Abstract
Background
There is an ongoing debate regarding the optimal criteria for return to sport after an acute hamstring injury. Less than 10% isokinetic strength deficit is generally recommended but this has never been documented in professional football players after rehabilitation. Our aim was to evaluate isokinetic measurements in MRI-positive hamstring injuries.
Methods
Isokinetic measurements of professional football players were obtained after completing a standardised rehabilitation programme. An isokinetic strength deficit of more than 10% compared with the contralateral site was considered abnormal. Reinjuries within 2 months were recorded.
Results
52 players had a complete set of isokinetic testing before clinical discharge. There were 27 (52%) grade 1 and 25 (48%) grade 2 injuries. 35 of 52 players (67%) had at least one of the three hamstring-related isokinetic parameters that display a deficit of more than 10%. The percentage of players with 10% deficit for hamstring concentric 60°/s, 300°/s and hamstring eccentric was respectively 39%, 29% and 28%. There was no significant difference of mean isokinetic peak torques and 10% isokinetic deficits in players without reinjury (N=46) compared with players with reinjury (N=6).
Conclusions
When compared with the uninjured leg, 67% of the clinically recovered hamstring injuries showed at least one hamstring isokinetic testing deficit of more than 10%. Normalisation of isokinetic strength seems not to be a necessary result of the successful completion of a football-specific rehabilitation programme. The possible association between isokinetic strength deficit and increased reinjury risk remains unknown.
Keywords: Hamstring injuries, Isokinetics, Soccer
Introduction
A progressive criteria-based rehabilitation programme composed of valid and reliable assessment tools is desirable when managing athletes with acute hamstring injuries.1 2 Unfortunately, the specific criteria for progression through a rehabilitation programme are infrequently described and rarely validated. As a result, the clinician managing hamstring strain injuries in athletes is frequently required to progress rehabilitation in the absence of an adequate evidence base.1 2 Arguably, the most important and most complex stage of a progressive rehabilitation programme is the decision to return to sport (RTS), as this will impact athlete availability and risk for reinjury. As with all elements of the rehabilitation programme, there remains little evidence base to this decision.
In all athletes, but particularly in professional athletes, functional sports-specific field testing is widely accepted as an appropriate and practical approach to evaluate whether injuries are sufficiently resolved and to allow RTS.1–3 However, a 2005 systematic review of RTS guidelines concluded that there was insufficient evidence for RTS criteria and it was recommended that prospective studies should focus on assessing the value of isokinetic strength testing in guiding RTS decisions.3 Despite this recommendation, there remains a lack of consensus whether isokinetic assessment is useful in assisting RTS decisions.4
In Creighton et al's5 three-step decision-based RTS model, medical factors including an isokinetic evaluation and sport-specific field testing comprise the first step of RTS evaluation. Applying this approach, evidence of an injured muscle returning or nearly returning to preinjury strength levels, or attaining equivalence with the contralateral uninjured limb (5–10% is generally considered to be an acceptable threshold),6 may typically be recommended prior to progressing through the decision-based RTS model.
Only one report has prospectively evaluated isokinetic data of an injured hamstring compared with an uninjured leg at RTS.7 In that study, 25 recreational athletes were cleared for RTS when distinct functional criteria were met, including a not further specified sport-specific movement programme. They reported a 10% deficit in peak torque compared with the uninjured leg at RTS, with restoration of the isokinetic deficit observed 6 months after RTS. Unfortunately, the proportion of players who had full restoration of isokinetic peak torque when compared with the contralateral uninjured leg was not reported. Hence, it remains unclear whether completion of rehabilitation and functional field testing (FFT) results in normalisation of isokinetic function in hamstring muscle injuries.
Our hypothesis was that despite completing a progressive criteria-based rehabilitation programme and demonstrating a full clinical recovery, normalisation of isokinetic function is not always achieved. Our aim was to prospectively evaluate isokinetic variables in a cohort of MRI-positive hamstring-injured professional football players who had completed a six-stage rehabilitation programme including functional sports-specific rehabilitation.
