Supplemental Digital Content is available in the text.
Abstract
Background:
Advanced thumb carpometacarpal arthritis is widely treated with trapeziectomy and tendon interposition despite donor-site morbidities. Trapeziectomy alone leaves a postresection space, leading to proximal metacarpal migration and scaphoid/trapezoid impingement. Prosthetic implants have been unsuccessful due to particulate debris, silicone synovitis, osteolysis, and migration. Recent studies have shown successful use of allograft for interposition material in the posttrapeziectomy space both in animal and human models. To obviate the need for autologous tissue, maintain thumb length, and reduce the risk of scaphoid impingement, the senior author developed an interposition arthroplasty technique using a spacer constructed from human acellular dermal matrix (HADM).
Methods:
Sixteen patients with Eaton stage III–IV thumb carpometacarpal osteoarthritis received the above procedure from the 2 senior authors. HADM was imbricated to fill the posttrapeziectomy space and secured to the volar capsule and metacarpal base. Pre- and postoperative trapezial space on radiograph, pain scores, and grip strength were recorded.
Results:
Six months postoperatively, radiographs showed an average joint space loss of 11%. Heights postoperatively were not significantly different from immediate postoperative heights (P ≥ 0.01). At 6 months, patients had improved pain and grip strength (P ≤ 0.01). No infections, foreign body reactions, or other complications occurred.
Conclusions:
HADM has been used extensively in other forms of reconstruction and has been shown to incorporate into surrounding tissues through neovascularization. Our early results illustrate that HADM can safely fill the dead space left by trapeziectomy.
There is no consensus regarding treatment for late-stage thumb carpometacarpal (CMC) arthritis.1 Trapeziectomy alone leaves a postresection space that allows proximal metacarpal migration and possible scaphoid/trapezoid impingement, with a markedly narrow joint space under stress.2 Although scaphoid impingement is a commonly cited but rarely documented complication, a reduced joint space is universally expected and loss of strength and stability and pain are known complications.3,4
Numerous studies show satisfactory functional outcomes for autologous tissue interposition with or without ligament reconstruction. Joint height is reduced by 50% after 12–36 months although patients tend to have adequate pain relief for many years.5,6 Using the flexor carpi radialis tendon for ligament reconstruction and tendon interposition improves strength, pain, function, and patient satisfaction,7–9 and loss of arthroplasty height was 11–13% on long-term follow-up.10,11 Patients occasionally reported increased postoperative pain, FCR tendonitis, and decreased fatigue strength.6 Autograft procedures require more operative time and extensive dissection that leads to increased scarring and pain and altered wrist kinetics. Naidu et al12 showed that FCR tendon harvesting leads to a significant decrease in the wrist flexion extension torque ratio and wrist flexion fatigue resistance.
Variations of the LRTI include recreating of the volar beak ligament with the abductor pollicis longus13,14 or abductor pollicis brevis.15 Although these studies cite satisfactory pain improvement, trapezial space loss was 14–32%. “Double arthroplasty” using the distal FCR and flexor carpi ulnaris has significant morbidity from tendon harvests. Costochondral allograft technique by Trumble et al16 had a trapezial height loss of over 21% after 1 year. Without clear benefits, complex autograft interposition can be associated with increased donor-site morbidity, scar tenderness, tendon adhesion or rupture, sensory changes, and complex regional pain syndrome.17
Early attempts at nonautologous interposition grafts were unsuccessful. A 2001 human trial of trapeziectomy alone versus trapeziectomy with porcine xenograft (Permacol) interposition was aborted because 6 of the 13 interposition patients developed joint space collapse with subluxation and adduction deformities. Patients had persistent histologically proven graft reactions, resulting in greater pain and erythema.18 In 1998, Muermans and Coenen19 used Gore-Tex (expanded polytetrafluroethylene) and Marlex (polypropylene) as interposition material to find that 3 out of 10 patients with Gore-Tex had significant synovitis requiring explantation. Although the Marlex group had similar results to the tendon interposition control group, Marlex is not regularly used today.
Newer allografts have shown promise. Trapezial space preservation was successful in a rabbit model in 2007 using acellular dermal matrix20 and in a monkey model21 in 2000 using allogenic tendon. In humans, Kokkalis et al22 used human acellular dermal matrix (HADM) to reconstruct the palmar beak ligament and fill the posttrapeziectomy space. At 12 months, they reported significantly less pain, increased grip and pinch strength (16% and 19%, respectively), 31% reduction in arthroplasty space, and no adverse reactions. Adams et al23 described arthroscopic debridement of the first CMC joint with HADM interposition arthroplasty. They reported improved pain and grip strength and high patient satisfaction with no complications. Taghinia et al24 proposed a suspended cadaveric fascia lata interposition allograft but reported a 50% loss of trapezial height despite satisfactory functional outcomes. To address current allograft shortcomings, the senior author proposes a novel technique.
