of diabetic foot ulceration: a role for the microcirculation? Diabet Med. 1992;9:320–329. doi: 10.1111/j.1464-5491.1992.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 4.Chao CYL, Cheing GLY. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. 2009;25:604–614. doi: 10.1002/dmrr.1004. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg S, Alex M, Joshi RA, et al. Nonatheromatous peripheral vascular disease of the lower extremity in diabetes mellitus. Diabetes. 1959;8:261–273. doi: 10.2337/diab.8.4.261. [DOI] [PubMed] [Google Scholar]
- 6.Hile C, Veves A. Microcirculation of the diabetic foot. Diabetes and Cardiovascular Disease. 2005:403–418. In: Johnstone MT, Veves A, eds. [Google Scholar]
- 7.LoGerfo FW, Coffman JD. Vascular and microvascular disease of the foot in diabetes. N Engl J Med. 1984;311:1615–1619. doi: 10.1056/NEJM198412203112506. [DOI] [PubMed] [Google Scholar]
- 8.Ditzel J. Functional microangiopathy in diabetes mellitus. Diabetes. 1968;17:388–397. doi: 10.2337/diab.17.6.388. [DOI] [PubMed] [Google Scholar]
- 9.Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des. 2005;11:2301–2309. doi: 10.2174/1381612054367328. [DOI] [PubMed] [Google Scholar]
- 10.Parving HH, Noer I, Deckert T, et al. The effect of metabolic regulation on microvascular permeability to small and large molecules in short-term juvenile diabetics. Diabetologia. 1976;12:161–166. doi: 10.1007/BF00428983. [DOI] [PubMed] [Google Scholar]
- 11.Sandeman DD, Shore AC, Tooke JE. Relation of skin capillary pressure in patients with insulin-dependent diabetes mellitus to complications and metabolic control. N Engl J Med. 1992;327:760–764. doi: 10.1056/NEJM199209103271103. [DOI] [PubMed] [Google Scholar]
- 12.Tooke JE. Microvascular function in human diabetes. A physiological perspective. Diabetes. 1995;44:721–726. doi: 10.2337/diab.44.7.721. [DOI] [PubMed] [Google Scholar]
- 13.Tilton RG, Faller AM, Burkhardt JK, et al. Pericyte degeneration and acellular capillaries are increased in the feet of human diabetic patients. Diabetologia. 1985;28:895––900. doi: 10.1007/BF00703132. [DOI] [PubMed] [Google Scholar]
- 14.Singleton JR, Smith AG, Russell JW, et al. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52:2867–2873. doi: 10.2337/diabetes.52.12.2867. [DOI] [PubMed] [Google Scholar]
- 15.Uccioli L, Mancini L, Giordano A, et al. Lower limb arterio-venous shunts, autonomic neuropathy and diabetic foot. Diabetes Res Clin Pract. 1992;16:123–130. doi: 10.1016/0168-8227(92)90083-4. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan S, Rayman G. Microcirculation and diabetic foot. The Foot in Diabetes. 2006:41–50. In: [Google Scholar]
- 17.Boulton AJ, Scarpello JH, Ward JD. Venous oxygenation in the diabetic neuropathic foot: evidence of arteriovenous shunting? Diabetologia. 1982;22:6–8. doi: 10.1007/BF00253861. [DOI] [PubMed] [Google Scholar]
- 18.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 19.del Guercio R, Leonardo G, Arpaia MR. Evaluation of postischemic hyperemia on the skin using laser Doppler velocimetry: study on patients with claudicatio intermittens. Microvasc Res. 1986;32:289–299. doi: 10.1016/0026-2862(86)90066-x. [DOI] [PubMed] [Google Scholar]
- 20.Rossi M, Carpi A. Skin microcirculation in peripheral arterial obliterative disease. Biomed Pharmacother. 2004;58:427–431. doi: 10.1016/j.biopha.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Harris LM, Faggioli GL, Shah R, et al. Vascular reactivity in patients with peripheral vascular disease. Am J Cardiol. 1995;76:207–212. doi: 10.1016/s0002-9149(99)80066-6. [DOI] [PubMed] [Google Scholar]