Methods
Subjects
This study was part of a randomised controlled trial on the effect of platelet-rich plasma in hamstring injuries (ClinicalTrial.gov number NCT01812564). For this isokinetic study, we included all consecutively injured football players with MRI-positive hamstring injury who underwent a progressive six-stage criteria-based rehabilitation programme, including successfully completing football-specific FFT (box 1). Isokinetic measurements were obtained after successfully finishing the FFT.
Box 1. Eligibility criteria.
Inclusion criteria
Age 18–50 years
Professional football player
Acute onset of posterior thigh pain
Presenting and MRI within 5 days from injury
MRI confirmed grade I or II hamstring lesion
Male gender
Able to perform five sessions of physiotherapy a week at our clinic
Exclusion criteria
Contraindication to MRI
Reinjury or chronic hamstring injury
Concurrent other injury inhibiting rehabilitation
Unwilling to comply with follow-up
Needle phobia
Overlying skin infection
Diabetes, immune-compromised state
Medication with increasing bleeding risk
Medical contraindication to injection
Data were collected between November 2009 and May 2013 from consecutive participants meeting the inclusion criteria
In the randomised controlled trial on platelet-rich plasma, participants were randomised into three groups: one group received an injection of 3 mL platelet-rich plasma (Biomet Recover, USA),8 one group received an injection of 3 mL platelet-poor plasma and one group received no injection. The injections were performed using a sterile ultrasound-guided technique into the region of maximal muscle injury, as determined by the initial MRI of the injury. To reach optimal local concentration, three separate depots of 1 mL were injected into the injured area of the muscle.
MRI
MRI was performed on the hamstring muscles using a 1.5-T magnet system (Magnetom Espree, Siemens) with the use of a body matrix coil. First coronal and transversal fast-spin echo proton density (PD)-weighted images (TR/TE of 3000/32 ms, filed of view (FOV) of 240 mm, slice thickness of 5 mm and a 333×512 matrix) with an echo train length (ETL) of 9 for the coronal images and 6 for the transverse. Subsequent coronal and transversal fast-spin echo PD fat saturation images (TR/TE of 3000/32 ms, FOV of 240 mm, slice thickness of 3.5 mm, a 326×512 matrix for the coronal images and TR/TE of 3490/27 ms, FOV of 320 mm, slice thickness of 3.5 mm, a 333×512 matrix for the transversal images) with an ETL of 6.
The radiologist was blinded to the clinical status of the injury and scored using an MRI modification of Peetrons’ grading; grade 0: no abnormalities; grade I: oedema without architectural distortion; grade II: oedema with architectural disruption; grade III: complete tear.9 10 When more than one muscle was involved, the muscle with the most extensive oedema or disruption was scored.
Standardised physical therapy programme
The participants completed a standardised physiotherapy programme, including range of motion exercises, progressive strengthening exercises, core stability training and agility exercises.
Rehabilitation was performed at one location by three sports physical therapists with 7–25 years of experience treating elite-level athletes, who were blinded to the intervention. As the effect of acute injection platelet rich plasma (PRP) on the time course of healing for muscle injury is unknown, functional, criteria-based progressions (as opposed to time-based progressions) were utilised for the six-stage rehabilitation protocol. The specifics of the rehabilitation programme are described in table 1.
Table 1.
