Abstract
Background:
Even though vascular malformations are well categorized, further details are relatively unknown. Of treated patients regarding the frequency, demographic distributions, and other related factors by multivariate regression analyses in proportion to total vascular malformations, methods of treatment and how to manage them have not been elucidated thoroughly.
Methods:
From January 2006 to March 2012, consecutively treated patients with vascular anomalies were included in this investigation at least 1-year follow-up.
Results:
Of the total of 123 cases, 86 females and 37 males, the mean follow-up was 3.5 ± 1.68 years, and the frequency of treatment was 1–8 times (1.8 ± 1.30). Surgery was performed for 22 cases (17.9%) of venous malformations and arteriovenous malformations. In multivariate regression, the frequency of treatment was significantly correlated with the length of follow-up (P < 0.001), age (P < 0.05), and type of malformations (P < 0.05) (R2 = 0.18). Need for surgery was significantly increased with age at odds ratio (OR) of 1.06 [95% confidence interval (CI), 1.03–1.80] (P < 0.001), and head/face/neck, and upper limb are more performed at OR of 0.24 (95% CI, 0.07–0.85) (P < 0.05). The satisfaction score varied from 1 to 5 (3.9 ± 0.68). Complications occurred in 3 cases (2.4%). In logistic regression of complications, the OR of the satisfaction score was 0.13 (95% CI, 0.02–0.80) (P < 0.05).
Conclusions:
Treatment of vascular malformations is an integral part of multidisciplinary approaches. Venous malformations are more frequent in combination surgery, and if there are fewer complications, the patients’ satisfaction increases.
Vascular malformations include capillary malformation (CM), venous malformation (VM), lymphatic malformation (LM), arteriovenous malformation (AVM), and their combinations,1 and they are distinct from vascular tumors regarding clinical appearance, imaging, and histopathological characteristics. VM is the most common type of vascular malformation, and the overall incidence of VM is reportedly 65% of the total 1.5% of all congenital vascular malformations found in a general population.2 AVMs occur with equal frequency in men and in women, with 40–60% of lesions apparent at birth and an additional 30% becoming obvious during childhood.3 AVMs are categorized into 4 different stages proposed by Schobinger and accepted by the International Society for the Study of Vascular Anomalies.4 The natural progression and recurrence of AVMs in children are likely to occur before adulthood and may exacerbate with resection either with or without embolization.5 LMs are developmental anomalies of the lymphatic system that result in defects in lymphatic flow. Both macrocystic and microcystic LMs are often present at birth. Macrocystic LMs are more frequently located in the neck and axilla and are often referred to incorrectly as cystic hygroma,6 whereas microcystic LMs become apparent after complications of infection or bleeding and are predominantly located in the proximal limbs, upper extremities, axillae, and the chest. CM is a slow-flow anomaly, occurs in 0.3% of infants, and is sometimes diagnosed as bruising or erythema from birth trauma. CM is a congenital anomaly that is believed to be a vasculature developmental error and can be clinically diagnosed. CM may be mimicked by cutaneous erythema overlying deeper AVMs. Doppler ultrasound, computed tomography, and magnetic resonance imaging (MRI) may be helpful in diagnosing arteriovenous fistula or shunting.7 Despite the availability of embolo/sclerotherapy for vascular malformations,8 the role of surgical removal and subsequent reconstruction is relatively low due to the risk of bleeding during surgery and inaccessibility for excision of the entire vascular malformation, which may result in deterioration of future treatment. In patients undergoing embolo/sclerotherapy, the incidence of soft-tissue injury and neuropathy was reported to be 11.9% and 8.6%, respectively, in 573 patients, and surgical treatment such as escharectomy, skin grafting, and amputation was employed for 41.2% of the soft-tissue injury cases to rescue and restore the defects.9 We analyze all the treated patients for frequency, demographic distributions, and other related factors by multivariate regression analyses in proportion to total vascular malformations, and methods of treatment and management are discussed.
MATERIALS AND METHODS
From January 2006 to March 2012, consecutive treated patients were included in this investigation of vascular anomalies.
