Abstract
Background:
Conventional autologous skin grafts are associated with significant donor-site morbidity. This study was conducted to determine feasibility, safety, and efficacy of a new strategy for skin grafting based on harvesting small columns of full-thickness skin with minimal donor-site morbidity.
Methods:
The swine model was used for this study. Hundreds of full-thickness columns of skin tissue (~700 µm diameter) were harvested using a custom-made harvesting device, and then applied directly to excisional skin wounds. Healing in donor and graft sites was evaluated over 3 months by digital photographic measurement of wound size and blinded, computer-aided evaluation of histological features and compared with control wounds that healed by secondary intention or with conventional split-thickness skin grafts (STSG).
Results:
After harvesting hundreds of skin columns, the donor sites healed rapidly without scarring. These sites reepithelialized within days and were grossly and histologically indistinguishable from normal skin within 7 weeks. By contrast, STSG donor sites required 2 weeks for reepithelialization and retained scar-like characteristics in epidermal and dermal architecture throughout the experiment. Wounds grafted with skin columns resulted in accelerated reepithelialization compared with ungrafted wounds while avoiding the “fish-net” patterning caused by STSG.
Conclusion:
Full-thickness columns of skin can be harvested in large quantities with negligible long-term donor-site morbidity, and these columns can be applied directly to skin wounds to enhance wound healing.
Skin wounds that are too extensive to heal by primary closure often require reconstruction by autologous grafting or flap transplantation,1 which in turn requires harvesting of donor skin, causing morbidities—including pain, risk of infection, discoloration, and scarring—that are frequently more troublesome for patients than the primary wounds themselves.2,3 Split-thickness skin grafting (STSG), the current “gold standard” for closing large skin wounds,4 involves harvesting the epidermis and the upper portion of dermis from donor sites, and then transplanting the grafts onto the wound(s). STSGs are generally less damaging to donor sites (although donor-site morbidities are still substantial), and have higher “take” rates, than full-thickness grafts. However, because deep dermal structures such as hair follicles and sweat glands are not harvested, the STSG is aesthetically and functionally abnormal and scar-like. In addition, STSGs are usually meshed and expanded before grafting, which increases area coverage and facilitates fluid drainage but also causes a permanent, unsightly “fish-net” appearance in the grafted skin. Much effort has been invested in finding/developing exogenous graft materials, such as cadaveric skin, xenografts, and artificial skin substitutes.5 At present, these options can only provide temporary wound coverage, and eventual autologous grafting remains necessary. Alternative wound closure technologies that minimize donor-site morbidity, provide STSG-like graft reliability without unsightly texture, and can be performed easily in practice would be very beneficial.
Scarring at the donor and/or graft site is one of the most problematic morbidities associated with autologous skin grafting. Scarring is nature’s way of quickly filling large voids in tissue, with a haphazard arrangement of connective tissue elements. Scar tissue is stiff, often painful, dysfunctional, and tends to contract over time, causing deformities. The arrangement of extracellular matrix and specialized dermal structures that confer function to normal tissue is missing.6 In contrast to scarring, a process that haphazardly replaces grossly missing tissue, remodeling is a process that replaces tissue while maintaining tissue architecture on the microscopic scale. Remodeling occurs in every organ as we age and grow, and it is abundantly clear that almost every tissue has the capacity for local remodeling without scarring. While scarring is stimulated by large-scale tissue damage, remodeling is stimulated by microscopic tissue damage. This principle became clear when we developed fractional photothermolysis (FP),7 which is now in widespread clinical use to improve photoaged skin and various lesions including wound scars.8 In FP, laser microbeams are used to produce hundreds to thousands of microscopic thermal burns per cm2 of skin surface, creating very thin columns of thermal damage or tissue removal, called “microthermal zones.” Microthermal zones less than about 300 µm in diameter heal rapidly without scarring.9 The differences between FP and third-degree skin burns are striking. Both involve extensive, full-thickness (ie, including complete epidermis and dermis) thermal damage, but third-degree burns heal slowly with scarring, whereas after FP the epidermis closes within 1 day, and the dermal injury is repaired in about 2 weeks, followed by continued tissue remodeling without scarring.9 Amazingly, up to 50% of the volume of normal skin can be killed or removed by FP—followed by rapid replacement of the lost tissue by remodeling, resulting in new skin tissue that is both functionally and aesthetically normal.9
Since our experience with FP established that millions of small, full-thickness columns of skin tissue can be removed without causing scarring, we hypothesized that similarly large numbers of full-thickness microscopic skin tissue columns (MSTCs) could be harvested from healthy skin with negligible donor-site morbidity and that these MSTCs could serve as a graft to improve skin wound healing. The current study was performed to test these hypotheses in the swine, a commonly used model for human skin.10,11
METHODS
Harvesting Device
Harvesting needles were produced by honing standard hypodermic needles to have 2 cutting edges. When the needle is inserted through full-thickness skin and withdrawn, a column of tissue is extracted. A fluidic device was then constructed, in which each extracted column is removed from the harvesting needle by negative pressure, and transported through a tube of flowing air and normal saline into a collection basket.
