Abstract
Background:
Hyaluronic acid (HA) fillers have become the most popular tool for wrinkle treatment and volumization, although HA is generally absorbed within 6–12 months and requires repeated treatments to maintain the effects.
Methods:
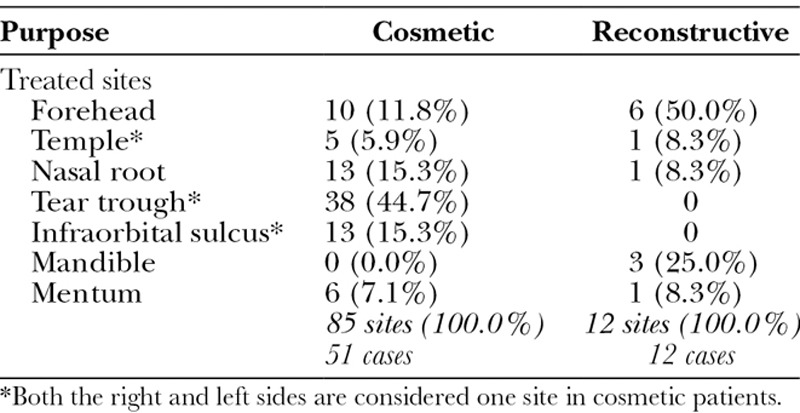
HA was injected onto the bone for volumization with a small 30-gauge needle to examine the long-lasting effects. Of the 63 Japanese patients with 97 treated sites followed up more than 12 months, 51 had HA injections for cosmetic purposes and 12 were treated for reconstructive volumization of facial deformity such as localized scleroderma and postsurgical bony deformity. Treated sites included the forehead, temple, nasal root, mentum, tear trough, and infraorbital sulcus.
Results:
After long-term follow-up (12–93 months, mean = 21.6), persistent volumizing effects were observed in most patients. In fact, 86.6% of the treated sites showed >50% volume retention and 49.5% showed >75% retention. Magnetic resonance imaging analyses revealed that the injected space was well maintained, capsulated, and filled with heterogeneous content. Magnetic resonance imaging quantitative T2 maps indicated that much of the injected HA was replaced with other materials. Together with clinical inspection, these findings suggest that onlay injection of HA on the bone induced formation of capsule, fibrosis, and/or calcification/ossification, which contributed to persistent volumization.
Conclusions:
Semipermanent volumizing effects can be achieved by HA injection if the target area has an underlying bony floor. Periosteal stem cells may be activated by HA injection and may contribute to persistent volumizing effects. This treatment may be a much less invasive alternative to fat or bone grafting.
Absorbable fillers have become increasingly popular to reverse signs of aging on the face. Since bovine collagen fillers received Food and Drug Administration approval in 1981, these fillers gained popularity for more than a decade1; however, bovine collagen had the potential for allergic reactions and required skin testing before the first treatment. Since then, non–animal-based hyaluronic acid (HA), which had been used for intra-articular joint injection and ophthalmologic procedures for many years with a very good safety profile,2 was introduced and has become the most commonly used facial filler over the past several years. HA fillers show excellent efficacy not only in correcting wrinkles but also in restoring tissue volume with minimal downtime. These fillers are easy to use, allergy-free, and enzymatically degradable using an injection of hyaluronidase in case of a bad result.3 Thus, HA fillers have become a key tool in aesthetic surgery and medicine, and numerous products are commercially available.
Degradability of HA has 2 aspects. HA fillers exhibit an excellent safety profile because any unfavorable HA effects are temporary. On the other hand, virtually the only downside of HA fillers is that any beneficial HA effects are also temporary. Many HA products officially indicate that the materials are resolved within 6–12 months.4 Thus, patients require repeated injections to maintain satisfactory results. Because any synthetic permanent fillers result in long-term complications such as foreign body granuloma and sustained inflammation,5 achieving both safety and longevity of the effects with filler treatments is quite challenging.
Here, we report an easy HA filler injection technique that leads to semipermanent volumizing effects. This technique simply involves injection of HA into the deepest layer on the bone with a small, sharp needle. This article describes the procedure and clinical outcomes and discusses the indications of the treatment and possible mechanisms of the long-lasting effects.