METHODS
The senior authors began using HADM interposition with trapeziectomy in 2010. Sixteen patients with Eaton stage III or IV thumb CMC osteoarthritis qualified for this procedure and all were reviewed. All patients had 6 months of postoperative follow-up and had failed medical management of their osteoarthritis, including splinting, steroid injections, and nonsteroidal anti-inflammatory medications. Pre- and postoperatively, the trapezial space was measured with fine calipers on Rolando radiographs. All radiographs were taken on the same machine with the same specialty-trained technicians, who have 30 years of combined experience in hand films. Pain scores, pinch strength, and grip strength were recorded pre- and postoperatively.
Statistical analyses were performed using computer software (Stata/SE. V11.0 for Windows; StataCorp LP, College Station, Tex.). Paired t test was used to determine statistical differences between the pre- and postoperative measurements. P values were 2-tailed, and values equal to or less than 0.01 were defined as statistically significant. An institutional review board approval from Cedars Sinai Medical Center and the Keck School of Medicine of the University of Southern California is in place to follow-up the patients in this study for 2 additional years.
Surgical Procedure
A longitudinal dorsoradial incision is made from the base of the first metacarpal to the radial styloid for access to the thumb CMC joint (Fig. 1). Trapeziectomy is performed piecemeal using sharp and blunt dissection (Fig. 2). The base of the first metacarpal is then decorticated with a sagittal saw. A 4 × 7 cm sheet of 0.23-mm HADM (FlexHD, Ethicon) is fashioned by suture imbrication into the size and shape of the patient’s trapezium, keeping the dermal side exposed as much as possible (Figs. 3 and 4). The allograft is secured with multiple interrupted 3-0 Ticron sutures and the bundle is placed into the posttrapeziectomy space. Additional suture fixation is performed between the HADM and the volar capsule of the trapezium and base of the thumb metacarpal using 4-0 Mersilene sutures (Fig. 5). The dorsal capsule is closed with 4-0 Mersilene sutures. Intraoperative imaging is performed to confirm satisfactory radiographic position of the first metacarpal in line with the scaphoid (See Video 1, Supplemental Digital Content 1, which highlights technical aspects of creating and securing the interposition graft, available in the “Related Videos” section of the full-text article on PRSGO.com and http://links.lww.com/PRSGO/A10).
Fig. 1.
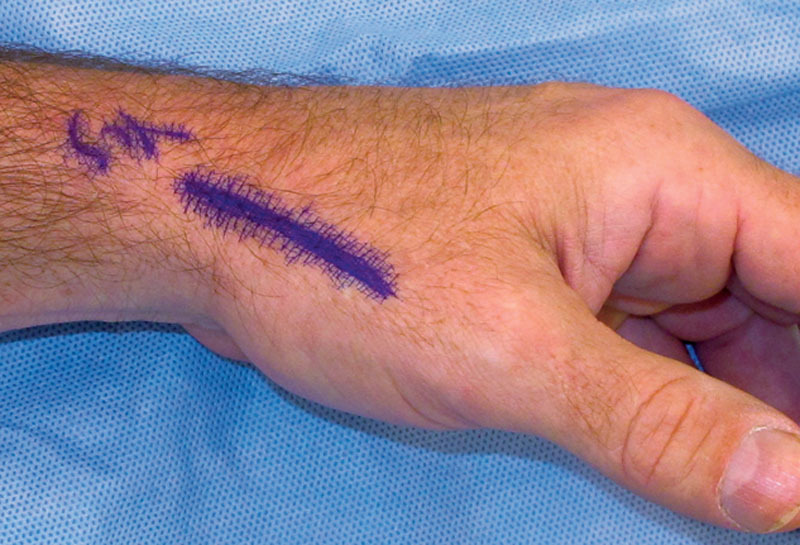
Incision for access to the thumb CMC joint.
Fig. 2.
