Abstract
Background
Previous studies have shown that MRI of fresh hamstring injuries have diagnostic and prognostic value. The clinical relevance of MRI at return to play (RTP) has not been clarified yet. The aim of this study is to describe MRI findings of clinically recovered hamstring injuries in amateur, elite and professional athletes that were cleared for RTP.
Methods
We obtained MRI of 53 consecutive athletes with hamstring injuries within 5 days of injury and within 3 days of RTP. We assessed the following parameters: injured muscle, grading of injury, presence and extent of intramuscular signal abnormality. We recorded reinjuries within 2 months of RTP.
Results
MRIs of the initial injury showed 27 (51%) grade 1 and 26 (49%) grade 2 injuries. Median time to RTP was 28 days (range 12–76). On MRI at RTP 47 athletes (89%) had intramuscular increased signal intensity on fluid-sensitive sequences with a mean longitudinal length of 77 mm (±53) and a median cross-sectional area of 8% (range 0–90%) of the total muscle area. In 22 athletes (42%) there was abnormal intramuscular low-signal intensity. We recorded five reinjuries.
Conclusions
89% of the clinically recovered hamstring injuries showed intramuscular increased signal intensity on fluid-sensitive sequences on MRI. Normalisation of this increased signal intensity seems not required for a successful RTP. Low-signal intensity suggestive of newly developed fibrous tissues is observed in one-third of the clinically recovered hamstring injuries on MRI at RTP, but its clinical relevance and possible association with increased reinjury risk has to be determined.
Keywords: Hamstring injuries, MRI, Muscle damage/injuries
Introduction
MRI has been validated for the diagnosis and prognosis of acute hamstring injuries and is frequently used in these common injuries, especially in the elite athlete.1–9 Follow-up MRI has been suggested to monitor recovery after injury and support decisions for return to play (RTP), but has not been validated yet.7 10 Hamstring injuries are characterised by a high reinjury rate of 14–63% within 1 year.5 11–17 The reinjury is often more severe and associated with a longer absence from play.15 It has been hypothesised that reinjuries may be related to altered muscle mechanics due to fibrous tissue formation, reduced strength due to disuse atrophy, pain and/or reflex inhibition or to a premature RTP.11 18–21 Although there is no consensus as to when an athlete can safely RTP, in clinical practice an athlete is typically regarded as being ready once full range of motion, full strength and functional sport-specific activities (eg, sprinting, jumping and cutting) can be performed asymptomatically.10 18 22 Despite this conventional approach, the decision whether an athlete can safely RTP remains challenging.18 23 This is reflected in the high number of reinjuries that occur shortly after RTP, as it has been reported that 59% occur within the first month after RTP.24 Obviously, there is a need for assessment tools, which can discriminate between those athletes ready and athletes not ready for RTP.
Imaging modalities may have a role in assisting a safe RTP.7 10 Little is known about the value of MRI in monitoring recovery and RTP decisions. Connell et al2 found that increased signal intensity on fluid-sensitive sequences consistent with oedema may persist after resolution of clinical symptoms with 36% (15/42) having persistent abnormal findings on MRI at 6 weeks after the onset of injury. Similarly, Askling et al3 reported increased signal intensity on fluid-sensitive sequences on MRI 6 weeks after the onset of injury in 17 of 18 athletes. Long-term MRI observations on hamstring injuries have been reported by Silder et al,21 where 5–23 months after hamstring injury, increased low-signal intensities, suggestive of fibrous tissues, were found in 11 of 14 participants. These studies did not relate the MRI observations to RTP.
Two previous studies have reported MRI findings at RTP. Sanfilippo et al25 found at RTP in 25 athletes, that on average 20% of the muscles’ cross-sectional area still showed increased signal intensity on fluid-sensitive sequences on MRI. In the second study of the same research group Silder et al26 reported that none of 21 athletes had complete resolution of the increased signal intensity on MRI after being cleared to RTP and that most participants showed early signs of scar tissue formation. Detailed information regarding the presence and extent of fibrous tissues at RTP is not reported. If MRI is to be used in facilitating RTP decisions then observations, which can discriminate between a successful and unsuccessful RTP, should be identified.
Our hypothesis is that normalisation of increased signal intensities on fluid-sensitive sequences on MRI is not required for a successful RTP and that low-signal intensity suggestive of fibrous tissues may be observed in the majority of clinically recovered hamstring injuries at RTP. The aim of this study is to describe MRI findings of hamstring muscles in athletes, who have clinically recovered from an acute non-contact hamstring injury, and were cleared for RTP.
Methods
Subjects
At inclusion, informed consent was obtained from all patients. Approval was obtained from the Regional Ethical Committee of South West Holland and the Ethical Committee of Aspetar, Qatar Orthopaedics and Sports Medicine Hospital.