Criteria-based rehabilitation programme for hamstring injury
| Stage | Content | Criteria to progress |
|---|---|---|
| Stage 1 | All activity to be pain-free 2 leg squat, or if able, single leg squat Maintain pelvis control, hip and knee alignment, squat to 45°, hold, return to start Supine Bridge—2 leg 2 s up, 2 s down (4 s total per rep.) Begin at 45°. Must reach knee-hip-shoulder in alignment. 4×15 supine isometric heel digs In supine, painlessly pull heel into bed through range. Can bias with tibial IR/ER when painless. Exercise bike Upright or recumbent, can substitute with elliptical trainer. Isometric manual-resisted hamstring Therapist applied resistance isometrically in varying angles in prone Soft tissue massage Proximal and distal to injury site, lymphatic drainage. Active range of motion exercises Supine active knee flexion and extension then Prone active flexion and extension |
Criteria to progress to stage 2:
|
| Stage 2 | Any exercise from stage 1 permitted, additionally: Supine bridge—1 leg Same rate as for 2 legs, other knee in full extension, thighs parallel throughout exercise. 4×15 Walk-Jog Walk 20 m corners, jog the 30 m straight, painless. Begin at 25% (self-rated) jog, progress to max 70%. Triple extension walk 100 m laps, every third step triple extension—ie, alternating legs. ‘A’ drill Walking late swing knee extension, painless. Alternating legs, 100 m lap. Soft tissue massage Can massage injured area. Maximum allowed pain VAS: 4/10. Therapist uses caution with any report of discomfort, monitor symptoms, adjust accordingly Stretching Hamstring (supine, 90° hip flexion, knee extension); SLR (supine to onset of discomfort add ankle DF) Initially active, patient-controlled, progress to passive, end range. SLR mobilisation if indicated. Resisted hamstring Note tibial rotation as indicated. 4×15 repetitions, aiming for fatigue |
Criteria to progress to stage 3:
|
| Stage 3 | Any exercises from stages 1 and 2, additionally: Single leg bridge 1 s repetition, 2 s recovery. 4×8 repetitions. Single leg bridge, foot on Swiss ball 2 s up, 2 s down. 4×8 repetitions. Interval running 20 m jog 30 m run. Begin running at 70% (patient rated), progressing by 10% steps, painlessly. At 90%, progress by 5%. Monitor performance by hand timing. Modified T-Drill Direction changing running over T-Drill course. Begin at patient rated 70%, progress as able by 10% until 90%, then by 5%. Monitor performance by hand timing. Eccentric exercises Nordic Hamstrings, manual-resisted eccentric, prone catches, Arabesque (single leg stance, trunk flexion) |
Criteria to progress to stage 4 (sport-specific rehab):
|
| Stage 4 | Any exercises from stages 1–3, additionally on-field, football-specific drills: Direction change drills With and without the ball, 40 min Jumping drills 10–15 min |
Criteria to progress to stage 5 (sport-specific rehab): 1. Painless completion of stage 4 |
| Stage 5 |
Passes and run Long passes progression Crosses (static) Corner kicks Crosses (dynamic) |
Criteria to progress to stage 6 (sport-specific rehab): 1. Painless completion of stage 5 |
| Stage 6 |
Passes and run Shooting scenarios Competitive 1 versus 1 drills Shooting scenarios Scoring scenarios |
Criteria to progress to medical review for return to sport: 1. Painless completion of stage 6 |
DF, dorsiflexion; ER, external rotation; IR, internal rotation; Modified T-Drill, (always) forward running over the course of the Agility t test; ROM, range of motion; SLR, straight leg raise.
Football-specific FFT
After successfully finishing the first three stages of the physiotherapy programme, the final stages of a football-specific FFT was supervised by a sports rehabilitator with 11 years of practical experience in elite football, who was also blinded to the intervention. The programme consisted of staged progression of volume and intensity of direction changes, sprints, jumps, (cross-) passes, shooting, interval running, one-on-one attacking and defence drills, mimicking muscle fatigue and competitiveness during football training and game situations. Successful completion of the FFT was defined as full sports-specific functional ability without obvious limitation and/or symptoms and evaluated by the sports physician on the day of FFT.3
Isokinetic testing
After completion of the FFT, athletes underwent an isokinetic evaluation of the knee flexors and extensors (System 3, Biodex, New York, USA). Prior to testing, the athletes were instructed as to the nature and purpose of the isokinetic testing. The athletes performed a standardised warm-up procedure comprising 6 min on a stationary exercise bike (Technogym, Italy) at a resistance (in Watts) equivalent to 1.5 times their bodyweight (in kg) at their chosen cadence (typically this was approximately 85 rpm).11 They were then instructed to perform a minimum of a further 4 min warm-up of their choosing. Typically, this comprised dynamic running, agility drills and self-stretching. Prior to each isokinetic test, the athlete was instructed as to the mode of testing, and given a minimum of three repetitions practice, and testing was not started unless the athlete thought they were ready to do so. The order (ie, left, right) was randomised, and this was maintained for each of the three modes and speeds for that athlete. During the testing, vigorous verbal encouragement was provided. Testing comprised three modes and speeds. First, the athletes were tested over five repetitions at 60°•s-1 concentric knee flexion and extension (concentric quadriceps (Q conc 60°•s-1) | hamstrings, (H conc 60°•s-1)). This was followed by 10 repetitions at 300°•s-1 concentric knee flexion/extension (concentric quadriceps (Q conc 300°•s-1) | hamstrings (H conc 300°•s-1)). Finally, they performed five repetitions at 60°•s-1|180°•s-1 eccentric knee extension/flexion (eccentric hamstrings (H ecc) | concentric hamstrings). Duration between the injury and completion of isokinetic testing was documented.
