Abstract
Background:
Capsular contracture is the most common complication following primary augmentation mammoplasty. It remains poorly understood but is attributed to subclinical infection, immunologic response to breast implants, and chronic inflammatory changes caused by the presence of the implants. The infectious theory of contracture has lead to the practice of irrigating implant pockets with a triple antibiotic solution. The purpose of this study was to determine if antibiotic irrigation reduced the incidence and severity of capsular contracture compared with saline irrigation.
Methods:
A cohort study enrolling all patients having undergone primary augmentation mammoplasty performed by surgeon A and surgeon B between 2011 and 2012 for all women satisfying inclusion and exclusion criteria was conducted. The only difference in surgical technique was the use of antibiotic irrigation by surgeon B. A chi-square test and analysis of variance with predetermined 95% confidence intervals were performed.
Results:
Fifty-five patients were operated on. Twenty-eight of surgeon A’s patients were included, ranging in age from 22 to 50 with a mean follow-up time of 1.8 years. Twenty-seven of surgeon B’s patients were included, ranging in age from 22 to 56 with a mean follow-up time of 1.6 years. Rate of capsular contracture was 3.6% (surgeon A) and 3.7% (surgeon B). Chi-square statistic was found to be 0.0014 (P = 0.97) and analysis of variance F value was 1 (P = 0.39).
Conclusions:
Triple antibiotic breast irrigation is not associated with a significant reduction in the incidence or severity of capsular contracture compared with sterile saline when high-quality surgical technique is used.
Capsular contracture concerns all surgeons involved in breast augmentation and is the most common complication following cosmetic breast augmentation.1 It is a poorly understood progressive postoperative complication occurring after breast augmentation that some largely attribute to the patient’s immunological response to the breast implants.2 Current research suggests that an immune response to the implant is likely responsible with bacterial contamination suspected as playing a role in initiating an inflammatory response leading to capsular contracture.3
Many different in situ preparations of the breast implant pocket have been reported in attempts to reduce the rate of capsular contracture. Reported techniques include copious irrigation with sterile normal saline, in situ irrigation with saline followed by antibiotic instillation,4–6 and in situ glucocorticoid instillation.7 Current research focuses on the texturing of implants,8–11 using in situ biogels and collagen scaffolds to reduce the rate of capsular contraction,9 and limiting the body’s immune response to the implants.10 Additional studies have been reported using rabbits as a model for capsule formation to guide the development of more efficacious breast implants.12 Unfortunately, there are little published data to guide surgeons on how to best prepare the implant pocket to reduce the rate of capsular contracture.
Data establishing that in situ antibiotic treatment of the implant pocket in humans reduces the rate of contracture in clinical practice are limited.3,13 Available data supporting the instillation of a triple antibiotic solution into the implant pocket are generated from observational or cross-sectional studies without directly comparing this method of pocket preparation with other methods to ascertain the effect that in situ antibiotics have in reducing the rate of capsular contracture. Frequently cited publications regarding the instillation of triple antibiotic solution to reduce capsular contracture do not control for several confounding factors, include outcomes from Food and Drug Administration-banned solution using povidone-iodine, and use a highly heterogeneous population.3,13
To increase the available literature on this topic, a cohort study was undertaken to evaluate if the instillation of triple antibiotic solution into the implant pocket reduces the rate and severity of capsular contracture compared with irrigating the implant pocket with sterile saline.
METHODS
A cohort study enrolling all patients satisfying the inclusion and exclusion criteria from 2011 to 2012 was initiated. This study was approved by the Mercy Medical Center-Des Moines Institutional Review Board. Patients selected for participation in the study were notified in writing and consent obtained before inclusion in the study.
Database
Data from patients enrolled in the study were prospectively recorded in a database. The data recorded included dates of surgery, incision, implant type and style, the use of antibiotic irrigation, the presence of capsular contracture, and the grade of capsular contracture noted on examination.
Patient Selection
All patients who underwent cosmetic breast augmentation in 2011 and 2012 were identified at the time of the study initiation. The study population was further refined by identifying cases satisfying inclusion and exclusion criteria.