The patients in this study consist of cohorts of two ongoing double-blind randomised controlled trials (RCTs) on the effect of platelet-rich plasma in hamstring injuries: Dutch trial register number 2771 and ClinicalTrial.gov number NCT01812564. The first multicentre RCT started in February 2011 and was performed at the sports medicine departments of a large general district hospital, a university hospital and the medical centre of the national football association (Dutch cohort). In this study participants were randomised into an intervention group or a control group. The intervention group received two injections of 3 mL PRP; Autologous Conditioned Plasma, Biocore, Arthrex Inc, Karlsfeld, Germany) and the control group received two injections of 3 mL saline at the site of the injury. The first injection was performed within 5 days of the injury and the second injection 5–7 days later. The other RCT started in November 2009 and was performed in a specialised orthopaedic and sports medicine hospital (Qatar cohort). In this study participants were randomised into three groups: one group received an injection of 3 mL PRP (Biomet Recover, USA), one group received an injection of 3 mL platelet-poor plasma and one group received no injection. The injections were given using a sterile ultrasound-guided technique into the region of maximal muscle injury, as determined by the initial MRI. Three separate depots of 1 mL were injected.27 All participants completed a standardised physiotherapy programme, including a range of motion exercises, progressive strength exercises, core stability training and agility exercises.
The eligibility criteria for the present study are presented in table 1. In the first cohort the clinical diagnosis of the hamstring injury was made by six registered sports medicine physicians with 3–25 years of clinical experience in medical care of professional club and national team athletes in sports where hamstring injuries are common (football, futsal (indoor football), rugby, field hockey and squash). The functional criteria-based rehabilitation programme was supervised by a sports physiotherapist and clearance was given for RTP once they successfully and asymptomatically completed the physiotherapy programme, including functional sport-specific activities. In the second cohort the clinical diagnosis of the hamstring injury and the clearance for RTP was performed by eight registered sports medicine physicians with 7–20 years clinical experience, covering medical care of professional club and national team athletes where hamstring injuries are common (predominantly football, rugby, track and field). The guideline criteria to assist RTP decision included: successfully and asymptomatically completing the functional criteria-based four-staged physiotherapy programme, including a final supervised sport-specific (outdoor) training phase and less than 10% side-to side-difference at isokinetic strength testing. After RTP clearance, athletes were advised to complete 5 days of team training before participating in partial match play.
Table 1.
Eligibility criteria
| Dutch cohort | Qatar cohort |
|---|---|
| Inclusion criteria | |
|
|
| Exclusion criteria | |
|
|
RTP, return to play.
Magnetic resonance imaging
In each participant, MRI of the injury was performed twice: within 5 days from initial injury and within 3 days of RTP. The MRI of the initial injury was performed prior to the injection procedure.
MRI protocol
Two comparable MRI protocols were used. The protocol in the first RCT was a modified version of the protocol described by Askling et al.3 To locate the area of the injury the entire hamstring of the injured limb was visualised by obtaining coronal and sagittal short-tau inversion recovery (STIR) images from the ischial origin of the hamstring muscles to insertion on the fibula and the tibia (repetition time/echo time (TR/TE) of 3500/31 ms, field of view (FOV) of 300 mm and a 256×320 matrix). Subsequently, transversal STIR (TR/TE of 3500/31 ms, FOV of 300 mm and a 205×256 matrix), T1-weighted (TR/TE of 500/12 ms, FOV of 300 mm and a 355×448 matrix) and T2-weighted (TR/TE of 4080/128 ms, FOV of 300 mm and a 355×448 matrix) images were obtained from the injured area. The thickness of the slices for all sequences was 5 mm. MRI were obtained with a 1.5-T magnet system (Magnetom Essenza, Siemens) with the use of a body matrix coil.
In the second RCT MRI were obtained of the hamstring muscles with a 1.5-T magnet system (Magnetom Espree, Siemens) with the use of a body matrix coil. First coronal and transversal fast-spin echo proton density (PD) weighted images (TR/TE of 3000/32ms, FOV of 240 mm, slice thickness of 5 mm and a 333×512 matrix) were obtained. Subsequently coronal and transversal fast-spin echo PD fat saturation (PD-FS) images (TR/TE of 3000/32ms, FOV of 240 mm, slice thickness of 3.5 mm, a 326×512 matrix for the coronal images and TR/TE of 3490/27ms, FOV of 320 mm, slice thickness of 3.5 mm, a 333×512 matrix for the transversal images) were obtained.
