Abstract
Background:
With the reintroduction of shaped silicone gel implants in the United States, questions regarding indications and outcomes for each are likely. The purpose of this article is to review the author’s early experience using shaped and round implants for breast reconstruction over a 14-month consecutive interval.
Methods:
Breast reconstruction using shaped or round implants was performed on 69 women that included shaped silicone gel devices in 49 and round devices in 20. Patients were evaluated based on nipple-sparing vs skin-sparing mastectomy, 1-stage vs 2-stage, radiation therapy, unilateral vs bilateral, occurrence of complications, and follow-up.
Results:
Of the 49 patients (78 breasts) who had shaped implants, reoperation was necessary in 6 patients (12.2%) and in 7 breasts (9%). This was secondary to infection in 2 breasts, capsular contracture in 2 breasts, incisional dehiscence in 1 breast, asymmetry in 1 breast, and exposure in 1 breast. Of the 20 patients (28 breasts) who had round implants, reoperation was necessary in 2 patients (10%) and 2 breasts (7.1%) and included the removal of the device secondary to a late infection in 1 patient and the correction of a malposition (double bubble deformity) in 1 patient. There were no malpositions involving the shaped silicone gel implants.
Conclusions:
Both shaped and round silicone gel devices can result in natural aesthetic outcomes. Shaped devices are preferred for contouring the upper pole and for optimizing breast projection. Round devices are preferred when the upper pole is not deficient and the patient desires softer breasts. Longer follow-up studies will be necessary.
With the recent reintroduction of shaped cohesive silicone gel implants for breast reconstruction, plastic surgeons once again have an elevated level of control when considering breast shape and contour. Between 1991 and 2005, most plastic surgeons in the United States were only able to use round or shaped saline implants because of the moratorium placed on silicone gel devices by the Food and Drug Administration (FDA). In 2005, the use of round silicone gel implants was permitted as the FDA lifted the moratorium. Round saline and silicone gel implants continue to be useful; however, the degree of control with regard to breast shape and contour can be limited in some situations.
With the recent approval of shaped silicone gel implants by the FDA in 2012 (Sientra, Santa Barbara, Calif.) and 2013 (Allergan, Irvine, Calif.) (Mentor, Santa Barbara, Calif.), all 3 manufacturers are able to distribute these devices to plastic surgeons across the United States. Using these shaped devices, plastic surgeons can select the appropriate implant to optimally match the “footprint” of the natural breast and achieve better control of height, width, and projection. However, because these devices are shaped and textured, proper use of these implants requires certain technical maneuvers that must be appreciated to deliver an outcome that is predictable, reproducible, and aesthetically desirable.1–3
This article will review the author’s early experience using shaped silicone gel implants as well as round silicone gel and saline implants for primary and secondary breast reconstruction with an emphasis on indications and outcome.
METHODS
Over a 14-month period (August 2012– September 2013), a total of 69 patients had breast reconstruction or revision using shaped or round implants. Of these, 49 patients had implantation of shaped silicone gel implants and 20 had implantation of round implants. The shaped implants were stratified based on the manufacturer: Sientra (Santa Barbara, Calif.) and Allergan (Irvine, Calif.). Use of the Sientra devices began in August 2012 and use of Allergan devices began in February 2013. The Mentor (Santa Barbara, Calif.) shaped devices were not used because they had not been approved for routine use by the FDA during the specified time interval. Included within the shaped implant cohort were 35 patients who received a Sientra implant (19 bilateral, 16 unilateral) and 14 patients who received an Allergan implant (8 bilateral, 6 unilateral). Thus, a total of 76 breasts were reconstructed with a shaped implant.
During the same interval, 20 patients had breast reconstruction or revision using a round implant. These were also stratified based on manufacturer that included Mentor (8 patients, 6 unilateral, 2 bilateral), Allergan (8 patients, 4 unilateral, 4 bilateral), and Sientra (4 patients, 2 unilateral, 2 bilateral). Thus, a total of 28 breasts were reconstructed using a round implant.
Patient demographics and complications for the Allergan, Sientra, and round device cohorts are listed in Tables 1–3 and include patient age, type of implant, 1-stage vs 2-stage, nipple-sparing mastectomy (NSM) vs skin-sparing mastectomy, unilateral vs bilateral, radiation therapy, and follow-up.
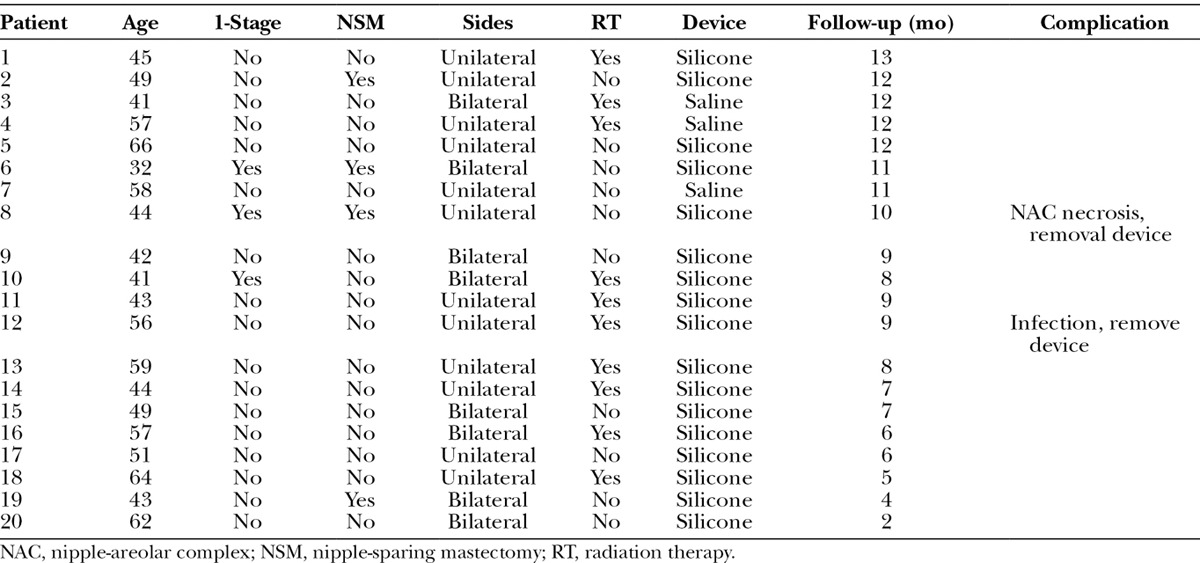
Table 1.