Animal Procedures
All animal procedures were performed in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. Under general anesthesia and aseptic conditions, a series of 1.5 × 1.5 cm2, full-thickness skin wounds were produced on adult female Yorkshire swine (~50 kg) by excising skin down to the panniculus carnosus. Wound sites were separated from each other by at least 3 cm of unwounded skin. The corners and sides of each wound were tattooed for wound margin identification. In clinical settings, depending on the characteristics of the individual wound, skin grafts are either applied to freshly excised wound beds or to granulation tissue formed days to weeks after the initial wounding event. To model these 2 scenarios, 2 sets of wound conditions were tested: one set of wounds was grafted immediately after wound excision, while a second set was allowed to granulate for 1 week, then debrided and grafted (Table 1). The effects of applying MSTCs to the wound beds were compared to controls of 1) ungrafted wounds (negative control); and 2) meshed STSG grafted onto similar full-thickness wounds (positive control). Wound treatments were randomly assigned, with different treatments placed in adjacent sites to minimize intra-animal variation. MSTCs were harvested from distant skin sites of the same animal, using the harvesting device described above, with 19G harvesting needles. To enable subsequent identification of MSTC harvest sites, sterile India ink was applied to some of the MSTC donor sites at the time of harvesting. Fifty MSTCs, corresponding to ~14% of the excised tissue mass, were spread evenly over each wound surface (Fig. 1A). STSGs were prepared by harvesting split-thickness skin tissue from the same animal using an electric-powered dermatome (Nouvag USA, Lake Hughes, CA), set to a cutting depth of 0.55 mm. The split-thickness skin was meshed, cut into 1.5 × 1.5 cm2 pieces, and sutured onto wound beds (Fig. 1B). Eight repeats were performed for each set of experimental conditions. The excision of full-thickness skin and the preparation and grafting of STSGs were performed by an experienced plastic surgeon (Y.W.), following standard surgical practice. The wounds were dressed with ADAPTIC Non-Adhering Dressing (Systagenix, Gargrave, North Yorkshire, UK), TELFA (Tyco Healthcare, Mansfield, MA), and Tegaderm film dressing (3M Health Care, St. Paul, MN). The wounds were inspected and photographed weekly, and skin biopsies were collected at various times for histological study.
Table 1.
Experimental Groups
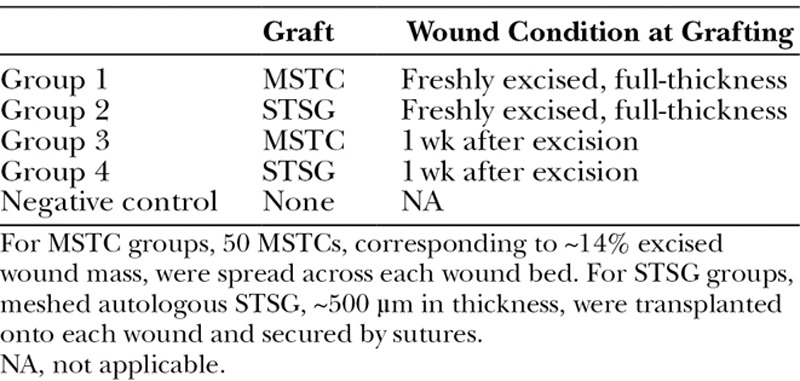
Fig. 1.
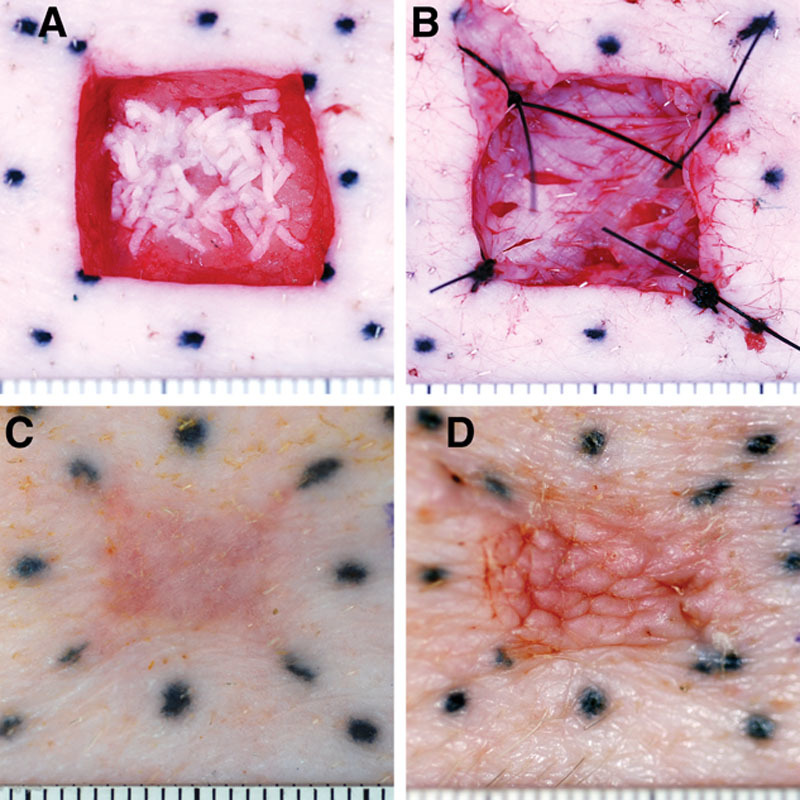
Full-thickness wound model. Full-thickness wounds were treated by spreading MSTCs evenly over the wound beds (A) or with conventional STSGs (B). Wound sites were marked by a series of tattoos (black dots in each image). C and D, Photographs of MSTC- and STSG-treated wounds, respectively, taken 5 wk later. STSG-treated wounds healed with marked creases on their surfaces (D) due to the meshing pattern of the grafts, whereas MSTC-treated wounds healed with a smooth, even surface (C). Each mark on the rulers spans 1 mm.