MRI assessment
Each MRI was assessed by one of two radiologists, each with more than 9 years of experience in musculoskeletal radiology (EA and MM). The radiologists were blinded for the information on whether the MRI was of the initial injury or at RTP. For assessment of the MRIs we used standardised scoring forms based on the literature.2 4 6 21 28 We measured the increased T2-signal intensity for the affected hamstring muscle in craniocaudal, transverse and anterior–posterior dimensions on the fluid-sensitive sequences (STIR or PD-FS). We recorded the longitudinal length (craniocaudal) and calculated the involved cross-sectional area as a percentage of the total muscle cross-sectional area in the transversal plane. We measured the extent of low signal on T1-weighted images similarly in the three planes. We recorded the involved muscle(s) and performed grading of the injury using a modification of Peetrons classification4 28: grade 1—increased signal intensity on fluid-sensitive sequences without evidence of a macroscopic tear, grade 2—increased signal intensity on fluid-sensitive sequences with a partial tear, and grade 3—total muscle or tendon rupture. Increased signal intensity was defined as an abnormal intramuscular increased signal compared to the unaffected surrounding muscle tissues. Identically, the low-signal intensity was defined as an abnormal intramuscular low-signal intensity compared to the surrounding muscle tissues. Good to excellent interobserver and intraobserver reliability was found for the used MRI parameters in a previous study.29
Reinjury
We recorded acute hamstring injuries that occurred within 2 months after RTP at the same site as reinjuries.15
Analysis
We performed all statistical analysis with SPSS software (V.20.0; SPSS, Chicago, Illinois, USA). We analysed frequencies of the presence of intramuscular signal abnormalities, involved muscles and extent of intramuscular signal abnormalities using descriptive statistics. We tested the normality of the data with the Kolmogorov-Smirnov test: when p>0.05 we considered data normally distributed. We analysed differences in the extent of the intramuscular increased signal intensity on fluid-sensitive sequences over time using the dependent t test when normally distributed and the Wilcoxon's signed-rank test when there was non-parametric distribution.
Results
We included 53 consecutive patients in the analysis. The patient characteristics are presented in table 2. The median time to RTP was 28 days (range 12–76 days). The median time between injury and the first MRI was 2 days (range 1–5 days). The median time between the second MRI and RTP was 2 days after RTP (range 3 days before—3 days after RTP). The median time between the last injection and the MRI at RTP was 23 days (range 5–71 days).
Table 2.
Patient characteristics
| Median age (minimum–maximum) | 27 (18–46) |
| Gender, male/female | 53/0 |
| Sports | |
| Football | 40 |
| Futsal (indoor football) | 6 |
| Field hockey | 5 |
| Athletics | 1 |
| Squash | 1 |
| Level of sports | |
| Professional | 24 |
| Competitive | 19 |
| Recreational | 10 |
MRI findings
MRIs of the initial injury showed 27 (51%) grade 1 and 26 (49%) grade 2 injuries. Intramuscular increased signal intensity on fluid-sensitive sequences was present in 89% of the MRIs at RTP (figures 1 and 2). The characteristics of intramuscular abnormal increased signal intensity on fluid-sensitive MRI sequences of the initial injury as well as at RTP are presented in table 3.
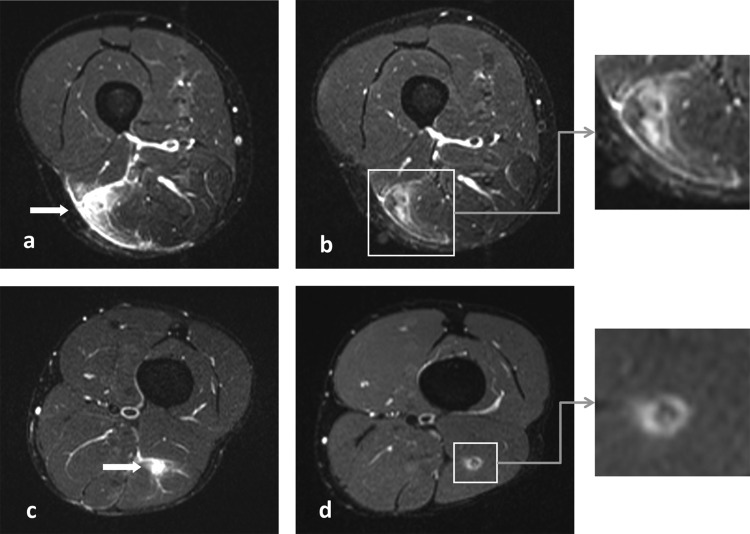
Figure 1.
(A and C) Short-tau inversion recovery (STIR) images of the initial injuries showing increased signal intensity of the musculus biceps femoris (arrow). (B and D) STIR images at return to play showing increased signal intensity around a centre of low signal at the site of the injury, indicating oedema and fibrous tissues.
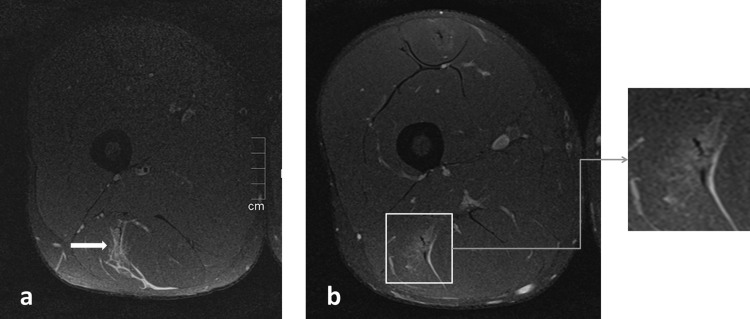
Figure 2.
(A) Proton density fat saturation (PD-FS) image of the initial injuries showing increased signal intensity of the musculus biceps femoris (arrow). (B) PD-FS image at return to play showing increased signal intensity at the site of the injury.