Study inclusion criteria were female patients over the age of 22 who underwent cosmetic breast augmentation in 2011 or 2012, use of Allergan Naturelle silicone breast implants (Allergan, Irvin, Calif.), inframammary incision, and subpectoral implant placement. Various sizes of implants (Allergan) were selected on the basis of patient preference.
Exclusion criteria were age less than 22 years, previous breast surgery, implant exchange, breast reconstruction, a history of cancer, vascular disease, diabetes, chest radiotherapy, and smoking.
Surgical Technique and Perioperative Care
Inframammary incisions were marked on all patients before surgery. All patients were positioned supine and both breasts cleansed with povidone-iodine and draped creating a sterile field. At the time of the first incision, 12 mg of dexamethasone was given intravenously to decrease the rate of scar formation.14
Incisions were made along markings within the inframammary fold. The implant pocket was created using the surgical procedure as described by Adams et al.13 The sterile sizing implant was inserted using “No-touch Technique” via a Keller Funnel. The same procedure was repeated for the contralateral breast. The patient was then placed in a semi-Fowler’s position by raising the head of the table and an assessment of the breasts with sizers in place was completed. Following the intraoperative assessment of the sized breasts, the table was lowered, patient brought into supine position, and the sizers removed.
At this point, surgeon B has the implant placed in a bath of triple antibiotic solution and irrigated the pocket with approximately 250 mL slurry of triple antibiotic solution. Surgeon B used a triple antibiotic solution containing 1 g of cefazolin, 80 mg of gentamicin, 50,000 IU of bacitracin, and 500 mL of normal saline.
Surgeon A performs this surgery identical to surgeon B except he does not use a triple antibiotic solution. Surgeon A irrigates the pocket with only normal saline before implant insertion and bathes the implant in sterile room-temperature saline. This is the only difference in operative technique between the 2 surgeons.
The implants are inserted using the “No-touch Technique” via a new Keller Funnel and the breasts reassessed for proper placement of the implants, symmetry, and overall appearance. Lastly, the pocket is irrigated by both surgeons with 1% lidocaine containing 1:1000 epinephrine.
Incisions were closed with running 3-0 vicryl suture placed in the subcutaneous tissues. Skin was closed with 4-0 vicryl deep subdermal sutures and followed by subcuticular closure using 4-0 prolene suture. Steri-Strips (3M, St. Paul, Minn.) were placed and removed 2 weeks postoperatively.
All patients were fitted with a surgical brassiere and instructed to wear the device for 4 weeks postoperatively. Additionally, patients were instructed to avoid underwire brassieres, lifting objects over head, carrying anything in excess of 5 pounds, and strenuous physical activity for 6 weeks after surgery. All patients were prescribed 500 mg of cephalexin 3 times daily by mouth for 1 week. Pain control was accomplished with oxycodone/acetaminophen if needed.
Postoperative Care
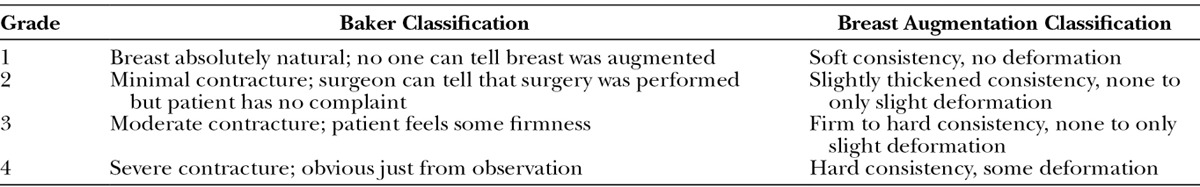
Patients were evaluated postoperatively at 5 days, 2 weeks, 6 weeks, 3 months, 6 months, 12 months, and yearly thereafter for 3 years postoperatively. All examinations were performed by 2 healthcare providers. Patients were assessed for the presence of capsular contracture and capsular contracture graded according to the Baker Classification as displayed in Table 1 by the 2 examining providers present. A mean Baker Grade was assigned to each patient after each examiner assessed the patient at the time of the examination (Table 1).