All the patients were followed for at least 1 year after the last treatment. The pretreatment before including in our study was divided into 4 subcategories: 0 = no previous treatment, 1 = 1- to 3-time treatment, 2 = 4- to 10-time treatment, and 3 = over 10-time treatment. The subjective satisfaction score (1, lowest to 5, highest) was collected at the last outpatient clinic for each patient. The agent used for embolization was mainly N-butyl cyanoacrylate, and for large and high-input lesions, a platinum-feathered coil was used, while absolute ethanol was used for sclerotherapy at 0.1 mL/injection under the echo guidance at maximum of 0.6 mL/body weight (kg). When embolization and sclerotherapy were combined, a 24- to 72-hour interval was employed. Surgery could be performed at the time of sclerotherapy.
When the lesion is so obviously small and well-defined, after sclerotherapy, surgically resection is followed. Or redundant tissue may be suspended after sclerotherapy. Surgical reconstruction was followed if the lesion is considered completely resected.
Clinical assessment and imaging examinations such as Doppler ultrasonography, computed tomography, MRI, and angiography preceded any treatment such as transcutaneous embolization and percutaneous sclerotherapy for AVMs or sclerotherapy alone for other vascular malformations. When surgery was implemented, all were followed by embolization and sclerotherapy (embolo/sclerotherapy) or sclerotherapy.
Patient Consent and Ethical Disclosure
All treatments in this clinical series were approved by the Internal Review Board of Nagasaki University (approved number 10032690), and informed consent forms are included in the internal review board format.
Statistics
Results are expressed as the mean ± SD. The multiple linear regression test and logistic regression test were used for multiple variable analysis. Statistical significance was defined as P < 0.05. All analyses were performed using IBM SPSS Statistics, version 21 (Japan IBM Co. Ltd, Tokyo, Japan).
RESULTS
All consecutive cases were 123: 86 female (69.9%) and 37 male (30.1%). There were 85 cases of VMs, 19 AVMs, 8 LMs, 4 CMs, 2 capillary and venous malformations (CVM), and 5 lymphatic and venous malformations (LVMs), and patients were treated in the Department of Plastic and Reconstructive Surgery, Nagasaki University Hospital. The distribution of anatomical locations was 59 cases (48.0%) in the head/face/neck, 18 cases (14.6%) in the upper limbs, 11 cases (8.9%) in the torso, 34 cases (27.6%) in the lower limbs, and 1 case (0.8%) in the head/face/neck and torso (Table 1).
Table 1.
Follow-up, Frequency of Treatment, and Number of Surgeries
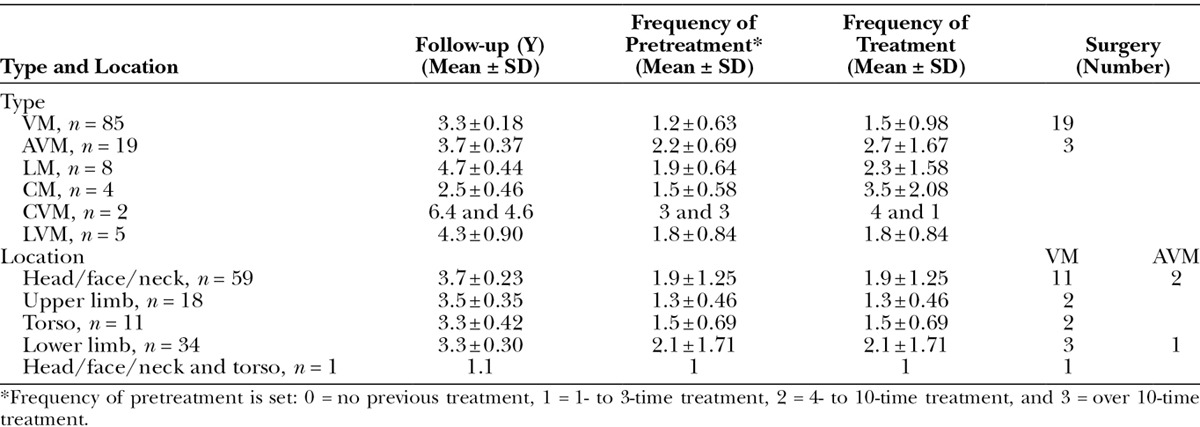