Table 3.
Characteristics of intramuscular increased signal intensity on fluid-sensitive MRI sequences of initial injury and at return to play (RTP)
| Initial injury | RTP | |||
|---|---|---|---|---|
| Intramuscular increased signal intensity | ||||
| Present | 53/53 | 100% | 47/53 | 89% |
| Absent | 0/53 | 0% | 6/53 | 11% |
| Involved muscles | ||||
| Biceps femoris long head | 44/53 | 83% | 39/47 | 83% |
| Biceps femoris short head | 0/53 | 0% | 0/47 | 0% |
| Semitendinosus | 2/53 | 4% | 1/47 | 2% |
| Semimembranosus | 9/53 | 17% | 8/47 | 17% |
| Grades | ||||
| 1 | 27/53 | 51% | 37/47 | 79% |
| 2 | 26/53 | 49% | 10/47 | 21% |
| 3 | 0/53 | 0% | 0/47 | 0% |
| Extent of increased signal intensity | ||||
| Mean longitudinal length (SD) | 132 mm | ± 62 | 77 mm | ±53* |
| Median involved cross-sectional muscle area (minimum–maximum) | 28% | 1–100 | 8% | 0–90* |
*Statistically significant difference between initial injury and RTP: p=0.000.
Intramuscular abnormal low-signal intensity was present in 42% of the MRIs at RTP (figure 3). The characteristics of intramuscular abnormal low-signal intensity on MRI, measured on T1-weighted images, of the initial injury as well as at RTP are presented in table 4. Three participants (6%) showed no intramuscular signal abnormality on MRI at RTP.
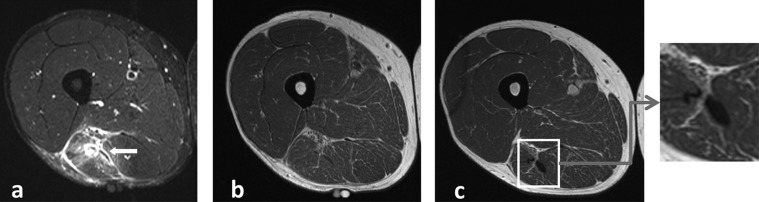
Figure 3.
(A) Short-tau inversion recovery (STIR) image of the initial injury showing extensive increased signal intensity at the musculotendinous junction of the musculus biceps femoris (arrow). (B) T1-weighted image of the same initial injury showing no abnormality. (C) T1-weighted image at return to play showing an increased area of low-signal intensity at the site of the injury, indicating fibrous tissue formation.
Table 4.
Characteristics of intramuscular abnormal low-signal intensity on MRI (fibrous tissues) of initial injury and at return to play (RTP)
| Initial injury | RTP | |||
|---|---|---|---|---|
| Intramuscular fibrosis | ||||
| Present | 4/53 | 8% | 22/53 | 42% |
| Absent | 49/53 | 92% | 31/53 | 58% |
| Involved muscles | ||||
| Biceps femoris long head | 3/4 | 75% | 20/22 | 91% |
| Biceps femoris short head | 0/4 | 0% | 0/22 | 0% |
| Semitendinosus | 0/4 | 0% | 3/22 | 14% |
| Semimembranosus | 1/4 | 25% | 1/22 | 5% |
| Extent of fibrosis | ||||
| Median longitudinal length (minimum–maximum) | 78 mm | 72–88 | 48 mm | 8–190 |
| Median length on axial view (minimum–maximum) | 9.2 mm | 5.4–12.9 | 8.5 mm | 2.8–22.9 |
| Median width on axial view (minimum–maximum) | 4.4 mm | 1.3–9.0 | 4.5 mm | 1.5–20.6 |
MRI, magnetic resonance imaging; RTP, return to play.
Reinjury
We recorded five (9%) reinjuries within 2 months after RTP. The reinjuries occurred at 2, 4, 5, 7 and 38 days after RTP. The presence and extent of increased signal intensity on fluid-sensitive sequences and fibrosis on MRI at RTP of participants with reinjuries compared to participants without reinjuries are presented in table 5.
Table 5.
Intramuscular increased signal intensity and fibrosis on MRI at return to play (RTP) of participants without reinjury compared to participants with reinjury within 2 months after RTP
| No reinjury (n=48) | Reinjury (n=5) | |||
|---|---|---|---|---|
| Increased signal intensity present | 43/48 | 90% | 4/5 | 80% |
| Extent of increased signal intensity | ||||
| Median longitudinal length (minimum–maximum) | 73 mm | 0–220 | 65 mm | 0–94 |
| Median involved cross sectional muscle area (minimum–maximum) | 8% | 0–90 | 14% | 0–31 |
| Intramuscular fibrosis present | 18/48 | 38% | 4/5 | 80% |
| Extent of fibrosis | ||||
| Median longitudinal length (minimum–maximum) | 88 mm | 8–190 | 48 mm | 15–130 |
| Median length on axial view (minimum–maximum) | 9.4 mm | 3.3–20.1 | 5.7 mm | 2.8–22.9 |
| Median width on axial view (minimum–maximum) | 4.9 mm | 2.1–10.1 | 3.1 mm | 1.5–20.6 |
MRI, magnetic resonance imaging; RTP, return to play.