Patient Demographics, Treatment, and Outcome Variables for the Patients Having Shaped Sientra Devices
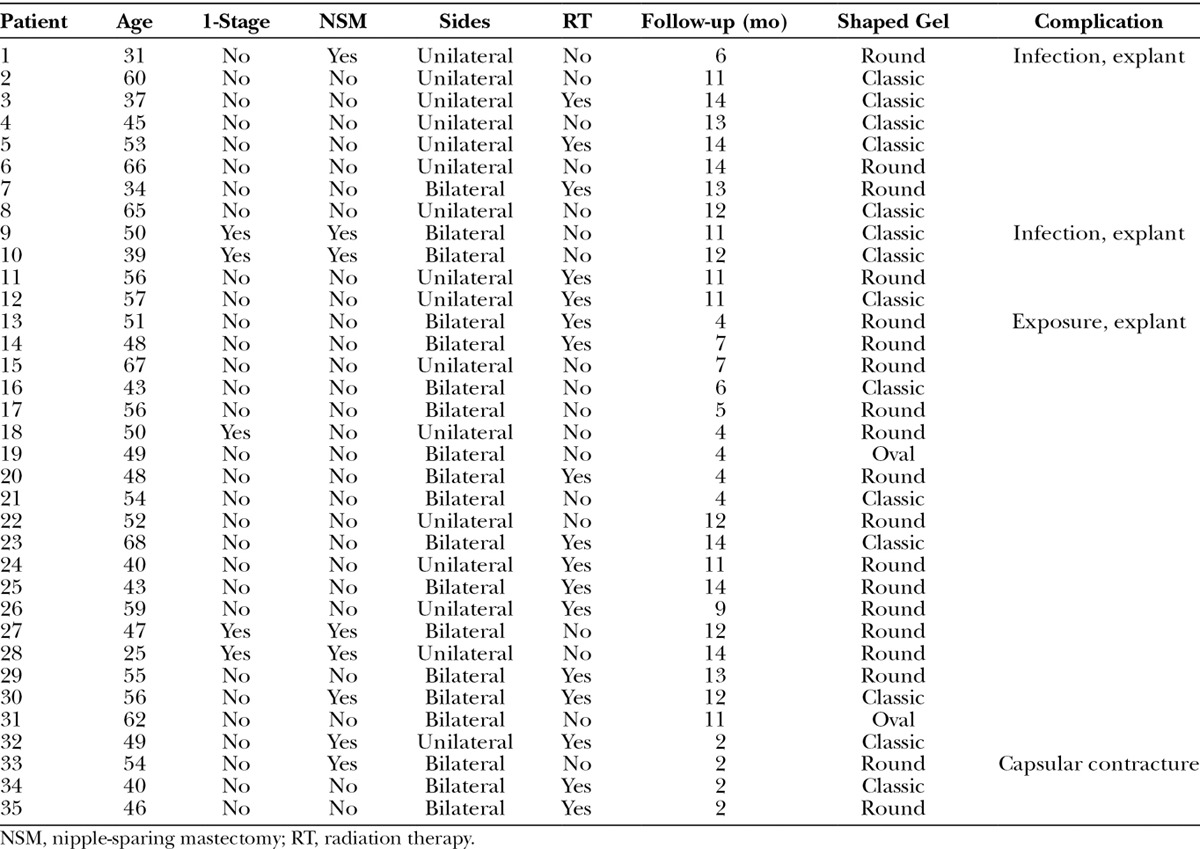
Table 3.
Patient Demographics, Treatment, and Outcome Variables for the Patients Having Round Devices
Technique
The technique for using a shaped or round implant is different and due, in part, to differences in cohesivity, surface texturing, and periprosthetic pocket dimensions. Shaped implants are highly cohesive, less deformable, and more firm than round implants. Shaped implants are always textured to minimize the incidence of malrotation, whereas round devices can be smooth or textured. Using the shaped devices, strict attention to the dimensions of the breast pocket is necessary to minimize the risk of malrotation.
The indications for using a shaped device are variable. In general, a shaped device is preferred in women with a long torso that need additional volume in the upper pole to minimize the scalloping deformity that sometimes occurs. Shaped devices are also useful in women who desire increased projection. Of the available shaped silicone gel devices, the Sientra round base provides the most projection for a given volume. Round devices are typically inserted in patients with a shorter torso that do not need additional upper pole fill volume. They are also useful in women who desire a softer breast. All patients are given the opportunity to examine and feel a shaped and a round device to facilitate the decision-making process.
The technique for using shaped silicone gel devices is different than that of round devices. In cases where a tissue expander is being exchanged for a shaped implant, the shaped implant must be placed in a pocket that configures to a “hand-in-glove” fit. This may entail performing a capsulorrhaphy with or without reinforcement using an acellular dermal matrix (ADM). In cases where a shaped implant is used for 1-stage reconstruction, the use of an ADM has proven very useful to control the dimensions of the pocket and for tissue support.4,5 In all cases of 1- and 2-stage prosthetic reconstruction, an ADM was used at the initial operation for tissue support, compartmentalization, and to prevent “window-shading” of the pectoralis major muscle.
The orientation of the device is critical when inserted into the breast pocket to prevent malrotation. With the Sientra device, the orientation line is aligned vertically. With the Allergan device, the orientation pads are vertically aligned. The positioning of both devices is important and is typically achieved by placing the inferior base of the device at the desired inframammary fold. In 3 patients (5 breasts), an ADM was used to support a capsulorrhaphy performed at the time of device exchange to a shaped silicone gel implant. In most cases, a closed suction drain is usually used.
With round devices, the pocket dimensions are important but not as critical. In cases where a tissue expander is being exchanged for a round implant, an upper capsulotomy is usually performed to allow for better redraping of the soft tissues over the implant. Smooth devices are designed for some degree of movement within the pocket. Textured devices are less likely to move but may have benefit in reducing the incidence of capsular contracture.6 The silicone gel in a round implant is less cohesive than the shaped implants; thus, they are softer. A closed suction drain is sometimes used in cases where extensive capsulotomy, capsulectomy, or capsulorrhaphy has been performed. When a round implant is used for 1-stage reconstruction, the use of an ADM has also been very useful.
RESULTS
Of the 35 patients who had Sientra shaped devices, 19 (54.3%) were bilateral and 16 (45.7%) were unilateral for a total of 54 devices. Table 1 represents a list of the 35 patients with the associated demographic and treatment variables. The mean age for this cohort was 50.1 years (range, 25–68 y). A 1-stage, direct-to-implant reconstruction was performed in 5 of 35 patients (14.2%) and an NSM was performed in 8 of 35 patients (22.9%). Radiation therapy was necessary in 17 of 35 patients (48.6%). The mean follow-up for this cohort was 6.2 months (range, 2–14 mo). Reoperation was necessary in 4 patients (5 breasts, 9.3%). This included an infection in 2 patients (2 breasts, 3.7%), exposure in 1 patient (1 breast, 1.9%), and capsular contracture in 1 patient (2 breasts, 3.7%). Both infected devices occurred in the setting of NSM and required removal. The exposure and explant occurred in a woman who had previous breast conservation and radiation therapy. The capsular contracture occurred early following placement of tissue expanders and persisted following aggressive capsulotomy and exchange to a shaped silicone gel implant. No patient developed a malrotation or seroma. Figures 1–6 illustrate a patient following bilateral mastectomy, tissue expansion, and exchange for shaped Sientra silicone gel implants.
Fig. 1.
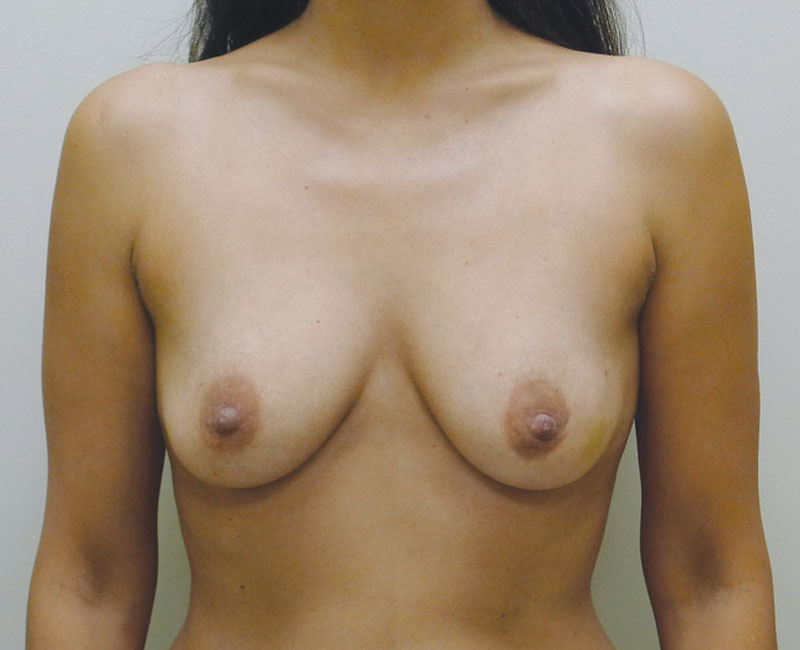
Preoperative image before bilateral mastectomy.
