Abstract
Background:
In aesthetic medicine, the most promising techniques for noninvasive body sculpturing purposes are based on ultrasound-induced fat cavitation. Liporeductive ultrasound devices afford clinically relevant subcutaneous fat pad reduction without significant adverse reactions. This study aims at evaluating the histological and ultrastructural changes induced by ultrasound cavitation on the different cell components of human skin.
Methods:
Control and ultrasound-treated ex vivo abdominal full-thickness skin samples and skin biopsies from patients pretreated with or without ultrasound cavitation were studied histologically, morphometrically, and ultrastructurally to evaluate possible changes in adipocyte size and morphology. Adipocyte apoptosis and triglyceride release were also assayed. Clinical evaluation of the effects of 4 weekly ultrasound vs sham treatments was performed by plicometry.
Results:
Compared with the sham-treated control samples, ultrasound cavitation induced a statistically significant reduction in the size of the adipocytes (P < 0.001), the appearance of micropores and triglyceride leakage and release in the conditioned medium (P < 0.05 at 15 min), or adipose tissue interstitium, without appreciable changes in microvascular, stromal, and epidermal components and in the number of apoptotic adipocytes. Clinically, the ultrasound treatment caused a significant reduction of abdominal fat.
Conclusions:
This study further strengthens the current notion that noninvasive transcutaneous ultrasound cavitation is a promising and safe technology for localized reduction of fat and provides experimental evidence for its specific mechanism of action on the adipocytes.
Despite the overall satisfaction of patients and surgeons for liposuction for body shaping purposes,1 there is an increasing demand for noninvasive fat reduction methods that are similarly effective, yet comfortable and safe, with minimal downtime.2 A variety of physical treatments, including mechanical massage, electric stimulation, radiofrequency emission, and “cold” low-level laser irradiation, have been investigated for the ability to induce localized fat reduction.2–5 However, when tested clinically, most of them have failed to meet the expectations and some have also raised safety concerns.6
In a clinical perspective, the most effective instruments for noninvasive fat reduction use ultrasounds7–10: through the generation of compression and depression cycles at appropriate frequency, they cause cavitation phenomena at the fat droplet-watery cytoplasm interface and eventually adipocyte rupture and triglyceride release.7,9 Ultrasonic energy can be delivered by nonfocused or focused waves. With the nonfocused mode, due to depth-related ultrasound attenuation, the superficial skin is exposed to maximum energy intensity. By contrast, focused ultrasound can be concentrated in a defined subcutaneous area to produce clinically relevant fat lysis11,12 while limiting damage to blood vessels, nerves, connective tissue, and the underlying organs. However, the thermal effects of focused ultrasound energy may result in adipocyte necrosis in the treatment area.13 The most recent devices for liporeductive purposes have been specifically designed to prevent unwanted tissue injury. Contour I (UltraShape, Yoqneam, Israel), a focused ultrasound emitter, was first demonstrated to achieve selective adipocyte lysis and clinically relevant reduction of the volume of subcutaneous fat pad, in the absence of significant adverse reactions.8,14 On the other hand, Med2Contour (General Project, Montespertoli, Italy) uses a different approach, as it is based on 2 angled nonfocused transducers that create a weakly focused ultrasound field within the subcutaneous fat pad at the point where the beams overlap.15
Despite their undoubted clinical efficacy, the biological mechanisms underlying the observed liporeductive effects are not fully understood. It has been shown that adipose cell cavitation can induce focal alterations of the plasma membrane and lipid leakage,15 but little is known about the possible noxious effects of ultrasound on adipocytes, other cell types of the adipose tissue (blood vessels and mast cells), and the neighboring tissues crossed by the ultrasound beam (epidermis and dermis).
The current study was designed to evaluate and quantify the histological and ultrastructural changes induced by ultrasound cavitation on the different cell components of human subcutaneous fat—that is, adipocytes, blood vessel cells, and perivascular mast cells—and upper skin tissues, that is, dermal fibroblasts and epidermal keratinocytes. To this aim, ex vivo full-thickness skin samples taken at surgery and subcutaneous biopsies of sham-treated and ultrasound-treated skin areas from individual patients were used.
MATERIALS AND METHODS
This study complied with the guidelines of the Declaration of Helsinki, as amended in Edinburgh, 2008. It was approved by the Ethical Committee of the Faculty of Medicine, University of Florence, Italy. All subjects gave written informed consent to their participation in the study.
Ex Vivo Study
In a first ex vivo experimental set, abdominal full-thickness biopsies of normal skin (n = 3), approximately 15 mm thick and including the epidermis, dermis, and subcutaneous adipose tissue, were taken at surgery from 3 patients. Each biopsy was cut in 2 parts of similar size and weighed. Then, each specimen was placed in a Petri dish on ice, the subcutaneous tissue facing downward, and 2 ml of preoxygenated incubation medium (phenol red-free Dulbecco’s modified Eagle medium; Gibco Invitrogen, Milan, Italy) was added. A first specimen was subjected to ultrasound cavitation using Med2Contour (General Project, Montespertoli, Italy), a nonfocused, dual-transducer device, with the following settings: 3 W power output, 20 kHz frequency, and pulsed mode (2 pulses, 6 s each, separated by a 10-s pause). A single transducer of the Med2Contour was placed in direct contact with the epidermis through a thin layer of Aquasonic Clear ultrasound gel (Parker, Fairfield, CT). The above power and frequency settings were adopted because they were similar to those yielding best clinical performance,8,14 while the timing protocol was chosen to avoid tissue overheating, considering that the skin explants lacked blood flow-related temperature homeostatic mechanisms. In some experiments, tissue temperature was continuously monitored with a digital thermometer and found not to exceed 38°C. The other specimen was sham-treated (ie, subjected to the same handling procedure but with no ultrasound emission) and used as control. After the treatments, the specimens were incubated for up to 1 h at room temperature and small volumes (500 ml) of incubation medium were taken at 1, 15, 30, and 60 minutes for measurement of triglyceride release. At the end of the experiments, fragments of adipose tissue and cutis were taken from the central part of the treated and control specimens, fixed in isotonic 4% glutaraldehyde and 1% OsO4, dehydrated, and embedded in Epon epoxy resin (Fluka, Buchs, Switzerland) for light and electron microscopic studies.