Table 1.
Baker Classification of Capsular Contracture
End Points
The primary end point of this study is overall rate of capsular contracture of any grade. The secondary end point is the severity of capsular contracture as determined by Baker Classification of capsular contracture.
Statistical Analysis
Descriptive statistics generated included mean age and duration of follow-up, overall rate of capsular contracture, and total number of breasts with clinically significant capsular contracture. A chi-square analysis with one degree of freedom and a 95% confidence interval was performed to determine if irrigation of the implant pocket with antibiotics was correlated with a reduced rate of capsular contracture. Lastly, analysis of variance (ANOVA) was performed to evaluate the correlation of in situ antibiotics and Baker Grade of capsular contracture. A 95% confidence interval was used for ANOVA. All calculations and statistical analyses were performed in Microsoft Excel 2007 (Redmond, Wash.) with the statistics add-on package.
RESULTS
A total of 55 cases, 28 from surgeon A and 27 from surgeon B, were identified as having satisfied the inclusion and exclusion criteria and included in this analysis. Table 2 contains surgeon of record, patient age, duration of follow-up, presence of capsular contracture, and Baker Grade for all cases included in this study. The mean patient age for surgeon A was found to be 34.8 years with a range from 22 to 50 years. Surgeon B’s average patient age was found to be 33.6 years with patients ranging from 22 to 56 years. Each surgeon was found to have one case of bilateral grade III contracture. The relative rate of capsular contracture was found to be 3.6% and 3.7% for surgeon A and surgeon B, respectively (Fig. 1).
Table 2.
Case Characteristics and Outcomes for Primary Augmentation Mammoplasty Using Naturelle Silicone Implants
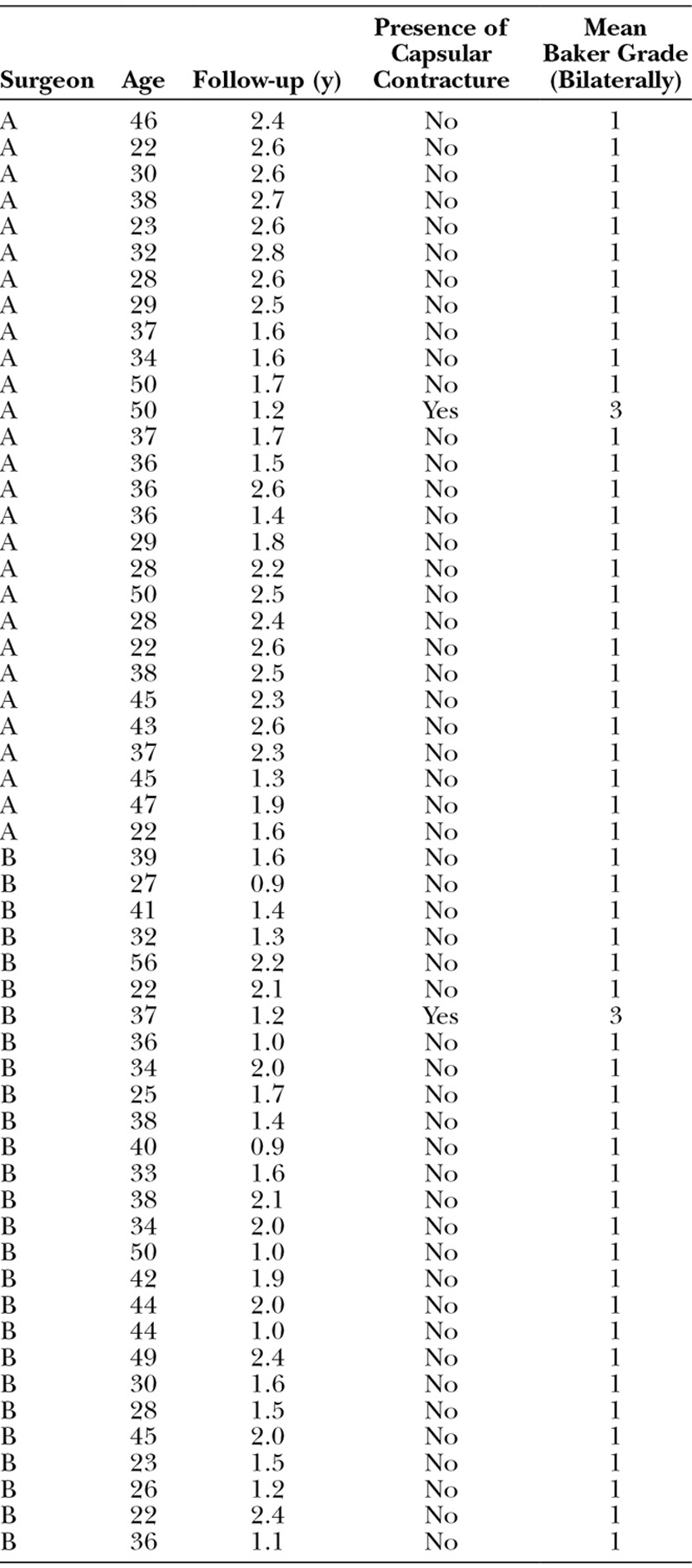
Fig. 1.
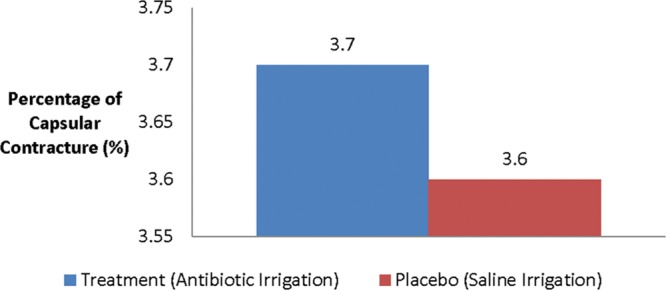
Relative rate of capsular contracture among cohorts.
The descriptive statistics are displayed and summarized in Table 3. The average length of follow-up was 1.8 years for surgeon A and 1.6 years for surgeon B. The results of the chi-square test revealed χ2 = 0.0014 (P = 0.97) (Fig. 2). ANOVA for the Baker Grade was conducted and yielded an F(1,6) = 1 (P = 0.39) (Fig. 3).
Table 3.
Primary Outcome Measures of Surgeons A and B
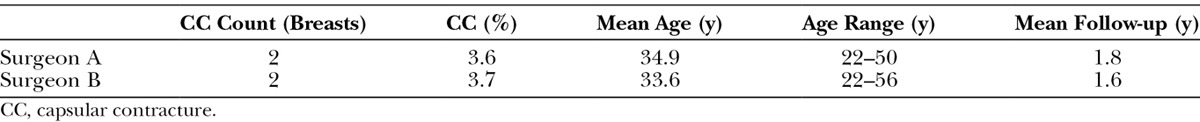
Fig. 2.
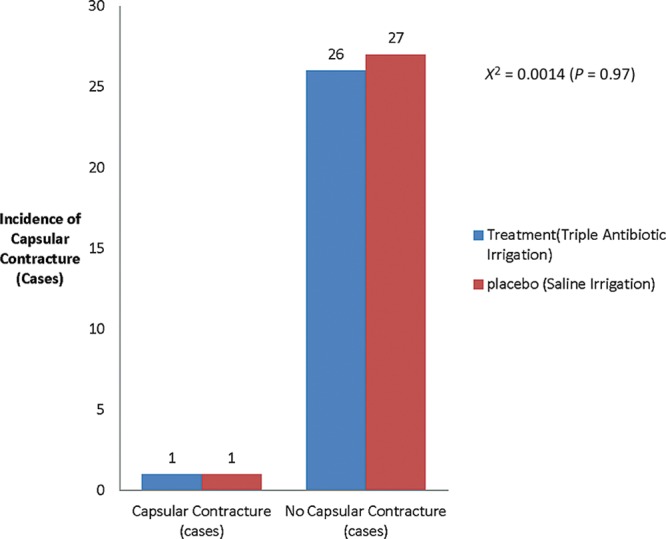
Absolute capsular contracture rate among treatment and placebo cohorts.
