Abstract
Summary:
What is wrong with the current understanding of etiopatho genesis of androgenic alopecia (AGA)? What is the most important question to ask to understand AGA? Why is that question skimped? Which findings are interpreted incorrectly? Is there anything that has not been discerned about AGA until today? What are the deceptive factors for investigators? Is it possible to snap the whole view uninterruptedly in one clear picture? Answers and an overview with fresh perspectives are provided.
The reader would probably agree that the following are generally recognized as safe to claim with regard to androgenic alopecia (AGA) and contribute to androgenic theory:
5-alpha reductase enzyme activity, which converts testosterone to dihydrotestosterone (DHT), increases in balding scalp,1
Number of DHT receptors on the hair follicles increases in balding scalp,4
Blocking conversion of testosterone to DHT delays progression of AGA.5
Based on these findings, DHT is held responsible for miniaturization of hair follicles in AGA.6 However, as DHT is a more potent form of testosterone and androgens are expected to convert hair follicles from vellus to terminal not the other way around, this inference gives rise to a paradox. In a review article, Randall says, “How one type of circulating hormone has such contrary effects on a single tissue depending on its body site is not clear; this biological paradox alone makes androgen action in hair follicles very intriguing.”7 It is indeed so.
Androgen-dependent/independent and androgen-sensitive/insensitive hair follicle concepts have been used to explain the opposite effects of androgens on hair follicles at different body sites.6–8 According to these concepts, nonbalding scalp hair is designated as androgen independent and androgen insensitive, whereas balding scalp hair as androgen independent but androgen sensitive.8 Here, there is a point that deserves attention. Androgen-dependent/independent hair follicle concept treats the whole scalp as a single body site, which stands to reason. Scalp hair, either balding or nonbalding, is considered androgen independent because they grow in the absence of androgens as opposed to, for example, axillary or pubic hair. However, androgen-sensitive/insensitive hair follicle concept divides the scalp into 2 sites as androgen-sensitive and androgen-insensitive hair-bearing areas. This arbitrary division of scalp is discomforting in the first place and inappropriateness of the concept becomes evident as it is questioned further.
As stated above, DHT is accepted to be the androgen that binds to androgen receptors and effects follicular miniaturization. Along with other findings, this conclusion is based on the finding of increased levels of DHT in balding scalp compared with nonbalding scalp. Unless DHT levels in balding scalp are equal to DHT levels in nonbalding scalp, follicular miniaturization in AGA cannot be considered a matter of sensitivity to androgens. The amount of androgen is not the same in the so-called androgen-insensitive area.
In a relatively recent review article, the role of androgens in the pathophysiology of AGA is documented by Kaufman.6 Kaufman says, “In men with MPHL (male pattern hair loss), follicular miniaturization is caused by an inherited sensitivity of scalp hair follicles to normal levels of circulating androgens.” “Thus, it appears that in balding men DHT binds to androgen receptors in susceptible hair follicles and, by an unknown mechanism, activates genes responsible for follicular miniaturization.”
Kaufman tries to put it right saying sensitivity to “normal levels of circulating androgens” to no avail. It helps correct the terminology from one point of view only and obviously does not bring in an explanation to the opposite effects of androgens on balding scalp hair follicles vs hair follicles at other body sites. If there is sensitivity to normal levels of circulating androgens and the resulting product of this sensitivity is DHT, an overall pronounced effect is expected not an opposite effect. DHT is a stronger form of testosterone.
Androgen sensitivity concept is not only an inept concept to explain the role of androgens in AGA but it is also confusing and misleading its adopters after some dubious “genes responsible for follicular miniaturization” and “an unknown mechanism” that activates them. Worst of all, it skimps the most critical question and prevents it from standing forward, which is:
Why does DHT (or 5-alpha reductase enzyme activity) increase in balding scalp?
This question requires a solid answer and has priority over the other crucial questions that are as follows:
How does DHT cause hair loss while exactly the opposite effect is expected?
Why does balding (or increase in DHT levels) occur only at the top of the head?
If these questions are considered fair questions, a valid theory on the etiology of AGA has to be able to answer all of these questions.