Systemic vascular malformations, such as Klippel-Trenaunay syndrome,10 Parkes-Weber syndrome,11 and Sturge-Weber syndrome,12 were excluded from this study. The mean age was 27.6 ± 21.16 (1–88 years old) for VMs, 40.9 ± 19.89 (11–80 years old) for AVMs, 19.8 ± 20.74 (2–61 years old) for LMs, 54.0 ± 4.97 (49–60 years old) for LMs, 33.0 ± 12.73 (24–42 years old) for CVMs, and 13.0 ± 7.21 (6–24 years old) for LVMs. The mean follow-up was 3.5 ± 1.68 years. Mean follow-up for VM, AVM, LM, CM, CVM, and LVM was 3.3 ± 0.18 years (1–7.2), 3.7 ± 0.37 years (1.2–6.7), 4.7 ± 0.44 years (2.9–7.0), 2.5 ± 0.46 years (1.2–3.3), 5.5 ± 0.90 years (4.6–6.4), and 4.3 ± 0.60 years (1.9–5.2), respectively. Mean follow-up for the head/face/neck, upper limbs, torso, lower limbs, and head/face/neck and torso was 3.7 ± 0.23 years (1.0–7.0), 3.5 ± 0.35 years (1.1–6.5), 3.3 ± 0.42 years (1.2–5.2), 3.3 ± 0.30 years (1.0–7.2), and 1.1 years, respectively.
The frequency of treatment was 1–8 times (1.8 ± 1.30): 1.5 ± 0.98 (1–6) times for VM, 2.7 ± 1.67 (1–8) times for AVM, 2.3 ± 1.58 (1–5) times for LM, 3.5 ± 2.08 (1–6) times for CM, 2.5 ± 2.12 (1–4) times for CVM, and 1.8 ± 0.84 (1–3) times for LVM. The frequency of treatment was 1.9 ± 1.25 (1–6) times for the head/face/neck, 1.3 ± 0.46 (1–2) times for upper limbs, 1.5 ± 0.69 (1–3) times for the torso, 2.1 ± 1.71 (1–8) times for lower limbs, and once for the head/face/neck and torso.
Surgery was performed for 22 cases (17.9%), subdivided into 19 of 85 cases (22.4%) for VM and 3 of 19 cases (15.8%) for AVM. Thirteen of 59 cases (22.0%) in the head/face/neck, 2 of 18 cases (11.1%) in upper limbs, 2 of 11 cases (18.2%) in the torso, 4 of 34 cases (11.8%) in lower limbs, and 1 of 1 case (100%) in the head/face/neck and torso underwent surgery. In VMs, 11 of 19 cases (58.0%) were in the head/face/neck, 2 of 19 (10.5%) in upper limbs, 2 of 19 (10.5%) in the torso, 3 of 19 (15.8%) in lower limbs, and 1 of 19 (5.3%) in the head/face/neck and torso. In contrast, in AVMs, 2 of 3 (66.7%) cases were in the head/face/neck and 1 of 3 cases was in lower limbs (Table 1). Considered complete surgical resections were 14 cases and 1 case for AVM and 13 cases for VMs. An AVM case of the temporal head was reconstructed with free-vascularized anterolateral thigh flap. Nine cases of head/face/neck VMs are considered completely resected; 2 cases of upper limb VMs and 2 cases of torso VMs are considered completely resected. The mean follow-up for surgical cases was 3.4 ± 0.46 (1.0–7.1) years, whereas 3.5 ± 0.16 years for nonsurgical cases.
In multivariate regression analysis, when the frequency of treatment was set as a criterion variable and explanatory variables were defined for age, location, type, and sex, age (P < 0.05), type (P < 0.05), and follow-up (P < 0.001) demonstrated the significant difference (R 2 = 0.18, F = 2).
In multivariate regression analysis, when follow-up was set as a criterion variable and frequency and explanatory variables were defined for age, location, type, and sex, statistical significance was observed for frequency (P < 0.001) (R 2 = 0.13, F = 2).
