Abstract
Non-motor symptoms are a key component of Parkinson's disease, possibly representing a clinical biomarker of its premotor phase. The burden of non-motor symptoms can define a patient's health-related quality of life. Non-motor symptoms substantially increase the cost of care—requiring increased hospitalisation and treatment—and pose a major challenge to healthcare professionals. However, clinicians often regard non-motor symptoms and their management as peripheral to that of the motor symptoms. Here, we address the clinical issues and unmet needs of non-motor symptoms in Parkinson's disease.
Keywords: PARKINSON'S DISEASE, NON MOTOR SYMPTOMS, NMSQuest
Introduction
I have Parkinson's. I would like you to address the following symptoms that bother me the most: sleep, pain and then my movement disorder.
A patient with Parkinson's disease, 10 October 2013
James Parkinson recognised the implications and importance of motor Parkinson's disease (PD) in 1817 and highlighted key non-motor symptoms, such as sleep dysfunction, dysautonomia, cognitive and neuropsychiatric issues. However, it was almost 150 years before the importance of the burden of non-motor symptoms on the lives of the people with Parkinson's and the carers became apparent. Many studies have addressed cognition, dementia, sleep disorders and depression in PD. More recently, holistic tools, such as the self-rated Non-Motor Symptoms Questionnaire (NMSQuest)1 and the Non-Motor Symptoms Scale (NMSS)2 3 have allowed clinicians to quantify the overall burden of these additional symptoms in PD and their impact on quality of life.
The problems
Clinicians frequently overlook non-motor symptoms and do not discuss important symptoms like depression, anxiety, fatigue and sleep disturbance.4 The UK's National Institute for Health and Care Excellence—a non-departmental public body of the Department of Health—has recognised non-motor symptoms and their management as an important area of unmet need in PD.5 However, despite their 2006 guidance, an international survey in 2010 showed that up to 62% of PD patients do not declare symptoms such as apathy, pain, sexual difficulty, bowel incontinence or sleep disorder, either through embarrassment or being unaware that their symptoms link to PD.6 Furthermore, clinicians themselves may not realise that these symptoms need addressing. Non-motor symptoms are common even at first presentation. In 2013, a German study assessed them (by the NMSQuest) in untreated PD patients at presentation: these symptoms presented a significant burden—even at this early untreated stage—compared with age-matched controls.7 A UK-based study reported similar findings, highlighting the important need for an awareness of non-motor symptoms right at the onset of ‘motor’ PD.8 Their under-reporting has important therapeutic and societal implications as most are treatable. Left untreated, non-motor symptoms detrimentally affect quality of life and frequently cause hospitalisation and institutionalisation,9 10 increasing the cost of PD care by fourfold.6
Initiatives to address management of non-motor symptoms
Parkinson's UK—the major UK-based patient charity—commissions an annual audit of Parkinson services. The 2011 report showed non-motor symptoms recorded in only 21% of elderly care and 9% of neurology services in the UK. This deficiency mainly relates to lack of time during consultation and also to not using self-declaration tools, which would allow patients to ‘flag’ symptoms. This outcome led to a national policy for data collection (motor and non-motor) of patients being referred to hospital.11
The neuropathological basis of non-motor Parkinson's
For the nigrostriatal dopaminergic disorder of PD, one pathological process clearly does not fit all! Jellinger stated that Parkinson's can no longer be considered a complex motor disorder characterised by extrapyramidal symptoms, but as a progressive multisystem disease—or more correctly, multiorgan disease—with variegated neurological and non-motor deficiencies.12
The traditional concept that the first neuropathological insult leading to PD is the degeneration of neuromelanin-containing neurones in the pars compacta of the substantia nigra (resulting in depleted levels of the dopamine) has been challenged. Many studies, spearheaded by the Braak theory, suggest that a non-dopaminergic process is key to the non-motor symptoms of PD, many of which start well before the motor Parkinson's features emerge.12–14 Interestingly, Friedrich Lewy (1913) first described Lewy bodies in the dorsal motor nucleus of the vagus, a site implicated in Braak stage 2.15
Table 1 summarises the growing evidence that in PD the degeneration of non-dopaminergic neurones occurs well before dopaminergic motor symptoms start. There is also clear evidence of differential neuronal degeneration involving several neuropeptide pathways in the brain in PD.13 14 Furthermore, there is neuropathological heterogeneity between early-onset and late-onset PD,13 which manifests clinically as subtypes within both motor PD and (more recently recognised) non-motor PD.16
Table 1.
Non-dopaminergic involvement in PD
| Evidence of non-dopaminergic involvement in PD | Implications on stage of PD | Author/year |
|---|---|---|
| Lewy bodies first described in non-dopaminergic neurones | Premotor and early motor | Forno, 199617 |
| Neuronal loss in dorsal motor nucleus of the vagus is as marked as in the substantia nigra | Premotor and early motor | Jellinger, 198718 Hirsch, 198719 Halliday, 199013 |
| Cholinergic pediculopontine nucleus neurones and substance P-containing neurones suffer 77% loss in dorsal motor nucleus of the vagus while tyrosine hydroxylase-immunoreactive neurones appear spared (<5% loss) | Premotor and early motor | Jellinger, 198718 Hirsch, 198719 Halliday, 199013 |
| Complete sparing of medullary dopaminergic neurones reported | Premotor and early motor | Saper, 199120 |
| Lewy body degeneration is prominent in the non-dopaminergic anterior olfactory nucleus | Premotor and early motor | Wakabayashi, 199721 |
| Non catecholaminergic neurones severely depleted in PD in the autonomic system: spinal intermediolateral nucleus 30–40% loss of preganglionic autonomic neurones | Premotor and early motor | Wakabayashi, 199721 |
| Lewy bodies are frequent in the vasoactive intestinal peptide neurones of the enteric nervous system but rare in catecholaminergic cells | Premotor and early motor | Wakabayashi, 199721 |
| Lewy bodies present in both tyrosine hydroxylase+and tyrosine hydroxylase—cells in the cardiac plexus | Premotor and early motor | Wakabayashi, 199721 Iwanaga, 199922 |
| Lewy body degeneration developing in lower brainstem neurones well before the substantia nigra | Premotor and early motor | Braak, 200315 |
| Incidental Lewy bodies identified within pontomedullary neurones in the absence of substantia nigra pathology, but not vice versa | Premotor and early motor | Braak, 200423 |
PD, Parkinson's disease.