Discussion
The major finding of this study is that in 89% of clinically recovered grades 1 and 2 hamstring injuries we observed intramuscular increased signal intensity on MRI on fluid-sensitive sequences at RTP. Low-signal intensity, suggestive of fibrous tissues, was observed in 42% of the clinically recovered grades 1 and 2 hamstring injuries on MRI at RTP.
The present study provides a detailed description of the MRI findings at RTP after hamstring injuries and is the largest series currently published on this topic. Two previous published studies found that increased signal intensity is still present on T2-weighted images in athletes cleared for RTP.25 26 These findings are consistent with the findings of the present study. The present study provides additional information on the presence and extent of increased signal intensity on fluid-sensitive sequences (oedema) as well as decreased signal intensity (fibrous tissues).
Several published clinical guidelines incorporated follow-up imaging to monitor progression after hamstring injury.7 10 It is unknown as to what extent intramuscular increased signal on fluid-sensitive sequences on MRI, found in 89% of the athletes reflects ongoing muscle damage in recovering muscle. While the extent of the muscle signal intensity alteration on fluid-sensitive sequences is decreased at RTP compared to the initial injury, there is an overlap between the extent of the signal intensity alteration found in the initial injury and at RTP. Thus, there are clinically recovered athletes in which the amount of the increased signal intensity on fluid-sensitive sequences in the muscle at RTP exceeds that of other athletes at the time of initial injury. This suggests that the extent of the increased signal intensity seen on fluid-sensitive sequences does not delineate an injured from a recovered hamstring muscle.
As almost all athletes that are clinically recovered and successfully returned to play showed increased signal intensities on fluid-sensitive sequences, some even with extensive signal abnormalities, RTP decisions after hamstring injuries should not depend on MRI features. Schneider-Kolsky et al9 reported only moderate correlation between clinical assessment using functional tests and MRI findings and showed that functional testing was more accurate than MRI assessment for predicting time required to RTP in fresh injuries. These studies support that functional performance of the athlete should be leading in rehabilitation and RTP decisions, rather than time dependent related to imaging findings.10 30 MRI of an acute injury has a role in determining the involved muscle(s) and the location of the injury, but should not be used in RTP decisions.
In the present series five athletes sustained a reinjury, of which four (80%) had increased signal intensity on fluid-sensitive sequences observed on MRI at RTP compared to 90% of the participants that did not sustain a reinjury. The extent of this increased signal intensity reveals a similar pattern in the reinjured as well as non-reinjured athletes (table 5). Although there is insufficient statistical power to study the association between increased signal intensity on fluid-sensitive sequences and reinjury risk, the observation that in 90% of the athletes without a reinjury increased signal intensities on fluid-sensitive sequences are present on MRI at RTP, suggests that normalisation of this increased signal intensity is not required for a successful RTP.
Increased signal intensity on fluid-sensitive sequences on MRI is considered to reflect increased intracellular or extracellular free water (typically ‘muscle oedema’).31 32 However, there remains limited understanding of the pathophysiological significance of either increased signal intensity on fluid-sensitive sequences, or oedema in acute muscle injuries, a point recently highlighted by an expert’s consensus statement on muscle injuries.33 Muscle damage is associated with an inflammatory response as well as oedema, both of which can result in increased signal intensity on fluid-sensitive sequences.34 The long-term persistence of this increased signal after injury does not seem to fit with the temporal changes of inflammation and oedema.35 What this increased signal intensity exactly reflects, at the initial muscle injury as well as during recovery, is unclear. This should be clarified in future studies. Importantly, our observations suggest that the increased signal intensity seen on fluid-sensitive sequences on MRI in clinically recovered hamstring injuries has no relevance for a successful RTP and hence the clinical relevance of increased signal intensity is dubious.
In the present study 42% of the cases had low-signal intensity, indicating fibrous tissues, at RTP. Although, as pathological correlation is lacking, the exact nature of this low-signal intensity is unknown. In four cases the low-signal intensity was present at the initial injury, suggesting a previous injury or other mechanism. Thus, 18 of 53 (34%) injured athletes developed new low-signal intensity at the site of the injury. Clinical studies showed that the low-signal intensity of fibrous tissues could persist in the long-term on MRI.2 21 The formation of fibrous tissues alters muscle stiffness and is frequently reported as a risk factor of reinjury,20 21 36 37 although evidence from clinical studies confirming that fibrosis increases the risk of reinjury is absent. In the present series five participants sustained a reinjury, of which four (80%) had fibrosis observed on MRI at RTP compared to 38% of the participants that did not sustained a reinjury. At first sight there seems to be a tendency that the fibrosis at RTP, seen as low-signal intensity on MRI, is associated with an increased risk of reinjury. Given its multifactorial origin, the limited number of reinjuries and insufficient power, it remains however unclear if there is an association between fibrosis on MRI and reinjury risk. Future studies with more participants are needed to examine the prognostic value of fibrosis for reinjuries.