RTS and reinjury
Athletes were clinically reviewed by a sports physician blinded to the intervention on a weekly basis and immediately prior to RTS. The guidelines for making the final RTS decision included a number of elements. The first stage in the decision-based RTS pathway was consideration of medical factors including the successful and asymptomatic completion of the progressive criteria-based rehabilitation programme described above, and the results of isokinetic assessment. In keeping with the decision-based RTS model, the physician's final decision was guided, but not determined by these medical factors, but included consideration of sport risk modifiers (step 2) and decision modifiers (step 3).5 Accordingly, the isokinetic test was only one element of a comprehensive RTS process. If during the Sport Physician's assessment, any factor was established that did not allow the player to return to full participation, rehabilitation was resumed and reassessment performed prior to ultimate RTS. Following RTS, players were monitored on a monthly basis with a phone call, and in the event of a clinical suspicion of reinjury, the player was advised to immediately consult the sports physician. Acute hamstring strain injuries at the same site, that occurred within 2 months from RTS, were classified as reinjuries.9 12
Analysis
We performed all statistical analysis with SPSS software (V.20.0; SPSS, Chicago, Illinois, USA). Continuous variables were expressed as means with SDs. We analysed differences of binomial data between groups with unpaired t test or Fisher's exact data test. Our analysis was primarily based on detecting a 10% isokinetic deficit. Significance was assumed if the p value was less than 0.05. In addition, we calculated the percentage of players with 5%, 15%, 20% and 25% isokinetic deficits.
Results
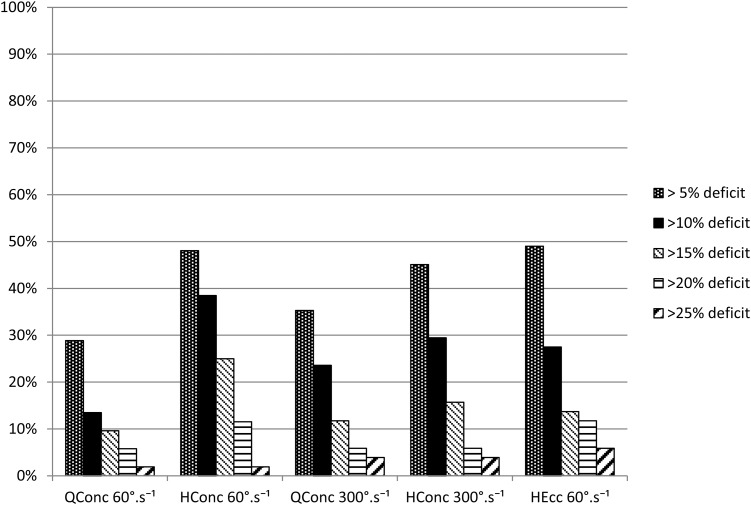
Of 59 players meeting the inclusion criteria, we excluded 7 from the analysis because of an incomplete set of isokinetic data. For the 52 players included, the mean age was 24.9 years (range 18–38). Using MRI-based grading, there were 27 (52%) grade 1 and 25 (48%) grade 2 injuries. The mean time between injury and isokinetic test was 21 days (range 7–43). After completing the isokinetic tests and following evaluation by the sports physician, RTS was delayed in two players: one player reported minor hamstring-related pain during the FFT and one player experienced insufficient subjective readiness. Both underwent further rehabilitation. A total of 59%, 14%, 19%, 6% and 4% had isokinetic testing within 1, 2, 3, 4 and 6 days, respectively, of finishing the FFT. Isokinetic characteristics for the 52 players are listed in table 2 and figures 1 and 2. The percentage of players with 10% deficit for hamstring concentric 60°/s, 300°/s and hamstring eccentric were, respectively, 39%, 29% and 28%. Table 3 presents the isokinetic data for the reinjured cohort in comparison with the remainder of the cohort. There was no significant difference in either mean isokinetic peak torque (for any mode of testing) or for number of players with a least one isokinetic deficit greater than 10%, when comparing players without reinjury (N=46; 88.5%) with players with reinjury (N=6; 11.5%).