Animal models addressing non-motor symptoms of PD are important. We discuss these in online supplementary material, and box 1 shows a snapshot of existing animal models.
Box 1. Parkinsonian animal models with possible expression of non-motor symptoms and exploration of pathophysiology.
6-OHDA lesioned rodents24
Sensorimotor
Olfaction
Sensory/pain threshold
Sleep/wakefulness
Circadian rhythms
Cognitive function
- Also possible to study:
- Altered cardiovascular function
- Bladder hyperactivity
- Altered motility of gastro-intestinal tract
α-Synuclein overexpressor (ASO=Thy1-aSYN) mice25
Olfaction
Autonomic
Constipation
Sleep
Cognition
MPTP-treated primates24
Bladder hyper-reflexia
Constipation
Drooling
Altered cardiovascular function
Sleep disturbance
Cognitive disturbance
Mice model of intragastric rotenone administration26
α-Synuclein accumulation in dorsal vagal nucleus
Potential for investigating autonomic symptoms such as constipation
Göttingen minipigs (Ellegaard Minipigs ApS)27
Cognition/sleep
OHDA, hydroxydopamine; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
What are the non-motor symptoms of PD?
James Parkinson referred to sleep disturbance, constipation, dysarthria, dysphonia, dysphagia, sialorrhoea, urinary incontinence and ‘at the last, constant sleepiness with slight delirium’ in his essay.28 The widespread neuropathology of PD gives a wide range of symptoms from gastrointestinal to sleep disorders, from cognitive to apathy and depression. In addition, some symptoms relate to drug therapy. It is thus difficult to ‘lump’ non-motor symptoms into a single category, and we suggest the following classification:
- Related to the disease process or pathophysiology
- Dopaminergic origin
- Non-dopaminergic origin
Related to a partial non-motor origin (usually brainstem autonomic impairment with motor end result, such as constipation or diplopia)
- Related to non-motor fluctuations (cognitive, autonomic and sensory subtypes)
- Fluctuating
- Constant
- Related to PD drug therapy
- Specific symptoms (eg, hallucinations, delirium)
- Syndromes—impulse control disorders, dopamine agonist withdrawal syndrome, Parkinson's hyperpyrexia syndrome (thermoregulatory failure, delirium)
- Possibly genetically determined
- Dementia in cases with glucocerebrosidase mutation
- Depression and sleep disorders in cases with leucine-rich repeat kinase-2 mutation
Some symptoms may overlap: for instance, hallucination as part of the advancing disease, or non-motor fluctuations in PD.
While some non-motor symptoms dominate in the early or even in the ‘premotor’ phase of PD, others complicate the clinical picture throughout the disease (pain, fatigue) and especially in its advanced stages (dementia, apathy, dysautonomia), as shown in the Sydney multicentre study report at 20 years.29 The ‘Parkinson's at risk syndrome’ study tries to identify the premotor non-motor risk factors for developing the motor syndrome of PD. There is now also an attempt to redefine PD, moving away from the typical brain bank-defined motor diagnostic criteria.30–32
PD can be divided into a preclinical phase (supported by molecular or imaging markers), a premotor phase (with non-motor symptoms, table 2) and the motor phase, the ‘tip of the iceberg’. The key is to develop robust biomarkers and also to define the specific predictive value of the premotor non-motor symptoms. Table 2 shows examples of non-motor symptoms reported in the premotor stage.
Table 2.
Non-motor symptoms in the premotor PD
| Commonly associated—with reasonable evidence base | |
| Hyposmia (usually of late onset and idiopathic) | 10 times increase in risk of developing PD;+abnormal DATScan—43% develop motor PD in 4 years33 |
| Rapid eye movement sleep behaviour disorder | 25–40% risk of developing a synucleinopathy at 5 years; 40–65% risk of developing a synucleinopathy at 10 years34 |
| Constipation | 2.7–4.5 times increased risk of PD35 |
| Depression | 2.4 times increased risk of developing PD36 |
| Described associations | |
| Excessive daytime sleepiness | 3.3 times increased risk of PD37 |
| Fatigue (a sense of exhaustion as opposed to sleepiness) | In 45%—a premotor symptom38 |
| Pain (often unilateral and in affected limb) | 34% increased risk of PD39 |
| Erectile dysfunction | 3.8 times increased risk of PD40 |
PD, Parkinson's disease.
Epidemiology
The NMSQuest and the NMSS each assesses their global burden in PD. Table 3 lists the studies that have used them to assess non-motor symptoms in unselected PD populations across the world and shows the importance of these symptoms in PD irrespective of cultural background, racial origin and hospital settings. Three controlled studies compared NMSQuest data in PD with age-matched healthy controls: all show that while some symptoms, such as insomnia, urinary difficulties and memory issues, are common in ‘normal’ subjects, the severity and frequency of these non-motor symptoms are more severe in PD. Furthermore, some symptoms, such as fatigue, dribbling of saliva and excessive daytime sleepiness, are highly significantly more common in PD.1 7 8
Table 3.
| Non-motor symptoms | Mean (%) | Range (%) |
|---|---|---|
| Cognitive | ||
| Memory | 45.8 | 37.9–62.5 |
| Concentration | 38.7 | 29.6–50.0 |
| Depression | ||
| Sadness | 42.5 | 22.5–56.0 |
| Anxiety | 43.4 | 30.7–55.8 |
| Sleep | ||
| Excessive daytime somnolence | 30.5 | 21.2–37.1 |
| Insomnia | 40.9 | 17.6–52.5 |
| REM sleep behaviour disorder | 34.2 | 29.6–38.7 |
| Restless legs syndrome | 35.8 | 27.7–41.1 |
| Fatigue | 41.5 | 31.1–58.1 |
| Pain | 31.1 | 18.2–45.9 |
| Gastrointestinal | ||
| Swallowing | 25.4 | 16.1–30.3 |
| Constipation | 46.5 | 27.5–71.7 |
| Urinary | ||
| Urgency | 53.4 | 35–61 |
| Nocturia | 53.8 | 26.4–66.7 |
| Global comparison | ||
| NMSQ-PD | 8.3 | 4–19 |
| NMSQ-C | 3.5 | 2–12 |
Data taken from studies by Chaudhuri et al;1 Chaudhuri et al;6 Mollenhauer et al;7 Khoo et al;8 Martinez-Martin et al;41 Barone et al;42 Crosiers et al;43 Chen et al;44 and expresses mean values of non-motor symptoms.