Fig. 6.
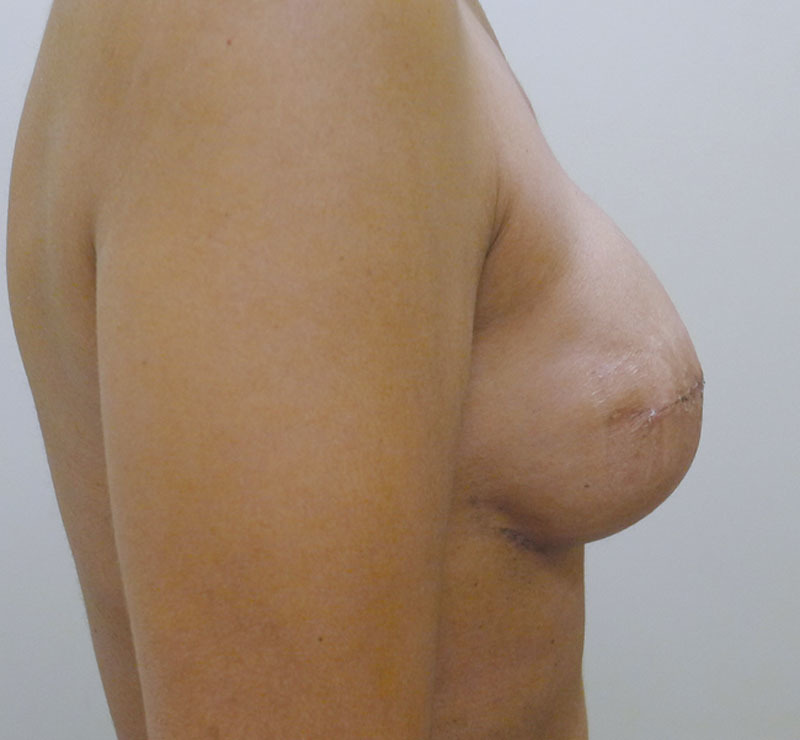
Right lateral postoperative view.
Fig. 2.
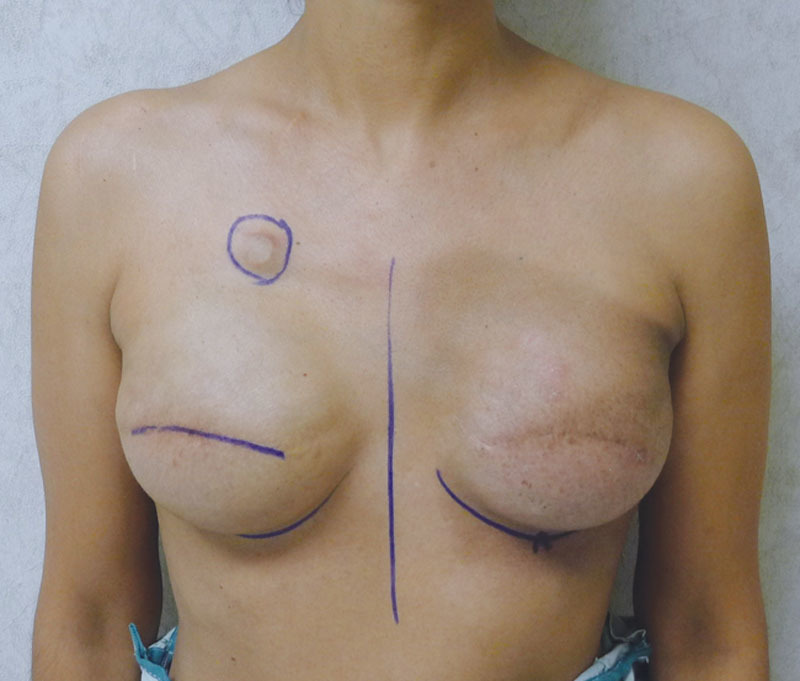
Postoperative image with bilateral tissue expanders following left radiation therapy.
Fig. 3.
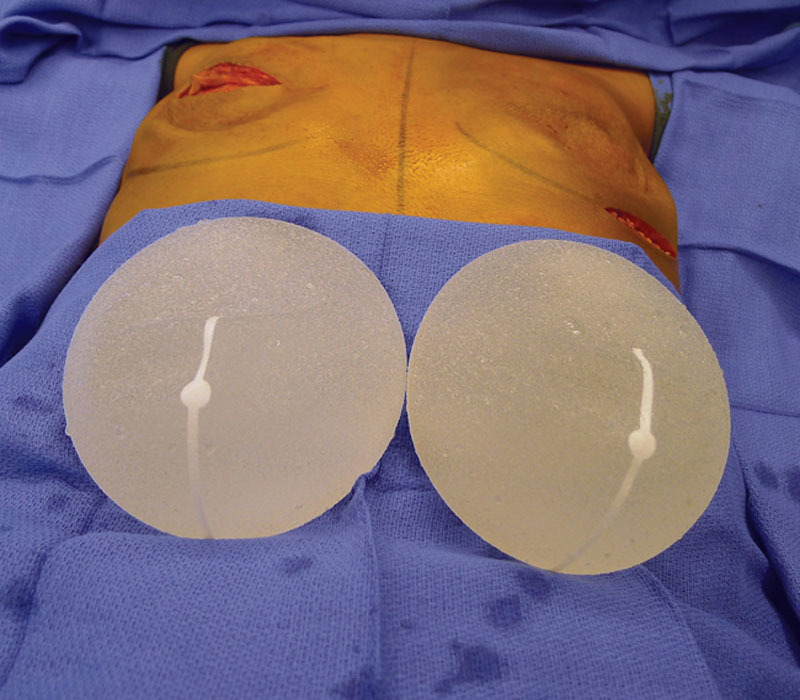
Intraoperative image of the Sientra shaped silicone gel devices.
Fig. 4.
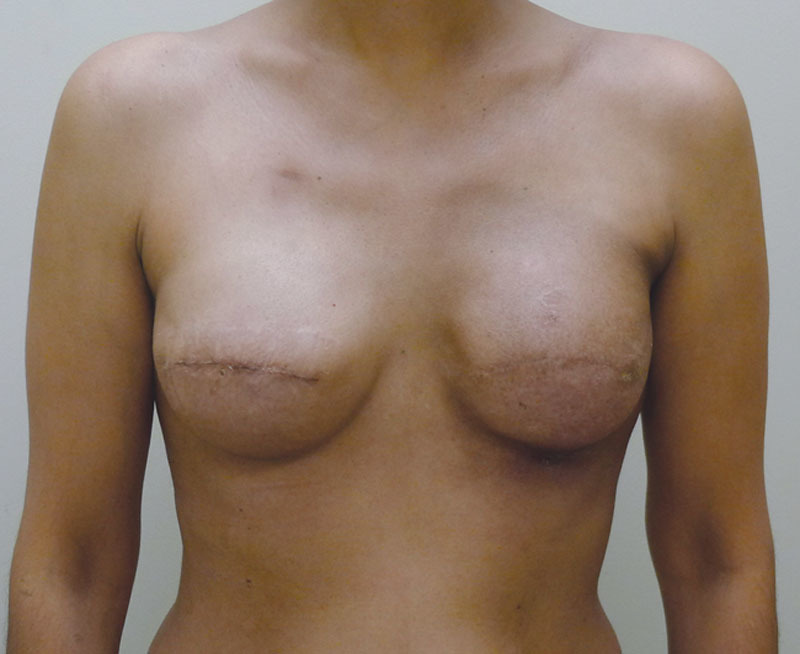
Postoperative image following insertion of the shaped silicone gel implants.