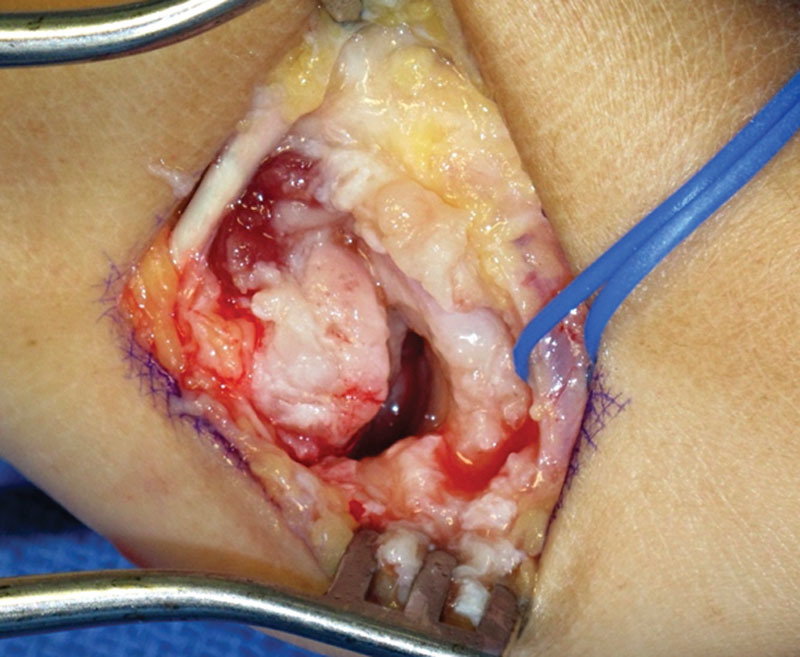
Piecemeal trapeziectomy.
Fig. 3.
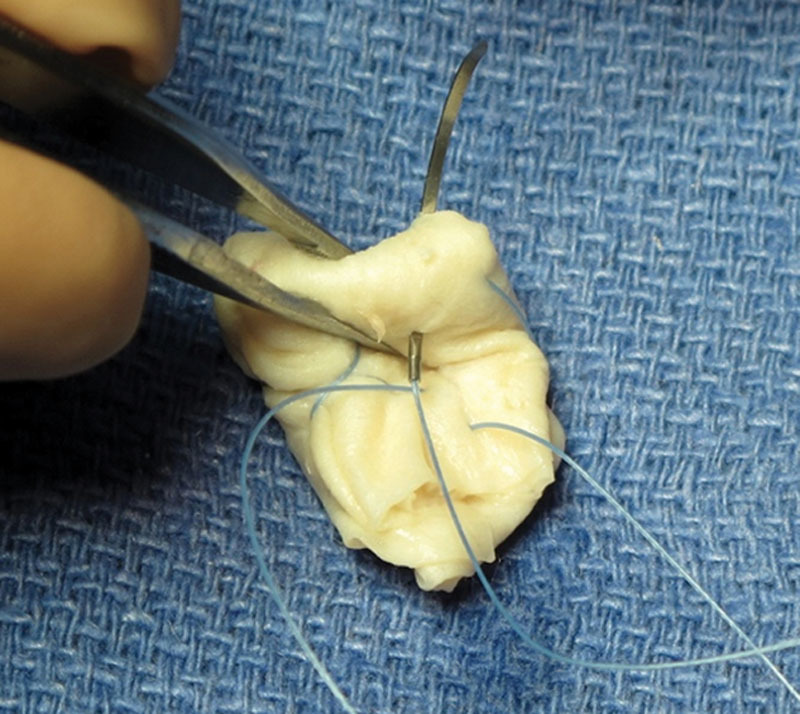
HADM imbricated with sutures.
Fig. 4.
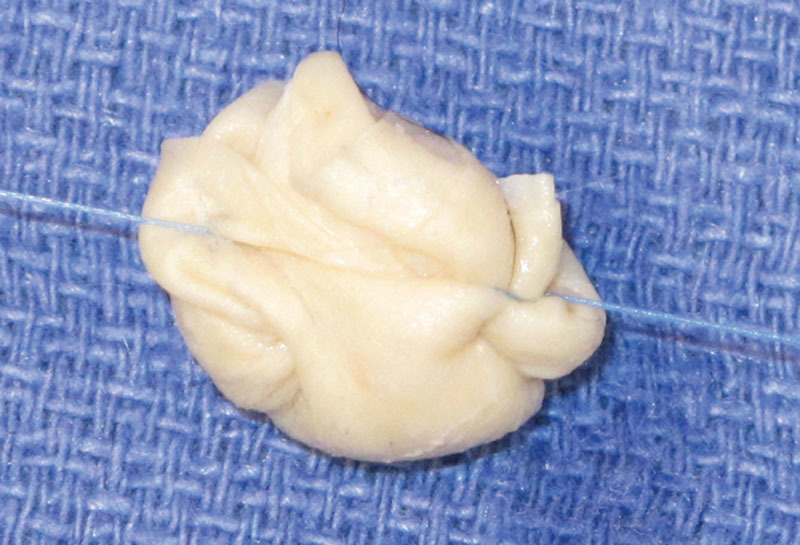
Dermal side of HADM exposed as much as possible and final construct shaped into size and contour of the patient’s trapezium.