PD, Parkinson's disease; NMSQ-PD, Non-Motor Symptoms Questionnaire in PD patients; NMSQ-C, NMSQ—in controls.
Biomarkers
There are several suggested biomarkers, both tissue and imaging, but all remain investigational. A controlled study from Germany comparing untreated PD with age-matched controls suggested that clinical tools such as the NMSQuest and a validated bedside autonomic scale (SCOPA-autonomic), combined with laboratory tests such as the smell-identification test, reach the sensitivity and specificity (>0.9) for a biological marker of PD at presentation.7 Table 4 lists other possible biomarkers in PD.
Table 4.
Some reported possible biomarkers in the early diagnosis of PD
| Biochemical/histopathology markers | |
| Rectal/colonic biopsy | Phosphorylated α-synuclein-positive Lewy neurites; α-synuclein-positive nerve fibres (figure 1) |
| Skin biopsy | α-Synuclein accumulation |
| Gastric biopsy | Phosphorylated α-synuclein-positive Lewy neurites |
| Low uric acid | Proposed |
| Low-density lipoprotein/serum cholesterol | Proposed |
| Genetic markers | |
| Leucine-rich repeat kinase 2 mutation (G2019S) | |
| Glucocerebrosidase mutation | |
| Imaging markers | |
| Transcranial sonography | |
| DAT scan | |
| The above two can be used in conjunction with clinical symptoms such as hyposmia or REM sleep behaviour disorder | |
| Clinical markers combined with imaging | |
| NMSQuest, SCOPA AUT, smell-identification test (figure 2) and transcranial sonography—can be used as marker with 95.8% sensitivity in PD7 | |
PD, Parkinson's disease; NMSQ, Non-Motor Symptoms Questionnaire; SCOPA AUT, SCOPA autonomic scale.
Some probable biomarker tests and kits
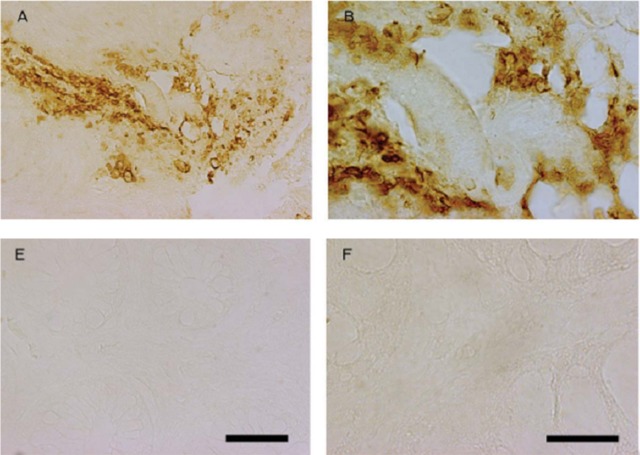
Figure 1.
(A) Showing 10 untreated Parkinson patients; all positive for α-synuclein and 3-nitro-tyrosin (a marker for mitochondrial stress) on sigmoidoscopy and rectal biopsy (A,B). From Shannon et al.45 Copyright 2011 Movement Disorder Society. Reproduced with permission from John Wiley and Sons, Inc. (B) Controls show no relevant staining (E,F). From Shannon et al.45 Copyright 2011 Movement Disorder Society. Reproduced with permission from John Wiley and Sons, Inc.
Figure 2.

University of Pennsylvania smell-identification test for testing olfaction in the clinic.
The clinical scenario: a possible solution to the neglect of non-motor symptoms in clinic
First, tools such as NMSQuest do not consume clinical interactions; the patient completes them and they help direct the consultation with nurse specialist or consultant. The clinician can then grade the non-motor symptoms burden numerically (box 2). Patients are then usually asked to flag the most bothersome symptoms. These are then addressed either through pharmacological, allied health specialist therapies, neuropsychological or neuropsychiatric input (figure 3). A yearly documentation of non-motor symptoms score helps to chart the progress of these symptoms. Pharmaceutical companies have developed and distributed alternative self-completed tools—such as the Parkinson well-being map—but have not been validated in PD. Figure 4 shows the importance of using such tools in the clinic. Two newly diagnosed patients, untreated and with similar motor disability, completed NMSQuests (figure 4A,B). The first patient reports only five non-motor symptoms: mainly problems with dribbling of saliva at night, urgency to pass urine, unexplained pain and dizziness. The second patient reports a wider range of non-motor symptoms (19/30): problems related to sleep, dribbling of saliva and other autonomic symptoms. Each of them needs a different management plan for their non-motor symptoms, as well as the treatment of the motor symptoms. These observations have been confirmed by two recent studies in untreated PD7 and early PD8 (compared with controls).
Box 2. Non-motor Parkinson's disease burden grading using Non-Motor Symptoms Scale (NMSS) and Non-Motor Symptoms Questionnaire (NMSQ) (Kings ISCIII grading).