Fig. 5.
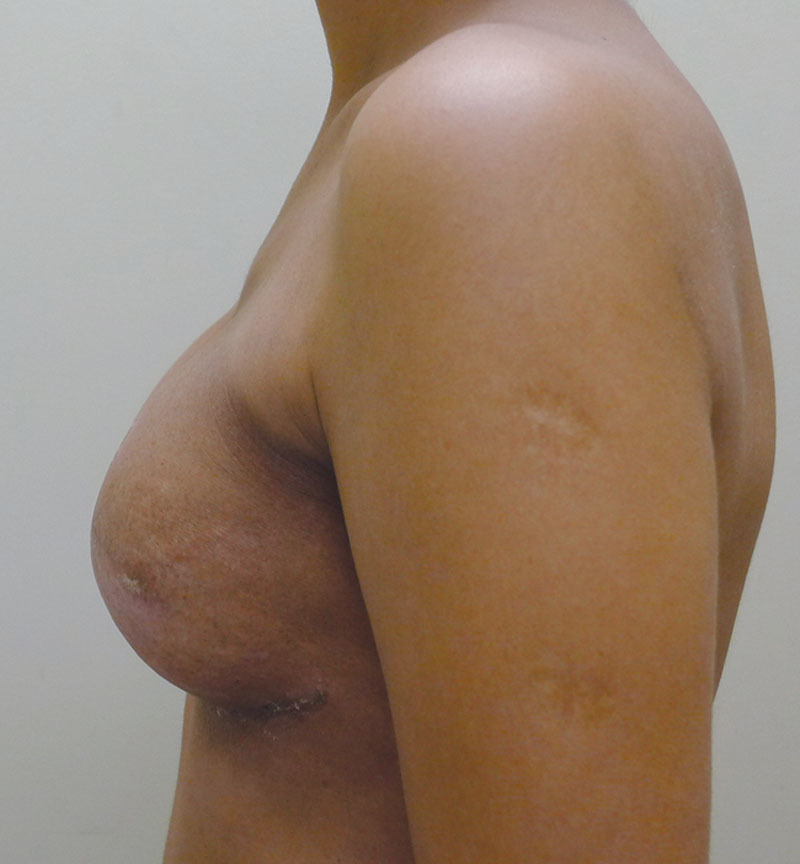
Left lateral postoperative view.
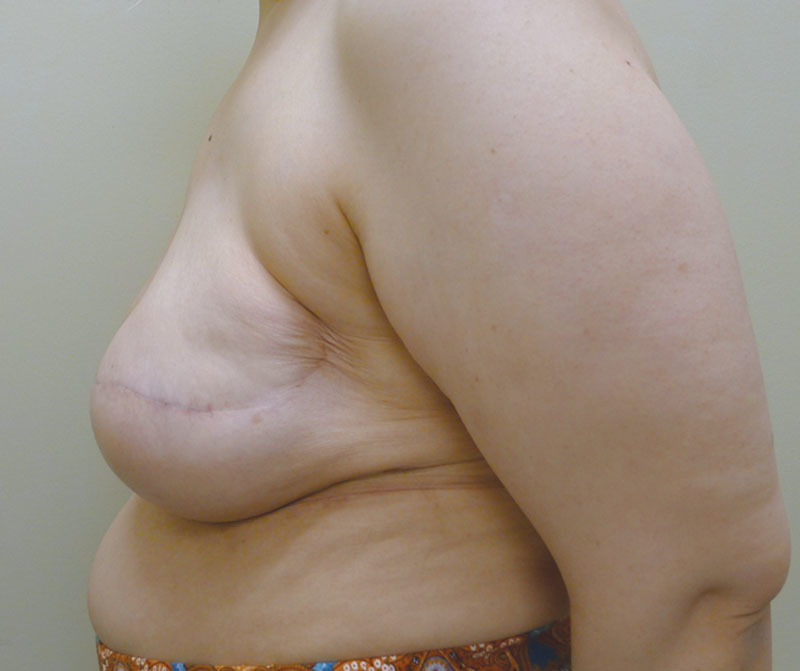
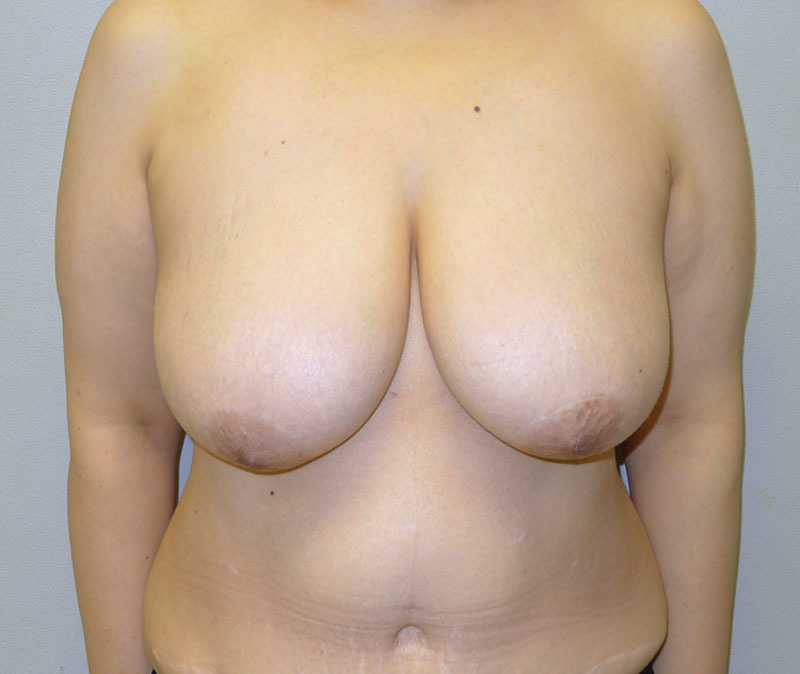
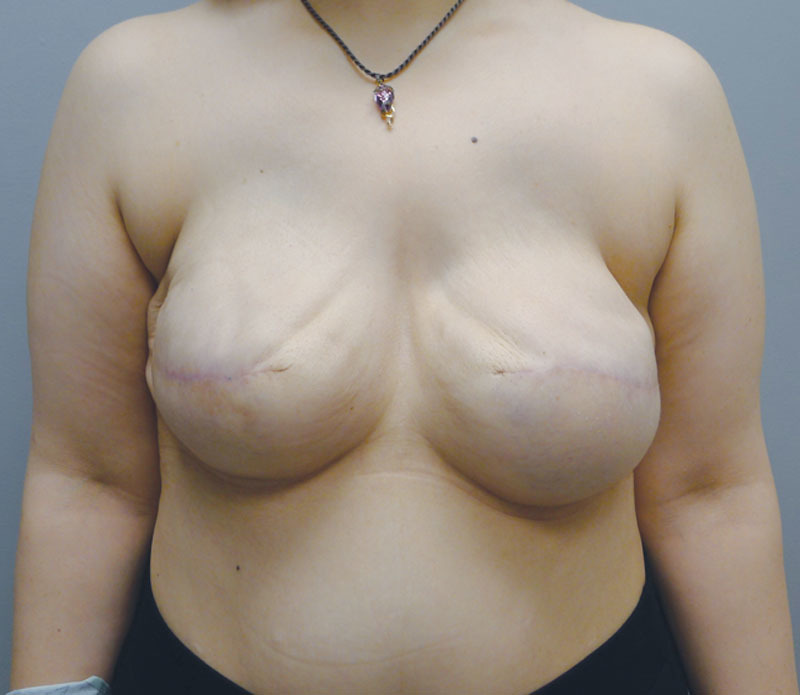
Of the 14 patients who had Allergan shaped silicone gel, all were with the style 410 full height full projection devices. Table 2 represents a list of the 14 patients with the associated demographic and treatment variables. The mean age for this cohort was 52.4 years (range, 33–69 y). The reconstruction was bilateral in 8 patients and unilateral in 6 patients, thus a total of 22 implants were used. All reconstructions were performed in 2 stages in which the preexisting tissue expander was exchanged for the shaped silicone gel device. NSM was performed in 2 unilateral patients (14.3%). Radiation therapy was a factor in 3 patients (21.4%). The mean follow-up for this cohort was 4.2 months (range, 1–8 mo). Reoperation was necessary in 2 patients (14.3%) and 2 breasts (9.1%) that included an incisional dehiscence requiring debridement and device exchange as well as an asymmetry requiring exchange to a larger shaped device. To date, there have been no cases of capsular contracture, seroma, infection, or implant malposition. Figures 6–12 illustrate a patient following bilateral mastectomy and tissue expansion that had conversion of the round silicone gel implants for shaped Allergan silicone gel implants.