For morphometric analysis of adipocyte size, digital photomicrographs of semithin sections, 2 µm thick, were taken under a light microscope (Microstar IV, Reichert, Seefeld, Germany) equipped with an Eurekam-9 high-resolution videocamera and software (BEL Engineering, Monza, Italy). From each specimen, 10 randomly chosen micrographs, each corresponding to a test area of 65,700 µm2, were collected. The surface area of cross-sectioned adipocyte lipid vacuoles was measured using ImageJ 1.33 image analysis program (http://rsb.info.nih.gov/ij), upon setting an appropriate threshold to include only the osmiophilic lipid vacuoles of the adipocytes. Vacuolar profiles ≤ 1000 µm2, consistent with polar cross-sections, were excluded. Data were reported as mean values (± SEM) of the control and treated groups.
For transmission electron microscopy, ultrathin sections were stained with aqueous uranyl acetate and alkaline bismuth subnitrate, viewed and photographed under a JEM 1010 transmission electron microscope (Jeol, Tokyo, Japan) equipped with a MegaView III high-resolution digital camera and imaging software (Jeol). The different components of subcutaneous adipose tissue, that is, adipocytes, blood microvessels, and interstitial stoma, in the control and ultrasound-treated samples were carefully examined and compared.
Measurement of triglyceride release was performed in small volumes of conditioned medium taken at 1, 15, 30, and 60 minutes after ultrasound or sham treatment of the skin explants (n = 3). To this purpose, a TRIG assay (ADVIA Chemistry, Bayer, CH) was used. Values, in mg/dl, were normalized to weight of the skin specimen and expressed as µg/mg tissue (wet weight), assuming P ≤ 0.05 as significant.
In Vivo Study
In a second in vivo experimental set, 3 overweight volunteers scheduled for abdominal liporeductive surgery were subjected to ultrasound-induced cavitation, using the Med2Contour. Being equipped with a dual-transducer handpiece, the 2 nonfocused beams can create a focused ultrasound field within the adipose tissue where the beams overlap. Settings were as follows: 2 W power output, pulsed mode, 20 kHz frequency, and 15-minute treatment. In each patient, the right hypogastrium was the test area, whereas the left hypogastrium was the sham-treated area. In 2 patients, the treatment was repeated 4 times, namely, 27 days, 20 days, 12 days, and 1 day before surgery. In the other patient, the treatment was repeated 3 times, namely, 27, 20, and 12 days before surgery: this protocol aimed at studying whether the ultrasound effects were maintained with time. Further 3 overweight patients (2 males, 1 female; age, 30–33) were enrolled for the assessment of subcutaneous adipose tissue mass in control and ultrasound-treated abdominal skin. They were subjected to 4 weekly ultrasound treatments, as described above. Just before each treatment and 1 week after the last treatment, the thickness of subcutaneous fat pad in the control and test areas was assessed by measuring the depth of skin folds with a caliper (Holtain Ltd., Crymych, UK). To normalize individual differences, the values were expressed as percent changes over the initial measures.
At surgery, fragments of subcutaneous adipose tissue and cutis were taken from the central part of the test and control areas: part of them was processed for electron microscopy, as above, while others were fixed in buffered 4% formaldehyde, dehydrated, and embedded in paraffin for light microscopy. Similar to the ex vivo experiment, morphometry of adipocyte size was performed on semithin sections and ultrastructural analysis on ultrathin sections of the Epon-embedded specimens. Histological sections, 6 µm thick, from the paraffin-embedded specimens were used to evaluate adipocyte apoptosis by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, using a FragEL–Klenow DNA Fragmentation Detection Kit (Oncogene, San Diego, CA) according to the manufacturer’s instructions. Apoptotic nuclei were recognized by the presence of dark brown staining, while those of viable cells appeared pale brown or green. From each sample, 10 microscopic fields (20× objective) were chosen at random. The overall number of adipocyte nuclei was counted and the percentage of the positive ones was calculated. An average of 100 nuclei was counted in each sample. Two observers carried out the measurements on the same microscopic fields and the individual values were then averaged.
Statistical Analysis
The assayed quantitative parameters were statistically analyzed assuming the individual patients as sample units (n = 3). For adipocyte morphometry and TUNEL assay, statistical significance of differences between groups was assessed by unpaired Student’s t test. Time-course curves of triglyceride levels in the conditioned medium of the control and ultrasound-treated specimens and of plicometric measurements of the in vivo study were compared by two-way analysis of variance. In both cases, Graph Pad Prism 4.03 statistical software (GraphPad, San Diego, CA) was used. A P value ≤ 0.05 was considered significant.