The participants in the present study were participants of two RCTs on the effect of PRP. As a part of these double-blind placebo-controlled studies participants received either no injection or intramuscular injections with PRP, platelet-poor plasma or normal saline. The effect of these injections on hamstring muscle healing is still unknown. A potential limitation of this study is that the injections given 5–71 days before the MRI at RTP might influence the findings of the MRI. It could be hypothesised that the needle and/or the injected fluid increase the increased signal intensity on fluid-sensitive sequences. However, histological studies in animals show that intramuscular saline injections result in only minimal oedematous changes within the first 2 days.38 39 It therefore seems unlikely that the saline injections substantially influence the MRI findings at RTP. Little is known about the effect of PRP injections on muscle oedema. A recent histological study on animals found that healthy muscle tissues injected with PRP showed an inflammatory response with oedema and necrosis followed by fibrosis, similar to the traditional acute healing response in injured muscles.40 MRI analysis of recovering muscle injuries after PRP injections have been reported previously.27 41 In a pilot study Wright-Carpenter et al41 reported nearly completed regression of the muscle increased signal intensity on fluid-sensitive sequences in 18 athletes with muscle injuries treated with PRP injections at 14–16 days after injury. Their treatment regime consisted a mean of 5.4 injections of 5 mL PRP with 2 days between the injections. In a case report Hamilton et al27 found a resolution of increased signal intensity on fluid-sensitive sequences in 17 days after hamstring injury treated with a single 3 mL PRP injection. Although controlled trials comparing MRI analysis after PRP injections with no injections are lacking, the findings in these reports suggest accelerated reduction of the increased signal intensity on fluid-sensitive sequences after injection of PRP rather than prolongation. For generalisation to populations without PRP injections the present study might underestimate the amount of increased signal intensity on fluid-sensitive sequences found on MRI of clinically recovered hamstring injuries.
Another limitation is that we analysed two different cohorts with some differences in criteria for clearance for RTP in this study. There are however, still no validated criteria to ascertain whether an athlete is recovered from the injury and ready to RTP. This lack of validated criteria is emphasised by the differences of definitions and criteria used in scientific research as well as clinical practice.18 22 42 Our study reflects this common clinical challenge. On the other hand, our data on RTP are comparable with other studies and representative for a prospective cohort of acute hamstring injuries.1 2 4 6 9 Furthermore, this heterogeneity increases the external validity and generalisability for daily clinical practice where heterogeneous RTP criteria are used.
A second minor limitation of the analysis of two cohorts is that two slightly different MRI protocols were used: STIR and T1-weighted images in the first cohort and PD-FS and PD images in the second cohort. In scientific research and clinical practice, however, these sequences are all used in MRI of muscle injuries.43 This heterogeneity can be considered a minor weakness of present study, although it increases the external validity and generalisability for clinical practice where both protocols are used.
With our cohort we cannot exclude possible gender and age bias. Generalisation of the findings to female athletes and athletes under the age of 18 years should therefore be made with caution.
In summary, we reported the MRI findings of 53 consecutive athletes, after acute non-contact grades 1 and 2 hamstring injury, who were cleared for RTP. Eighty-nine per cent of the injuries showed intramuscular increased signal intensity on fluid-sensitive sequences on MRI at RTP. Normalisation of this increased signal intensity on MRI does not seem required for a successful RTP. Low-signal intensity suggestive of newly developed fibrous tissues at the site of the injury was observed in 34% at RTP. Five reinjuries were recorded. Given this limited number and insufficient power, the possible association of the MRI observations at RTP with increased reinjury risk has yet to be determined.
What are the new findings?
The majority (89%) of the clinically recovered hamstring injuries showed intramuscular increased signal intensity on MRI at return to play (RTP).
Low-signal intensity suggestive of newly developed fibrous tissues at the site of the injury is observed in one-third of the clinically recovered hamstring injuries on MRI at RTP.
How might it impact on clinical practice in the near future?
- The findings of present article support clinicians with the interpretation of follow-up MRI after acute hamstring injury:
- Normalisation of the increased signal intensity (oedema) on MRI is not required for a successful return to play (RTP).
- Increased signal intensity (oedema) on MRI of clinically recovered hamstring injuries does not seem to have clinical relevance for RTP decisions.
- Low-signal intensity, suggestive of fibrous tissues, might be associated with increased reinjury risk, but its clinical relevance has yet to be determined.
Acknowledgments
The authors thank the medical staff of Aspetar, Medical Center The Haghue, Medical Center of the Royal Dutch Football Association and University Medical Center Utrecht for their contribution to the study. The authors thank Sirine Boukarroum, Kees Baak, Mike van Os, Hazel Kluvers and Chantal Hebing for their efforts in the data collection.