Table 2.
Patient Demographics, Treatment, and Outcome Variables for the Patients Having Shaped Allergan Devices
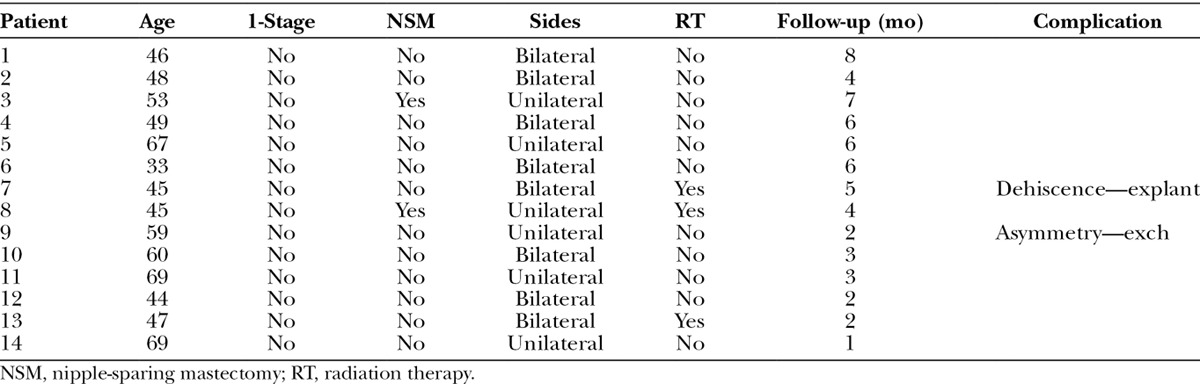
Fig. 12.
Left lateral postoperative view.
Fig. 7.
Preoperative image before mastectomy.
Fig. 8.
Postoperative image following bilateral mastectomy, tissue expansion, and exchange for round silicone gel implants. Note the extensive rippling.
Fig. 9.
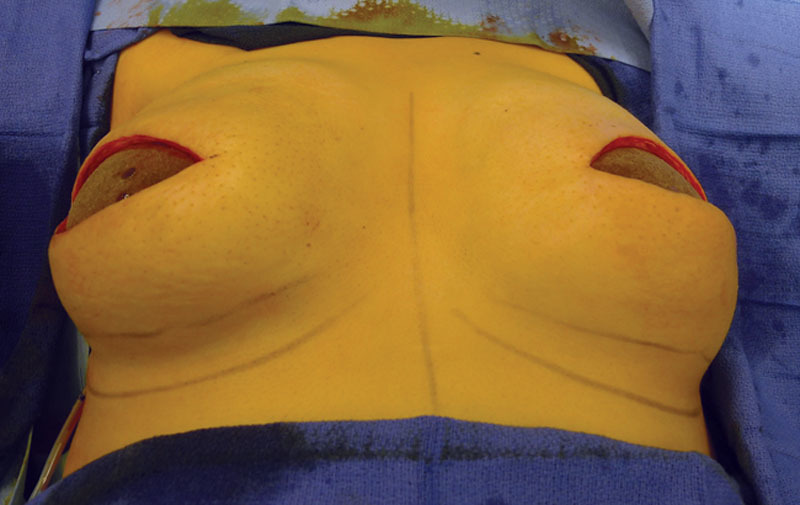
Intraoperative image of the Allergan shaped silicone gel implants following insertion.
Of the 20 patients who had a round nonshaped permanent implant, 8 were Mentor, 8 were Allergan, and 4 were Sientra. Table 3 represents a list of the 20 patients and the associated demographic and treatment variables. The mean age for this cohort was 50.2 years (range, 32–66 y). The reconstruction was bilateral in 8 patients and unilateral in 12 patients, thus a total of 28 devices were used. Of the 28 implants, 4 were low-profile smooth saline implants that were placed under flaps and 24 were high-profile silicone gel implants that were placed at time of the exchange from the tissue expander to the implant. The reconstruction was performed as a single stage in 3 patients and was nipple-areolar complex sparing in 4 patients. Radiation therapy was a factor in 10 patients (50%). The mean follow-up for this cohort was 9.2 months (range, 2–13 mo). Reoperation was necessary in 2 patients (10%) and included the removal of the device secondary to a late infection in 1 patient and the correction of a malposition (double bubble deformity) in 1 patient. One patient has developed a mild capsular contracture following radiation therapy (follow-up 8 mo). No patient developed seroma. Figures 13–17 illustrate a patient following bilateral mastectomy, tissue expansion, and round high-profile silicone gel implants.
Fig. 13.
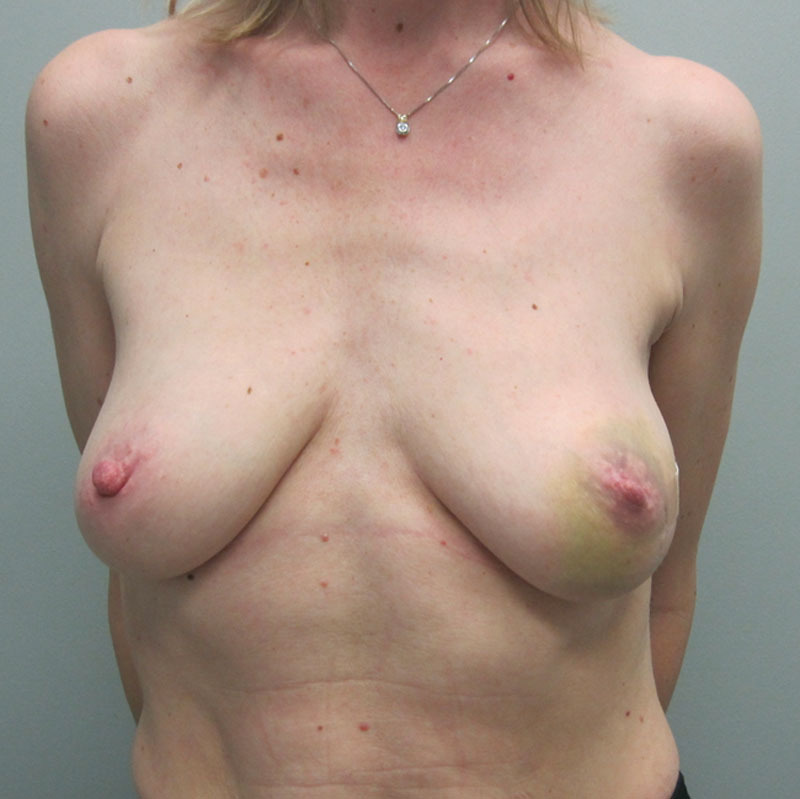
Preoperative image before bilateral mastectomy.
