Abstract
Summary:
Hair transplantation techniques have changed in the last decades. Partial longitudinal follicular unit transplantation is a new hair transplantation technique, which differs from all other hair transplantation techniques by the size of the graft and therefore much more vulnerable grafts compared to the conventional hair transplantation grafts. In this study, we reveal the influence of the preservation solution on the viability of the grafts. We have extracted 15 hair transplantation grafts of 0.6 mm and 15 hair transplantation grafts of 0.7 mm from 3 different patients and investigated the influence of 2 commercially available preservation media, saline solution (Braun, Melsungen, Germany) and Ringer’s lactate (Braun), on the viability of grafts and compared these solutions with the preservation solution developed by Hair Science Institute with trypan blue. The grafts stored in the preservation solution developed by Hair Science Institute showed a significant better viability compared with the 2 commercially available preservation media saline solution and Ringer’s lactate. This study shows that a preservation solution could influence the viability of the grafts which could be essential for hair transplantations with small grafts such as in partial longitudinal follicular unit transplantation.
Earlier studies revealed that in conventional hair transplantation techniques, such as the strip method, the preservation solution could influence the survival rate after implantation1 and could reduce apoptosis in the grafts.2 Crisóstomo3 described the presence of oxidative stress in hair transplants. These studies showed that hair transplantation grafts are vulnerable, but do not reveal that in conventional hair transplantation procedures, the preservation solution is essential, since grafts, usually stored for a minimum period of 2 hours in saline solution before implantation, will regenerate new hairs.4 However, hair transplantation techniques have changed dramatically in the last decades from full punch grafts via the strip method to follicle unit transplantations, which automatically means that the grafts become smaller and smaller.
Partial longitudinal follicular unit transplantation (PL-FUT) is a new hair transplantation technique, which enables us to transplant hairs with the preservation of the donor site. PL-FUT differs from all other hair transplantation techniques by the size of the graft.5 The grafts used in PL-FUT are approximately 0.5–0.6 mm in diameter (surface area of 0.95 mm2) and are approximately 2.5 times smaller than the micrografts of 0.8–1.0 mm in diameter (surface area of 2.54 mm2) used in conventional hair transplantations. Consequently, the PL-FUT grafts are much more vulnerable compared to the conventional hair transplantation grafts.
This problem was already recognized in 2006 by Er et al,6 who recommended not to implant sectioned hair follicle parts. He stated that the survival rate of the transected hair follicles is directly related to the level of transection and that the growth rate of the sectioned parts is not satisfactory and he stated that the hairs are thinner than the original follicles.6
Although Er et al6 did not recommend to implant partial hair follicles because of the changed characteristics, we try to reveal the influence of the preservation solution on the viability of the grafts and, therefore, the characteristics of the regrown hairs. In PL-FUT, we transplant a considerably smaller amount of tissue compared to conventional hair transplantation procedures; in our opinion, the preservation solution will influence the survival rate of the grafts and therefore hair growth. This hypothesis is based on:
The small size of the graft (0.5–0.6 mm).
Long-time interval between extraction and implantation (2–6 h).
MATERIALS AND METHODS
In this study, we have extracted grafts from 3 different hair transplantation patients. Per patient, 15 hair transplantation grafts of 0.6 mm and 15 hair transplantation grafts of 0.7 mm were extracted (Table 1).
Table 1.
The Viability of Grafts in Hair Transplantation Surgery
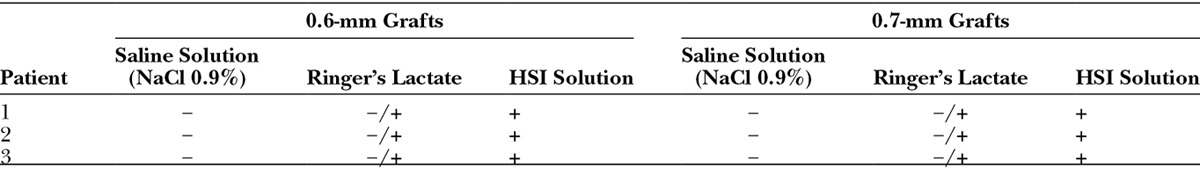
We investigated the influence of 2 commercially available preservation media, saline solution (Braun, Melsungen, Germany) and Ringer’s Lactate (Braun), on the viability of grafts and compared these solutions with the preservation solution developed by Hair Science Institute (HSI). This preservation solution contains sodium chloride, potassium chloride, magnesium sulfate, sodium phosphate, calcium chloride, glucose, sodium bicarbonate, sodium lactate, sodium pyruvate, human serum albumin, insulin, and the following ingredients: bis(maltolato)oxovanadium (BMOV) and monohydroxy ethylrutoside (HSI, Maastricht, The Netherlands).