I introduced a new theory in 2008.9 It does accomplish this hard task adroitly; moreover, it is in agreement with all findings in connection with AGA. The mechanism of AGA is thought to be highly complex.10 However, this theory provides a new viewpoint from which it seems to be quite simple. According to the theory, pressure on the hair follicles created by the weight of the scalp is the cause of AGA. Total weight of the skin, subcutaneous connective tissue, and galea are operative. With sandwiched fat tissue and fibrous connections between the skin and galea, all of these components of the scalp form a combined structure that sits on the cranial bones much like a separate structure movable on the cranial bones due to the intervening loose areolar tissue. Hair follicles are compressed by the skin against the calvarial bones. This theory is uniquely capable of explaining all related phenomena and paradoxes.
In summary, the theory points out that the pressure on the hair follicles is buffered by the surrounding subcutaneous fat tissue and young dermis that is capable of keeping itself well hydrated. As one ages, thickness of the subcutaneous fat tissue and the volume of the dermis decreases,11,12 that is, the buffer decreases and consequently the pressure on the hair follicles increases. Another factor that is well known to cause thinning of subcutaneous fat tissue much more rapidly than aging is testosterone.13–16 With the onset of puberty, subcutaneous fat tissue starts to decrease instantaneously at an early age in the male due to increase in testosterone levels.17,18 Estrogen protects the cushioning tissues until after menopause in the female.19–24 And, testosterone effected reduction in subcutaneous fat tissue normally does not happen in the female at all.
On the other hand, it has been shown that the downward growth of early anagen follicle occurs by growth pressure.25,26 It has to work against the compressive force described above. As the cushion reduces, the hair follicle needs to strive against a higher pressure to reach its terminal follicle size. More androgen is demanded to promote the growth. This is a local demand, and there is a mechanism for increasing the effect of androgens locally without raising systemic androgen levels. 5-alpha reductase enzyme activity increases at the locale and converts more testosterone to DHT, which has severalfold greater affinity for androgen receptors than testosterone. And, DHT increases locally. The sequence of reactions does not end here. Increased DHT causes further erosion of the subcutaneous fat tissue around the hair follicle.27–29 A vicious circle is created (Fig. 1).
Fig. 1.
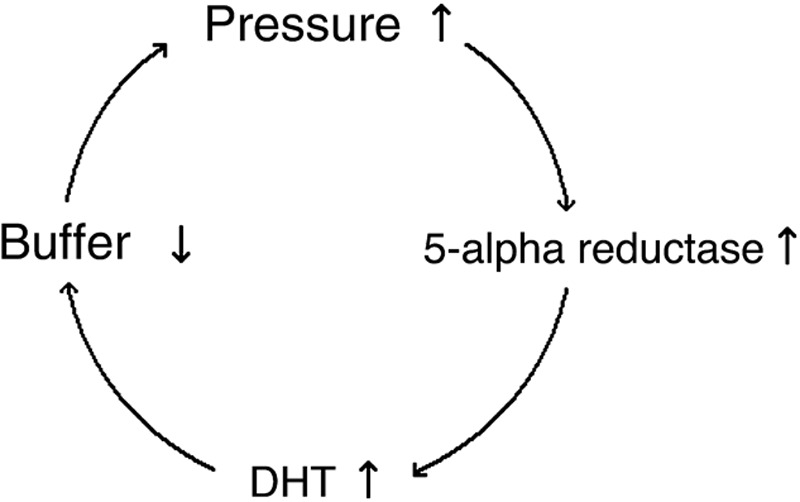
The vicious circle in AGA.