Footnotes
Contributors: GR designed the study, monitored data collection, analysed and interpreted the data and drafted the article. GJG interpreted the data and revised the article. JLT designed the study, interpreted the data and revised the article. EA and MM analysed the MRIs, interpreted the data and revised the article. MHM, AW, JANV and BH interpreted the data and revised the article. All authors gave final approval for the version to be published.
Funding: The Dutch randomised controlled trial was supported by the Royal Dutch Football Association and Arthrex Medizinische Instrumente GmbH.
Competing interests: None.
Ethics approval: Regional Ethical Committee of South West Holland and the Ethical Committee of Aspetar, Qatar Orthopaedics and Sports Medicine Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional unpublished data are available on request by contacting the corresponding author. The unpublished data contain the detailed measurements of the extent of the abnormal MRI signal and the location of the injury within the hamstring muscles.
References
- 1.Verrall GM, Slavotinek JP, Barnes PG, et al. Diagnostic and prognostic value of clinical findings in 83 athletes with posterior thigh injury: comparison of clinical findings with magnetic resonance imaging documentation of hamstring muscle strain. Am J Sports Med 2003;31:969–73 [DOI] [PubMed] [Google Scholar]
- 2.Connell DA, Schneider-Kolsky ME, Hoving JL, et al. Longitudinal study comparing sonographic and MRI assessments of acute and healing hamstring injuries. AJR Am J Roentgenol 2004;183:975–84 [DOI] [PubMed] [Google Scholar]
- 3.Askling CM, Tengvar M, Saartok T, et al. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med 2007;35:197–206 [DOI] [PubMed] [Google Scholar]
- 4.Ekstrand J, Healy JC, Waldén M, et al. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med 2012;46:112–17 [DOI] [PubMed] [Google Scholar]
- 5.Gibbs NJ, Cross TM, Cameron M, et al. The accuracy of MRI in predicting recovery and recurrence of acute grade one hamstring muscle strains within the same season in Australian Rules football players. J Sci Med Sport 2004;7:248–58 [DOI] [PubMed] [Google Scholar]
- 6.Slavotinek JP, Verrall GM, Fon GT. Hamstring injury in athletes: using MR imaging measurements to compare extent of muscle injury with amount of time lost from competition. AJR Am J Roentgenol 2002;179:1621–8 [DOI] [PubMed] [Google Scholar]
- 7.Kerkhoffs GMMJ, van Es N, Wieldraaijer T, et al. Diagnosis and prognosis of acute hamstring injuries in athletes. Knee Surg Sports Traumatol Arthrosc 2013;21:500–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comin J, Malliaras P, Baquie P, et al. Return to competitive play after hamstring injuries involving disruption of the central tendon. Am J Sports Med 2013;41:111–15 [DOI] [PubMed] [Google Scholar]
- 9.Schneider-Kolsky ME, Hoving JL, Warren P, et al. A comparison between clinical assessment and magnetic resonance imaging of acute hamstring injuries. Am J Sports Med 2006;34:1008–15 [DOI] [PubMed] [Google Scholar]
- 10.Mendiguchia J, Brughelli M. A return-to-sport algorithm for acute hamstring injuries. Phys Ther Sport 2011;12:2–14 [DOI] [PubMed] [Google Scholar]
- 11.Malliaropoulos N, Isinkaye T, Tsitas K, et al. Reinjury after acute posterior thigh muscle injuries in elite track and field athletes. Am J Sports Med 2011;39:304–10 [DOI] [PubMed] [Google Scholar]
- 12.Koulouris G, Connell DA, Brukner P, et al. Magnetic resonance imaging parameters for assessing risk of recurrent hamstring injuries in elite athletes. Am J Sports Med 2007;35:1500–6 [DOI] [PubMed] [Google Scholar]
- 13.Verrall GM, Slavotinek JP, Barnes PG, et al. Assessment of physical examination and magnetic resonance imaging findings of hamstring injury as predictors for recurrent injury. J Orthop Sports Phys Ther 2006;36:215–24 [DOI] [PubMed] [Google Scholar]
- 14.Sherry MA, Best TM. A comparison of 2 rehabilitation programs in the treatment of acute hamstring strains. J Orthop Sports Phys Ther 2004;34:116–25 [DOI] [PubMed] [Google Scholar]
- 15.Ekstrand J, Hägglund M, Waldén M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med 2011;39:1226–32 [DOI] [PubMed] [Google Scholar]
- 16.Orchard J, Seward H. Epidemiology of injuries in the Australian Football League, seasons 1997–2000. Br J Sports Med 2002;36:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Visser HM, Reijman M, Heijboer MP, et al. Risk factors of recurrent hamstring injuries: a systematic review. Br J Sports Med 2012;46:124–30 [DOI] [PubMed] [Google Scholar]
- 18.Heiderscheit BC, Sherry MA, Silder A, et al. Hamstring strain injuries: recommendations for diagnosis, rehabilitation, and injury prevention. J Orthop Sports Phys Ther 2010;40:67–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orchard J, Best TM. The management of muscle strain injuries: an early return versus the risk of recurrence. Clin J Sport Med 2002;12:3–5 [DOI] [PubMed] [Google Scholar]