PATIENTS AND METHODS
Injection Materials and Devices
Two types of HA fillers were used: Restylane (Q-MED AB, Uppsala, Sweden), which was used in almost all cases, and Macrolane, which was used in a small number of cases who required a large amount of injection. A sharp 30-gauge needle was used for Restylane, and Macrolane was injected through a sharp 27-gauge needle after transfer to a luer-lock 1-ml syringe.
Treatment Procedures
Treatment was performed by injection of an HA filler on the bone in the target area. The injection needle was inserted through the skin until it made contact with the bone, and HA was introduced directly onto the bone to place small HA droplets (0.02–0.15 ml each). Local anesthesia was conducted by applying a frozen gel (for several seconds), topical lidocaine cream (for 1 hour), local injection of lidocaine, or nerve block. Care was taken not to inject away from the bone by confirming the attachment of the needle tip to the bone or scratching the periosteum with the needle tip. HA was supposed to be injected just above (or under) the periosteum (Fig. 1).
Figure 1.
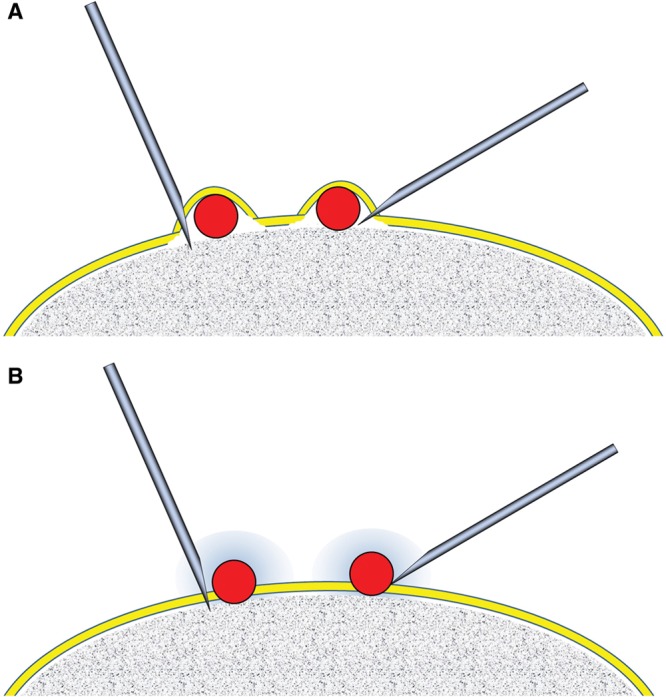
Concept and procedure of HA treatment. (A) The treatment was originally designed to inject HA below the periosteum for osteoinduction from the underlying bone, although this procedure is not usually practical. A very small (30- to 34-gauge) sharp needle is preferable. (B) HA is actually injected on the periosteum in most cases. The injury and persisting inflammatory changes around the injected HA particles are expected to activate periosteal stem cells and contribute to the induction of tissue neogenesis, such as formation of capsule, fibrosis, and calcification/ossification during the HA absorption process.
Patients
The onlay injection of HA to the bone has been performed since 2002, and 63 patients (97 treated sites) were followed up for more than 12 months. Fifty-one patients had the treatment for cosmetic volumization and 12 patients for reconstructive volumization for concave facial deformity suffered from localized scleroderma, lupus erythematosus profundus, congenital anomaly, or prior cranial/maxillofacial surgery. All but 3 patients were females. Patient age at the start of the treatment ranged from 20 to 83 years (59.8 ± 14.1; mean ± SD), and the follow-up period ranged from 12 to 93 months (21.6 ± 20.8; mean ± SD). Patient data are summarized in Table 1. There was no control group with subcutaneous or intramuscular injection; it is well established that HA injected intradermally, subcutaneously, or intramuscularly disappears over time, typically fewer than 12 months.6,7
Table 1.