There is not another theory that reasonably and satisfactorily explains hair loss in AGA without ascribing a function to DHT that is opposite to its known function. DHT increases to help the hair follicle forge ahead deeper to reach its normal terminal follicle size in the face of increased pressure due to decrease in cushioning tissues. As long as the pressure on the follicle is adequately buffered, a base androgen level is enough and required for healthy hair growth.30 As the cushion decreases, the balance is lost at some point and the vicious circle is initiated. Increased DHT promotes hair growth probably mainly by stimulating mitosis in the early anagen follicle. However, increased growth pressure due to advanced mitosis cannot overcome the compressing pressure on the hair follicle but speeds up and shortens the anagen phase. Hair follicle cannot grow to its full size and becomes smaller and smaller with each cycle along with the increasing pressure on the hair follicle. Hair follicle miniaturization, that is, terminal to vellus conversion, takes place, anagen to telogen ratio reduces, and hair loss increases.
A reaction-dubbed microinflammation around the bulge region of individual hair follicles in balding scalp has been presumed a causative factor in AGA,31,32 but most likely it is evidence for the new theory. A reaction that is much milder than the inflammation seen in a typical inflammatory scarring alopecia and takes place at the site of mitosis suggests scavenging of products resulting from inefficient mitosis rather than a factor in the etiology of AGA.
Finally, if the pressure created by the weight of the scalp causes the hair loss in AGA, which is claimed by the theory, it is expected that the hair at the top of the head is lost. This is exactly what happens in AGA. Although the conformity is manifest and there is an appreciable relation between the shape of the cranium and the hair loss area in AGA, a few points have to be observed so as not to get confused.
It is better to examine only the last-stage AGA cases observing and evaluating the hair loss area from this theory point of view for the first time. AGA is a progressive condition, and there are several factors that can be effective on where hair loss starts and how it progresses in different individuals. Observing more hair loss at presumedly lower pressure areas than higher pressure areas in intermediate-stage AGA cases can be deceptive and is the most common source of confusion.
An example of the relation between the shape of the cranium and the hair loss area is shown in Figure 2. Outlines of the side views and back views of 2 heads are seen. The only difference between the 2 heads is the shape of the back of the calvarium. One of them is rather rounded in shape (Fig. 2A), whereas the other is more like 2 oblique surfaces that meet at an obtuse angle (Fig. 2C). Hair loss area extends down to where the back of the upright head contacts with a vertical line (red line in the drawing). The pressure is relieved below this contact site as the scalp turns away from the vertical direction. Most of the time the point of pressure relief is located at a lower level in the latter type (Fig. 2D) than in the former (Fig. 2B), so that looking from behind the person a bigger bald area is seen in the latter. This finding is a strong revealing finding for the new theory but may be misleading if it is not interpreted correctly.
Fig. 2.
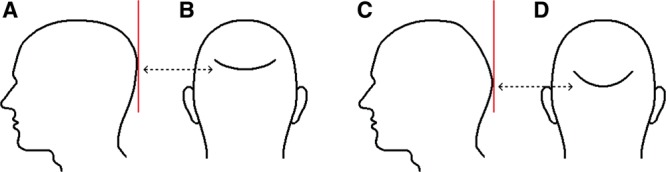
The relation between the shape of the cranium and the hair loss area in AGA is a proof for the validity of the new theory. Hair loss extends to where a vertical line makes contact with the back of the upright head. Depending on the shape of the back of the head, this point may be at a higher (A, B) or lower (C, D) location.
Also, the structure of the scalp has to be given due consideration during the evaluation of hair loss area in relation to the shape of the cranium. For example, even if the back of the head is precisely straight and vertical when the head is upright, the weight of the scalp still creates pressure on the hair follicles at the back of the head although the direction of the gravitational force is vertical, that is, parallel to the back of the head (the same applies for the hair follicles within the contact site with the vertical line in the previous example). Galea aponeurotica is a tough nonelastic structure and there are dense, nonelastic fibrous attachments between the galea and the skin of the scalp. Downward pull in the vertical direction on the skin of the back of the head is opposed by these nonelastic fibrous attachments. The resulting net force is toward the calvarium and it compresses the hair follicles (Fig. 3). Therefore, in such cases, hair loss area is expected to extend down usually to the level of the border of the galea with the occipitalis muscle at the back of the head.
Fig. 3.