In a multivariate regression analysis, when the pretreatment was set as a criterion variable and explanatory variables were defined for age, follow-up, location, type, frequency, and sex, statistical significance was observed in frequency (P < 0.001), location (P < 0.05), type (P < 0.05), and sex (P < 0.02) (R 2 = 0.85, F = 2). More pretreatment leads to more frequent interventions in this study, head/face/neck location demonstrates more significant than other locations, CVM and AVM are more than other types, and females are more frequent in pretreatment.
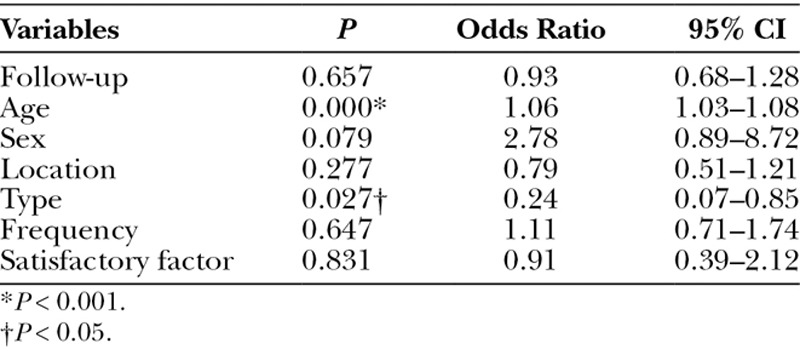
In multivariate logistic regression analysis, backward linear regression step-wise regression with or without surgery, explanatory variables were set for follow-up, age, frequency of the treatment, location, type, and sex, and the odds ratios (OR) of age and type were 1.06 [95% confidence interval (CI), 1.03–1.80] (P < 0.001) and 0.24 (95% CI, 0.07–0.85) (P < 0.05), respectively. Surgery was only performed for VM and AVM and showed significantly greater frequency in VM compared with AVM (Table 2). More multidisciplinary treatment with embolo/sclerotherapy and surgery are utilized in AVM.
Table 2.
Multivariate Logistic Analyses of Variables to Predict Surgery
The satisfaction scores ranged from 1 to 5 (3.9 ± 0.68): 3.9 ± 0.07 (2–5) for VM, 3.6 ± 0.19 (1–5) for AVM, 4.3 ± 0.25 (3–5) for LM, 3.5 ± 0.29 (3–4) for CM, 4 for CVM, and 4 for LVM. The satisfactory scores for location were 4.0 ± 0.08 (2–5) for the head/face/neck, 3.6 ± 0.18 (1–4) for upper limbs, 4.0 ± 0.19 (3–5) for the torso, 3.8 ± 0.11 (2–5) for lower limbs, and 3 for the head/face/neck and torso.
Complications occurred in 3 cases (2.4%), of which 1 case was in AVMs and showed tissue necrosis after sclerotherapy and 2 were in VMs, one of which demonstrated incomplete facial nerve paralysis and the other suffered massive bleeding and required a blood transfusion to recover, because systemic anticoagulation and antihemorrhage capacities were limited due to massive and multiple malformations. In multivariate logistic regression analysis, backward LR step-wise regression with or without complications, explanatory variables were set for follow-up, age, satisfactory score, frequency of the treatment, location, type, and sex, and the OR of the satisfaction score was 0.13 (95% CI, 0.02–0.80) (P < 0.05). When there were fewer complications, the satisfaction score increased.