Histology
Fresh tissue samples were fixed in 4% formaldehyde, embedded in paraffin, and then cut into 5 µm sections and stained with hematoxyin and eosin (H&E) for general histology, Gömöri’s Trichrome stain for collagen, or Verhoeff’s stain for elastin. Expression of alpha-smooth muscle actin (α-SMA) was determined by immunohistochemistry: tissue sections were deparaffinized in CitriSolv (Fisher Scientific, Pittsburgh, PA), rehydrated through graded alcohol washes, incubated in Cytomation Target Retrieval solution (DAKO, Carpinteria, CA) at 98°C for 30 minutes for antigen retrieval, permeabilized with 0.1% Triton X-100 in Tris-buffered saline for 15 minutes, blocked with a solution of 10% goat serum, 3% bovine serum albumin, 0.1% Tween-20 in Tris-buffered saline for 30 minutes to minimize nonspecific binding, and incubated with a rabbit anti-α-SMA antibody (ab5694, 1:100 dilution, Abcam, Cambridge, MA) overnight at 4°C. Binding of the primary antibody was detected with the MACH 2 Rabbit AP-Polymer and the Vulcan Fast Red Chromogen Kit (Biocare Medical, Concord, CA), according to the manufacturer’s instructions, followed by counterstaining with hematoxylin.
Quantification and Statistical Analysis
Wound sites were photographed weekly, with a ruler included in every image to enable quantification of wound dimensions. Wound areas were determined from the photographs using a custom-written MATLAB (MathWorks, Natick, MA) script.
Histological characteristics for donor wounds at 7 weeks postharvest were quantified using computer-assisted image analysis. For epidermal morphology, 1-mm-long regions of interest were selected randomly, and the number of rete ridges in each ROI was counted, by a dermatologist who was blinded toward experimental conditions, while the average epidermal thickness was calculated by measuring the cross-sectional area of the epidermis in each ROI using NDP.view (Hamamatsu, Bridgewater, NJ), then dividing by the ROI length (1 mm). For dermal cellularity and elastin content, 500 µm × 500 µm ROI were selected randomly, and the number of cells within each ROI was obtained by counting the number of nuclei, while the length of elastin fibers was determined by tracing the fibers within each ROI, then quantifying the total length using a custom-written MatLab script. At least 10 independent ROIs, with at least 1 mm separation from each other, were analyzed for each treatment group. The extent of dermal collagen disorganization was also evaluated using NDP.view: areas with disorganized collagen were measured and normalized either by the total cross-sectional skin area (for MSTC donor) or by the cross-sectional area of the top 550 µm of skin (for STSG donor)—different bases for normalization were used because while MSTCs were harvested from the entire depth of the dermis, STSGs were only harvested to 550 µm deep. For each donor type, this analysis was aggregated over 300 mm2 of skin area from 7 independent tissue samples. Results between the different experimental groups were compared statistically using the Mann-Whitney U test.
RESULTS
Column Harvesting
Using our single-needle, fluid-assisted harvesting device, MSTCs were consistently harvested at a rate of approximately 1 Hz, that is, MSTCs needed to graft each 1.5 × 1.5 cm2 wound were harvested in about 1 minute. The diameter of MSTCs was controllable by choosing the diameter of the harvesting needle: 25 to 19G harvesting needles could be used to collect MSTCs ranging from 200 to 700 µm in diameter. For the rest of this study, we used MSTCs of 700 µm diameter harvested with 19G needles. Each MSTC consisted of full-thickness skin tissue including epidermis, dermis, and some subcutaneous fat—with the dermal layer containing a random sampling of adnexal structures.
Wound Healing
Wounds that were left ungrafted contracted rapidly, which is characteristic of full-thickness skin wounds in swine (and most other commonly used animal models, but not in humans). In the swine model, wound contraction is inhibited when a full epithelium forms. Implantation of MSTCs and STSGs both significantly slowed the rate of wound contraction (Table 2), indicating that epithelial coverage was restored sooner in these wounds than the untreated wounds. This finding was confirmed by histology showing rapid reepithelialization of the wounds grafted with either MSTCs or STSGs (Fig. 2). Consistent with rapid proliferation, the epidermis in MSTC-treated wounds was initially thickened and lacked rete ridges at earlier time points, then normalized over time (Fig. 2C) to produce an apparently normal epidermis.
Table 2.