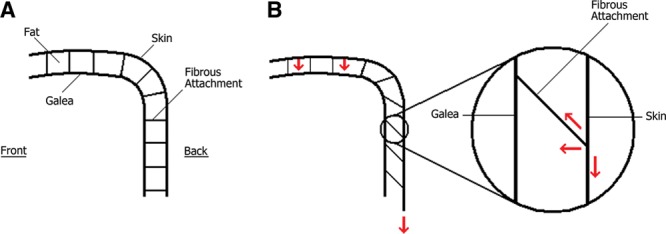
Fat tissue and fibrous bands between the skin and galea of the scalp in a hypothetical case wherein the back of the calvarium is vertical and straight are depicted. (A), If there were no gravity, there would not be a downward pull, whereas (B) in the presence of gravity, downward pull on the skin at the back of the head is directed inward because of the inelastic fibrous bands connecting the skin to the inelastic galea and resisting the gravity.
One more important point that should be regarded is that the force of downward pull caused by the gravity on the scalp skin is not distributed equally around the circumference of the head (Fig. 4). As the ears are firmly fixed to the temporal bones, they interrupt the soft-tissue continuity, shore up the soft tissues above and around them, and assume the pull of the soft tissues below. By contrast, scalp skin is continuous with the skin of the face between the ears and eyes on both sides of the face, so that the weight of the facial soft tissues adds to the pressure in the frontal part of the scalp. The circumstances are similar at the back of the head in terms of effective weights. The weight of the soft tissues below the ear level at the back of the head similarly adds to the pressure in the vertex area as an extra weight compared with the area above the ears. In most of the AGA cases, hair loss starts at the frontal and/or vertex areas.
Fig. 4.
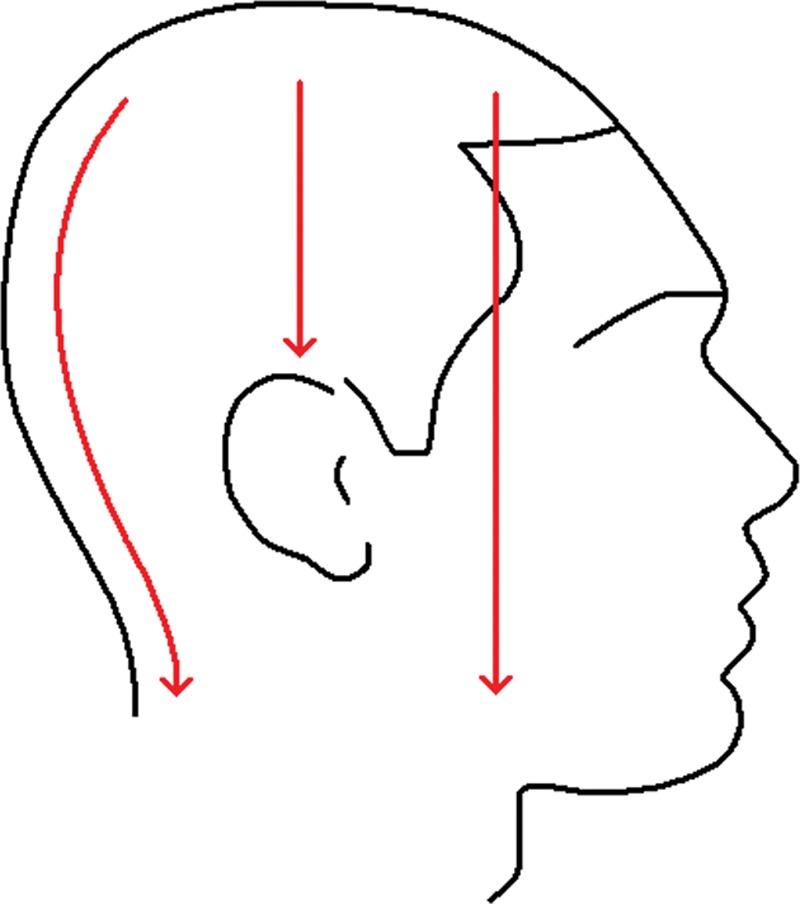
Anatomy is a determining factor on the magnitude of the downward pull at different parts of the scalp. Middle top of the scalp is supported by the ears in contrast with the frontal and vertex regions. Soft-tissue loads on the frontal and vertex areas of the scalp are more than the soft-tissue load on the middle top.