- 20.Silder A, Reeder SB, Thelen DG. The influence of prior hamstring injury on lengthening muscle tissue mechanics. J Biomech 2010;43:2254–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silder A, Heiderscheit BC, Thelen DG, et al. MR observations of long-term musculotendon remodeling following a hamstring strain injury. Skeletal Radiol 2008;37:1101–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orchard J, Best TM, Verrall GM. Return to play following muscle strains. Clin J Sport Med 2005;15:436–41 [DOI] [PubMed] [Google Scholar]
- 23.Askling CM, Nilsson J, Thorstensson A. A new hamstring test to complement the common clinical examination before return to sport after injury. Knee Surg Sports Traumatol Arthrosc 2010;18:1798–803 [DOI] [PubMed] [Google Scholar]
- 24.Brooks JHM, Fuller CW, Kemp SPT, et al. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med 2006;34:1297–306 [DOI] [PubMed] [Google Scholar]
- 25.Sanfilippo JL, Silder A, Sherry MA, et al. Hamstring strength and morphology progression after return to sport from injury. Med Sci Sports Exerc 2013;45:448–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silder A, Sherry MA, Sanfilippo J, et al. Clinical and morphological changes following 2 rehabilitation programs for acute hamstring strain injuries: a randomized clinical trial. J Orthop Sports Phys Ther 2013;43:284–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton B, Knez W, Eirale C, et al. Platelet enriched plasma for acute muscle injury. Acta Orthop Belg 2010;76:443–8 [PubMed] [Google Scholar]
- 28.Peetrons P. Ultrasound of muscles. Eur Radiol 2002;12:35–43 [DOI] [PubMed] [Google Scholar]
- 29.Hamilton B, Whiteley R, Almusa E, et al. Excellent reliability for MRI grading and prognostic parameters in acute hamstring injuries. Br J Sports Med 2014;48:1385–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorborg K. Why hamstring eccentrics are hamstring essentials. Br J Sports Med 2012;46:463–5 [DOI] [PubMed] [Google Scholar]
- 31.McMahon CJ, Wu JS, Eisenberg RL. Muscle edema. AJR Am J Roentgenol 2010;194:W284–92 [DOI] [PubMed] [Google Scholar]
- 32.May DA, Disler DG, Jones EA, et al. Abnormal signal intensity in skeletal muscle at MR imaging: patterns, pearls, and pitfalls. Radiographics 2000;20:S295–315 [DOI] [PubMed] [Google Scholar]
- 33.Mueller-Wohlfahrt H-W, Haensel L, Mithoefer K, et al. Terminology and classification of muscle injuries in sport: the Munich consensus statement. Br J Sports Med 2013;47:342–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathur S, Vohra RS, Germain SA, et al. Changes in muscle T2 and tissue damage after downhill running in mdx mice. Muscle Nerve 2011;43:878–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marqueste T, Giannesini B, Fur YL, et al. Comparative MRI analysis of T2 changes associated with single and repeated bouts of downhill running leading to eccentric-induced muscle damage. J Appl Physiol 2008;105:299–307 [DOI] [PubMed] [Google Scholar]
- 36.Gharaibeh B, Chun-Lansinger Y, Hagen T, et al. Biological approaches to improve skeletal muscle healing after injury and disease. Birth Defects Res C Embryo Today 2012;96:82–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedair HS, Karthikeyan T, Quintero A, et al. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med 2008;36:1548–54 [DOI] [PubMed] [Google Scholar]
- 38.Thuilliez C, Dorso L, Howroyd P, et al. Histopathological lesions following intramuscular administration of saline in laboratory rodents and rabbits. Exp Toxicol Pathol 2009;61:13–21 [DOI] [PubMed] [Google Scholar]
- 39.Sutton SC, Evans LA, Rinaldi MT, et al. Predicting injection site muscle damage. I: evaluation of immediate release parenteral formulations in animal models. Pharm Res 1996;13:1507–13 [DOI] [PubMed] [Google Scholar]
- 40.Harris NL, Huffer WE, von Stade E, et al. The effect of platelet-rich plasma on normal soft tissues in the rabbit. J Bone Joint Surg Am 2012;94:786–93 [DOI] [PubMed] [Google Scholar]
- 41.Wright-Carpenter T, Klein P, Schäferhoff P, et al. Treatment of muscle injuries by local administration of autologous conditioned serum: a pilot study on sportsmen with muscle strains. Int J Sports Med 2004;25:588–93 [DOI] [PubMed] [Google Scholar]
- 42.Reurink G, Goudswaard GJ, Tol JL, et al. Therapeutic interventions for acute hamstring injuries: a systematic review. Br J Sports Med 2012;46:103–9 [DOI] [PubMed] [Google Scholar]
- 43.Lee JC, Mitchell AWM, Healy JC. Imaging of muscle injury in the elite athlete. Br J Radiol 2012;85:1173–85 [DOI] [PMC free article] [PubMed] [Google Scholar]