Fig. 3.
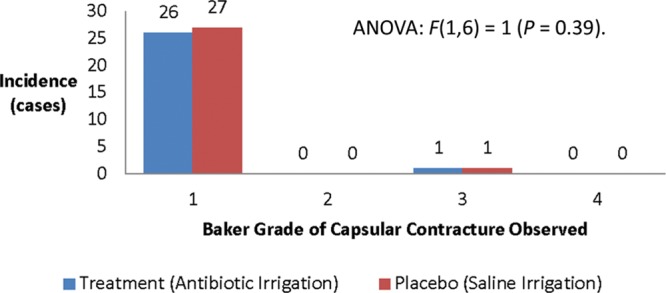
Severity of capsular contracture among cohorts.
DISCUSSION
Breast augmentation is one of the most common surgical procedures performed by plastic surgeons in the United States. More than 270,000 cosmetic augmentation mammoplasties and 80,000 breast reconstructions using breast prostheses were performed in 2012.15 Reported rates of capsular contracture are highly variable, ranging from 5% to 30% in some case series.16 The estimated number of women suffering grade III/IV capsular contracture ranges from 22,000 to 44,000 annually.15
Capsular contracture is a poorly understood phenomenon that continues to plague both patients and surgeons following breast surgery. There is no universally agreed upon mechanism responsible for it. Subclinical bacterial infection has been correlated with an increased rate of capsular contracture in many published articles.3 This line of reasoning has lead to a widespread practice of in situ antibiotic irrigation of the implant pocket with a triple antibiotic solution on the basis of several observational studies.3,13,16 Unfortunately, several studies indicate that prophylactic use of antibiotics may lead to an increased risk of acquiring iatrogenic antibiotic-resistant bacterial infections.17–19
Recent studies correlate contracture with the development of a biofilm around the implant.20 Antibiotics are ineffective at treating biofilms because they cannot penetrate the biofilm and destroy the bacteria.21–24 The only effective treatment for an infection mediated by a biofilm is debridement or in the case of capsular contracture removal of the compromised implant. Interestingly, bacteria isolated from cultures of intraoperative wounds do not correspond to bacteria cultured from biofilms.25 This finding indicates that the bacteria in intraoperative wounds are not responsible for the biofilm creation despite the presence of bacteria near the implant. Thus, selecting antibiotic prophylaxis based on the results of intraoperative wound cultures may not target the bacteria responsible for forming biofilms and causing capsular contracture.
Further complicating antibiotic selection is the recent work26 that concluded that “current perioperative antibacterial prophylaxis may be suboptimal to prevent bacterial seeding” and that breast ductules likely harbor bacteria seeding the implants. Antibiotic irrigation is unlikely to reach bacteria contained within breast ductules as it only bathes cells exposed by the dissection, not the breast ductules containing bacteria because it is not absorbed systemically. Additionally, using suboptimal dosing or delivery methods of antibiotics selects for bacteria prone to biofilm formation while creating difficult to treat infections.27 Theoretically, antibiotic instillation may lead to an increased rate of capsular contracture by selecting for bacteria prone to forming biofilms by failing to eradicate the bacteria in the breast and leaving the implant exposed to bacteria due to a subtherapeutic dose of antibiotics.28 This created the need for a controlled study of antibiotic instillation to determine the effect it has on the rate of capsular contracture.
The practice of instilling a triple antibiotic solution into the breast implant pocket was established by studies that were predominately observational or used historical controls and did not compare the solution to a placebo, such as sterile saline.3,13,16 No controlled cohort or randomized controlled studies existed demonstrating that in situ antibiotics are more or less effective at reducing the rate of capsular contracture than irrigation with sterile saline alone.