The new theory’s unparalleled ability to explain even the details of the hair loss process and the formation of the pattern in AGA is apparent.
In his review Trüeb10 states that genetic involvement in AGA is pronounced, but no specific gene has been identified yet and genetic predisposition to AGA remains poorly understood. He continues, “We probably deal with a polygenic inheritance, dependent on a combination of mutations, e.g. in or around the AR (androgen receptor) gene affecting the expression of the AR, and other genes controlling androgen levels.” However, systemic androgen levels are normal in AGA, DHT increases locally and the enzyme that converts testosterone to DHT is 5-alpha reductase. In the same review, Trüeb acknowledges that the genes encoding the two 5-alpha reductase isoenzymes have been shown not to be associated with AGA by Ellis et al.33 That is, although there are many findings that suggest genetic involvement in AGA, DHT increase in AGA is not an occurrence directly determined by genes.
It comes to the same question again: Why does DHT increase in balding scalp? This is the crux of the matter.
Since its introduction, the new theory has been regarded with notable skepticism and resistance. Simplifying a very complicated problem is probably the only disadvantage of the theory. AGA has been one of the biggest and most challenging problems of the humankind. It has affected so many lives throughout the human history and has been a devastating condition for so many of the afflicted. It is difficult to settle for any mechanism less than highly complex. However, all natural phenomena that seem to be complex look simpler if viewed from the right standpoint.
Footnotes
Disclosure: The author holds a patent on prevention and treatment of male pattern baldness (U.S. Patent No.7914548). The Article Processing Charge was paid for by the author.
REFERENCES
- 1.Sawaya ME, Price VH. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997;109:296–300. doi: 10.1111/1523-1747.ep12335779. [DOI] [PubMed] [Google Scholar]
- 2.Schweikert HU, Wilson JD. Regulation of human hair growth by steroid hormones. I. Testerone metabolism in isolated hairs. J Clin Endocrinol Metab. 1974;38:811–819. doi: 10.1210/jcem-38-5-811. [DOI] [PubMed] [Google Scholar]
- 3.Dallob AL, Sadick NS, Unger W, et al. The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness. J Clin Endocrinol Metab. 1994;79:703–706. doi: 10.1210/jcem.79.3.8077349. [DOI] [PubMed] [Google Scholar]
- 4.Hibberts NA, Howell AE, Randall VA. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol. 1998;156:59–65. doi: 10.1677/joe.0.1560059. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, Part 1):578–589. doi: 10.1016/s0190-9622(98)70007-6. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman KD. Androgens and alopecia. Mol Cell Endocrinol. 2002;198:89–95. doi: 10.1016/s0303-7207(02)00372-6. [DOI] [PubMed] [Google Scholar]
- 7.Randall VA. Androgens and human hair growth. Clin Endocrinol (Oxf) 1994;40:439–457. doi: 10.1111/j.1365-2265.1994.tb02483.x. [DOI] [PubMed] [Google Scholar]
- 8.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 9.Ustuner ET. Baldness may be caused by the weight of the scalp: gravity as a proposed mechanism for hair loss. Med Hypotheses. 2008;71:505–514. doi: 10.1016/j.mehy.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Trüeb RM. Molecular mechanisms of androgenetic alopecia. Exp Gerontol. 2002;37:981–990. doi: 10.1016/s0531-5565(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 11.Levakov A, Vucković N, Dolai M, et al. Age-related skin changes. Med Pregl. 2012;65:191–195. [PubMed] [Google Scholar]
- 12.Caso G, McNurlan MA, Mileva I, et al. Peripheral fat loss and decline in adipogenesis in older humans. Metabolism. 2013;62:337–340. doi: 10.1016/j.metabol.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu XF, De Pergola G, Björntorp P. Testosterone increases lipolysis and the number of beta-adrenoceptors in male rat adipocytes. Endocrinology. 1991;128:379–382. doi: 10.1210/endo-128-1-379. [DOI] [PubMed] [Google Scholar]