Table 2.
Isokinetic peak torques characteristics of the injured leg for 52 professional football players at clinical discharge
| Q conc 60°•s-1 (%) | H conc 60°•s-1 (%) | Q conc 300°•s-1 (%) | H conc 300°•s-1 (%) | H ecc (%) | |
|---|---|---|---|---|---|
| Mean | 98.9 | 96.3 | 96.8 | 102.3 | 98.6 |
| Median | 98.8 | 97.1 | 96.3 | 104.8 | 99.6 |
| SD | 9.7 | 11.0 | 10.8 | 12.8 | 14.4 |
| Minimum | 75.8 | 78.4 | 72.3 | 81.5 | 75.0 |
| Maximum | 115.2 | 115.0 | 121.5 | 127.8 | 133.8 |
Characteristics (%) of the injured leg compared with the uninjured leg are presented.
H conc, hamstrings concentric, H ecc, hamstrings eccentric; Q conc: quadriceps concentric.
Figure 1.
Percentage of players (y-axis) not achieving the five different peak torque criteria: 5%, 10%, 15%, 20% and 25% (x-axis). H conc, hamstrings concentric; H ecc, hamstrings eccentric; Q conc, quadriceps concentric.
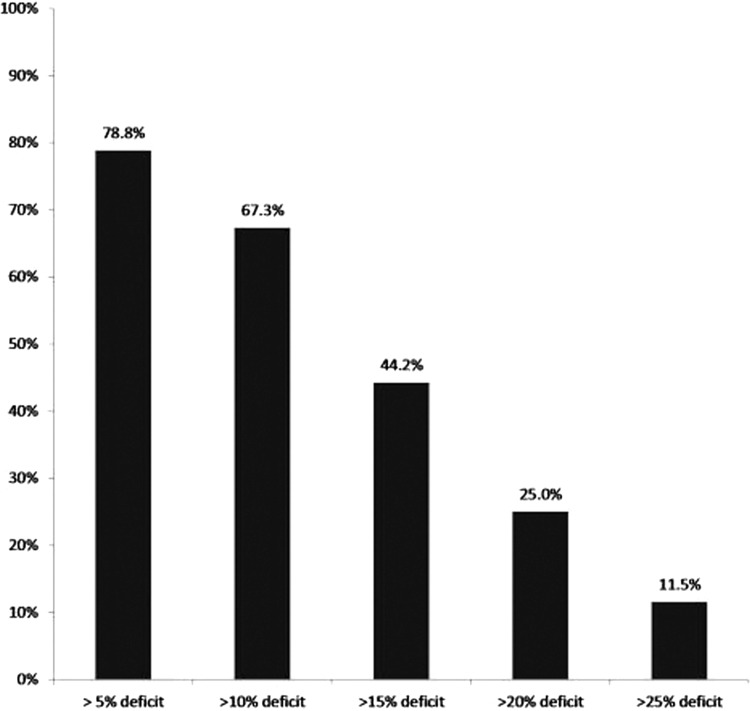
Figure 2.
Percentage of players (y-axis) who failed to meet any one of the three hamstring-related criteria with varying cut-points from 5% side-to-side deficit up to 25% side-to-side deficit (x-axis). Hamstring-related criteria were: knee flexion concentric at 60°•s-1° and 300°•s-1°, as well as eccentric at 60°•s-1.
Table 3.