Fig. 17.
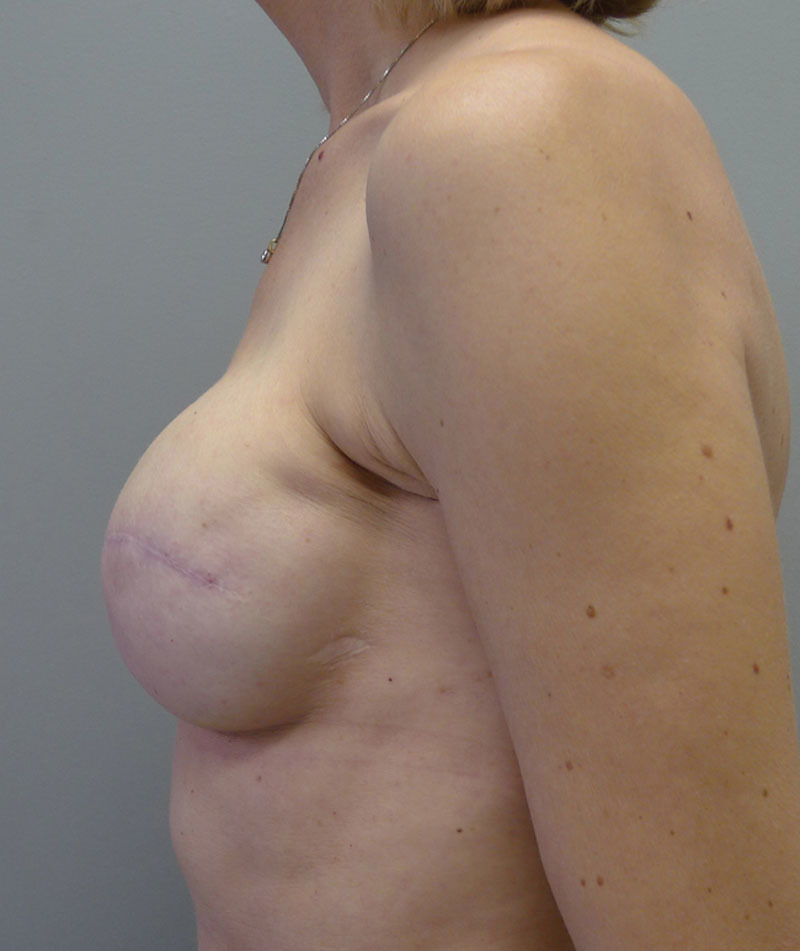
Left lateral postoperative view.
Fig. 10.
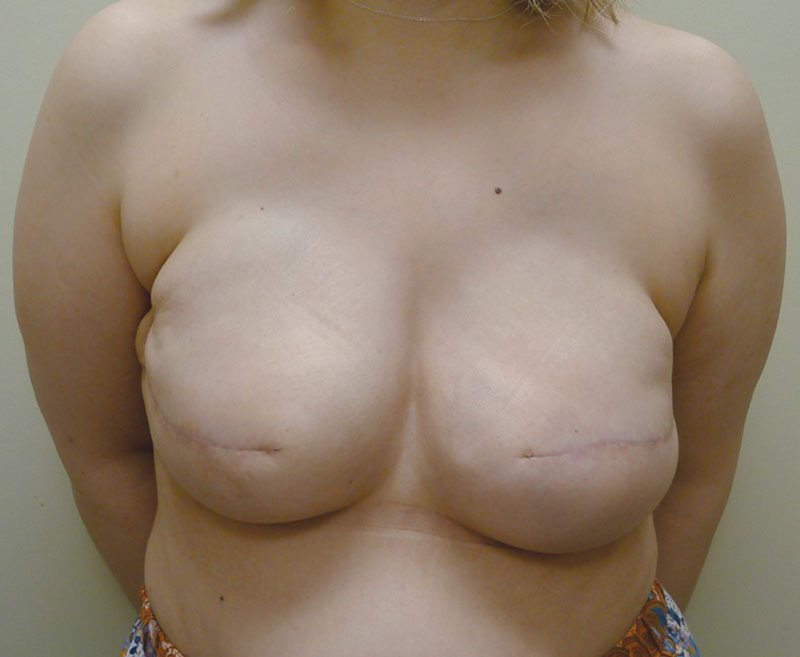
Postoperative image following insertion of the shaped silicone gel implants.
Fig. 11.
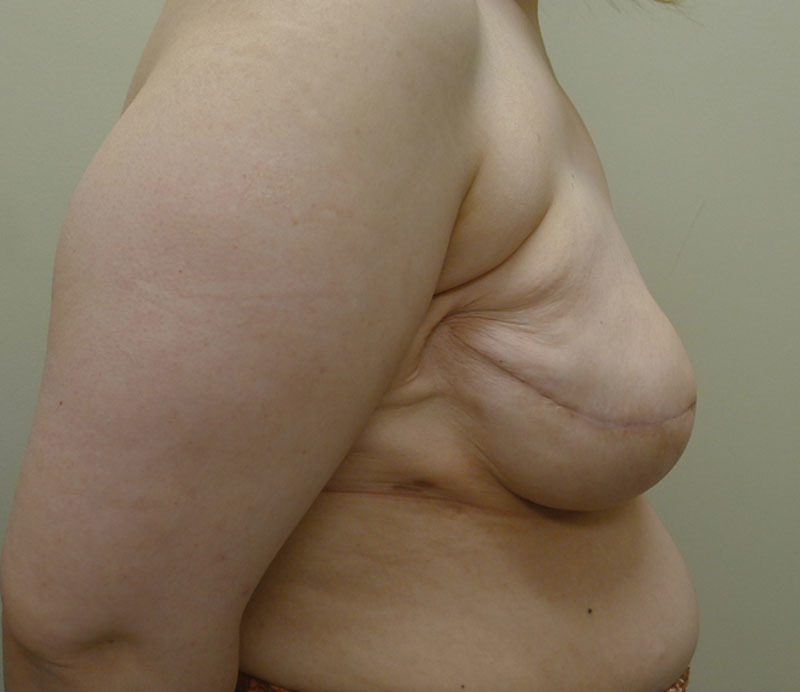
Right lateral postoperative view.
Fig. 14.