Fig. 5.
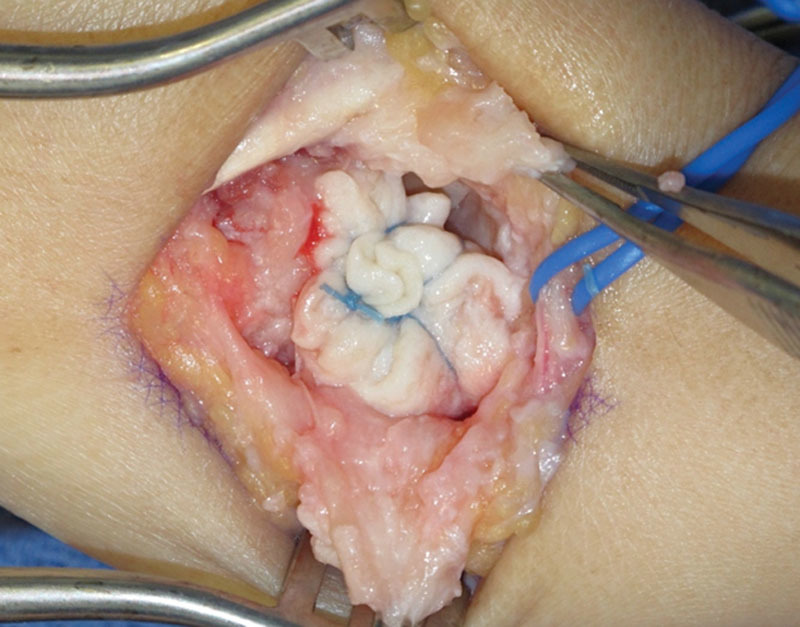
Additional suture fixation between the HADM and the volar capsule of the trapezium and base of the thumb metacarpal.
Video 1.
See video, Supplemental Digital Content 1, which shows technical highlights of creating and securing the FlexHD interposition graft, available in the “Related Videos” section of the full-text article on PRSGO.com, and http://links.lww.com/PRSGO/A10.
Postoperatively, the wrist is immobilized in a plaster short-arm thumb spica splint. One week later, a removable hand-based thumb spica splint is placed for 4 weeks. During this time, a hand therapist initiates gentle range of motion exercises. At 4 weeks, progressive hand therapy occurs until satisfactory range of motion and strength returns. Patients follow up at the postoperative appointment, 4 weeks, 3 months, 6 months, and every 6 months thereafter as needed.
RESULTS
Sixteen patients received the above procedure with a minimum follow-up time of 6 months. Average follow-up time was 7.7 months. Pre- and postoperatively, average trapezial space was 11.5 mm (SD, 2.7 mm) and 10.3 mm (SD, 2.7 mm), respectively. Six months postoperatively, radiographs showed that trapezial space height loss ranged from 0% to 27% with a mean of 11% (Table 1). Height 6 months postoperatively was not significantly different from immediate post-op height (P ≥ 0.01). Figures 6A–C highlight radiographic changes pre- and postoperatively. Figure 6A shows preoperative destruction of the thumb CMC joint space, whereas Figure 6B shows preservation of the trapezial space at 6 months using HADM. Figure 6C shows complete preservation of the space at 9 months. Fourteen patients had pre- and postoperative visual analog pain scores recorded; pain levels decreased from an average of 93% to 14% (P ≤ 0.01). At 6 months, all patients subjectively reported improved pain and quality of life. Thirteen patients had pre- and postoperative pinch strength recorded; 6 months after surgery, average strength improved from 4.2 kg to 8.5 kg, a 102% improvement (P ≤ 0.01). Ten patients had pre- and postoperative grip strength recorded. Grip strength increased from an average of 37.7 kg to 46.5 kg, a 23% improvement (P ≤ 0.01). No infections, foreign body reactions, or other complications occurred.
Table 1.