- 14.Frederiksen L, Højlund K, Hougaard DM, et al. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur J Endocrinol. 2012;166:469–476. doi: 10.1530/EJE-11-0565. [DOI] [PubMed] [Google Scholar]
- 15.Dieudonne MN, Pecquery R, Leneveu MC, et al. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology. 2000;141:649–656. doi: 10.1210/endo.141.2.7293. [DOI] [PubMed] [Google Scholar]
- 16.Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol. 2007;192:585–594. doi: 10.1677/joe.1.07070. [DOI] [PubMed] [Google Scholar]
- 17.Shen W, Punyanitya M, Silva AM, et al. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutr Metab (Lond) 2009;6:17. doi: 10.1186/1743-7075-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enzi G, Gasparo M, Biondetti PR, et al. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr. 1986;44:739–746. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- 19.Brincat MP, Baron YM, Galea R. Estrogens and the skin. Climacteric. 2005;8:110–123. doi: 10.1080/13697130500118100. [DOI] [PubMed] [Google Scholar]
- 20.Hall G, Phillips TJ. Estrogen and skin: the effects of estrogen, menopause, and hormone replacement therapy on the skin. J Am Acad Dermatol. 2005;53:555–568. doi: 10.1016/j.jaad.2004.08.039. quiz 569–572. [DOI] [PubMed] [Google Scholar]
- 21.Raine-Fenning NJ, Brincat MP, Muscat-Baron Y. Skin aging and menopause: implications for treatment. Am J Clin Dermatol. 2003;4:371–378. doi: 10.2165/00128071-200304060-00001. [DOI] [PubMed] [Google Scholar]
- 22.Shu YY, Maibach HI. Estrogen and skin: therapeutic options. Am J Clin Dermatol. 2011;12:297–311. doi: 10.2165/11589180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Archer DF. Postmenopausal skin and estrogen. Gynecol Endocrinol. 2012;28(Suppl 2):2–6. doi: 10.3109/09513590.2012.705392. [DOI] [PubMed] [Google Scholar]
- 24.Van Pelt RE, Gozansky WS, Hickner RC, et al. Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity (Silver Spring) 2006;14:2163–2172. doi: 10.1038/oby.2006.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magerl M, Tobin DJ, Müller-Röver S, et al. Patterns of proliferation and apoptosis during murine hair follicle morphogenesis. J Invest Dermatol. 2001;116:947–955. doi: 10.1046/j.0022-202x.2001.01368.x. [DOI] [PubMed] [Google Scholar]
- 26.Paus R, Müller-Röver S, Van Der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 27.Gupta V, Bhasin S, Guo W, et al. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol. 2008;296:32–40. doi: 10.1016/j.mce.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, De Pergola G, Björntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology. 1990;126:1229–1234. doi: 10.1210/endo-126-2-1229. [DOI] [PubMed] [Google Scholar]
- 29.Singh R, Artaza JN, Taylor WE, et al. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 30.Glaser RL, Dimitrakakis C, Messenger AG. Improvement in scalp hair growth in androgen-deficient women treated with testosterone: a questionnaire study. Br J Dermatol. 2012;166:274–278. doi: 10.1111/j.1365-2133.2011.10655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaworsky C, Kligman AM, Murphy GF. Characterization of inflammatory infiltrates in male pattern alopecia: implications for pathogenesis. Br J Dermatol. 1992;127:239–246. doi: 10.1111/j.1365-2133.1992.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 32.Mahé YF, Michelet JF, Billoni N, et al. Androgenetic alopecia and microinflammation. Int J Dermatol. 2000;39:576–584. doi: 10.1046/j.1365-4362.2000.00612.x. [DOI] [PubMed] [Google Scholar]
- 33.Ellis JA, Stebbing M, Harrap SB. Genetic analysis of male pattern baldness and the 5alpha-reductase genes. J Invest Dermatol. 1998;110:849–853. doi: 10.1046/j.1523-1747.1998.00224.x. [DOI] [PubMed] [Google Scholar]