RESULTS
Visual examination of semithin sections and morphometrical analysis of subcutaneous adipose tissue of ex vivo skin explants (Fig. 1) showed that ultrasound cavitation induced a marked, statistically significant decrease (−23%) in the size of lipid vacuoles in adipocytes (control: 15,654 ± 942 µm2; cavitation: 11,423 ± 558 µm2; P < 0.001; n = 3). Similar findings were obtained by comparison of subcutaneous fat biopsies taken at surgery from sham- or ultrasound-pretreated abdominal skin (Fig. 2). In patients 1 and 2 (biopsies were taken 1 d after the last ultrasound application), the treatment caused a significant reduction (−26%) of the size of adipocyte lipid vacuoles (control: 11,908 ± 373 µm2; cavitation: 8637 ± 530 µm2; P < 0.001). In patient 3 (biopsies were taken 12 d after the last ultrasound application), the treatment still induced a significant reduction (−47%) of adipocyte lipid vacuoles (control: 11,864 ± 422 µm2; cavitation: 6323 ± 200 µm2; P < 0.001). No differences were observed among the control biopsies from the 3 patients (data not shown). The TUNEL assay, performed to detect and quantify apoptotic adipocytes (Fig. 3), showed no significant differences in the nuclear labeling/unlabeling ratio between the control and test adipose tissue areas (control: 0.09 ± 0.01; cavitation: 0.11 ± 0.03; merged values from the 3 patients).
Fig. 1.
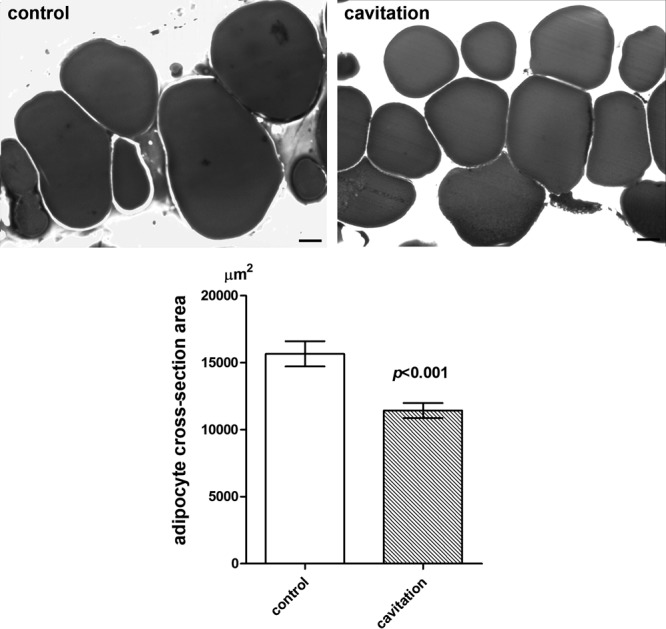
Histological and morphometrical findings of subcutaneous adipocytes from ex vivo skin explants. Ultrasound cavitation causes a statistically significant reduction of mean cross-section surface area of lipid vacuoles, related to adipocyte overall volume (Student’s t test, n = 3 biopsies per experimental group). OsO4 fixation/staining and toluidine blue counterstaining. Bars = 10 µm.
Fig. 2.
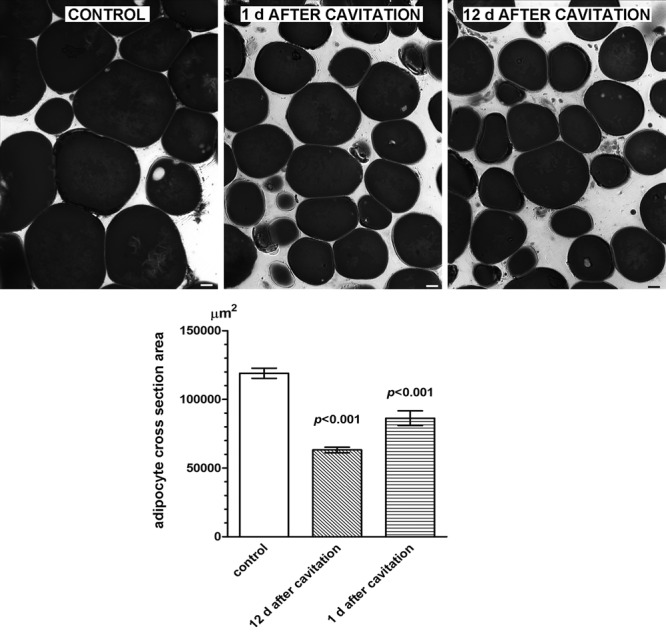
Histological and morphometrical findings of subcutaneous adipocytes from control or ultrasound-pretreated abdominal skin biopsies taken at the noted times after the treatment. Ultrasound cavitation causes a statistically significant reduction of mean cross-section surface area of lipid vacuoles, related to adipocyte overall volume (Student’s t test, n = 10 microscopical fields per biopsy). No differences were observed between the control samples from the 2 patients. OsO4 fixation/staining and toluidine blue counterstaining. Bars = 10 µm.
Fig. 3.