Kings-ISCIII grading by NMSQuest (can be performed by healthcare professional and based on patient responses using NMSQ)46
Stage 0 NMSQ—0 no non-motor symptoms
Stage 1 NMSQ—1–5 mild
Stage 2 NMSQ—6–12 moderate
Stage 3 NMSQ—13–20 severe
Stage 4 NMSQ—21–30 very severe
Grading by NMSS (to be used for clinical and research based studies)47
Non-motor symptoms burden level 0 NMSS—0
Non-motor symptoms burden level 1 NMSS—1–20
Non-motor symptoms burden level 2 NMSS—21–40
Non-motor symptoms burden level 3 NMSS—41–70
Non-motor symptoms burden level 4 NMSS—≥71
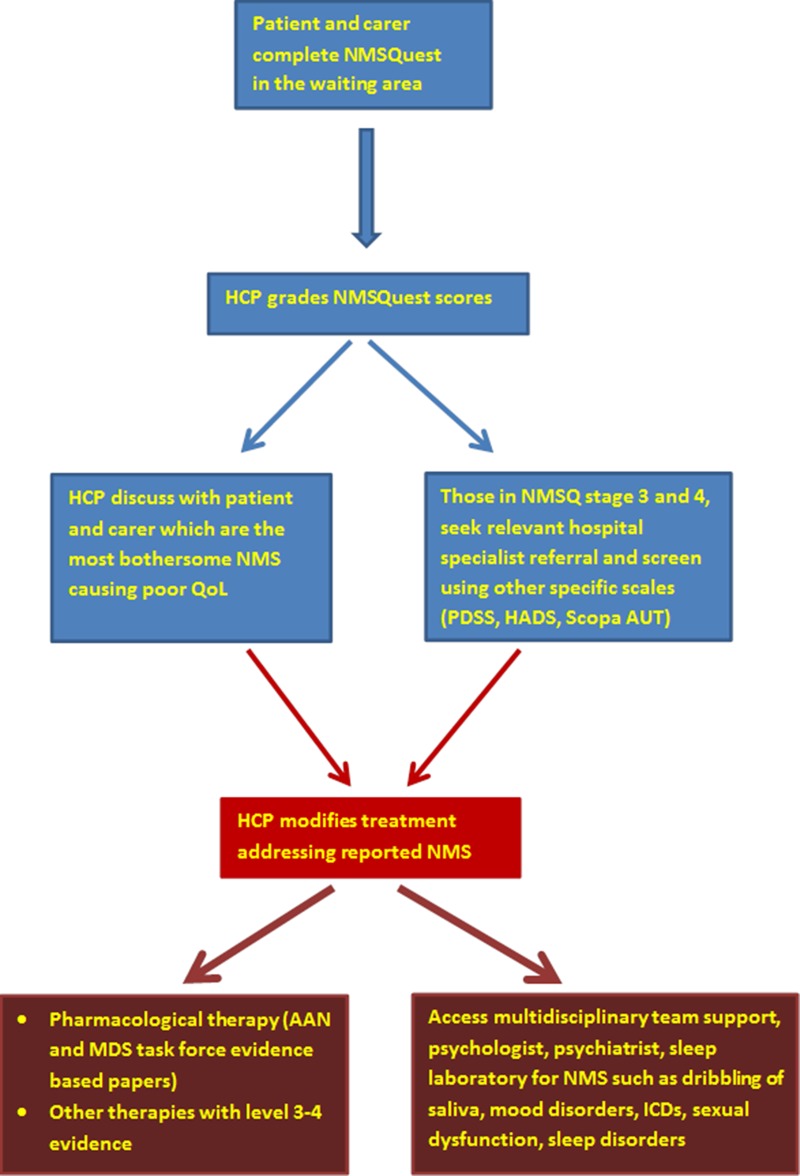
Figure 3.
Suggested algorithm of addressing non-motor symptoms in clinic (modified from Chaudhuri et al).48 HCP, healthcare professional; QoL, quality of life; PDSS, Parkinson's Disease Sleep Scale; HADS, Hospital Anxiety and Depression Scale; Scopa AUT, Scales for Outcomes in Parkinson’s disease—Autonomic; ICD, impulse control disorders.
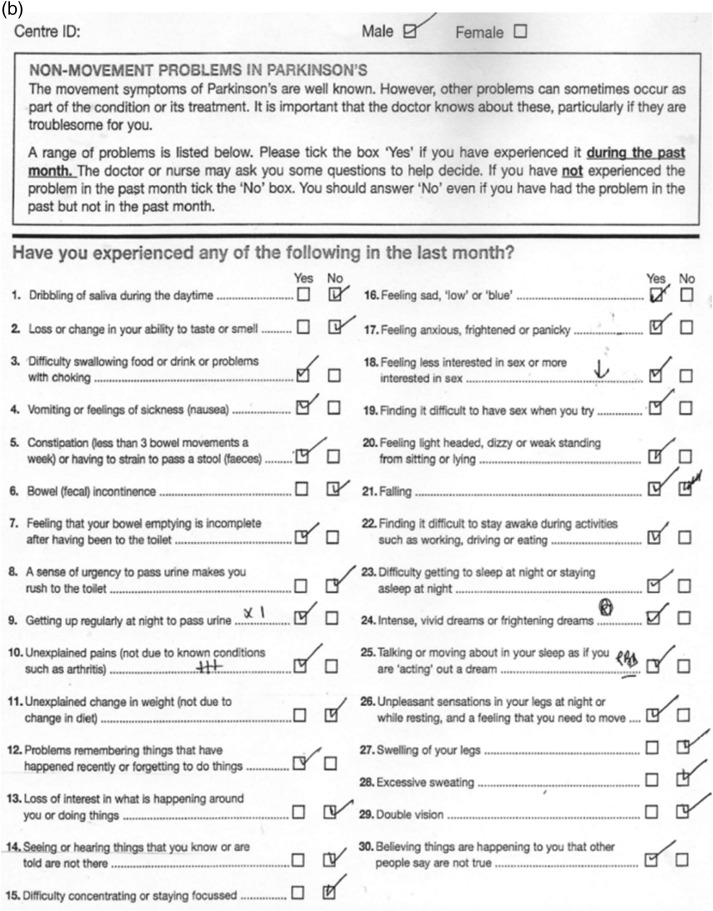
Figure 4.
(A) Non-motor symptoms (NMS) Quest, drug-naïve patient, H&Y 1, 5/30.
Figure 4.
(B) NMS Quest, drug-naïve patient, H&Y 1, 19/30.
A simple grading using patient-completed NMSQuest has stages of severity burden of non-motor symptoms ranging between levels 1 and 4 (proposed as the Kings-ISCIII grading).46 This classification has been validated using the NMSS, the latter being particularly aimed towards research-based studies. NMSQ grading of the burden can influence treatment and screening in primary care is particularly recommended. For instance, for the patient cited in figure 4B, Hoehn and Yahr stage 1 would have NMSQ grade 4 and should be referred for treatment more promptly than the patient cited in figure 4A. Both NMSQ and NMSS burden grades have highly significant inverse correlations with HRQol of patients.47
Non-motor classification and subtypes
The classification based on NMSS has also given rise to the growing concept of non-motor dominant subtypes within PD. Just as in multiple sclerosis and motor neurone disease, clinical subtyping in PD is important (although some argue against this). Subtyping helps give a clearer definition of the clinical symptoms of PD as well as helping with inclusion criteria of patients for clinical trials. It also makes sense given the heterogeneity of neuropathological process underlying PD.