Changes in Wound Area for the Different Treatments, Expressed as % Area of the Same Wound at Time of Grafting
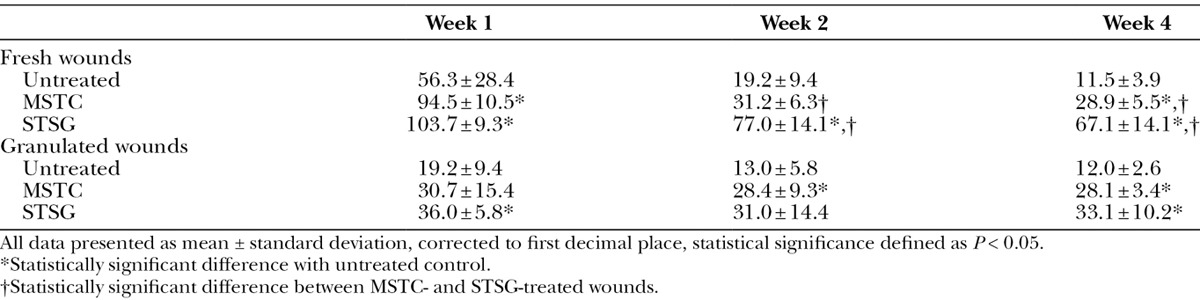
Fig. 2.
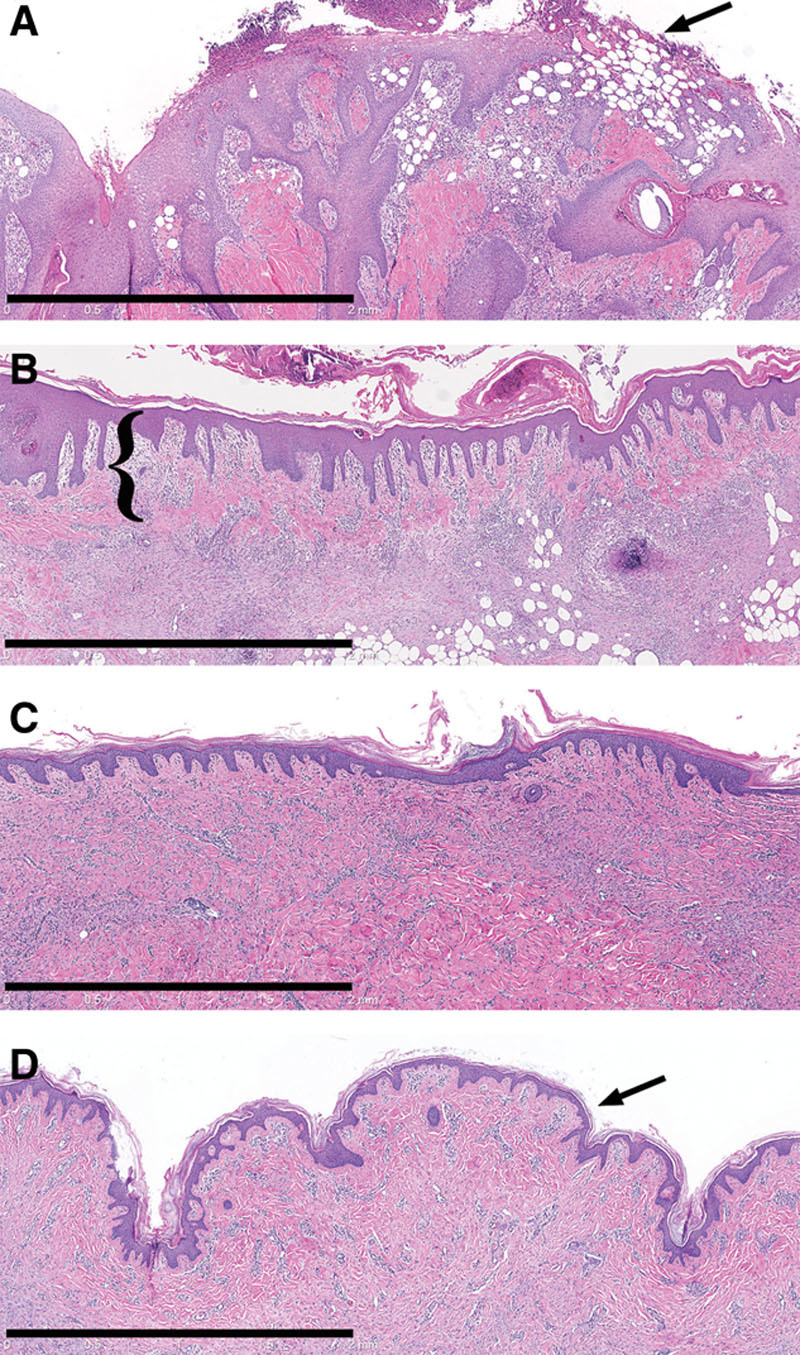
Histology (H&E) of wounds treated with MSTCs (A, C) or STSG (B, D), at 1 (A, B) and 5 wk (C, D) after treatment. One week after treatment, wounds treated with MSTCs were partially reepithelialized, with intermittent gaps still remaining in the new epithelial layer (A, arrow) at the wound surface. By contrast, STSG provided immediate coverage (B, STSG highlighted by bracket), with granulation tissue developing under the graft. By 2 wk after treatment, MSTC-treated wounds were also completely reepithelialized, and epidermal remnants were no longer seen in the dermal region (not shown). The uneven surface that is characteristic of meshed STSGs were also evident in histology as prominent folds in the epidermal and upper dermal layers (D, arrow), and these persisted through the course of the experiment. Scale bars: 2 mm.