Trapezial Space 6 Months Postoperatively
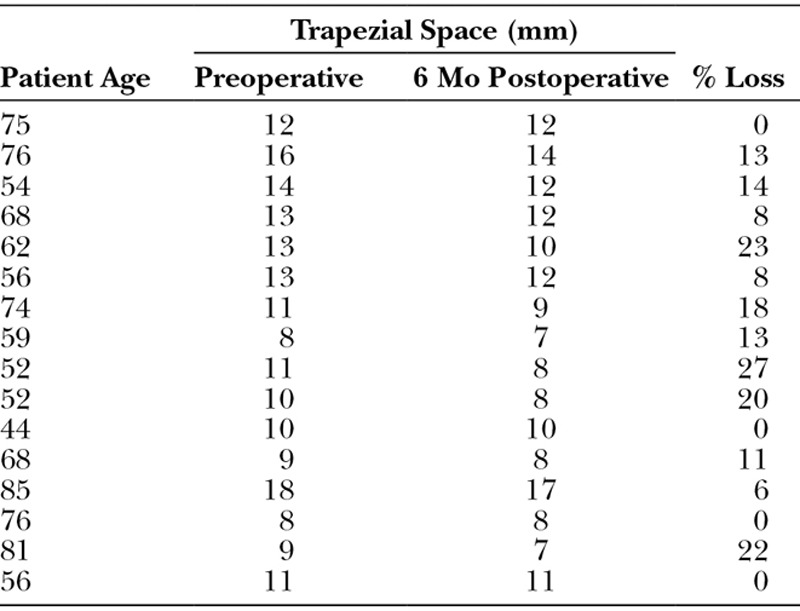
Fig. 6.
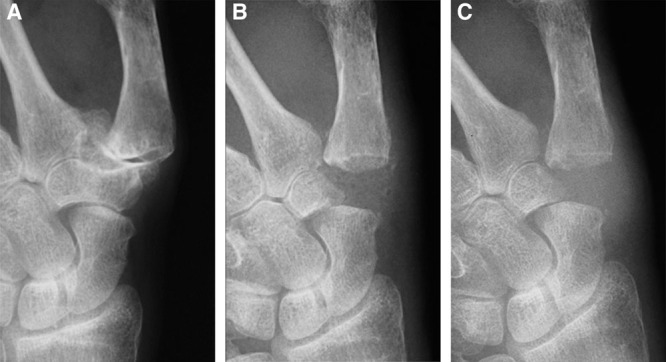
A, Preoperative destruction of the thumb CMC joint space. B, Trapezial space preservation at 6 mo. C, Trapezial space preservation at 9 mo.
DISCUSSION
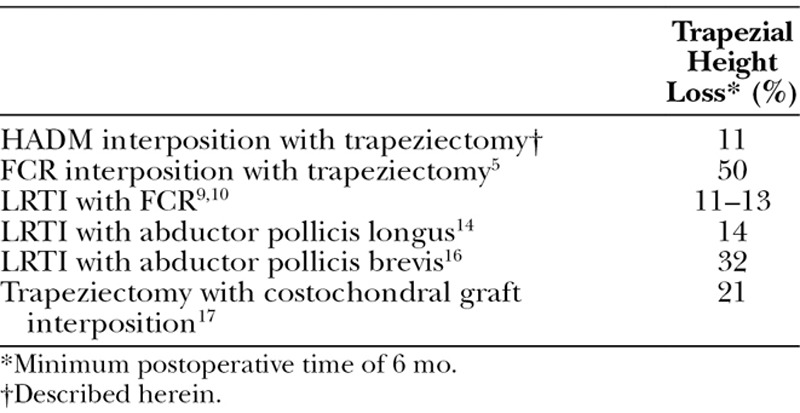
Although our procedure has an average joint height reduction of 11%, height reduction after tendon interposition alone and LRTI is 50%5,6 and 11–13%,10,11 respectively. Improved pain and strength in this study are comparable or superior to previously published results for tendon interposition and LRTI, but with no complications regarding FCR tendonitis, decreased fatigue strength, and increased postoperative pain. However, given the retrospective nature of our study, 19% of patients did not have pre- and postoperative pinch strength measurements, and 38% did not have grip strength measurements.