The groups of motor subtypes of PD include
tremor dominant
akinetic dominant
postural instability and gait disturbances
mixed.
Based on the dichotomy between non-motor symptom scores and motor scores in PD, non-motor classification of PD has been proposed, with five levels of non-motor symptoms burden47 (box 2), as well as non-motor subtyping.47 A recent study identified four distinct clusters within PD: two were non-motor-dominant and benign mixed motor/non-motor (with prominent clinical expression of non-motor symptoms); the other two groups were motor-dominant and benign pure motor.16 We have proposed specific PD subtypes based on the predominance of key symptoms relevant to the early stage of PD; in the advanced stages, many of these subtypes may merge, as with motor subtypes.47 However, the natural history of these proposed subtypes at the moment remains unknown.
Evidence that non-motor symptoms are a key determinant of quality of life
Several studies have shown that the non-motor symptoms burden is a key determinant of quality of life of PD and carers.42 49 Patient-related questionnaires also show a growing awareness that these symptoms need addressing since they may adversely affect the lives of patient and carer. Improvement in non-motor symptoms, particularly the dopaminergic therapy-responsive symptoms, translates to robust improvements in quality of life.50 51
Non-motor fluctuation in PD
Apart from motor fluctuation, patients with PD also experience ‘non-motor fluctuations’,52 53 which often develop in tandem with motor fluctuations. Some non-motor symptoms are accentuated during ‘off’ periods while others occur only in ‘off’ periods (below).
Non-motor symptoms that worsen during ‘off’ periods
fatigue
depression
anxiety
inner restlessness
impaired concentration/attention.
Non-motor symptoms occurring only/exclusively during ‘off’ periods
fatigue
depression
anxiety
impaired concentration
inner restlessness.
Anxiety, depression, fatigue and pain had a negative impact on health-related quality of life.52
Treatment
There are diverse treatments to address non-motor symptoms in PD. Both the American Academy of Neurology and the Movement Disorders Society have issued task force documents for managing non-motor symptoms in PD.54 55 Also, several open-label or comparative studies have addressed the effect of advanced therapies in PD on non-motor symptoms (apomorphine, intrajejunal levodopa infusion and rotigotine transdermal patch).56–58 However, the evidence base is poor, with few level 1 studies supporting any specific therapy. However, there was a recent randomised double-blind controlled study providing class I evidence that paroxetine and venlafaxine XR treat depression effectively in PD.59 A few randomised clinical trials have addressed key non-motor symptoms in PD, such as pain (oxycodone with naloxone: the PANDA study) and excessive daytime sleepiness (caffeine, pitolisant, adenosine receptor antagonists). Several novel non-dopaminergic targets using non-dopaminergic agents have been described for managing some motor (mainly dyskinesias) and non-motor (excessive daytime sleepiness, pain, constipation, depression and cognition) symptoms (table 5). At present, these remain investigational.60 We still await robust evidence for treatment of non-motor symptoms in PD; this remains a key unmet need.
Table 5.
Treatment suggestions for some non-motor symptoms
| Non-motor symptoms | Commonly used strategies (where possible based on randomised controlled studies) | Investigational or reported treatment options (based on open label or observational reports) |
|---|---|---|
| Sleep disorders | ||
| Excessive daytime sleepiness | Sleep hygiene (regular daytime exercise, avoiding stimulants at bedtime, regular hours of sleep at night) Modafinil (subjective improvement) |
Caffeine intake (contradictory data and tablets may be used) Sodium oxybate—taken at night, only under specialist supervision (potential for abuse) Selective histamine H3 receptor inverse agonist ▸ Pitolisant (in trial) Adenosine receptor antagonists—Istradefylline, Tozadenant (in setup) |
| Insomnia | Sleep hygiene Short-acting benzodiazepines Non-benzodiazepine hypnotics ▸ Zopiclone Tricyclic antidepressants ▸ Amitriptyline |
Night-time apomorphine infusion or Rotigotine patch (may help in cases of insomnia due to severe nighttime rigidity, restless legs syndrome and ‘off’ periods) |
| REM sleep behaviour disorder | Sleep in a safe environment while in bed, (remove all sharp and breakable objects) Clonazepam (usually used first line) Melatonin Pramipexole in combination with Clonazepam (one successful trial reported) |
Long-acting melatonin (use being investigated) |
| Mood disorders | ||
| Depression | ▸ Pramipexole—recommended by Movement Disorders Society ▸ Selective serotonin reuptake inhibitor – Paroxetine – Citalopram ▸ Serotonin and norepinephrine reuptake inhibitor) – Venlafaxine XR ▸ Tricyclic antidepressants—recommended by Movement Disorders Society – Nortriptyline – Desipramine Awareness of non-motor fluctuation related mood disorders |
If as part of non-motor fluctuations ▸ Trial of long acting dopamine agonists ▸ Infusional therapies – Apomorphine infusion – Intrajejunal levodopa infusion |
| Fatigue | Methylphenidate—recommended by American Academy of Neurology, although considerable side effect profile | Modafinil (weak evidence base) |
| Pain | No specific recommendations apart from analgesics and dopaminergic drugs for non motor fluctuation related pain such as off related dystonic pain Baclofen (muscular pain aggravated by rigidity, anecdotal evidence) Opiates (Tramadol) |
Central pain: Oxycodone with naloxone (PANDA study, randomised placebo-controlled study completed) |
| Cognitive dysfunction | ||
| Dementia | ▸ Rivastigmine—recommended by the Movement Disorders Society (oral or transdermal patch) ▸ Donepezil |
Memantine Galantamine |