Antibiotic irrigation was first reported in 2006 to reduce the rate of capsular contracture when compared with historical outcomes.13 This widely cited study did not compare antibiotic irrigation against saline irrigation alone and included data from 2 different antibiotic solutions (one of which was banned by the Food and Drug Administration), thus limiting generalizability of the study. Using 2 different antibiotic solutions in an observational study lacking a placebo, while using saline and silicone implants, multiple types of incisions and dissections introduced multiple confounding factors all of which can affect the rate of contracture development.2 Unfortunately, these results were not statistically corrected for with multivariate analysis.13 Ideally, confounding factors should be minimized in any scientific study so that reproducible results and generalizable conclusions are generated.29,30
To our knowledge, this is the first report that directly assesses the efficacy of in situ antibiotic irrigation in a controlled manner. Use of a lubricating and irrigating solution was necessary in using a no-touch surgical method. Sterile saline is an appropriate placebo because it lacks substantial antimicrobial properties and has been used in multiple surgeries as a placebo fluid. Our study is the first report that the authors are aware of that directly compares the efficacy of antibiotic irrigation with placebo while not using historical controls. In constructing our inclusion and exclusion criteria, the authors decided to eliminate many potential confounding variables to better study the effect that antibiotic instillation had on the development of capsular contracture. All patients included were of similar age, health status, and received the same model of silicone implant. The only difference in surgical technique between cohorts was the use of in situ antibiotic irrigation of the implant pocket.
By eliminating confounding variables, any reduction in rate and severity of capsular contracture observed in the treatment group can accurately be attributed to the instillation of antibiotics into the implant pocket. Eliminating confounding variables is an enormous strength of this study producing results that are reliable and thus are generalizable to plastic surgery practices nationwide.
The decision to limit the sample population to only inframammary incisions was done to eliminate the effect alternative incision choices may have on the rate of contracture development as periareolar, transaxillary, and transumbilical breast augmentation have different rates of capsular contracture.26 It is suspected that these alternative incisions expose the implant to greater amounts of breast stroma and endogenous breast bacterial flora leading to an increased rate of contracture, requiring multivariate analysis to correct for and thus increasing risk for error. Ergo, to improve the accuracy of our results, it was decided to eliminate other incisions and focus solely on the inframammary incisions. Doing such allows a more accurate determination of the effect that antibiotic irrigation has on subsequent development of contracture.
Our results indicate that in situ antibiotics are not correlated with a decreased rate of capsular contracture (P = 0.97). In situ antibiotics had a statistically insignificant 0.1% higher rate of contracture. Furthermore, ANOVA revealed that antibiotic irrigation does not decrease the severity of contracture formation (P = 0.39). This indicates a lack of benefit when a triple antibiotic solution is used compared with sterile saline.
The majority of capsular contractures occur 12 months postoperatively, making our mean follow-up times of 1.8 and 1.6 years for each cohort appropriate; the observed contractures were clinically diagnosed at 6 and 8 months postoperatively. Our follow-up time is consistent with other studies establishing the efficacy of antibiotic irrigation13 and consistent with literature indicating that 92% of contractures occur within 1 year of the original operation.2 Although contracture can occur years after surgery, the predominate concern is the bulk of cases that occur 1 year postoperatively. Late-onset contracture is suspected to be caused by chronic low-level inflammation, implant filler bleed, and chronic capsular maturation due to elastomer degradation.31,32
Regardless of the irrigation solution used, we observed no differences in the incidence of capsular contracture. If subclinical infection was the primary mechanism for inducing capsular contracture, one would expect the observed rate of contracture to be higher among the patients receiving only saline irrigation. However, there was no significant difference in the rate of contracture or the observed grade of contracture on examination between cohorts. A possible explanation is that high-quality surgical technique negates much the advantage of using an antibiotic solution in preventing bacterial seeding of the implant. These findings may support the hypothesis that an immunologic response to the breast prosthesis is responsible for contracture development regardless of the rapidity of development.