Trypan blue can be used to determine nonviable tissue.7 To compare the viability of the grafts, we stored the 0.6 mm and 0.7 mm micrografts in saline solution, Ringer’s lactate, or our preservation solution for 4 hours. Afterward, we have left the grafts in a 0.1% trypan blue solution for 15 minutes (Fig. 1). We have evaluated the viability of the grafts semiquantitatively in the following levels (Table 1):
Fig. 1.
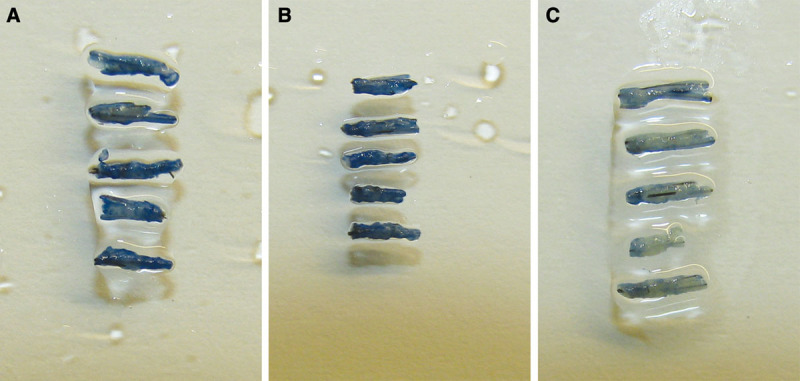
Grafts (0.7 mm) after 4 h in (A) saline solution, (B) Ringer’s lactate, and (C) preservation solution developed by HSI and 0.1% trypan blue solution for 15 min. The viability of the grafts is higher in Ringer’s lactate compared to the saline solution, but the viability of the grafts is the highest in the preservation solution developed by HSI.

RESULTS
In Figures 1 and 2, we can clearly see that the viability of the grafts is higher in Ringer’s lactate compared to the saline solution, but the viability of the grafts is the highest in the preservation solution developed by HSI.
Fig. 2.
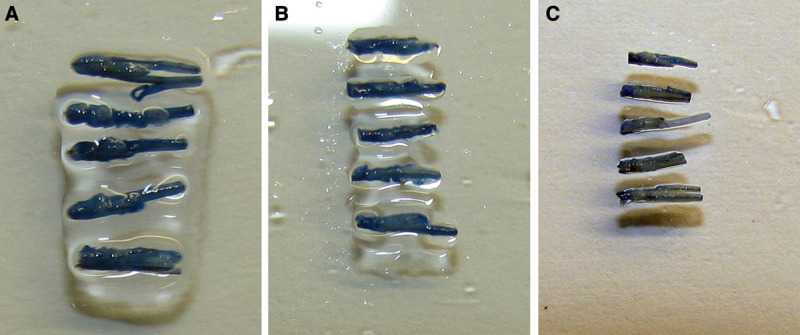
Grafts (0.6 mm) after 4 h in (A) saline solution, (B) Ringer’s lactate, and (C) preservation solution developed by HSI and 0.1% trypan blue solution for 15 min. The viability of the grafts is higher in Ringer’s lactate compared to the saline solution, but the viability of the grafts is the highest in the preservation solution developed by HSI.
DISCUSSION
Since Krugluger et al2 as described the presence of apoptosis and Crisóstomo3 revealed the relevance of oxidative stress in hair transplant grafts, the ingredients BMOV and monohydroxy ethylrutoside could have beneficial effects on the viability of the grafts. Lebeau et al8 and Ortolani et al9 revealed that different flavonoids, such as rutosides, have beneficial effects on ischemia and reperfusion injury. BMOV has many interesting physiological actions but probably exerts its main influence in the prevention of apoptosis by its ability to block the tyrosine phosphorylation pathway.10
We revealed that a considerably amount of tissue will become not viable (Fig. 1) in saline solution. However, when using large grafts, the amount of viable tissue seems to be sufficient to regenerate new hair growth in conventional hair transplantation techniques.4
This study clearly shows that a preservation solution is essential for hair transplantations with small grafts such as in PL-FUT. HSI’s preservation solution differs significantly from saline solution, Ringer’s lactate, and even from commercially available media used for keratinocyte culture. The addition of BMOV and rutosides will reduce oxidative stress, which is known to damage hair transplantation grafts.3 Any other addition such as growth factors seems to be unnecessary as viability tests have shown excellent survival of the grafts.