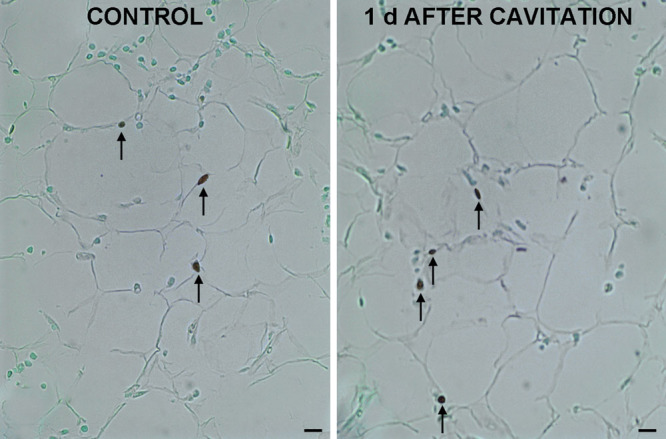
TUNEL assay performed to detect apoptotic adipocytes in subcutaneous biopsies from sham- or ultrasound-pretreated abdominal skin. Arrows point at apoptotic nuclei. No differences were observed or measured between the 2 groups. Bars = 10 µm.
Ultrastructural analysis of adipose tissue from sham-treated ex vivo skin explants demonstrated normal adipocytes, showing a large, osmiophilic lipid vacuole with a peripheral electron-lucent rim contiguous to a thin cytoplasmic layer containing scanty organelles, pinocytosis microvesicles and small lipid droplets (Fig. 4A). Cells were surrounded by a continuous basement membrane. The interstitial connective tissue was composed of a loose, electron-lucent matrix containing thin bundles of collagen fibers. Blood microvessels, mainly capillaries, also showed a normal appearance (Fig. 4B): the endothelial cells showed intact plasma membranes, contained numerous transcytosis microvesicles and were joined by junctional complexes to form a continuous endothelium; they were surrounded by a basement membrane harboring some pericytes. Normal features of adipocytes and blood capillaries were also observed in the adipose tissue biopsies taken from sham-treated areas of the 3 patients enrolled in the study (not shown).
Fig. 4.
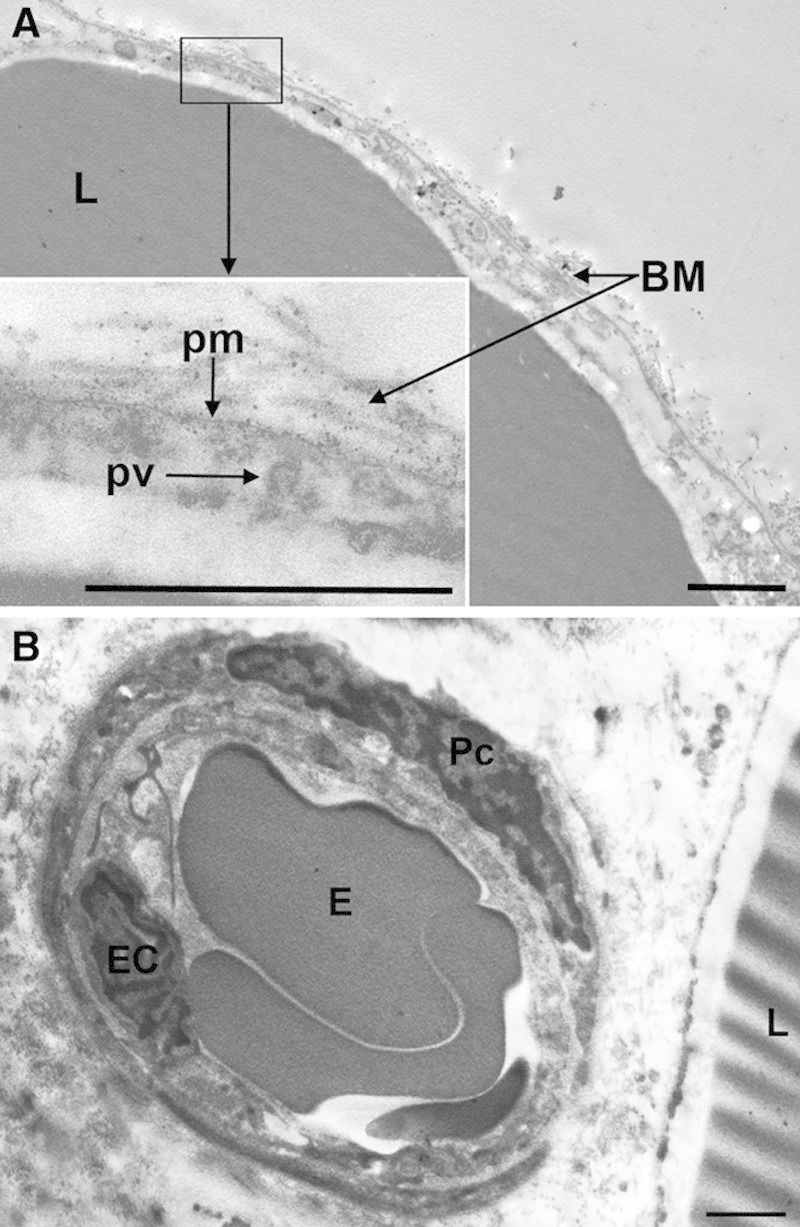
Representative transmission electron microscopy images of adipose tissue from sham-treated ex vivo skin explants. A, Adipocytes show normal features. B, Control adipose tissue interstitium also shows normal morphology. BM, basement membrane; E, erythrocyte; EC, endothelial cell; L, lipid vacuoles; Pc, pericyte; pm, plasma membrane; pv, pinocytosis vesicle. Bars = 1 µm.