The observed rate of contracture in this series is under the acceptable rate of 5% for experienced surgeons employing the “No-touch Technique.” The reason that the overall rate of contracture seems high is that a small sample size was employed, which is a limitation of our study. The rate of contracture in each surgeon’s practice is less than 1% for primary augmentation mammoplasty. However, from these retrospective results, one can determine a minimum rate of contracture with saline irrigation, which is critical in calculating alpha for eventually performing prospective clinical trials.
Despite the limitations of small sample size and cohort study, the authors believe that the results are applicable to other plastic surgery practices because the patients included in the analysis satisfied prespecified inclusion and exclusion criteria and the only difference in surgical technique was the addition of antibiotic irrigation used by surgeon B. The lack of blinded prospective randomization of the irrigation instilled is a major weakness as is the use of only 2 surgeons. The surgeons use identical surgical technique with the sole exception that surgeon A does not use antibiotic irrigation. However, this enabled the creation of control and treatment cohorts while allowing the authors to directly assess the efficacy of triple antibiotic irrigation without either surgeon altering their clinical care.
CONCLUSIONS
In an attempt to advance breast surgery and reduce the rate of capsular contracture, the authors undertook a cohort study that prospectively documented outcomes to determine if irrigating breast implant pockets with a triple antibiotic solution reduced the rate and severity of capsular contracture. We found that irrigating the breast implant pocket with only saline solution does not result in a significantly increased or unacceptable rate of capsular contracture or an increased severity of capsular contracture in primary augmentation mammoplasty relative to antibiotics. In this small study, triple antibiotic solution cannot be determined to be superior to sterile saline. Further study is needed to confirm these findings.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Pelosi MA, III, Pelosi MA., II Breast augmentation. Obstet Gynecol Clin North Am. 2010;37:533–546, viii. doi: 10.1016/j.ogc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Araco A, Caruso R, Araco F, et al. Capsular contractures: a systematic review. Plast Reconstr Surg. 2009;124:1808–1819. doi: 10.1097/PRS.0b013e3181bf7f26. [DOI] [PubMed] [Google Scholar]
- 3.Adams WP, Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;117:30–36. [PubMed] [Google Scholar]
- 4.Burkhardt BR, Eades E. The effect of Biocell texturing and povidone-iodine irrigation on capsular contracture around saline-inflatable breast implants. Plast Reconstr Surg. 1995;96:1317–1325. doi: 10.1097/00006534-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Burkhardt BR, Dempsey PD, Schnur PL, et al. Capsular contracture: a prospective study of the effect of local antibacterial agents. Plast Reconstr Surg. 1986;77:919–932. [PubMed] [Google Scholar]
- 6.Mladick RA. “No-touch” submuscular saline breast augmentation technique. Aesthetic Plast Surg. 1993;17:183–192. doi: 10.1007/BF00636260. [DOI] [PubMed] [Google Scholar]
- 7.Marques M, Brown S, Correia-Sá I, et al. The impact of triamcinolone acetonide in early breast capsule formation in a rabbit model. Aesthetic Plast Surg. 2012;36:986–994. doi: 10.1007/s00266-012-9888-z. [DOI] [PubMed] [Google Scholar]
- 8.Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117:2182–2190. doi: 10.1097/01.prs.0000218184.47372.d5. [DOI] [PubMed] [Google Scholar]
- 9.Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128:403e–410e. doi: 10.1097/PRS.0b013e31822b6637. [DOI] [PubMed] [Google Scholar]
- 10.Katzel EB, Koltz PF, Tierney R, et al. The impact of Smad3 loss of function on TGF-β signaling and radiation-induced capsular contracture. Plast Reconstr Surg. 2011;127:2263–2269. doi: 10.1097/PRS.0b013e3182131bea. [DOI] [PubMed] [Google Scholar]
- 11.Puskas JE, Luebbers MT. Breast implants: the good, the bad and the ugly. Can nanotechnology improve implants? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:153–168. doi: 10.1002/wnan.164. [DOI] [PubMed] [Google Scholar]
- 12.Adams WP, Jr, Haydon MS, Raniere J, Jr, et al. A rabbit model for capsular contracture: development and clinical implications. Plast Reconstr Surg. 2006;117:1214–1219; discussion 1220. doi: 10.1097/01.prs.0000208306.79104.18. [DOI] [PubMed] [Google Scholar]
- 13.Adams WP, Jr, Conner WC, Barton FE, Jr, et al. Optimizing breast pocket irrigation: an in vitro study and clinical implications. Plast Reconstr Surg. 2000;105:334–338; discussion 339. doi: 10.1097/00006534-200001000-00051. [DOI] [PubMed] [Google Scholar]
- 14.Lemperle G, Exner K. Effect of cortisone on capsular contracture in double-lumen breast implants: ten years’ experience. Aesthetic Plast Surg. 1993;17:317–323. doi: 10.1007/BF00437105. [DOI] [PubMed] [Google Scholar]
- 15.American Society of Plastic Surgeons Website. Available at: http://www.plasticsurgery.org/news-and-resources/2012-plastic-surgery-statistics.html. Accessed May 5, 2013.