| Psychosis (hallucinations/delusions) | ▸ Quetiapine (often used first line, based on clinical experience, despite of unconvincing trial data) ▸ Clozapine (needs specialised monitoring of blood count to monitor for agranulocytosis)– recommended by the Movement Disorders Society ▸ Exclusion of concomitant systemic infection or illness which may precipitate psychosis |
Pimavanserin (serotonin 2A receptor inverse agonist)—in clinical trial |
| Autonomic dysfunction | ||
| Dribbling of saliva | ▸ Oral atropine drops—2–3 times/day ▸ Botulinum toxin A and B—parotid and submandibular injections (under specialist supervision in centres with experience in technique)—recommended by the American Academy of Neurology and the Movement Disorders Society |
▸ Glycopyrrolate for short-term treatment—recommended by the Movement Disorders Society ▸ Ipratropium bromide spray (Atrovent)—1–2 doses per day sublingually |
| Constipation | ▸ Diet and lifestyle advise: – Fibre-rich diet – Ensure adequate fluid intake to avoid dehydration ▸ Medications: – Macrogol (Movicol in the UK)—recommended by the American Academy of Neurology and the Movement Disorders Society – Lactulose (Duphalac) – Senna (Senokot) – Avoid constipating opiates for pain |
Lubiprostone (Amitiza)—in clinical trial |
| Bladder dysfunction—urgency, nocturia | ▸ Anticholinergic agents (use with caution in patients with hallucinations and cognitive decline) – Oxybutynin – Tolterodine XL ▸ Desmopressin spray for troublesome nocturia (beware of nocturnal hypertension) |
▸ If during off state—adjust PD medications ▸ Exercise-based behavioural therapy |
| Erectile dysfunction | Phosphodiesterase-5 inhibitors (use with caution in patients with postural hypotension) ▸ Sildenafil—recommended by the American Academy of Neurology ▸ Tadalafil (Cialis) |
Apomorphine injection may be tried |
| Orthostatic hypotension | Non-pharmacological therapies ▸ Increased salt and water intake ▸ Waist-high support stockings ▸ Physical counter manoeuvres ▸ Avoid volume depleting drugs (diuretics, antihypertensives) Pharmacological therapy ▸ Fludrocortisone ▸ Ephedrine ▸ Midodrine |
Pharmacological therapies ▸ Domperidone in addition to fludrocortisone ▸ L-threo-3,4-dihidroxyphenylserine for refractory orthostatic hypotension |
PD, Parkinson's disease.
For some non-motor symptoms, such as anxiety, depression, pain and fatigue, clinicians must ascertain as far as possible whether these are part of non-motor fluctuations. If so, and if they are exclusively present in the ‘off’ period, then treatment may need to focus on alleviation of motor ‘off’ period with relevant anti-parkinsonian medication, rather than using antidepressants or antianxiety medications.
Multidisciplinary support
For symptoms such as dribbling of saliva, pain, sexual dysfunction, impulse control disorders and cognitive impairment, it is important and ideal to have the participation of a multidisciplinary team comprising PD nurse specialist, speech and language therapist, physiotherapist, occupational therapist, clinical psychologist and neuropsychiatrist. Speech and language therapy input in cases with disabling or severe dribbling of saliva is essential, for advice on swallow timers and head positioning. Experienced input from psychology and neuropsychiatry and a PD nurse specialist are essential in cases for managing severe depression, anxiety disorders and impulse control disorders. We also recommend, where possible, joint working with a member of the care of the elderly team with a special interest in PD with good access to multidisciplinary team support.
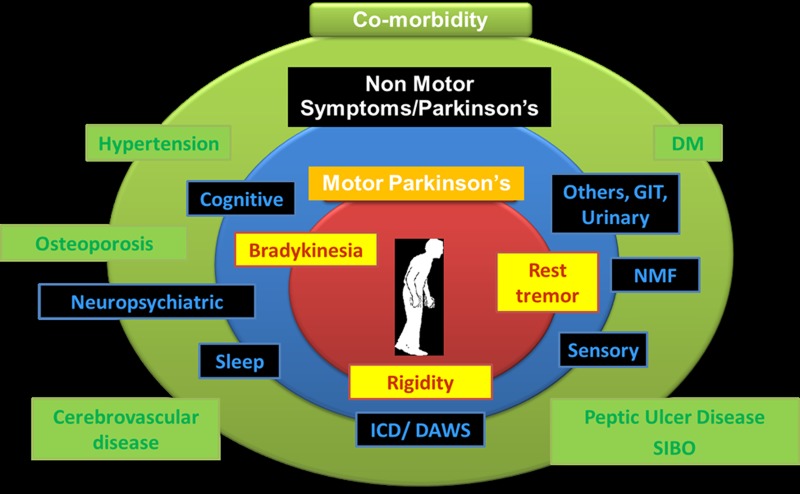
There is also increasing evidence suggesting that treating comorbidities, such as hypertension, diabetes mellitus, peptic ulcer disease, small intestinal bacterial overgrowth and osteoporosis, can ensure a complete treatment package for PD, in addition to monitoring for and managing drug-induced non-motor symptoms and non-motor syndromes (figure 5).
Figure 5.
The spectrum of the multimorbid Parkinson's disease. GIT, gastrointestinal; NMF, non-motor fluctuation; DM, diabetes mellitus; SIBO, small intestinal bacterial overgrowth; ICD, impulse control disorders; DAWS, dopamine agonist withdrawal syndrome.
The future and conclusions
Non-motor symptoms represent a huge challenge to the treating physicians and allied health colleagues and continue to be a key determinant of patients’ and carers’ quality of life at huge societal costs. Their diversity, their differential effects on quality of life and their possible progression patterns pose additional challenges. We need much more research to recognise and manage non-motor symptoms effectively in clinical practice.
Future biomarkers will probably define the premotor stages of PD as well as the clinical patterns of PD into specific endophenotypes. Classifications have hitherto focused on motor subtypes but will include specific non-motor subtypes with the potential for specific treatments. Extension of non-motor symptoms-based studies will also address hitherto unaddressed issues, such as the influence of ethnicity, and poorly explored issues, such as influence of genetics.
Greater awareness of non-motor PD symptoms and the development of validated rating scales have led to major advances in understanding their evolution and progression; experimental models to explore their pathogenesis and potential treatments have lagged behind. There are now opportunities using existing dopaminergic animal models, some of which are now gathering momentum but others, such as the investigation of dopamine dysregulation syndromes/compulsive behaviours and dopamine agonist withdrawal syndromes, are in their infancy. What would really help is a major push to solve the role of non-dopaminergic degenerative changes in the evolution of non-motor symptoms in PD. This is perfectly feasible as there are well-established tools to do this (eg, lesioning, toxin treatment), but so far there is no momentum for this.