Mean isokinetic peak torques (percentage compared with contralateral leg) and players with a greater than 10% deficit without reinjury compared with players with reinjury within 2 months of RTS
| No reinjury (n=46) | Reinjury (n=6) | p Value | |
|---|---|---|---|
| Mean peak torque | |||
| Q conc 60°•s-1 | 99.7% | 104.1% | 0.27 |
| H conc 60°•s-1 | 94.9% | 96.7% | 0.76 |
| Q conc 300°•s-1 | 98.4% | 103.8% | 0.31 |
| H conc 300°•s-1 | 98.1% | 94.7% | 0.54 |
| H ecc | 96.7% | 90.6% | 0.18 |
| 10% deficit | |||
| Q conc 60°•s-1 | 7 (15.2%) | 0 (0%) | 0.40 |
| H conc 60°•s-1 | 18 (39.1%) | 2 (33.3%) | 0.58 |
| Q conc 300°•s-1 | 12 (26.1%) | 0 (0%) | 0.19 |
| H conc 300°•s-1 | 13 (28.3%) | 1 (16.7%) | 0.48 |
| H ecc | 12 (26.1%) | 1 (16.7%) | 0.53 |
For the mean peak torques, the percentage is of the uninjured leg, that is, a value lower than 100% reflects a lower torque than the uninjured leg. For the 10% deficit category, the number (percentage) shown is for those athletes displaying a deficit of >10% with their uninjured leg for the individual mode of testing.
H conc, hamstrings concentric, H ecc, hamstrings eccentric; Q conc, quadriceps concentric; RTS, return to sport.
Discussion
In this series, 67% of hamstring-injured professional football players undertaking a criteria-based rehabilitation programme with strict functional and clinical RTS criteria showed at least one hamstring isokinetic testing deficit of more than 10%. Thus, normalisation of hamstring isokinetic function seems not to be an automatic outcome of completing a progressive football-specific rehabilitation programme.
Furthermore, analysis of those individuals who sustained a reinjury within 2 months of clinical discharge showed no difference in isokinetic strength parameters. However, due to the low reinjury rate (11.5%) and limited statistical power, it remains unknown if isokinetic functional deficit at the completion of rehabilitation is associated with an increased risk of reinjuries.
There are no comparable isokinetic studies available in professional athletes. In a smaller cohort of recreational athletes, Sanfilippo et al7 reported 10% peak torque deficit at RTS at group level but normalisation of isokinetic variables by 6 months. Given that the majority of hamstring-injured players return well before 6 months and do not reinjure, it can be questioned if complete restoration of isokinetic function is really required for a successful RTS. At this stage, there is insufficient evidence to support delaying RTS based on normalisation of isokinetic function.
Necessarily, RTS decisions are multifaceted, and as previously recognised, in professional sports, it might be preferable to have a player with a hamstring strain RTS at 3 weeks with a 10% risk of recurrence but playing in the key games, than returning at 8 weeks, having missed all the key games—but with a risk of recurrence of 0–5%.5 As long there remains a lack of quantifiable, valid and reliable determinants for RTS, there will persist a tension between early RTSs (primary outcome in most trials) and risk of recurrence (predominantly used as secondary outcome).
Isokinetic testing and FFT are elements of the decision-based RTS model.4
Other elements which may assist in RTS decision include questionnaires (personal medical history), specific functional tests (physical examination), additional diagnostics (particularly MRI) and sports-specific field tests. History taking represents an essential tool of our daily clinical decision-making process and may be enhanced by using patient-reported outcome (PRO) questionnaires. The PRO potentially reflects the self-reported readiness to RTSs, but has never been systemically investigated or validated. Currently, the only hamstring-specific PRO is the hamstring outcome score, originally developed as a risk factor screening tool, and further studies should focus on its validity as a tool for assisting RTS decisions.13
Analogous with RTS after anterior cruciate ligament (ACL) reconstruction, RTS after a hamstring strain injury is likely to be influenced by fear of reinjury and the subsequent psychological readiness of the injured athlete.14–16 In ACL reconstructions, the psychological readiness to RTSs has been successfully evaluated with the ACL RTS after injury.16 This scale measures the athlete's psychological state and has been shown to be associated with RTS and can potentially identify athletes at risk.14 16
For practitioners working with high-level athletes, psychological responses may be a crucial element of our RTS assessment, affecting the decision even when there is complete functional readiness and no symptoms reported on FFT. As in daily practice, the psychological evaluation could be complemented in future evidence-based RTS guidelines. When examining the player to assess RTS, the Askling-H RTS test has proven to be clinically applicable, relevant and reliable.13 17 Unfortunately, this test did not form part of our protocol, and hence was not evaluated against isokinetic function. By contrast, the limited value of additional imaging has recently been documented with convincing evidence that MRI observations cannot be used as criteria for successful RTS.18 Eighty-nine per cent of the clinically recovered injuries may still show persistent elevated signal intensity on T2 MRI with normalisation taking 6–12 months.7
A sports-specific field test is the ultimate test of the athlete's readiness to load the injured muscle as is required during (match) play, and subsequently it comes with its own risk of reinjury. Less rigorous field tests potentially reduce the reinjury risk during testing, but give rise to uncertainty as to whether the athlete is ready or not. We recommend that this final stage of sports-specific testing is not a standalone test, but should be preceded by a criteria-based rehabilitation programme to maximise the chances of a successful RTS.