- 22.Rossi M, Cupisti A, Perrone L, et al. Acute effect of exercise-induced leg ischemia on cutaneous vasoreactivity in patients with stage II peripheral artery disease. Microvasc Res. 2002;64:14–20. doi: 10.1006/mvre.2002.2393. [DOI] [PubMed] [Google Scholar]
- 23.Beckert S, Witte MB, Königsrainer A, et al. The impact of the Micro-Lightguide O2C for the quantification of tissue ischemia in diabetic foot ulcers. Diabetes Care. 2004;27:2863–2867. doi: 10.2337/diacare.27.12.2863. [DOI] [PubMed] [Google Scholar]
- 24.Knobloch K, Tomaszek S, Lichtenberg A, et al. Long-term palmar microcirculation after radial artery harvesting: an observational study. Ann Thorac Surg. 2006;81:1700–1707. doi: 10.1016/j.athoracsur.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Knobloch K, Grasemann R, Spies M, et al. Midportion achilles tendon microcirculation after intermittent combined cryotherapy and compression compared with cryotherapy alone: a randomized trial. Am J Sports Med. 2008;36:2128––2138. doi: 10.1177/0363546508319313. [DOI] [PubMed] [Google Scholar]
- 26.Knobloch K, Grasemann R, Spies M, et al. Intermittent KoldBlue cryotherapy of 3 × 10 min changes mid-portion Achilles tendon microcirculation. Br J Sports Med. 2007;41:e4. doi: 10.1136/bjsm.2006.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraemer R, Lorenzen J, Kabbani M, et al. Acute effects of remote ischemic preconditioning on cutaneous microcirculation—a controlled prospective cohort study. BMC Surg. 2011;11:32. doi: 10.1186/1471-2482-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraemer R, Lorenzen J, Knobloch K, et al. Free flap microcirculatory monitoring correlates to free flap temperature assessment. J Plast Reconstr Aesthet Surg. 2011;64:1353–1358. doi: 10.1016/j.bjps.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 29.Ladurner R, Feilitzsch M, Steurer W, et al. The impact of a micro-lightguide spectrophotometer on the intraoperative assessment of hepatic microcirculation: a pilot study. Microvasc Res. 2009;77:387–388. doi: 10.1016/j.mvr.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Forst T, Tarakci E, Forst S, et al. Reliability of lightguide spectrophotometry (O2C) for the investigation of skin tissue microvascular blood flow and tissue oxygen supply in diabetic and nondiabetic subjects. J Diabetes Sci Technol. 2008;2:1151. doi: 10.1177/193229680800200625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwase M, Imoto H, Murata A, et al. Altered postural regulation of foot skin oxygenation and blood flow in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2007;115:444–447. doi: 10.1055/s-2007-960499. [DOI] [PubMed] [Google Scholar]
- 32.Harrison DK, McCollum PT, Newton DJ, et al. Amputation level assessment using lightguide spectrophotometry. Prosthet Orthot Int. 1995;19:139–147. doi: 10.3109/03093649509167996. [DOI] [PubMed] [Google Scholar]
- 33.Rajbhandari SM, Harris ND, Tesfaye S, et al. Early identification of diabetic foot ulcers that may require intervention using the micro lightguide spectrophotometer. Diabetes Care. 1999;22:1292–1295. doi: 10.2337/diacare.22.8.1292. [DOI] [PubMed] [Google Scholar]
- 34.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 35.Dinh TL, Veves A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. Int J Low Extrem Wounds. 2005;4:154–159. doi: 10.1177/1534734605280130. [DOI] [PubMed] [Google Scholar]
- 36.Malik RA, Newrick PG, Sharma AK, et al. Microangiopathy in human diabetic neuropathy: relationship between capillary abnormalities and the severity of neuropathy. Diabetologia. 1989;32:92–102. doi: 10.1007/BF00505180. [DOI] [PubMed] [Google Scholar]