Wounds treated with meshed STSGs showed conspicuous creases at the skin surface that were apparent both grossly (Fig. 1D) and histologically (Fig. 2D). This creasing pattern gradually decreased over time, but was still evident by the end of the 11-week experiment. By contrast, MSTC-treated wound sites healed with a smooth skin surface texture (Fig. 1C). Figures 1 and 2 represent results from wounds treated immediately after excision—similar results were obtained from wounds that were treated after 1 week of granulation, except that reepithelialization was faster (completed within 1 wk) in MSTC-treated granulated wounds than in fresh wounds, and the surface creasing was less pronounced in STSG-treated granulated wounds. Other than the differences in surface profile, wound sites grafted with STSG and MSTC had similar histological features at the later time points: the epidermis appeared normal and dermal collagen was organized in crisscrossing bundles, but even at the latest data point (11 wk) there was no gross or histological evidence of adnexal structures, which is characteristic of scars.
Donor Sites
The small wounds caused by MSTC harvesting healed rapidly, without scarring. One week after harvesting, a small, punctate crust was present at each site where an MSTC was harvested. Thereafter, only mild erythema marked the MSTC donor sites, which otherwise had the appearance of normal skin. By 7 weeks after harvesting, the MSTC donor sites were no longer distinguishable by magnified gross inspection from untreated skin (Fig. 3). The MSTC donor wounds had completely reepithelialized within the first week, forming a new epidermal layer with prominent rete ridges (Fig. 4A). There was a minimal and perivascular dermal inflammatory infiltrate, consisting mainly of mononuclear cells, without apparent neovascularization or granulation tissue. By 7 weeks, the MSTC donor sites exhibited histological features of normal skin (Table 3): epidermal thickness and number of rete ridges in the MSTC donor region were the same as in untreated skin, while the dermal portion of the MSTC donor region showed collagen fibers arranged in the “basket-weave” pattern typical of normal dermal collagen (Fig. 4C). There was a small but statistically significant increase in cellularity at the MSTC harvesting sites. Fine elastin fibers were much more numerous in MSTC harvested sites than in STSG harvested sites (Figs. 4E, F). α-SMA expression in the dermal region of MSTC donor sites was sparse and largely limited to perivascular cells (Fig. 4G).
Fig. 3.
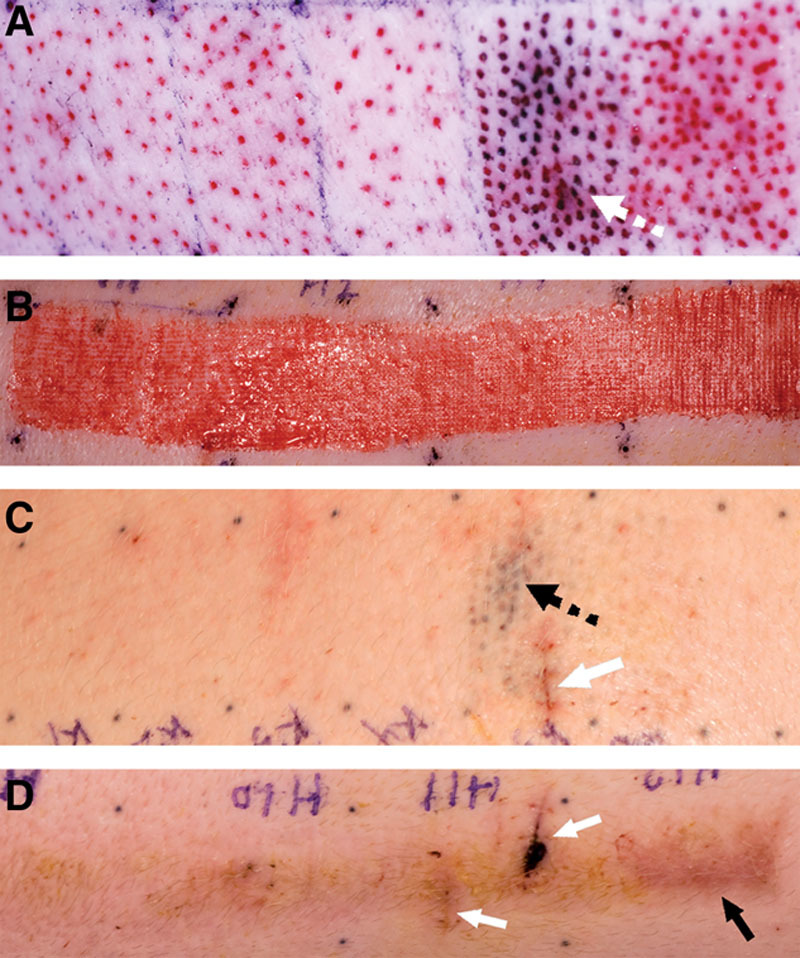
Gross appearance of donor sites for MSTC (A, C) and STSG (B, D) immediately after tissue harvesting (A, B) and 7 wk later (C, D). A portion of MSTC donor site was marked with Tattoo ink (A, C, dotted arrows). Donor-site edges were also marked by tattoos. MSTC donor sites healed quickly and were visually indistinguishable from surrounding skin by 7 wk after harvesting (C). The STSG donor site healed more slowly, and while its gross appearance also improved over time, it remained raised, stiff, and discolored (D, black arrow), compared to adjacent skin, through the course of the experiment. White arrows in C and D mark sites where biopsies were previously taken.