The conundrum created by our results is that if an RTS decision is clinically based, predicated on the successful completion of a criteria-based rehabilitation programme, isokinetic deficits will still be present in 67% of players. Thus, if one places any merit on the isokinetic findings, and a recent survey of physicians involved with elite footballers suggests that they do,5 the clinician will potentially be required to undermine an established rehabilitation pathway and their clinical judgement. However, the aetiology and significance of this residual deficit remains unclear, and greater numbers are required to evaluate whether this deficit actually affects either the risk of reinjury or the quality of the playing performance.
The strength of this study is that all players were blinded to the cointervention, followed a structured rehabilitation programme based on predefined criteria before performing a football-specific FFT. This increases the external validity when the same criteria-based protocol is applied. Owing to our study design the potential added value of FFT remains unknown. This requires an FFT randomisation protocol in an elite-athletes setting and remains a topic to be studied, rather than one that can be proved with our data.
This study has several features that may limit the generalisations of our findings. Our population consisted of professional male football players. The generalisation to other cohorts of athletes remains unknown. Although it is generally accepted that the contralateral leg is used as a reference value, we cannot exclude that side-to-side strength deficits were present before the injury occurred, as recently shown by Zvijac et al.19 We recommend that future studies compare any isokinetic deficits at RTP with preinjury baseline measurements. This will clarify whether the strength deficit was pre-existing or a result of the injury. As the isokinetic test was one of the RTS factors, it could have been a potential source of bias. However, none of the players was delayed in RTS because of the isokinetic results, as the physician's final decision was guided, but not determined by this factor.
In summary, we reported the isokinetic findings of 52 professional football players at clinical discharge following MRI-positive injury. When compared with the uninjured leg, two of the three clinically recovered hamstring injuries had at least one hamstring isokinetic variable with a deficit of more than 10%. Normalisation of isokinetic strength does not seem to be required for successful completion of a football-specific FFT. Owing to the low reinjury rate and limited statistical power, it remains unknown if an isokinetic functional deficit of 10% at the completion of rehabilitation is associated with an increased risk of reinjuries.
What are new findings?
Sixty-seven per cent of the clinically recovered hamstring injuries had at least one hamstring isokinetic testing deficit of the ipsilateral leg of more than 10%.
How might it impact on clinical practice in the near future?
The findings of the present paper assist clinicians in the interpretation of follow-up isokinetic strength measurements after an acute hamstring injury.
When compared with the uninjured leg, normalisation of isokinetic strength does not seem to be required for successful completion of a football-specific functional field test.
The possible association between isokinetic strength deficits and increased reinjury risk remains unknown.
Acknowledgments
The authors thank the staff of the Qatar National Sports Medicine Programme (Executive Director Dr Hakim Chalabi) for their contribution to the study, Sirine Boukarroum for her efforts in the data collection, Faten Smiley for leading the medical ethics approval process and Emad Almusa for scoring MRIs.