DISCUSSION
Treatment of vascular malformations is an integral part of percutaneous embolization, percutaneous sclerotherapy, and surgery. Preoperative embolo/sclerotherapy is beneficial for decreasing intraoperative bleeding and defining lesion margins to solidify lesions, especially in slow-flow vascular malformations. In the treatment of patients with vascular malformations, in a single-center analysis of 1130 cases of VM and LVMs and 329 AVMs, surgery was performed for VMs and LVMs (5% with embolo/sclerotherapy and 4% without), while 15% underwent embolo/sclerotherapy for AVMs.8 All of our cases in this study were preceded by presurgical embolo/sclerotherapy or sclerotherapy, with 22.4% surgeries for VMs and 15.8% for AVMs. Surgeries were performed only for VMs and AVMs because both VMs and AVMs are of concern regarding esthetics and restoring functions,5,13 and AVM patients who are surgically resected with or without embolization exhibit less re-expansion than those treated with embolization alone, and recurrence was less probable when the AVM stage (Schobinger staging) was lower in multivariate logistic regression analysis.5 Moreover, it may reflect that the cases are more defined and more easily accessible for surgical removal and reconstruction. Also, all the cases in this study underwent at least one treatment and the data settings may have been different. Therapeutic management of vascular malformations is an integral part. In our 123 series, only 5 cases are first time treatment cases and usually require multiple sessions and modalities. Previously treated cases sometime cause more complicated pathology as the presence of the scar tissue and altered microvasculature of the lesion. In VMs, surgical interventions are required for control of pain, bleeding, nerve impairment due to compression and extensively deterioration in function, and aesthetics. When the lesion is well surrounded, surgical resection and reconstruction is recommended.14
In our series, only 19 of 85 cases (22.4%) can lead to surgical resection and reconstruction, because the lesions are beyond the border of healthy deeper tissue and margin of the lesions is obscure to clarify, partly due to the previous treatment.
In AVMs, microvascular proliferation is correlated to the rate of the flow of the lesion regardless of the previous embolization15; thus, when the lesion seems active and expanding with fast flows, therapeutic interventions may be necessary to control of subsequent deterioration of the lesion or skeletal impacts to the pediatric patients.
In a follow-up of an average 3 years and 6 months, the mean frequency of treatment was 1.8 per case in our series. Surgery was performed in 17.9% of the cases in this study and according to logistic regression analysis. Complications occurred in 3 cases: 1 AVM and 2 VMs. In the AVM case, the complication was tissue necrosis due to fast flow leading to carriage of the injected absolute agent to peripheral sites; one massive facial VM resulted in an incomplete facial nerve, the buccal branch of the facial nerve, paralysis; and another VM case suffered continuous bleeding after sclerotherapy. Soft-tissue loss was observed in 68 of 573 cases (11.8%) and was greatest in AVMs, 42 of 143 cases (29.4%). Soft-tissue loss spontaneously recovered within 2.7 months, and the final rate was 28 of 573 cases (4.9%). Neuropathy was observed in 49 of 573 cases (8.6%), with VM demonstrating 30 of 273 cases (10.9%), and the final rate was 7 of 573 cases (1.2%) at 5.3 months.9 Our patients were assessed at least 1 year after the last treatment, and 1 of 19 AVMs (5.3%) demonstrated skin loss, in which the lesion lay along the digital vessels and injected absolute ethanol ran distally to the finger-tip. The rate of neuropathy in VM was 1 of 85 cases (1.2%), which was a massive and enlarged lesion, the frequency was 6 times, and appearing facial nerve courses may have been masked, with both rates being very comparable. There were no complications related to surgery. When the patient’s satisfaction was greater, the complication rate was inversely correlated, with an OR of 0.13. In a cross-sectional study of 158 VMs, female gender (OR, 4.49; 95% CI, 1.24–16.28), no or delayed visualization of drainage vein (OR, 9.22; 95% CI, 1.79–47.51), and a well-defined margin on MRI (OR, 13.38; 95% CI, 2.84–63.12) were independent predictors of good responders on multivariate analysis.16
In AVM, angiographic types and treatment are recommended according to different accessibility to the lesion, such as transarterial access, direct puncture, and transvenous access.17
CONCLUSIONS
Further objective analyses of the margin of lesions and drainage veins for VMs in surgery and appropriate combinations with different access and pretreated AVMs should be defined and appropriate follow-up and definitive removal of the lesion should be carefully determined in relation to the timing of the therapy and methods.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors, partly supported by Grant #25670751 from the Ministry of health, Labor and Welfare.