Fig. 4.
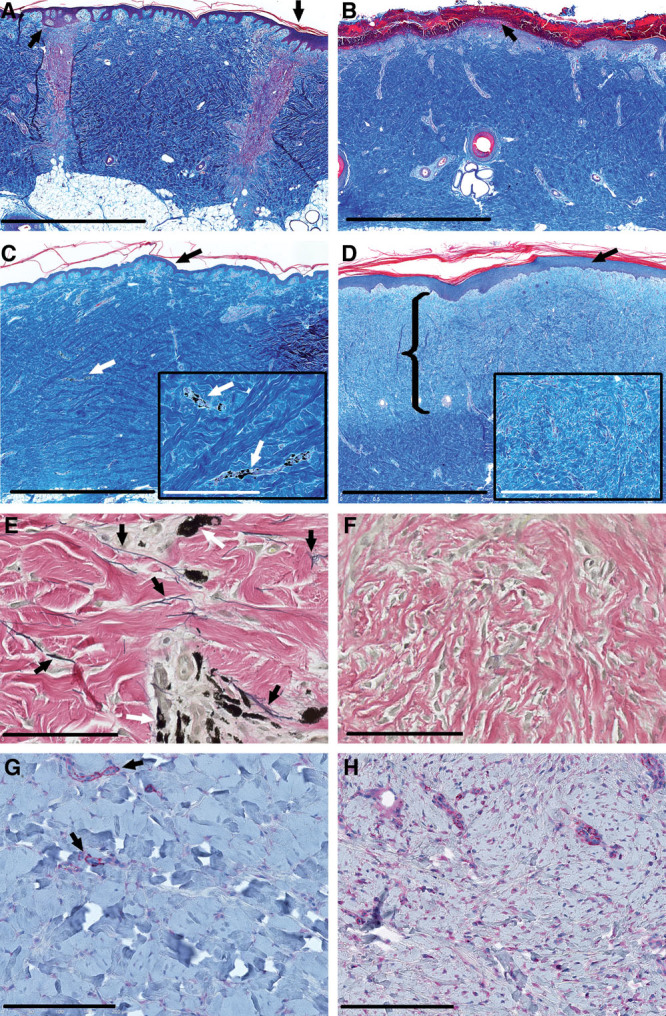
Histology of MSTC and STSG donor sites. Gömöri’s Trichome stain was used to enhance the visualization of dermal collagen (A–D), Verhoeff’s stain was used to identify elastin fibers (E, F), and immunohistochemical staining against α-SMA was used to evaluate myofibroblast infiltration (G, H). MSTC donor sites were completely reepithelialized within 1 wk, with prominent rete ridges in the epidermal layer (A, arrows). By contrast, most of the STSG donor site was covered by hemorrhagic crusting, with no epithelial coverage 1 wk after harvesting (B, arrow). The STSG donor site was reepithelialized after 2 wk (not shown). By 7 wk after harvesting, the MSTC donor sites (identified by tattoos, C, E white arrows) appeared to have recovered normal skin microarchitecture, including epidermis with prominent rete ridges (C, black arrow), collagen bundles with the characteristic “basket-weave” architecture (C, inset), and numerous elastin fibers (E, black arrows), while α-SMA expression was sparse, and mostly limited to perivascular cells (G, red color, highlighted by arrows). At the same 7-wk time point, the STSG donor site had a thickened epidermis with underdeveloped rete ridges (D, arrow), disorganized collagen (D, inset), hypercellularity, a near complete absence of elastin fibers (F), and extensive infiltration by α-SMA+ myofibrobasts (H, red color) in a substantial portion of the dermal layer (D, area highlighted by bracket). C and D (insets), Higher-powered views in the same area. The scar-like layer of the STSG donor site was diminished in thickness but still present after 11 wk (not shown). Scale bars: A–D: 2 mm; C, D insets: 400 µm; E, F: 100 µm; G, H: 200 µm.
Table 3.
Quantitative Comparison of Donor-site Histological Features
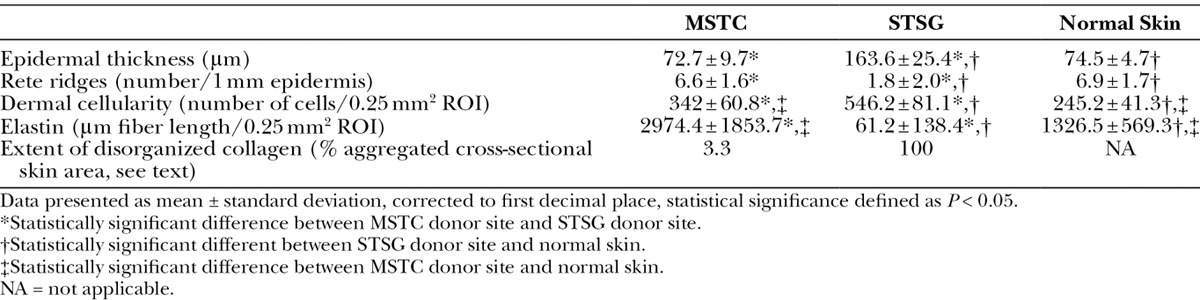
The STSG donor site healed much slower than MSTC donor sites, and with scarring, both grossly and histologically. The STSG donor site had pronounced hemorrhagic crusting and lacked epidermal coverage after 1 week (Fig. 4B) and remained grossly distinguishable from the surrounding untreated skin through the 11-week course of this experiment due to persistent discoloration (Fig. 3D), raised profile, and stiffness to the touch (not shown). Histological features in the STSG donor site, 7 weeks after harvesting, were consistent with scarring (Table 3): a hyperkeratotic epidermis that was over twice the normal thickness, loss of structure at the dermoepidermal junction with less than one-third the normal density of rete ridges (Fig. 4D), disorganized dermal collagen (Fig. 4D), marked hypercellularity, an almost complete absence of elastin fibers (Fig. 4F), and extensive infiltration by α-SMA+ myofibroblasts (Fig. 4H). This scar-like layer was most prominent at earlier time points, then gradually decreased in thickness, but was still present in the upper 500 µm (similar to the STSG thickness at initial harvest) of the dermal layer by the end of the 11-week experiment (not shown).