Footnotes
Contributors: JLT designed the isokinetic substudy, analysed and interpreted the data and drafted the paper. BH designed the study, interpreted the data and revised the paper. CE interpreted the data and revised the paper. PM and PJ monitored data collection and interpreted the data. RW designed the study, monitored data collection, analysed and interpreted the data and revised the paper. All authors gave final approval for the version to be published.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Obtained from the Institutional Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Thorborg K. Why hamstring eccentrics are hamstring essentials. Br J Sports Med 2012;46:463–5 [DOI] [PubMed] [Google Scholar]
- 2.Mendiguchia J, Brughelli M. A return-to-sport algorithm for acute hamstring injuries. Phys Ther Sport 2011;12:2–14 [DOI] [PubMed] [Google Scholar]
- 3.Orchard J, Best TM, Verrall GM. Return to play following muscle strains. Clin J Sport Med Off J Can Acad Sport Med 2005;15:436–41 [DOI] [PubMed] [Google Scholar]
- 4.Delvaux F, Rochcongar P, Bruyere O, et al. Return-to-play criteria after hamstring injury: actual medicine practice in professional soccer teams. Br J Sports Med 2013;47:e3. [PMC free article] [PubMed] [Google Scholar]
- 5.Creighton DW, Shrier I, Shultz R, et al. Return-to-play in sport: a decision-based model. Clin J Sport Med 2010;20:379–85 [DOI] [PubMed] [Google Scholar]
- 6.Croisier J-L, Forthomme B, Namurois M-H, et al. Hamstring muscle strain recurrence and strength performance disorders. Am J Sports Med 2002;30:199–203 [DOI] [PubMed] [Google Scholar]
- 7.Sanfilippo JL, Silder A, Sherry MA, et al. Hamstring strength and morphology progression after return to sport from injury. Med Sci Sports Exerc 2013;45:448–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy. JAMA 2010;303:144–9 [DOI] [PubMed] [Google Scholar]
- 9.Ekstrand J, Healy JC, Walden M, et al. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med 2011;46:112–17 [DOI] [PubMed] [Google Scholar]
- 10.Peetrons P. Ultrasound of muscles. Eur Radiol 2002;12:35–43 [DOI] [PubMed] [Google Scholar]
- 11.Whiteley R, Jacobsen P, Prior S, et al. Correlation of isokinetic and novel hand-held dynamometry measures of knee flexion and extension strength testing. J Sci Med Sport 2012;15:444–50 [DOI] [PubMed] [Google Scholar]
- 12.Fuller CW. Consensus statement on injury definitions and data collection procedures in studies of football (soccer) injuries. Br J Sports Med 2006;40:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Askling CM, Nilsson J, Thorstensson A. A new hamstring test to complement the common clinical examination before return to sport after injury. Knee Surg Sports Traumatol Arthrosc 2010;18:1798–803 [DOI] [PubMed] [Google Scholar]
- 14.Ardern CL, Taylor NF, Feller JA, et al. Psychological responses matter in returning to preinjury level of sport after anterior cruciate ligament reconstruction surgery. Am J Sports Med 2013;41:1549–58 [DOI] [PubMed] [Google Scholar]
- 15.Ardern CL, Taylor NF, Feller JA, et al. A systematic review of the psychological factors associated with returning to sport following injury. Br J Sports Med 2013;47:1120–6 [DOI] [PubMed] [Google Scholar]
- 16.Ardern CL, Taylor NF, Feller JA, et al. Fear of re-injury in people who have returned to sport following anterior cruciate ligament reconstruction surgery. J Sci Med Sport Sports Med Aust 2012;15:488–95 [DOI] [PubMed] [Google Scholar]
- 17.Askling CM, Tengvar M, Thorstensson A. Acute hamstring injuries in Swedish elite football: a prospective randomised controlled clinical trial comparing two rehabilitation protocols. Br J Sports Med 2013;47:953–9 [DOI] [PubMed] [Google Scholar]
- 18.Reurink G, Goudswaard GJ, Tol JL, et al. MRI observations at return to play of clinically recovered hamstring injuries. Br J Sports Med 2014;48:1370–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zvijac JE, Toriscelli TA, Merrick S, et al. Isokinetic concentric quadriceps and hamstring strength variables from the NFL scouting combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med 2013;41:1511–18 [DOI] [PubMed] [Google Scholar]