- 37.Jaap AJ, Shore AC, Stockman AJ, et al. Skin capillary density in subjects with impaired glucose tolerance and patients with type 2 diabetes. Diabetes Med. 1996;13:160–164. doi: 10.1002/(SICI)1096-9136(199602)13:2<160::AID-DIA36>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Rayman G, Malik RA, Sharma AK, et al. Microvascular response to tissue injury and capillary ultrastructure in the foot skin of type I diabetic patients. Clin Sci (Lond) 1995;89:467–474. doi: 10.1042/cs0890467. [DOI] [PubMed] [Google Scholar]
- 39.Cameron NE, Eaton SE, Cotter MA, et al. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- 40.Tesfaye S, Malik R, Ward JD. Vascular factors in diabetic neuropathy. Diabetologia. 1994;37:847–854. doi: 10.1007/BF00400938. [DOI] [PubMed] [Google Scholar]
- 41.Parving HH, Viberti GC, Keen H, et al. Hemodynamic factors in the genesis of diabetic microangiopathy. Metabolism. 1983;32:943––949. doi: 10.1016/0026-0495(83)90210-x. [DOI] [PubMed] [Google Scholar]
- 42.Fagrell B, Hermansson IL, Karlander SG, et al. Vital capillary microscopy for assessment of skin viability and microangiopathy in patients with diabetes mellitus. Acta Med Scand Suppl. 1984;687:25–28. doi: 10.1111/j.0954-6820.1984.tb08736.x. [DOI] [PubMed] [Google Scholar]
- 43.Shore AC, Jaap AJ, Tooke JE. Capillary pressure in patients with NIDDM. Diabetes. 1994;43:1198–1202. doi: 10.2337/diab.43.10.1198. [DOI] [PubMed] [Google Scholar]
- 44.Krug A, Medizintechnik L, Schlüsselwörter G. Mikrozirkulation und Sauerstoffversorgung des Gewebes. Phlebologie. 2007;36:300–312. [Google Scholar]
- 45.Nagase T, Sanada H, Takehara K, et al. Variations of plantar thermographic patterns in normal controls and non-ulcer diabetic patients: novel classification using angiosome concept. J Plast Reconstr Aesthet Surg. 2011;64:860–866. doi: 10.1016/j.bjps.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Bongard O, Fagrell B. Discrepancies between total and nutritional skin microcirculation in patients with peripheral arterial occlusive disease (PAOD). Vasa. 1990;19:105–111. [PubMed] [Google Scholar]
- 47.Wyss CR, Matsen FA, III, Simmons CW, et al. Transcutaneous oxygen tension measurements on limbs of diabetic and nondiabetic patients with peripheral vascular disease. Surgery. 1984;95:339–346. [PubMed] [Google Scholar]
- 48.Eickhoff JH. Forefoot vasoconstrictor response to increased venous pressure in normal subjects and in arteriosclerotic patients. Acta Chir Scand Suppl. 1980;502:7–14. [PubMed] [Google Scholar]
- 49.Fagrell B. Vital capillary microscopy. Scand J Clin Lab Invest. 1973;31:1–50. [Google Scholar]
- 50.Ubbink DT, Spincemaille GH, Reneman RS, et al. Prediction of imminent amputation in patients with non-reconstructible leg ischemia by means of microcirculatory investigations. J Vasc Surg. 1999;30:114–121. doi: 10.1016/s0741-5214(99)70183-7. [DOI] [PubMed] [Google Scholar]
- 51.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 52.Bailey CJ. Metformin: effects on micro and macrovascular complications in type 2 diabetes. Cardiovasc Drugs Ther. 2008;22:215–224. doi: 10.1007/s10557-008-6092-0. [DOI] [PubMed] [Google Scholar]
- 53.Wiernsperger NF. Review: 50 years later: is metformin a vascular drug with antidiabetic properties? Br J Diabetes Vasc Dis. 2007;7:204–210. [Google Scholar]
- 54.Stirban A, Salgin B, Koschinsky T, et al. Differential Role of Type 2 Diabetes and Cardiovascular Autonomic Neuropathy in Impaired Control of Skin Microcirculation. 2003.
- 55.Eickhoff JH, Engell HC. Transcutaneous oxygen tension (tcPO2) measurements on the foot in normal subjects and in patients with peripheral arterial disease admitted for vascular surgery. Scand J Clin Lab Invest. 1981;41:743–748. [Google Scholar]
- 56.Ghazanfari M, Vogt L, Banzer W, et al. Reproducibility of non-invasive blood flow measurements using laser Doppler spectroscopy. Phys Med Rehab Kuror. 2002;12:330–336. [Google Scholar]