HADM avoids past difficulties that have plagued prosthetic implants for the first CMC joint, for example, graft reactions, and persistent pain. Unlike allogenic material, in vivo studies show that HADM induces rapid fibroblast and vascular progenitor infiltration with minimal adverse inflammatory responses25 In vivo rat models show that vasculogenesis begins as early as day 7 after implantation.26 As HADM integrates into its biologic environment, the risk of adverse reactions decreases. Although our short (6 mo) follow-up time is a caveat, our series shows no infections and no foreign body reactions to our allograft. Cross-linked porcine dermis grafts induce inflammatory responses, which may explain the early failure of Adams and Steinmann.20 Less collagen cross-linking leads to fewer foreign body responses and improved remodeling while certain processing solvents induce superior biocompatibility.20 Therefore, the preparation for each brand’s dermal matrix will determine which is better suited for trapezium replacement. Our experience with FlexHD shows that it has the potential to integrate into the posttrapeziectomy space, barring any matrix degeneration at the core of the construct. Although early results show good joint space preservation, longer follow-up will elucidate whether robust neocellularization and matrix remodeling will continue.
HADM has been used experimentally to fill soft-tissue and bony defects in plastic, oral, and cranial surgeries. Defects in periodontal surgery are often filled with HADM after gingival, mucosal, bone, or tooth resection with good integration. In these patients, HADM successfully engenders cellular infiltration without adverse reactions.27,28 Cranial base defects and large scalp with calvarial defects have been successfully patched with layered HADM with full tissue integration.29,30
Three articles describe comparable procedures. Taghinia et al24 use GraftJacket for concurrent interposition and ligament reconstruction after trapeziectomy and report a 31% joint space reduction. They recreate the palmar beak ligament by suspending HADM, necessitating FCR dissection, drilling into the first metacarpal, and manipulating the intramedullary canal. Our procedure is simpler with shorter operating times and less soft tissue and bony trauma. As such, our pilot series shows results comparable to existing procedures with decreased donor-site morbidity (Table 2). The procedure by Capito et al26 is similar to our technique; however, their choice of allograft does not preserve the trapezial space as robustly. Adams et al reported a similar arthroscopic technique for debridement of the first CMC joint with HADM (GraftJacket) interposition. They reported favorable outcomes but treated less severe forms of osteoarthritis.25
Table 2.
Results Comparable to Existing Procedures with Decreased Donor-site Morbidity
As this is a pilot study, limitations include the short follow-up time and small cohort size. However, our results provide evidence that a more robust, long-term study is worthwhile. A large-scale prospective study that compares results of our procedure with trapeziectomy alone and trapeziectomy with LRTI has the potential to shift the standard of care for late-stage first CMC joint osteoarthritis. HADM is notably used in hand reconstruction for forearm muscle herniation, burns, contractures, and trauma. A comprehensive review by the senior author in the November 2012 Plastic and Reconstructive Surgery issue revealed that very few complications have occurred with HADM in the hand; when complications did occur, they usually involved an infected wound bed.31 Although the risk of immunogenic rejection or hyperactive inflammation is an inherent risk of HADM, it has not been reported in the literature on hand. In breast reconstruction, it is well known that HADM is associated with increased risks of seroma and infection.32 Therefore, a larger cohort is indicated to highlight potential risks of long-term HADM implantation.
CONCLUSIONS
Although trapeziectomy with LRTI is the most common procedure for treating late-stage first CMC joint osteoarthritis, many alternative procedures have been introduced in the literature. We provide an interposition option that avoids donor-site morbidity while better accommodating individual CMC joint space contours as HADM can be imbricated into custom shapes. Although our initial results are promising, further study with a larger prospective cohort is warranted to compare our procedure with trapeziectomy alone and trapeziectomy with LRTI.
Supplemental Digital Content
Footnotes
Paper “A Novel Surgical Technique: Trapeziectomy and Interposition Arthoplasty with an Acellular Human Dermal Matrix Spacer for Thumb Carpometacarpal Joint Arthritis” presented at 67th Annual Meeting of The American Society for Surgery of the Hand, September 6–8, 2012, Chicago, Ill. Paper “Pilot Study: Interposition Arthroplasty with Acellular Dermal Matrix for Thumb Carpometacarpal Joint Arthritis” presented at The American College of Surgeons Southern California Annual Scientific Meeting, January 31, 2011, Santa Barbara, Calif. Paper “Pilot Study: Interposition Arthroplasty with Acellular Dermal Matrix for Thumb Carpometacarpal Joint Arthritis” presented at California Society of Plastic Surgeons, 62nd Annual Meeting, May 25–28, 2012, Reno, Nev.