Supplementary Material
Acknowledgments
This paper presents independent research funded by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre and Dementia Unit at South London and Maudsley NHS Foundation Trust and King's College London. We acknowledge the help and support of the international Parkinson's non-motor group and in particular Professor Pablo Martinez-Martin and his team in the Institute of Neuroepidemiology, Madrid, for co-leading all studies related to NMSQuest and NMSS described in this paper. NMSQuest can be downloaded from the websites of the following: Parkinson's UK: NMSQuest: (http://www.parkinsons.org.uk/PDF/nms_questionnaire.pdf), The Movement Disorders Society: (http://www.movementdisorders.org/publications/rating_scales/). European Parkinson's Disease Association. Life With Parkinson's: (http://www.epda.eu.com/en/parkinsons/life-with-parkinsons/part-2/introduction/).
Footnotes
Contributors: KRC conceived the idea of the paper. The first draft was prepared by KRC, PJ and AT, and then circulated repeatedly among all authors for critical revision. AT produced the tables and figures and formatted the manuscript according to the requirements.
Funding: Aspects of research described in the review (NMSQuest studies, PD endophenotyping) have been supported by NIHR Biomedical Research Centre grants to Kings College, London.
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed. This paper was reviewed by Simon Lewis, Sydney, Australia, and by Nick Fletcher, Liverpool, UK.
References
- 1.Chaudhuri KR, Martinez-Martin P, Schapira AHV, et al. An international multicentre pilot study of the first comprehensive self-completed non-motor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord 2006;21:916–23 [DOI] [PubMed] [Google Scholar]
- 2.Chaudhuri KR, Martinez-Martin P, Brown RG, et al. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord 2007;22:1901–11 [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Martin P, Rodriguez-Blazquez C, Abe K, et al. International study on the psychometric attributes of the Non-Motor Symptoms Scale in Parkinson disease. Neurology 2009;73:1584–91 [DOI] [PubMed] [Google Scholar]
- 4.Shulman LM, Taback RL, Rabinstein AA, et al. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2002;8:193–7 [DOI] [PubMed] [Google Scholar]
- 5.Parkinson's Disease. National clinical guideline for diagnosis and management in primary and secondary care. NICE full guideline, 2006 [PubMed] [Google Scholar]
- 6.Chaudhuri KR, Prieto-Juvcynska C, Naidu Y, et al. The non declaration of non motor symptoms of Parkinson’s disease to healthcare professionals. An international survey using the NMSQuest. Mov Disord 2010;25:704–9 [DOI] [PubMed] [Google Scholar]
- 7.Mollenhauer B, Trautmann E, Sixel-Döring F, et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 2013;81:1–9 [DOI] [PubMed] [Google Scholar]
- 8.Khoo TK, Yarnall AJ, Duncan GW, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013;80:276–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000;69:308–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagell P, Nordling S, Reimer J, et al. Resource use and costs in a Swedish cohort of patients with PD disease. Mov Disord 2002;17:1213–20 [DOI] [PubMed] [Google Scholar]
- 11.Parkinson's best practice tariff. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/214902/PbR-Guidance-2013-14.pdf p 103, guidance d(access 21 Oct 2013)
- 12.Jellinger KA. Neuropathology of sporadic PD disease: evaluation and change of concepts. Mov Disord 2012;27:8–30 [DOI] [PubMed] [Google Scholar]
- 13.Halliday GM, Blumbergs PC, Cotton RGH, et al. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson's disease. Brain Res 1990;510:104–7 [DOI] [PubMed] [Google Scholar]
- 14.Kingsbury AE, Bandopadhay R, Silveira-Moriyama L, et al. Brain stem pathology in PD disease: an evaluation of the Braak staging model. Mov Disord 2010;25:2508–15 [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Del Tredici K, Rueb U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobio Aging 2003;24:197–210 [DOI] [PubMed] [Google Scholar]
- 16.Erro R, Vitale C, Amboni M, et al. Heterogeneity of early Parkinson's disease: a cluster analysis on newly diagnosed untreated patients. PLoS ONE 2013;8:e70244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol 1996;55:259–72 [DOI] [PubMed] [Google Scholar]
- 18.Jellinger K. Overview of morphological changes in Parkinson's disease. Adv Neurol 1987;45:1–18 [PubMed] [Google Scholar]
- 19.Hirsch EC, Graybiel AM, Duyckaerts C, et al. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson's disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA 1987;84:5976–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saper CB, Sorrentino DM, German DC, et al. Medullary catecholaminergic neurons in the normal human brain and Parkinson's disease. Ann Neurol 1991;29:577–84 [DOI] [PubMed] [Google Scholar]
- 21.Wakabayashi K, Takahashi H. The intermediolateral nucleus and Clarke's column in Parkinson's disease. Acta Neuropathol (Berl) 1997;94:287–9 [DOI] [PubMed] [Google Scholar]
- 22.Iwanaga K, Wakabayashi K, Yoshimoto M, et al. Lewy bodytype degeneration in cardiac plexus in Parkinson's and incidental Lewy body diseases. Neurology 1999;52:1269–71 [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Ghebremedhin E, Rub U, et al. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 2004;318:121–34 [DOI] [PubMed] [Google Scholar]
- 24. doi: 10.1111/j.1476-5381.2011.01426.x. Duty S, Jenner P. Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol 2011;164:1357–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. doi: 10.1007/s13311-012-0104-2. Chesselet MF, Richter F, Zhu C, et al. A progressive mouse model of Parkinson's disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics 2012;9:297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. doi: 10.1371/journal.pone.0008762. Pan-Montojo F, Anichtchik O, Dening Y, et al. Progression of Parkinson's disease pathology is reproduced by intragastric administration of Rotenone in mice. PLOS ONE 2010;5:e8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. doi: 10.1016/j.neubiorev.2007.02.003. Lind NM, Moustgaard A, Jelsing J, et al. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev 2007;31:728–51. [DOI] [PubMed] [Google Scholar]