In addition, we studied the survival rate of 0.6-mm grafts in patients. This study showed that all implanted grafts developed to full differentiated hairs with a normal diameter.5 In contrast to the experiments of Er et al,6 our grafts differ in the way that the part of the follicle is taken in a longitudinal way.5 As we have proven that follicular stem cells are located at different areas in the hair follicle, we believe that these hair transplantation grafts, which contain also stem cells, are responsible for complete regrowth of the hairs.
CONCLUSIONS
The use of a suitable preservation solution could be one of the crucial conditions in the success of all hair transplantation procedures with small grafts, such as PL-FUT.
Although we have not investigated the efficacy of the different individual ingredients and different combination of the ingredients, the described preservation solution works perfect for PL-FUT.
In theory, it can be possible that the same efficacy can be obtained with excluding one or more ingredients of the solution. It was not our aim to investigate the minimum composition, but only to reveal the influence of the preservation solution on the viability of the grafts. Nevertheless, in our opinion, we advise to use a suitable preservation solution for hair transplantation procedures with small grafts (under 0.8 mm).
Additional studies with (improved) preservation solutions need to be performed to evaluate the minimal quantity of tissue necessary to regenerate hair growth.
Footnotes
Disclosure: Dr. Gho is an employee of Hair Science Institute. Prof. Neumann declares to have no conflict of interest. This study was supported by institutional resources. The Article Processing Charge was paid for by the Hair Science Institute.
REFERENCES
- 1.Raposio E, Cella A, Panarese P, et al. Power boosting the grafts in hair transplantation surgery. Evaluation of a new storage medium. Dermatol Surg. 1998;24:1342–1345. discussion 1346. [PubMed] [Google Scholar]
- 2.Krugluger W, Moser K, Moser C, et al. Enhancement of in vitro hair shaft elongation in follicles stored in buffers that prevent follicle cell apoptosis. Dermatol Surg. 2004;30:1–5. doi: 10.1111/j.1524-4725.2004.30010.x. discussion 5. [DOI] [PubMed] [Google Scholar]
- 3.Crisóstomo MR, Guimarães SB, de Vasconcelos PR, et al. Oxidative stress in follicular units during hair transplantation surgery. Aesthetic Plast Surg. 2011;35:19–23. doi: 10.1007/s00266-010-9549-z. [DOI] [PubMed] [Google Scholar]
- 4.Rassman WR, Bernstein RM, McClellan R, et al. Follicular unit extraction: minimally invasive surgery for hair transplantation. Dermatol Surg. 2002;28:720–728. doi: 10.1046/j.1524-4725.2002.01320.x. [DOI] [PubMed] [Google Scholar]
- 5.Gho CG, Martino Neumann HA. Donor hair follicle preservation by partial follicular unit extraction. A method to optimize hair transplantation. J Dermatolog Treat. 2010;21:337–349. doi: 10.3109/09546630903359814. [DOI] [PubMed] [Google Scholar]
- 6.Er E, Kulahci M, Hamiloglu E. In vivo follicular unit multiplication: is it possible to harvest an unlimited donor supply? Dermatol Surg. 2006;32:1322–1326. doi: 10.1111/j.1524-4725.2006.32301.x. discussion 1325–1326. [DOI] [PubMed] [Google Scholar]
- 7.Komen J, Wolbers F, Franke HR, et al. Viability analysis and apoptosis induction of breast cancer cells in a microfluidic device: effect of cytostatic drugs. Biomed Microdevices. 2008;10:727–737. doi: 10.1007/s10544-008-9184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebeau J, Neviere R, Cotelle N. Beneficial effects of different flavonoids, on functional recovery after ischemia and reperfusion in isolated rat heart. Bioorg Med Chem Lett. 2001;11:23–27. doi: 10.1016/s0960-894x(00)00589-8. [DOI] [PubMed] [Google Scholar]
- 9.Ortolani O, Caggiano M, Mannelli R, et al. Protection from ischemia-reperfusion damage in patients with stroke: the role of rutin and GSH. Transplant Proc. 1995;27:2877–2878. [PubMed] [Google Scholar]
- 10.Liem DA, Gho CC, Gho BC, et al. The tyrosine phosphatase inhibitor bis(maltolato)oxovanadium attenuates myocardial reperfusion injury by opening ATP-sensitive potassium channels. J Pharmacol Exp Ther. 2004;309:1256–1262. doi: 10.1124/jpet.103.062547. [DOI] [PubMed] [Google Scholar]