Disclosure: Dr. Kulber has signed an agreement to be a consultant for the Musculoskeletal Transplant Foundation (MTF) but has to date not been compensated in any way and has not participated in any studies/projects. None of the other authors have a financial interest in any of the products, devices, or drugs mentioned in this manuscript. The Article Processing Charge was paid for by the Musculoskeletal Transplant Foundation.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Wolf JM, Delaronde S. Current trends in nonoperative and operative treatment of trapeziometacarpal osteoarthritis: a survey of US hand surgeons. J Hand Surg Am. 2012;37:77–82. doi: 10.1016/j.jhsa.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Varley GW, Calvey J, Hunter JB, et al. Excision of the trapezium for osteoarthritis at the base of the thumb. J Bone Joint Surg Br. 1994;76:964–968. [PubMed] [Google Scholar]
- 3.Kvarnes L, Reikerås O. Osteoarthritis of the carpometacarpal joint of the thumb. An analysis of operative procedures. J Hand Surg Br. 1985;10:117–120. doi: 10.1016/s0266-7681(85)80037-1. [DOI] [PubMed] [Google Scholar]
- 4.Dell PC, Brushart TM, Smith RJ. Treatment of trapeziometacarpal arthritis: results of resection arthroplasty. J Hand Surg Am. 1978;3:243–249. doi: 10.1016/s0363-5023(78)80088-4. [DOI] [PubMed] [Google Scholar]
- 5.Berger B, Linscheid RL. Arthroplasty in the hand and wrist. In: Green DP, Hotchkiss RN, Pederson WC, editors. Green’s Operative Hand Surgery. 5th ed. Amsterdam: Elsevier; 2007. [Google Scholar]
- 6.Lins RE, Gelberman RH, McKeown L, et al. Basal joint arthritis: trapeziectomy with ligament reconstruction and tendon interposition arthroplasty. J Hand Surg Am. 1996;21:202–209. doi: 10.1016/S0363-5023(96)80101-8. [DOI] [PubMed] [Google Scholar]
- 7.Burton RI. The arthritic hand. In: Evarts CM, editor. Surgery of the Musculoskeletal System. New York: Churchill Livingstone; 1983. pp. 670–681. [Google Scholar]
- 8.Tomaino MM, Pellegrini VD, Jr, Burton RI. Arthroplasty of the basal joint of the thumb. Long-term follow-up after ligament reconstruction with tendon interposition. J Bone Joint Surg Am. 1995;77:346–355. doi: 10.2106/00004623-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Burton RI, Pellegrini VD., Jr Surgical management of basal joint arthritis of the thumb. Part II. Ligament reconstruction with tendon interposition arthroplasty. J Hand Surg Am. 1986;11:324–332. doi: 10.1016/s0363-5023(86)80137-x. [DOI] [PubMed] [Google Scholar]
- 10.Tomaino MM, Burton RI, Pelligrini VD. Arthroplasty of the thumb basal joint: long-term follow-up of ligament reconstruction with tendon interposition. Orthop Trans. 1993;17:579. doi: 10.2106/00004623-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Burton RI, Pellegrini VD., Jr Surgical management of basal joint arthritis of the thumb. Part II. Ligament reconstruction with tendon interposition arthroplasty. J Hand Surg Am. 1986;11:324–332. doi: 10.1016/s0363-5023(86)80137-x. [DOI] [PubMed] [Google Scholar]
- 12.Naidu SH, Poole J, Horne A. Entire flexor carpi radialis tendon harvest for thumb carpometacarpal arthroplasty alters wrist kinetics. J Hand Surg Am. 2006;31:1171–1175. doi: 10.1016/j.jhsa.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Chang EY, Chung KC. Outcomes of trapeziectomy with a modified abductor pollicis longus suspension arthroplasty for the treatment of thumb carpometacarpal joint osteoarthritis. Plast Reconstr Surg. 2008;122:505–515. doi: 10.1097/PRS.0b013e31817d5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochevar AJ, Adham CN, Adham MN, et al. Thumb basal joint arthroplasty using abductor pollicis longus tendon: an average 5.5-year follow-up. J Hand Surg Am. 2011;36:1326–1332. doi: 10.1016/j.jhsa.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Heyworth BE, Jobin CM, Monica JT, et al. Long-term follow-up of basal joint resection arthroplasty of the thumb with transfer of the abductor pollicis brevis origin to the flexor carpi radialis tendon. J Hand Surg Am. 2009;34:1021–1028. doi: 10.1016/j.jhsa.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Trumble TE, Rafijah G, Gilbert M, et al. Thumb trapeziometacarpal joint arthritis: partial trapeziectomy with ligament reconstruction and interposition costochondral allograft. J Hand Surg Am. 2000;25:61–76. doi: 10.1053/jhsu.2000.jhsu025a0061. [DOI] [PubMed] [Google Scholar]