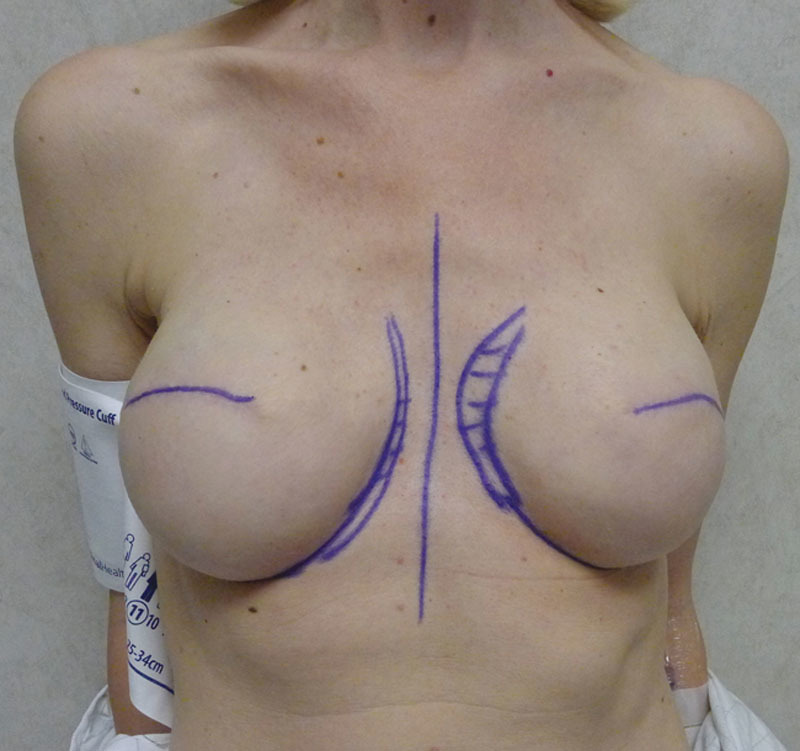
Postoperative image with bilateral tissue expanders.
Fig. 15.
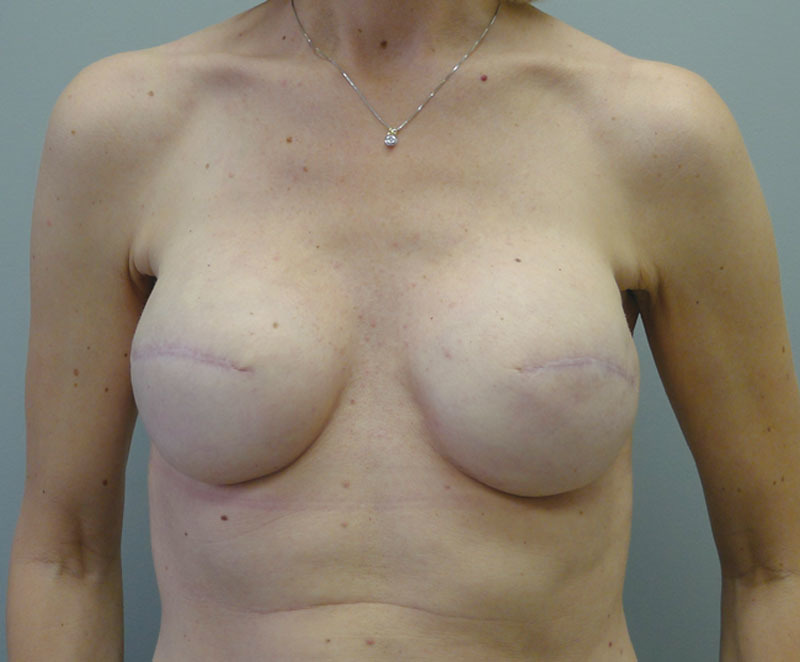
Postoperative image following insertion of the round silicone gel implants.
Fig. 16.
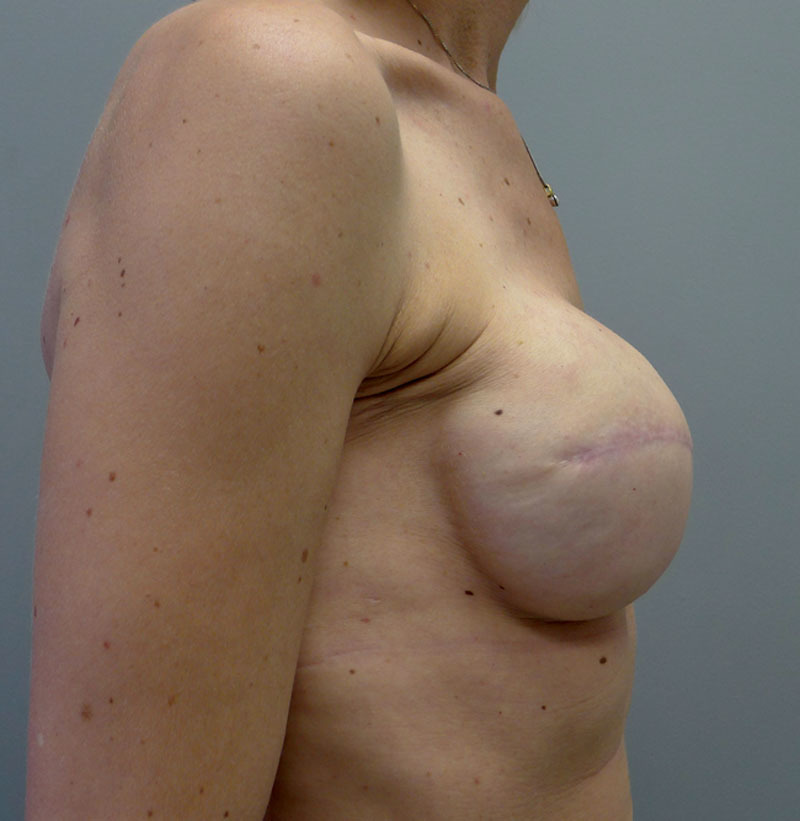
Right lateral postoperative view.
DISCUSSION
The use of shaped silicone implants has sparked the interest of many plastic surgeons who perform reconstructive and aesthetic breast surgery. For some plastic surgeons, the transition to shaped devices has been slow and associated with some degree of apprehension because of the risk of malrotation and increased firmness. Although these shaped silicone gel devices have been available for a short period of time and our initial experience has been limited by short follow-up, the early results of this study have demonstrated a similar complication profile to round devices and, more importantly, that malrotation is an infrequent occurrence assuming proper technique protocols.
The safe and effective use of shaped silicone gel devices requires that plastic surgeons pay close attention to the dimensions of the periprosthetic space. This is true for both immediate 1-stage reconstruction and the exchange procedure for 2-stage reconstruction. The use of ADM for 1-stage reconstruction and capsulorrhaphy techniques with or without ADM for the exchange procedure is effective means to achieve tight control of the pocket. A pocket that is too large relative to the dimensions of the implant creates a situation in which malrotation is more likely. The use of a closed suction drain is usually recommended to collapse the space and to prevent fluid accumulation that may facilitate malrotation. The results of this study have demonstrated that the Sientra and the Allergan shaped silicone gel devices are both effective at optimizing contour with low risk for malrotation.
The decision to use a round or shaped silicone gel device is usually based on the patient and breast characteristics. In this study, 76 of the 104 (73%) permanent devices used were shaped and 28 of the 104 were round (27%). With 1-stage reconstruction, the decision was usually based on the quality of the mastectomy skin flaps and the general “footprint” of the breast. When taller than wide, a shaped device was usually considered. A round implant was considered when there was no perceived upper pole deficiency. With 2-stage reconstruction, the dimensions of the expanded breast were appreciated. When there was a contour deficiency in the upper pole, a shaped silicone gel device was preferred. When increased projection was desired, a shaped device was preferred. With 2-stage reconstruction, the permanent device was usually 50–75 cm3 greater than the fill volume of the tissue expander to help maintain and fill the dimensions of the pocket.