Summary of Injected Sites
Evaluation of Results
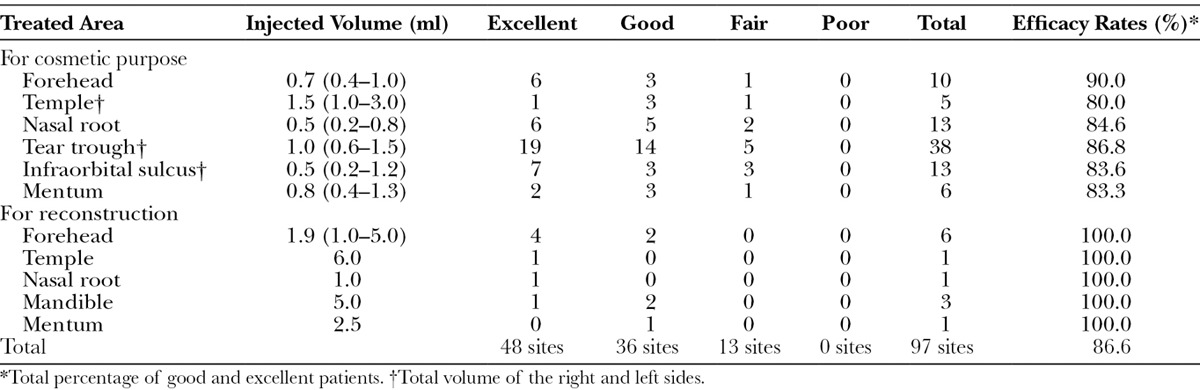
Clinical efficacy assessments were conducted by photographic method in 3 different directions (frontal, oblique, and submental oblique views) to evaluate the volume change in a three-dimensional structure. Photographs were taken for every patient before, immediately after, and long (at least 12 months) after treatment with a high-resolution digital camera (Canon EOS-D30, Osaka, Japan). Supposing the initial change between before treatment and immediately after treatment was 100%, the change between before treatment and at least 12 months after treatment was classified into 4 categories: “excellent” (75% or more remained), “good” (50% to less than 75%), “fair” (25% to less than 50%), and “poor” (0% to less than 25% or worse). Three blinded certified plastic surgeons, who did not perform the treatment, scored the volume change by evaluating the 3 photographs, before, immediately after, and long after treatment. Good and excellent patients were counted in the “clinical efficacy rate” in the table. We used the chi-square test for the correlation between clinical results and injection volume.
In addition, a smaller imaging study was carried out in 6 reconstructive patients who originally needed neurosurgical imaging follow-up, and we analyzed magnetic resonance imaging (MRI) or computed tomography (CT) findings at 12 months postbaseline for specific quality diagnosis.
RESULTS
Among the 63 patients (97 treated sites) with over 1-year follow-up, almost all patients showed persistent volumizing effects. None of the patients was the same as before treatment, and some exhibited similar results at follow-up to those just after injection. Excellent results were seen at 48 sites (49.5%), and good results were observed at 36 sites (37.1%). Thus, the efficacy rate (>50% retention) was 86.6% (Table 2). In 12 patients who were followed up for more than 3 years, both doctors and patients were clearly aware of the apparent continuing effects, suggesting that the volumizing effect observed at 1 year persists for years and can therefore be considered “semipermanent.”
Table 2.
Summary of Clinical Results
The total injected amount of HA filler varied by anatomical site and ranged from 0.2 to 10.0 ml. Statistical analysis showed no significant correlation between clinical results and injection volume (P > 0.05).
As for side effects, apparent bruising was observed postoperatively in 12 cases who had injections to the midface. No other serious complications, including embolization, skin necrosis, hematoma, infection, visible lumping or surface irregularity, asymmetry, and allergic reaction, occurred.
Cases
Five representative cases are shown as cases 1 and 2 (Figs. 2–5) and cases 3–6 (Figs. 6–9).
Figure 2.
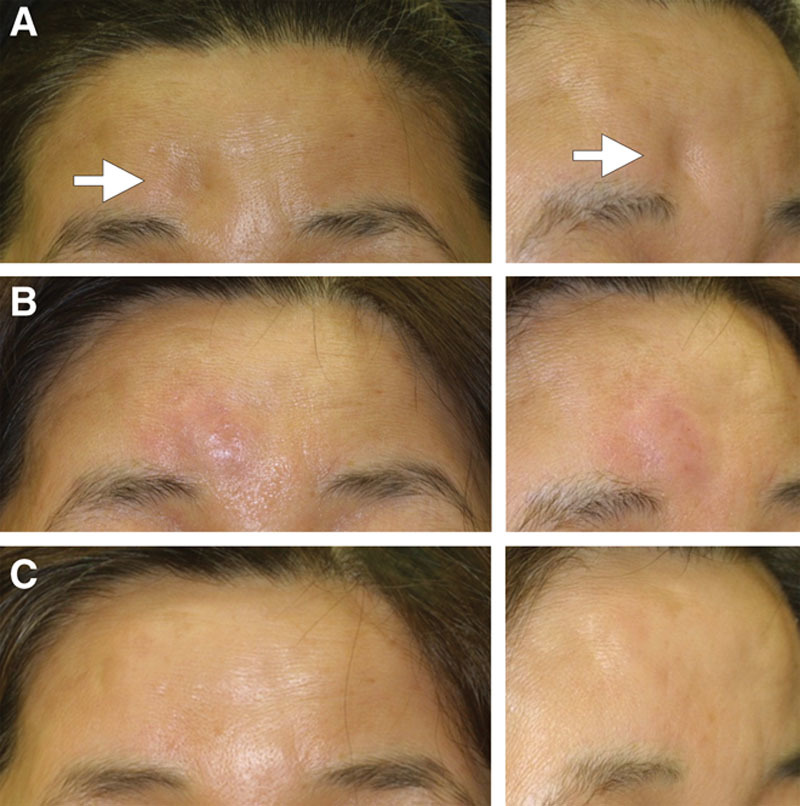
Case 1. HA was injected to correct postsurgical deformity on the forehead. (A) Before treatment, (B) immediately after injection, and (C) 12 months after treatment.