- 28.Alves G, Forsaa EB, Pedersen KF, et al. Epidemiology of Parkinson's. J Neurol 2008;255:18–32 [DOI] [PubMed] [Google Scholar]
- 29.Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–44 [DOI] [PubMed] [Google Scholar]
- 30.Siderowf A, Jennings D, Eberly S, et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov Disord 2012;27:406–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siderowf A, Lang A. Premotor Parkinson’s disease: concepts and definitions. Mov Disord 2012;27:608–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stern MB, Lang A, Poewe W. Toward a redefinition of Parkinson’s disease. Mov Disord 2012;27:54–60 [DOI] [PubMed] [Google Scholar]
- 33.Jennings D, Siderowf A, Stern M, et al. Evaluating phenoconversion to PD in the PARS prodromal cohort. Mov Disord 2013;28(Suppl 1):S59–60 [Google Scholar]
- 34.Postuma RB, Gagnon J-F, Montplaisir JY. REM sleep behavior disorder and prodromal neurodegeneration—where are we headed? Tremor Other Hyperkinet Mov 2013;3 http://tremorjournal.org/article/view/134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 2001;57:456–62 [DOI] [PubMed] [Google Scholar]
- 36.Leentjens AF, Van den AM, Metsemakers JF, et al. Higher incidence of depression preceding the onset of Parkinson's disease: a register study. Mov Disord 2003;18:414–18 [DOI] [PubMed] [Google Scholar]
- 37.Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 2005;65:1442–6 [DOI] [PubMed] [Google Scholar]
- 38.Kang SY, Ma HI, Lim YM, et al. Fatigue in drug-naïve Parkinson’s disease. Eur Neurol 2013;70:59–64 [DOI] [PubMed] [Google Scholar]
- 39.Lin CH, Wu RM, Chang HY, et al. Preceding pain symptoms and Parkinson's disease: a nationwide population-based cohort study. Eur J Neurol 2013;20:1398–404 [DOI] [PubMed] [Google Scholar]
- 40.Gao X, Chen H, Schwarzschild MA, et al. Erectile function and risk of Parkinson’s disease. Am J Epidemiol 2007;166:1446–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez-Martin P, Schapira AHV, Stocchi F, et al. Prevalence of non motor symptoms in PD disease in an international setting; study using non-motor symptoms questionnaire in 545 patients. Mov Disord 2007;22:1623–9 [DOI] [PubMed] [Google Scholar]
- 42.Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009;24:1641–9 [DOI] [PubMed] [Google Scholar]
- 43.Crosiers D, Pickut B, Theuns J, et al. Non-motor symptoms in a Flanders-Belgian population of 215 Parkinson’s disease patients as assessed by the Non-Motor Symptoms Questionnaire. Am J Neurodegener Dis 2012;1:160–7 [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W, Xu Z, Wang G, et al. Non-motor symptoms of Parkinson's disease in China: a review of the literature. Parkinsonism Relat Disord 2012;18:446–52 [DOI] [PubMed] [Google Scholar]
- 45.Shannon KM, Keshavarzian A, Mutluet E, et al. Alpha-Synuclein in colonic submucosa in early untreated Parkinson's disease. Mov Disord 2012;27:709–15 [DOI] [PubMed] [Google Scholar]
- 46.Chaudhuri KR, Martinez-Martin P, Sherman R, et al. The non motor staging of Parkinson's disease: results from an international pilot study. Neurology 2009;72(Suppl 3):A322 [Google Scholar]
- 47.Ray Chaudhuri K, Rojo JM, Schapira AHV, et al. A proposal for a comprehensive grading of Parkinson's disease severity combining motor and non-motor assessments: meeting an unmet need. PLoS ONE 2013;8:e57221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray Chaudhuri K, Odin P, Antonini A, et al. Parkinson's disease: the non-motor issues. Parkinsonism Relat Disord 2011;17:717–23 [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, et al. The impact of non motor symptoms on health-related quality of life of patients with Parkinson's disease. Mov Disord 2011;26:399–406 [DOI] [PubMed] [Google Scholar]
- 50.Reddy P, Martinez-Martin P, Todorova A, et al. The EuroInf study: a multi-centre European comparative study of apomorphine versus intrajejunal levodopa infusion in a real life cohort of Parkinson's patients. Mov Disord 2013;28(Suppl 1):S211 [Google Scholar]
- 51.Todorova A, Ray Chaudhuri K. Subcutaneous apomorphine and non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2013;19:1073–8 [DOI] [PubMed] [Google Scholar]
- 52.Storch A, Schneider CB, Wolz M, et al. Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology 2013;80:800–9 [DOI] [PubMed] [Google Scholar]
- 53.Seki M, Takahashi K, Uematsu D, et al. Clinical features and varieties of non-motor fluctuations in Parkinson's disease: a Japanese multicenter study. Parkinsonism Relat Disord 2013;19:104–8 [DOI] [PubMed] [Google Scholar]
- 54.Zesiewicz TA, Sullivan KL, Arnulf I, et al. Practice parameter: treatment of nonmotor symptoms of Parkinson disease. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010;74:924–31 [DOI] [PubMed] [Google Scholar]
- 55.Seppi K, Weintraub D, Coelho M, et al. The movement disorder society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord 2011;26:S42–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Martin P, Reddy P, Antonini A, et al. Chronic subcutaneous infusion therapy with apomorphine in advanced Parkinson's disease compared to conventional therapy: a real-life study of non-motor effect. J Parkinson's Dis 2011;1:197–203 [DOI] [PubMed] [Google Scholar]
- 57.Reddy P, Martinez-Martin P, Rizos A, et al. Intrajejunal levodopa versus conventional therapy in Parkinson disease: motor and nonmotor effects. Clin Neuropharmacol 2012;35:205–7 [DOI] [PubMed] [Google Scholar]
- 58.Trenkwalder C, Kies B, Rudzinska M, et al. Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord 2011;26:90–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richard IH, McDermott MP, Kurlan R, et al. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson's disease. Neurology 2012;78:1229–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rascol O, Lozano A, Stern M, et al. Milestones in Parkinson's disease therapeutics. Mov Disord 2011;26:1072–82 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.