Conversely, the subcutaneous adipose tissue of ultrasound-treated ex vivo skin explants displayed well-appreciable differences compared with the sham-treated specimens. In particular, many adipocytes showed peculiar abnormalities, consisting in microvesicle clustering and focal ruptures of the peripheral cytoplasmic rim (Fig. 5). Such ruptures were usually restricted to small areas of the cell surface, approximately 0.5–1.5 µm in diameter, but large enough to allow leakage of triglyceride droplets from the inner cytoplasmic vacuole to the extracellular space. Of note, no signs of adipocyte demise or cell remnants were observed. The interstitial stroma was composed of a loose, electron-lucent matrix containing thin bundles of collagen fibers. Small lipid droplets were often encountered (Fig. 5). Blood microvessels, mainly capillaries, showed a normal appearance (Fig. 6): endothelial cells showed intact plasma membranes, contained numerous transcytosis microvesicles and were joined by junctional complexes to form a continuous endothelium, and were surrounded by a continuous basement membrane harboring some pericytes. Intravascular erythrocytes also displayed normal features. Perivascular mast cells did not show signs of activation and granule discharge (not shown).
Fig. 5.
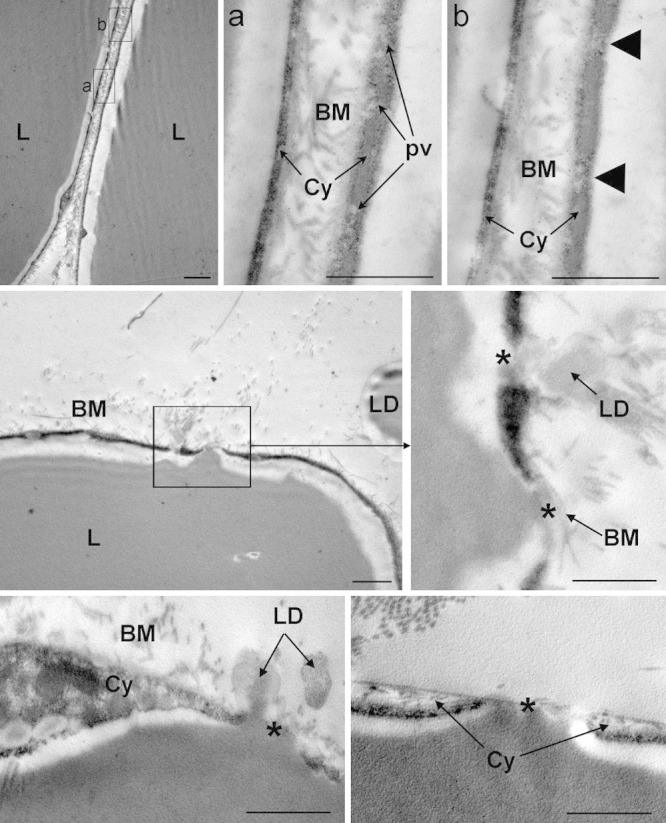
Representative transmission electron microscopy images of adipose tissue from ultrasound-treated ex vivo skin explants. Upper panels: Two adjacent adipocytes are shown. BM, basement membranes; Cy, cytoplasm; L, lipid vacuoles; pv, pinocytosis vesicles. The arrowheads in the inset (b) point at 2 areas, featuring microvesicular clusters, in which the cytoplasm appears to be about to rupture. Centre and lower panels: Abnormal adipocytes undergoing lipid release. L, lipid vacuoles; LD, lipid droplets in the extracellular matrix. The asterisks indicate focal cytoplasmic ruptures allowing leakage of triglyceride droplets in the interstitium. Bars = 1 µm.
Fig. 6.
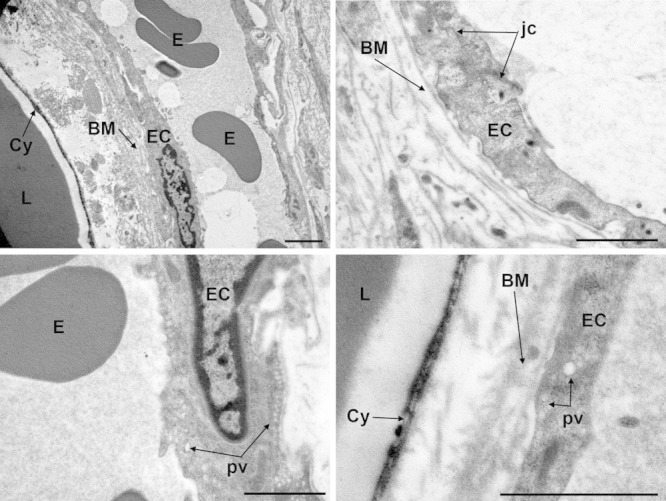
Representative transmission electron microscopy images of adipose tissue from ultrasound-treated ex vivo skin explants. Endothelial cells (EC) of blood microvessels show normal features. BM, basement membrane; Cy, adipocyte cytoplasm; E, erythrocytes; jc, junctional complex; L, lipid vacuoles; pv, pinocytosis vesicles. Bars = 1 µm.
Assay of triglyceride release in the conditioned medium of ex vivo skin explants showed a time-related increase in both the sham- and ultrasound-treated specimens (Fig. 7). Of note, cavitation induced a significantly higher release of triglycerides as compared with the controls, with a peak at 15 minutes (P < 0.05).
Fig. 7.