- 17.Davis TR, Brady O, Dias JJ. Excision of the trapezium for osteoarthritis of the trapeziometacarpal joint: a study of the benefit of ligament reconstruction or tendon interposition. J Hand Surg Am. 2004;29:1069–1077. doi: 10.1016/j.jhsa.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Belcher HJ, Zic R. Adverse effect of porcine collagen interposition after trapeziectomy: a comparative study. J Hand Surg Br. 2001;26:159–164. doi: 10.1054/jhsb.2001.0554. [DOI] [PubMed] [Google Scholar]
- 19.Muermans S, Coenen L. Interpositional arthroplasty with Gore-Tex, Marlex or tendon for osteoarthritis of the trapeziometacarpal joint. A retrospective comparative study. J Hand Surg Br. 1998;23:64–68. doi: 10.1016/s0266-7681(98)80222-2. [DOI] [PubMed] [Google Scholar]
- 20.Adams JE, Steinmann SP. Interposition arthroplasty using an acellular dermal matrix scaffold. Acta Orthop Belg. 2007;73:319–326. [PubMed] [Google Scholar]
- 21.Schmidt CC, McCarthy DM, Arnoczky SP, et al. Basal joint arthroplasty using an allograft tendon interposition versus no interposition: a radiographic, vascular, and histologic study. J Hand Surg Am. 2000;25:447–457. doi: 10.1053/jhsu.2000.7378. [DOI] [PubMed] [Google Scholar]
- 22.Kokkalis ZT, Zanaros G, Weiser RW, et al. Trapezium resection with suspension and interposition arthroplasty using acellular dermal allograft for thumb carpometacarpal arthritis. J Hand Surg Am. 2009;34:1029–1036. doi: 10.1016/j.jhsa.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Adams JE, Merten SM, Steinmann SP. Arthroscopic interposition arthroplasty of the first carpometacarpal joint. J Hand Surg Eur Vol. 2007;32:268–274. doi: 10.1016/J.JHSB.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Taghinia AH, Al-Sheikh AA, Upton J. Suture anchor suspension and fascia lata interposition arthroplasty for basal joint arthritis of the thumb. Plast Reconstr Surg. 2008;122:497–504. doi: 10.1097/PRS.0b013e31817d5456. [DOI] [PubMed] [Google Scholar]
- 25.Widgerow AD. Bioengineered matrices—part 2: focal adhesion, integrins, and the fibroblast effect. Ann Plast Surg. 2012;68:574–578. doi: 10.1097/SAP.0b013e31824b3d1c. [DOI] [PubMed] [Google Scholar]
- 26.Capito AE, Tholpady SS, Agrawal H, et al. Evaluation of host tissue integration, revascularization, and cellular infiltration within various dermal substrates. Ann Plast Surg. 2012;68:495–500. doi: 10.1097/SAP.0b013e31823b6b01. [DOI] [PubMed] [Google Scholar]
- 27.Batista EL, Jr, Batista FC, Novaes AB., Jr Management of soft tissue ridge deformities with acellular dermal matrix. Clinical approach and outcome after 6 months of treatment. J Periodontol. 2001;72:265–273. doi: 10.1902/jop.2001.72.2.265. [DOI] [PubMed] [Google Scholar]
- 28.Shi LJ, Wang Y, Yang C, et al. Application of acellular dermal matrix in reconstruction of oral mucosal defects in 36 cases. J Oral Maxillofac Surg. 2012;70:e586–e591. doi: 10.1016/j.joms.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Clark DW, Citardi MJ, Fakhri S. Endoscopic management of skull base defects associated with persistent pneumocephalus following previous open repair: a preliminary report. Otolaryngol Head Neck Surg. 2010;142:820–826. doi: 10.1016/j.otohns.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Kinsella CR, Jr, Grunwaldt LJ, Cooper GM, et al. Scalp reconstruction: regeneration with acellular dermal matrix. J Craniofac Surg. 2010;21:605–607. doi: 10.1097/SCS.0b013e3181d08cee. [DOI] [PubMed] [Google Scholar]
- 31.Ellis CV, Kulber DA. Acellular dermal matrices in hand reconstruction. Plast Reconstr Surg. 2012;130(5 Suppl 2):256S–269S. doi: 10.1097/PRS.0b013e318265a5cf. [DOI] [PubMed] [Google Scholar]
- 32.Israeli R. Complications of acellular dermal matrices in breast surgery. Plast Reconstr Surg. 2012;130(5 Suppl 2):159S–172S. doi: 10.1097/PRS.0b013e3182634e62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