The complication profiles for the shaped and round devices were very similar. Although there were 2 infections with the shaped Sientra devices, the circumstances were somewhat atypical. In 1 patient, the prescribed antibiotics were neither filled nor taken and the device was removed 3 weeks following insertion. The cultured bacterium was sensitive to the originally prescribed antibiotic. In the second patient, a late periprosthetic infection occurred requiring removal at 5 months. In both of these cases, the infection occurred in the setting of NSM. In the round implant cohort, there was one infection that also was late requiring removal at 6 months. No infections occurred in the Allergan cohort. Interestingly, the Allergan devices were used only in the setting of skin-sparing mastectomy performed in 2 stages and not NSM.
Other investigators have demonstrated a similar trend for complications using shaped silicone gel devices for breast reconstruction. Infection rates have ranged from 1.6% to 5.2% and devise malposition/rotation rates that required reoperation have ranged from 3.9% to 12.1%.7–9 Overall reoperation rates for any reason has ranged from 42.7% to 44.5% and overall rates for reoperation with implant removal or exchange has ranged from 21.8% to 31%.7–9 All of these values were with a minimum of 5-year follow-up. The reoperation rate in this series of patients was 9.3% and 14.3% for the Sientra and Allergan shaped devices, respectively. Although this may seem high at first glance, the reasons for reoperation were incisional dehiscence in 2 patients, infection in 2 patients, asymmetry requiring size change in 1 patient, and early capsular contracture in 1 patient. This represents the reality of prosthetic reconstruction. The reoperations were not because of a device flaw but rather due to other variables related to the surgeon (judgment), patients (radiation therapy), compliance (antibiotic use), and expectations (symmetry).
One of the considerations using highly cohesive shaped silicone gel devices is that the breast may be firmer with these devices compared with the less cohesive round silicone implants. In a review of 64 patients who had breast augmentation using Allergan Style 410 and the Eurosilicone Vertex shaped silicone gel implants, 24% of patients were demonstrated to have soft breasts, 53% had slightly firm breasts, and 23% had moderately firm breasts.10 In another study that reviewed 35 patients who had either breast augmentation (20 breasts) or breast reconstruction (31 breasts) using the Mentor contour profile gel shaped implant, 85% of women rated their breasts as soft.11 In a recent review of patient satisfaction using the BREAST-Q, Macadam et al12 found no difference in any category following prosthetic breast reconstruction other than patients felt that the shaped devices were firmer compared with the round. Although patients were not questioned about firmness in the present study, it is recognized that shaped highly cohesive devices are firmer than round; however, the contour advantages in some patients seem to negate the firmness. Another advantage that may be related to the added cohesivity of shaped implants is that there is less rippling and rippling both in the reconstructive and the aesthetic patients.3
The study has several limitations that are related to the small number of patients, limited follow-up interval, and lack of statistical analysis. The intent was to provide the readership with a review of my early experience and to be able to answer questions that may arise when trying to decide between shaped and round silicone gel devices in the setting of prosthetic breast reconstruction. Some may argue that this has been previously studied, but it should be noted that previous studies looked as a single device or a single company and not at the global experience using all devices. In addition, some of the current devices available are relatively new to the U.S. market. Whether the reconstruction is unilateral or bilateral really does not matter because the endpoints for analysis, for example, rotation, malposition, and infection, are all device related.
CONCLUSIONS
In summary, both the shaped and round silicone gel devices can result in natural aesthetic outcomes and have acceptable reoperation rates. The highly cohesive nature of the shaped devices permits less scalloping of the upper pole and may result in greater projection depending on the type of shaped device selected. The softer round silicone gel devices remain an excellent option for women who have adequate upper pole tissue and who desire a softer breast. The complication profiles for all devices in this study were similar. Malrotation of the shaped devices has not been observed within the specified time interval. The follow-up interval for this study is acknowledged to be short and long-term evaluation will be necessary to better assess performance over time. However, this current study has demonstrated that shaped silicone gel implants provide another option to obtain a desired shape and contour in the setting of reconstructive breast surgery.
Footnotes
Disclosure: Dr. Nahabedian is a speaker and consultant for LifeCell and Sientra and has been a speaker for Mentor and Allergan. He has received no financial support or assistance in the preparation of this article. The content of this article is based on his experience using devices from Sientra, Mentor, and Allergan. The Article Processing Charge was waived at the discretion of the Editor-in-Chief.
REFERENCES
- 1.Heden P, Bronz G, Elberg JJ, et al. Long-term safety and effectiveness of style 410 highly cohesive silicone breast implants. Aesthetic Plast Surg. 2009;33:430–436. doi: 10.1007/s00266-009-9360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salgarello M, Farallo E. Immediate breast reconstruction with definitive anatomical implants after skin-sparing mastectomy. Br J Plast Surg. 2005;58:216–222. doi: 10.1016/j.bjps.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Brown MH, Shenker R, Silver SA. Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg. 2005;116:768–779; discussion 780–781. doi: 10.1097/01.prs.0000176259.66948.e7. [DOI] [PubMed] [Google Scholar]
- 4.Nahabedian MY. Acellular dermal matrices in primary breast reconstruction: principles, concepts, and indications. Plast Reconstr Surg. 2012;130(Suppl 2):44S–53S. doi: 10.1097/PRS.0b013e31825f2215. [DOI] [PubMed] [Google Scholar]
- 5.Nahabedian MY, Spear SL. Acellular dermal matrix for secondary procedures following prosthetic breast reconstruction. Aesthet Surg J. 2011;31(7 Suppl):38S–50S. doi: 10.1177/1090820X11418093. [DOI] [PubMed] [Google Scholar]
- 6.Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: a five year Sientra study analysis using round, smooth and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132:1115–1123. doi: 10.1097/01.prs.0000435317.76381.68. [DOI] [PubMed] [Google Scholar]
- 7.Stevens WG, Harrington J, Alizadeh K, et al. Five-year follow-up data from the U.S. clinical trial for Sientra’s U.S. Food and Drug Administration-approved Silimed® brand round and shaped implants with high-strength silicone gel. Plast Reconstr Surg. 2012;130:973–981. doi: 10.1097/PRS.0b013e31826b7d2f. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell GP, Van Natta BW, Murphy DK, et al. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;32:709–717. doi: 10.1177/1090820X12452423. [DOI] [PubMed] [Google Scholar]
- 9.Hammond DC, Migliori MM, Caplin DA, et al. Mentor Contour Profile Gel implants: clinical outcomes at 6 years. Plast Reconstr Surg. 2012;129:1381–1391. doi: 10.1097/PRS.0b013e31824ecbf0. [DOI] [PubMed] [Google Scholar]
- 10.Niechaiev I, Jurell G, Lohielm L. Prospective study comparing two brands of cohesive gel breast implants with anatomic shape: 5-year follow-up evaluation. Aesthetic Plast Surg. 2007;31:697–710. doi: 10.1007/s00266-006-0057-0. [DOI] [PubMed] [Google Scholar]
- 11.Fruhstorfer BH, Hodgson EL, Malata CM. Early experience with an anatomical soft cohesive silicone gel prosthesis in cosmetic and reconstructive breast implant surgery. Ann Plast Surg. 2004;53:536–542. doi: 10.1097/01.sap.0000134508.43550.6f. [DOI] [PubMed] [Google Scholar]
- 12.Macadam SA, Ho AL, Lennox PA, et al. Patient-reported satisfaction and health-related quality of life following breast reconstruction: a comparison of shaped cohesive gel and round cohesive gel implant recipients. Plast Reconstr Surg. 2013;131:431–441. doi: 10.1097/PRS.0b013e31827c6d55. [DOI] [PubMed] [Google Scholar]