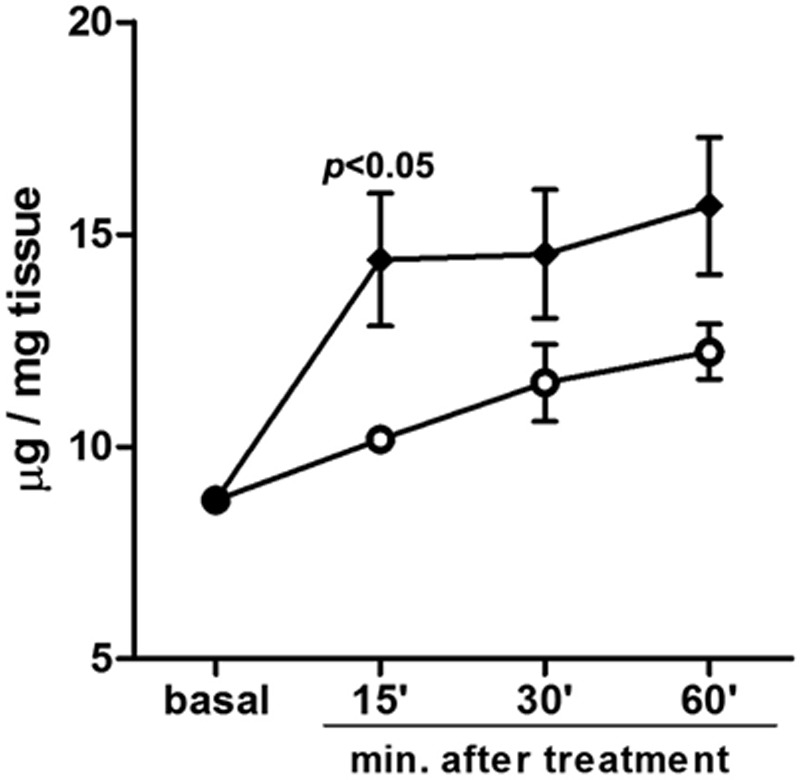
Assay of triglycerides released in the conditioned medium of ex vivo skin explants. A time-related increase is observed in both the sham- and ultrasound-treated specimens. Cavitation induced a higher release of triglycerides as compared with the controls, peaking at 15 min.
Ultrastructural examination of subcutaneous fat biopsies taken at surgery from ultrasound-pretreated abdominal skin areas showed different features from those of the ex vivo specimens. In all the patients examined, regardless the biopsies were taken 1 or 12 days after the last ultrasound application, images of triglyceride leakage from adipocytes were no longer detected and no appreciable changes were observed in the interstitial stroma and blood microvessels (not shown). However, adipocytes that had been exposed to ultrasounds consistently showed irregular, winding profiles and multiple lipid droplets clustered in the cytoplasmic rim (Fig. 8). These features were never found in the sham-treated adipocytes and are consistent with marked reduction of cell volume, conceivably related to triglyceride discharge. In keeping with this finding, sparse lipid droplets were often encountered in the adipose tissue stroma in the vicinity of blood vessels (Fig. 8).
Fig. 8.
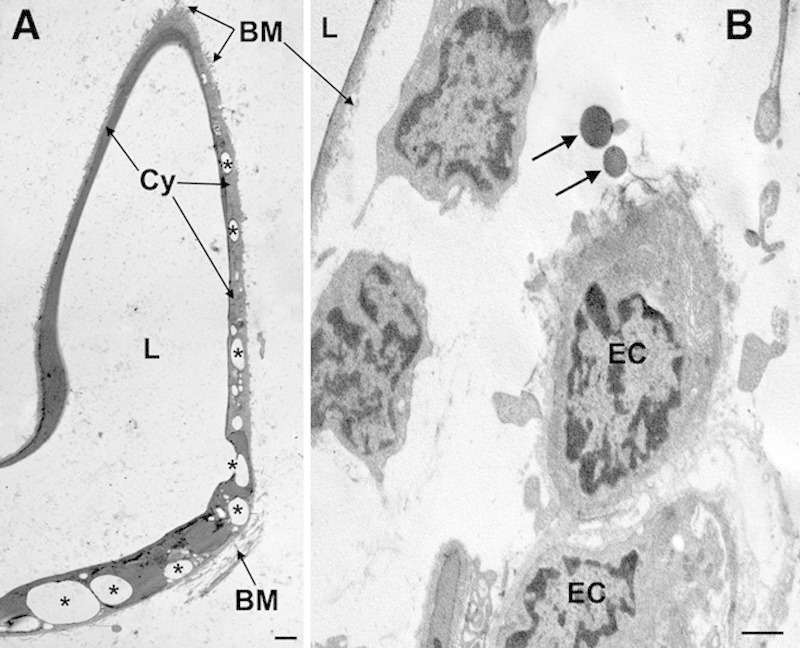
Representative transmission electron microscopy images of adipose tissue from ultrasound-pretreated (1 d) abdominal skin areas. A, An adipocyte showing irregular, winding profiles and multiple lipid droplets (some of which are labeled by asterisks) clustered in the cytoplasmic rim. B, Adipose tissue interstitium showing normal blood microvessels and free lipid droplets (arrows) in their proximity. BM, basement membrane; Cy, adipocyte cytoplasm; EC, endothelial cells; L, lipid vacuoles. Bars = 1 µm.
Assessment of subcutaneous adipose tissue mass by plicometry showed a time-related decrease of the measured values in the ultrasound-treated abdominal skin regions as compared with the corresponding control areas (Fig. 9).
Fig. 9.