- 16.Blount A, Martin M, Lineberry K, et al. Capsular contracture rate in low-risk population after primary augmentation mammaplasty—a retrospective review. Plast Reconstr Surg. 2011;128:9–10. [Google Scholar]
- 17.Goossens H. Antibiotic resistance and policy in Belgium. Verh K Acad Geneeskd Belg. 2000;62:439–469. [PubMed] [Google Scholar]
- 18.Ginzburg E, Namias N, Brown M, et al. Gram positive infection in trauma patients: new strategies to decrease emerging Gram-positive resistance and vancomycin toxicity. Int J Antimicrob Agents. 2000;16(Suppl 1):S39–S42. doi: 10.1016/s0924-8579(00)00305-8. [DOI] [PubMed] [Google Scholar]
- 19.Antoniadou A, Kanellakopoulou K, Kanellopoulou M, et al. Impact of a hospital-wide antibiotic restriction policy program on the resistance rates of nosocomial Gram-negative bacteria. Scand J Infect Dis. 2013;45:438–445. doi: 10.3109/00365548.2012.760845. [DOI] [PubMed] [Google Scholar]
- 20.Rieger UM, Mesina J, Kalbermatten DF, et al. Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013;100:768–774. doi: 10.1002/bjs.9084. [DOI] [PubMed] [Google Scholar]
- 21.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 22.Rosser BT, Taylor PA, Cix PA, et al. Methods for evaluating antibiotics on bacterial biofilms. Antimicrob Agents Chemother. 1987;31:1502–1506. doi: 10.1128/aac.31.10.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 24.Costerton JW, Lewandowski Z, Caldwell DE, et al. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson AR, Leid JG, Hunsaker D. Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope. 2006;116:1121–1126. doi: 10.1097/01.mlg.0000221954.05467.54. [DOI] [PubMed] [Google Scholar]
- 26.Bartsich S, Ascherman JA, Whittier S, et al. The breast: a clean-contaminated surgical site. Aesthet Surg J. 2011;31:802–806. doi: 10.1177/1090820X11417428. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan JB, Izano EA, Gopal P, et al. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. MBio. 2012;3:e00198–e00112. doi: 10.1128/mBio.00198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan JB. Antibiotic-induced biofilm formation. Int J Artif Organs. 2011;34:737–751. doi: 10.5301/ijao.5000027. [DOI] [PubMed] [Google Scholar]
- 29.Hennekens CH, Buring JE, Mayrent SL. Epidemiology in Medicine. Vol 515. Boston: Little Brown & Company; 1987. [Google Scholar]
- 30.Aickin M. Analysis of nonintervention studies: technical supplement. Perm J. 2012;16:e100–e120. doi: 10.7812/tpp/12-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caffee HH. The influence of silicone bleed on capsule contracture. Ann Plast Surg. 1986;17:284–287. doi: 10.1097/00000637-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Granchi D, Cavedagna D, Ciapetti G, et al. Silicone breast implants: the role of immune system on capsular contracture formation. J Biomed Mater Res. 1995;29:197–202. doi: 10.1002/jbm.820290209. [DOI] [PubMed] [Google Scholar]