DISCUSSION
This is the first description of the harvesting and use of MSTCs as an alternative to STSGs for wound repair. We also describe for the first time a fluid-assisted device for MSTC harvesting and show that it is possible to harvest large amounts of full-thickness skin tissue without scarring in the donor site. There are several advantages to this approach. There is minimal morbidity in the MSTC donor site, which heals rapidly without scarring—this result is consistent with previous reports that large numbers of small skin wounds can heal scarlessly after fractional laser resurfacing9 or micromechanical fractional skin rejuvenation.12 By contrast, the STSG donor site retained characteristics of scarring: a “flat” dermal-epidermal junction, nearly complete absence of elastin fibers, hypercellularity, disorganized collagen, and extensive infiltration by α-SMA+ myofibroblasts.6,13 Another advantage over STSG is the lack of the expanded-mesh-graft pattern that persists in STSG-grafted wounds, which causes a permanent disfiguring “fish-net” appearance in many patients. MSTC grafting is similar in some ways to several traditional skin grafting techniques, including pinch grafting,14 and minced skin grafting—a technique in use since the 1950s, in which conventionally harvested skin grafts are minced into fine pieces and applied to wounds.15–18 However, MSTC harvesting greatly reduces donor-site morbidity and potentially could transplant deep dermal tissue and adnexal structures. The wound sites in this study are smaller than the clinical wounds that typically require grafting. But we expect the general principle—that micron-size columns of skin tissue can contribute to, and accelerate, the process of wound reepithelialization—to be reproducible in larger wounds, especially because it has been demonstrated that minced grafts can be used successfully to treat large wound areas.15,19 We plan to perform human studies in the near future to identify optimal treatment parameters (MSTC diameter, harvesting and seeding densities, etc.) and to identify clinical wound care settings where MSTCs could replace STSG or where MSTCs could be used to augment healing in wounds that are typically unsuitable for grafting (eg, some postoperative wounds and chronic ulcers). The ease with which MSTCs can be harvested suggests that a “bedside” procedure, preformed with local anesthesia followed by simple wound care, can be performed.
There was an apparent lack of adnexal structures in MSTC-grafted skin wounds, even though the MSTCs included random fragments of skin adnexa from the donor site. Simply increasing the seeding density of MSTCs may increase the probability of transferring viable adnexal structures because in this study MSTCs placed in each wound corresponded to only 14% of the removed tissue mass. Terminal hair follicles are much larger in swine than in humans, and the eccrine and apocrine glands are located very deeply in the porcine dermis; therefore, in this study, we did not expect to transfer intact adnexa although that may not be necessary: a well-defined population of epithelial stem cells is located in the outer root sheath of hair follicles,20 and under favorable conditions, wounds can activate neogenesis of hair follicles.21 Fractional laser treatment has also been reported to induce hair growth in humans.22 Even if complete recapitulation of adnexal structures ultimately proves to be impossible with this technique, the transfer of dermal elements is still likely to be beneficial because components of adnexal structures (eg, follicular stem cells and sweat gland ductal cells) are known to contribute to wound healing.23,24
In this study, MSTCs were placed randomly into wounds without maintaining the epidermis-side-up orientation of normal skin tissue, which caused pieces of epidermis to be present (improperly) under the skin surface at the 1-week time point. These incorrectly oriented tissue fragments were no longer detectable by the second week (no evidence of cyst/milia formation was observed)—they may have been absorbed during the wound remodeling process; alternatively, the epithelial cells may have migrated toward the positive oxygen gradient. This finding is an interesting area for further studies as it is consistent with previous studies reporting that similar outcomes could be achieved by minced skin grafts regardless of the orientation of the grafted tissue.16,19
ACKNOWLEDGMENTS
We thank Dr. Garuna Kositratna and Ms. Maura Williams for excellent technical support and Dr. Jenny Zhao and the Wellman Center Photopathology Core for assistance with histological analysis.
Footnotes
Drs. Tam and Wang contributed equally to this work.
Part of the study described in this article was presented at the 2012 Military Health System Research Symposium (Fort Lauderdale, Fla.).