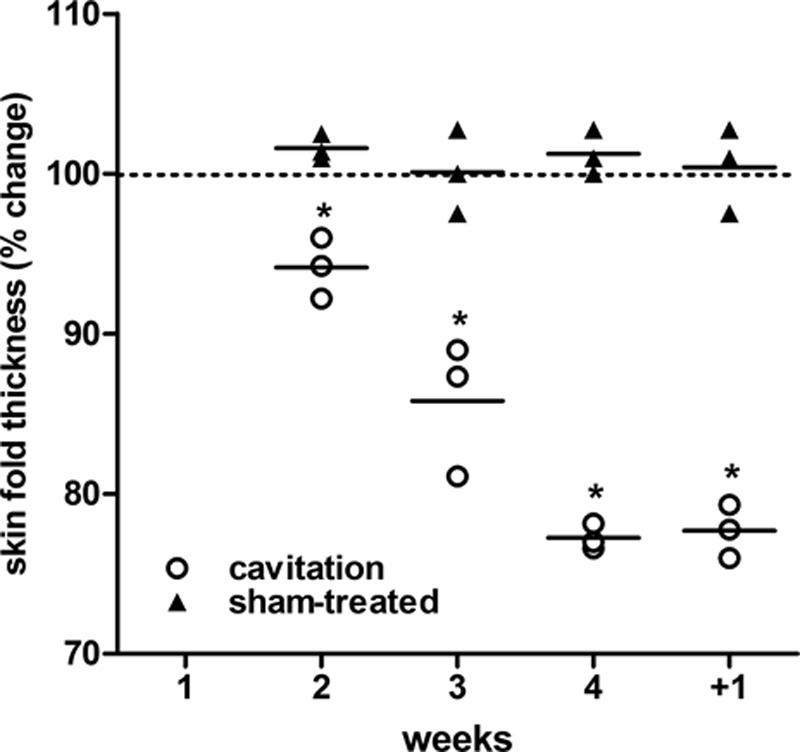
Percent changes of the thickness of sham- and ultrasound-treated abdominal skin folds measured by plicometry upon 4 weekly treatments and 1 wk after the last treatment (+1). A time-related, statistically significant decrease is observed in the ultrasound-treated skin areas as compared with the sham-treated controls. Two-way analysis of variance and Bonferroni’s posttest: n = 3, *P < 0.001.
DISCUSSION
The present findings indicate that ultrasound-induced cavitation of human skin, achieved by Med2Contour ultrasound device, can yield a substantial reduction of subcutaneous fat and adipose cell size, confirming the previous clinical observations of a marked liporeductive effect of this technique.8,14 This study also provides circumstantial evidence for the mechanism of action of ultrasounds on adipocytes. The histomorphometrical data indicate that exposure of full-thickness skin explants to 2 short ultrasound cycles (6 s each), with a similar energy output to that used for clinical purposes, resulted in a statistically significant shrinkage of subcutaneous adipocytes (−23%, P < 0.001). The ultrastructural analysis provided a mechanistic explanation for this phenomenon. In fact, cavitation seemed to induce destabilization of adipocyte cytoplasm and plasma membrane enveloping the lipid vacuole, possibly by coalescence of pinocytosis vesicles or generation of multivesicular clusters from the plasma membrane. In turn, this phenomenon causes focal ruptures of the adipocyte cytoplasm, approximately 0.5–1.5 µm in diameter, which allow leakage of triglyceride droplets from the lipid vacuole to the extracellular space. Consistent findings were observed in the adipose tissue biopsies taken from patients previously treated with ultrasound-induced cavitation. In particular, the mean size of subcutaneous adipocytes was markedly and significantly reduced as compared with the sham-treated areas. This effect was remarkable 1 day after the last treatment and remained well appreciable after 12 days. Ultrastructurally, images of triglyceride leakage from adipocytes were no longer observed in any of the patients under study, regardless tissue sampling was done 1 or 12 days after ultrasound delivery. However, the treated adipocytes showed ultrastructural features consistent with triglyceride emptying and scattered lipid droplets were often detected in the adipose stroma.
Of note, in both the ex vivo and in vivo experiments, lipid discharge was not accompanied by any morphological signs of adipocyte death and disruption or interstitial inflammation. Moreover, the noted cavitation-induced effects seem to be restricted to adipocytes, while blood microvascular cells—endothelial cells, pericytes, and mast cells—showed normal features, as in the sham-treated controls. These findings suggest that ultrasound-induced cavitation, at appropriate settings and timing, does not create local adverse conditions that may favor tissue injury and subsequent inflammatory/fibrotic reaction, in keeping with recent observations in swine skin in vivo.15 On the other hand, the integrity of the vascular components of the adipose tissue can favor the removal of interstitial fat droplets and putative proinflammatory mediators released from adipocytes, conceivably by lymphatic drainage.16
The above histomorphometrical and ultrastructural observations fit well with the results of triglyceride assay in the incubation medium of the ex vivo skin explants, indicating that ultrasound-induced cavitation causes a statistically significant release of triglycerides from the adipose tissue into the interstitial fluid, as previously reported.15,16
The present findings are in keeping with those of previous in vivo porcine studies with both Contour I and Med2Contour, in which ultrasound-induced cavitation was shown to cause selective adipose cell reduction without injury to skin, vessels, nerves, or connective tissue.7,15 Similar to the present findings, ultrasound treatment was reported to induce the formation of multiple small pores in the adipocytes, allowing dispersion of triglycerides into interstitial space and lymphatic vessels.7,15 It is conceivable that triglycerides can then be absorbed and metabolized by endogenous lipases to glycerol and free fatty acids and incorporated in the total lipoprotein pool. Of note, serum lipids were unchanged8,12,15 or slightly elevated, but still within the normal range,14 in experimental animals and in patients subjected to liporeductive ultrasound treatments, accounting for substantial safety of this procedure from a metabolic viewpoint. At variance with a previous report,16 we did not observe any signs of disarrangement of adipose tissue collagen network or induction of adipocyte apoptosis, but this discrepancy is reasonably due to the far longer (10 min) exposure of the skin samples to ultrasounds adopted in the noted study.16
CONCLUSIONS
In conclusion, this study further strengthens the current view that noninvasive transcutaneous ultrasound cavitation, one of the most sought-after plastic and aesthetic surgical procedures, is a promising technology for localized reduction of fat. Generalization of the meaning of our study is hampered by the fact that we enrolled a limited number of patients; however, the consistency of the observed findings provides support to the notion that Med2Contour, owing to its unique design yielding a weakly focused ultrasound field within the subcutaneous fat pad, can be an effective and safe tool for liporeductive purposes.