REFERENCES
- 1.Enjolras O, Mulliken JB. Vascular tumors and vascular malformations. Adv Dermatol. 1997;13:375–423. [PubMed] [Google Scholar]
- 2.Eifert S, Villavicencio JL, Kao TC, et al. Prevalence of deep venous anomalies in congenital vascular malformations of venous predominance. J Vasc Surg. 2000;31:462–471. [PubMed] [Google Scholar]
- 3.Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: part I. J Am Acad Dermatol. 2007;56:353–370; quiz 371. doi: 10.1016/j.jaad.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 4.Kohout MP, Hansen M, Pribaz JJ, et al. Arteriovenous malformations of the head and neck: natural history and management. Plast Reconstr Surg. 1998;102:643–654. doi: 10.1097/00006534-199809030-00006. [DOI] [PubMed] [Google Scholar]
- 5.Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrent after treatment. Plast Reconstr Surg. 2010;125:1185–1194. doi: 10.1097/PRS.0b013e3181d18070. [DOI] [PubMed] [Google Scholar]
- 6.Hassanein AH, Mulliken JB, Fishman SJ, et al. Evaluation of terminology for vascular anomalies in current literature. Plast Reconstr Surg. 2011;127:347–351. doi: 10.1097/PRS.0b013e3181f95b83. [DOI] [PubMed] [Google Scholar]
- 7.Dubois J, Garel L. Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr Radiol. 1999;29:879–893. doi: 10.1007/s002470050718. [DOI] [PubMed] [Google Scholar]
- 8.Kim YW. Proper selection of patients for percutaneous embolo-sclerotherapy in patients with congenital vascular malformations (CVMs). Eur J Vasc Endovasc Surg. 2010;39(Suppl 1):S49–S54. doi: 10.1016/j.ejvs.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Lee KB, Kim DI, Oh SK, et al. Incidence of soft tissue injury and neuropathy after embolo/sclerotherapy for congenital vascular malformation. J Vasc Surg. 2008;48:1286–1291. doi: 10.1016/j.jvs.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 10.Lee A, Driscoll D, Gloviczki P, et al. Evaluation and management of pain in patients with Klippel-Trenaunay syndrome: a review. Pediatrics. 2005;115:744–749. doi: 10.1542/peds.2004-0446. [DOI] [PubMed] [Google Scholar]
- 11.Giron-Vallejo O, Lopez-Gutierrez JC, Fernandez-Pineda I. Diagnosis and treatment of Parkes Weber syndrome: a review of 10 consecutive cases. Ann Vas Surg. 2013;27:820–825. doi: 10.1016/j.avsg.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Thomas-Sohl KA, Vaslow DF, Maria BL. Sturge-Weber syndrome: a review. Pediatr Neurol. 2004;30:303–310. doi: 10.1016/j.pediatrneurol.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Arneja JS, Gosain AK. Vascular malformations. Plast Reconstr Surg. 2008;121:195e–206e. doi: 10.1097/01.prs.0000304607.29622.3c. [DOI] [PubMed] [Google Scholar]
- 14.Gu JH, Jeong SH. Radical resection of a venous malformation in middle finger and immediate reconstruction using medial plantar artery perforator flap: a case report. Microsurgery. 2012;32:148–152. doi: 10.1002/micr.20969. [DOI] [PubMed] [Google Scholar]
- 15.Meijer-Jornal LB, van der Loos CM, de Boer OJ, et al. Microvascular proliferations in arteriovenous malformations related to high-flow characteristics, inflammation, and previous therapeutic embolization of the lesion. J Am Acad Dermatol. 2103;68:638–646. doi: 10.1016/j.jaad.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 16.Yun WS, Kim YW, Lee KB, et al. Predictors of response to percutaneous ethanol sclerotherapy (PES) in patients with venous malformations: analysis of patients self-assessment and imaging. J Vasc Surg. 2009;50:581–589. doi: 10.1016/j.jvs.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 17.Cho SK, Do YS, Shin SW, et al. Arteriovenous malformations of the body and extremities. J Endovasc Ther. 2006;13:527–538. doi: 10.1583/05-1769.1. [DOI] [PubMed] [Google Scholar]