Disclosure: Drs. Tam, Jiménez-Lozano, Franco, Sakamoto, Purschke, and Anderson and Mr. Farinelli are inventors in patent applications filed from MGH, based on the technology described in this article. This study was supported, in part, by the Defense Advanced Research Projects Agency (DARPA) and Army Research Office (ARO) (contract nos. W911NF-11-1-0122 and W911NF-12-1-0086). The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Zhang AY, Meine JG. Flaps and grafts reconstruction. Dermatol Clin. 2011;29:217–230, viii. doi: 10.1016/j.det.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Demirtas Y, Yagmur C, Soylemez F, et al. Management of split-thickness skin graft donor site: a prospective clinical trial for comparison of five different dressing materials. Burns. 2010;36:999–1005. doi: 10.1016/j.burns.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Voineskos SH, Ayeni OA, McKnight L, et al. Systematic review of skin graft donor-site dressings. Plast Reconstr Surg. 2009;124:298–306. doi: 10.1097/PRS.0b013e3181a8072f. [DOI] [PubMed] [Google Scholar]
- 4.Brusselaers N, Pirayesh A, Hoeksema H, et al. Skin replacement in burn wounds. J Trauma. 2010;68:490–501. doi: 10.1097/TA.0b013e3181c9c074. [DOI] [PubMed] [Google Scholar]
- 5.Priya SG, Jungvid H, Kumar A. Skin tissue engineering for tissue repair and regeneration. Tissue Eng Part B Rev. 2008;14:105–118. doi: 10.1089/teb.2007.0318. [DOI] [PubMed] [Google Scholar]
- 6.Profyris C, Tziotzios C, Do Vale I. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J Am Acad Dermatol. 2012;66:1–10; quiz 11. doi: 10.1016/j.jaad.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 7.Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426–438. doi: 10.1002/lsm.20048. [DOI] [PubMed] [Google Scholar]
- 8.Saedi N, Petelin A, Zachary C. Fractionation: a new era in laser resurfacing. Clin Plast Surg. 2011;38:449–461, vii. doi: 10.1016/j.cps.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Laubach HJ, Tannous Z, Anderson RR, et al. Skin responses to fractional photothermolysis. Lasers Surg Med. 2006;38:142–149. doi: 10.1002/lsm.20254. [DOI] [PubMed] [Google Scholar]
- 10.Mawafy M, Cassens RG. Microscopic structure of pig skin. J Anim Sci. 1975;41:1281–1290. doi: 10.2527/jas1975.4151281x. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto FH, Tannous Z, Doukas AG, et al. Porphyrin distribution after topical aminolevulinic acid in a novel porcine model of sebaceous skin. Lasers Surg Med. 2009;41:154–160. doi: 10.1002/lsm.20734. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes JR, Samayoa JC, Broelsch GF, et al. Micro-mechanical fractional skin rejuvenation. Plast Reconstr Surg. 2013;131:216–223. doi: 10.1097/PRS.0b013e3182789afa. [DOI] [PubMed] [Google Scholar]
- 13.Sidgwick GP, Bayat A. Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol. 2012;26:141–152. doi: 10.1111/j.1468-3083.2011.04200.x. [DOI] [PubMed] [Google Scholar]
- 14.Davis JS. The small deep graft: relationship to the true reverdin graft. Ann Surg. 1929;89:902–916. doi: 10.1097/00000658-192906000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boggio P, Tiberio R, Gattoni M, et al. Is there an easier way to autograft skin in chronic leg ulcers? ‘Minced micrografts’, a new technique. J Eur Acad Dermatol Venereol. 2008;22:1168–1172. doi: 10.1111/j.1468-3083.2008.02737.x. [DOI] [PubMed] [Google Scholar]
- 16.Svensjö T, Pomahac B, Yao F, et al. Autologous skin transplantation: comparison of minced skin to other techniques. J Surg Res. 2002;103:19–29. doi: 10.1006/jsre.2001.6331. [DOI] [PubMed] [Google Scholar]
- 17.Meek CP. Successful microdermagrafting using the Meek-Wall microdermatome. Am J Surg. 1958;96:557–558. doi: 10.1016/0002-9610(58)90975-9. [DOI] [PubMed] [Google Scholar]
- 18.Simizu R, Kishi K, Okabe K, et al. Recruited minced skin grafting for improving the skin appearance of the donor site of a split-thickness skin graft. Dermatol Surg. 2012;38:654–660. doi: 10.1111/j.1524-4725.2011.02266.x. [DOI] [PubMed] [Google Scholar]
- 19.Hackl F, Bergmann J, Granter SR, et al. Epidermal regeneration by micrograft transplantation with immediate 100-fold expansion. Plast Reconstr Surg. 2012;129:443e–452e. doi: 10.1097/PRS.0b013e318241289c. [DOI] [PubMed] [Google Scholar]
- 20.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 22.Beachkofsky TM, Henning JS, Hivnor CM. Induction of de novo hair regeneration in scars after fractionated carbon dioxide laser therapy in three patients. Dermatol Surg. 2011;37:1365–1368. doi: 10.1111/j.1524-4725.2011.01934.x. [DOI] [PubMed] [Google Scholar]
- 23.Levy V, Lindon C, Zheng Y, et al. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 24.Lu CP, Polak L, Rocha AS, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