ACKNOWLEDGMENTS
We acknowledge Dr. Moreno Naldoni, MSEE, for kindly providing the Med2Contour device, and Dr. Anna Paola Cellai, DSc, for skillful help in triglyceride assay.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by General Project Ltd., Montespertoli, Italy.
REFERENCES
- 1.Housman TS, Lawrence N, Mellen BG, et al. The safety of liposuction: results of a national survey. Dermatol Surg. 2002;28:971–978. doi: 10.1046/j.1524-4725.2002.02081.x. [DOI] [PubMed] [Google Scholar]
- 2.Coleman KM, Coleman WP, III, Benchetrit A. Non-invasive, external ultrasonic lipolysis. Semin Cutan Med Surg. 2009;28:263–267. doi: 10.1016/j.sder.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Neira R, Arroyave J, Ramirez H, et al. Fat liquefaction: effect of low-level laser energy on adipose tissue. Plast Reconstr Surg. 2002;110:912–922; discussion 923. doi: 10.1097/00006534-200209010-00030. [DOI] [PubMed] [Google Scholar]
- 4.Jackson RF, Dedo DD, Roche GC, et al. Low-level laser therapy as a non-invasive approach for body contouring: a randomized controlled study. Lasers Surg Med. 2009;41:799–809. doi: 10.1002/lsm.20855. [DOI] [PubMed] [Google Scholar]
- 5.Manuskiatti W, Wachirakaphan C, Lektrakul N, et al. Circumference reduction and cellulite treatment with a Tri-Polar radiofrequency device: a pilot study. J Eur Acad Dermatol Venereol. 2009;23:820–827. doi: 10.1111/j.1468-3083.2009.03254.x. [DOI] [PubMed] [Google Scholar]
- 6.de Felipe I, Del Cueto SR, Pérez E, et al. Adverse reactions after nonablative radiofrequency: follow-up of 290 patients. J Cosmet Dermatol. 2007;6:163–166. doi: 10.1111/j.1473-2165.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- 7.Zocchi ML. Clinical aspects of ultrasonic liposculpture. Perspect Plast Surg. 1993;7:153–174. [Google Scholar]
- 8.Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety and efficacy of the Contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779–789; discussion 790. doi: 10.1097/01.prs.0000270840.98133.c8. [DOI] [PubMed] [Google Scholar]
- 9.Brown SA, Greenbaum L, Shtukmaster S, et al. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124:92–101. doi: 10.1097/PRS.0b013e31819c59c7. [DOI] [PubMed] [Google Scholar]
- 10.Ascher B. Safety and efficacy of UltraShape Contour I treatments to improve the appearance of body contours: multiple treatments in shorter intervals. Aesthet Surg J. 2010;30:217–224. doi: 10.1177/1090820X09360692. [DOI] [PubMed] [Google Scholar]
- 11.Fatemi A, Kane MA. High-intensity focused ultrasound effectively reduces waist circumference by ablating adipose tissue from the abdomen and flanks: a retrospective case series. Aesthetic Plast Surg. 2010;34:577–582. doi: 10.1007/s00266-010-9503-0. [DOI] [PubMed] [Google Scholar]
- 12.Jewell ML, Baxter RA, Cox SE, et al. Randomized sham-controlled trial to evaluate the safety and effectiveness of a high-intensity focused ultrasound device for noninvasive body contouring. Plast Reconstruct Surg. 2011;128:253–262. doi: 10.1097/PRS.0b013e3182174278. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol. 2003;76:590–599. doi: 10.1259/bjr/17150274. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Moraga J, Valero-Altés T, Riquelme AM, et al. Body contouring by non-invasive transdermal focused ultrasound. Lasers Surg Med. 2007;39:315–323. doi: 10.1002/lsm.20478. [DOI] [PubMed] [Google Scholar]
- 15.Garcia O, Jr, Schafer M. The effects of nonfocused external ultrasound on tissue temperature and adipocyte morphology. Aesthet Surg J. 2013;33:117–127. doi: 10.1177/1090820X12469627. [DOI] [PubMed] [Google Scholar]
- 16.Palumbo P, Cinque B, Miconi G, et al. Biological effects of low frequency high intensity ultrasound application on ex vivo human adipose tissue. Int J Immunopathol Pharmacol. 2011;24:411–422. doi: 10.1177/039463201102400214. [DOI] [PubMed] [Google Scholar]