Figure 5.
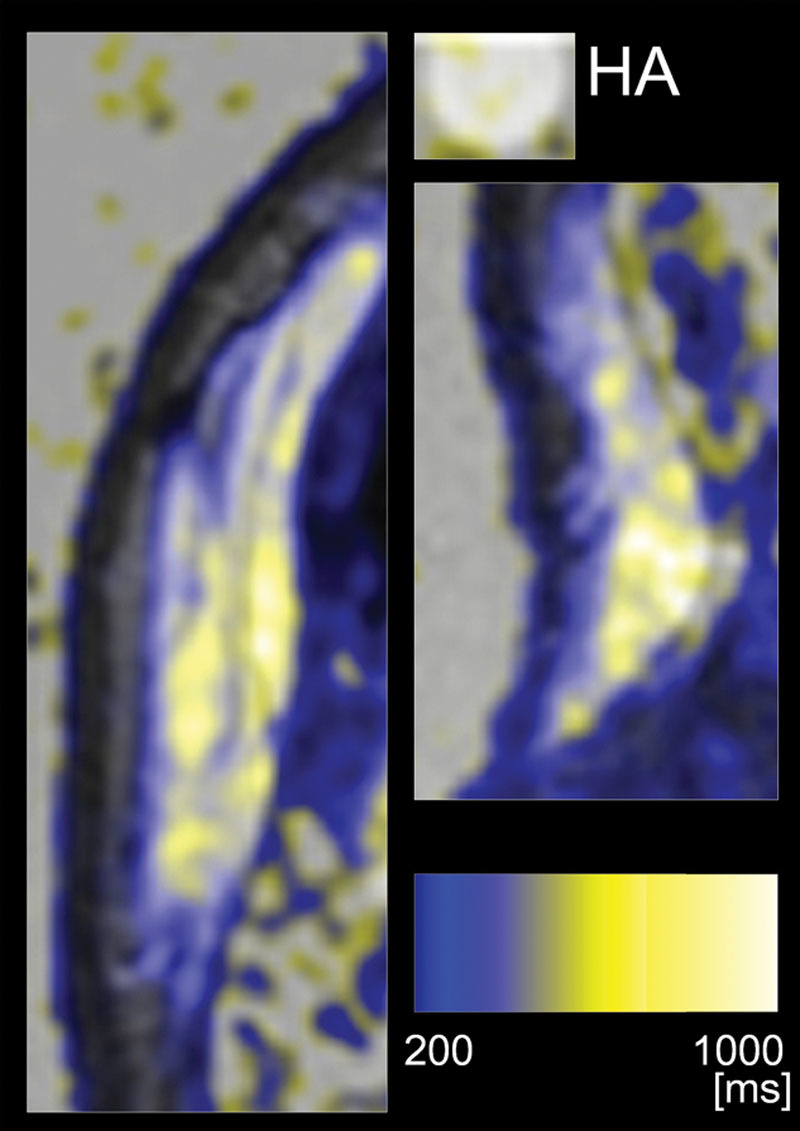
Magnetic resonance quantitative T2 maps overlaid on T2-weighted images of case 2. Left and right panels are the forehead and nasal root lesions, respectively. An HA syringe was also analyzed as a control. The T2 value was visualized according to the differential color bar. HA generally showed high intensity (>700 ms) and was expressed in white.
Figure 6.
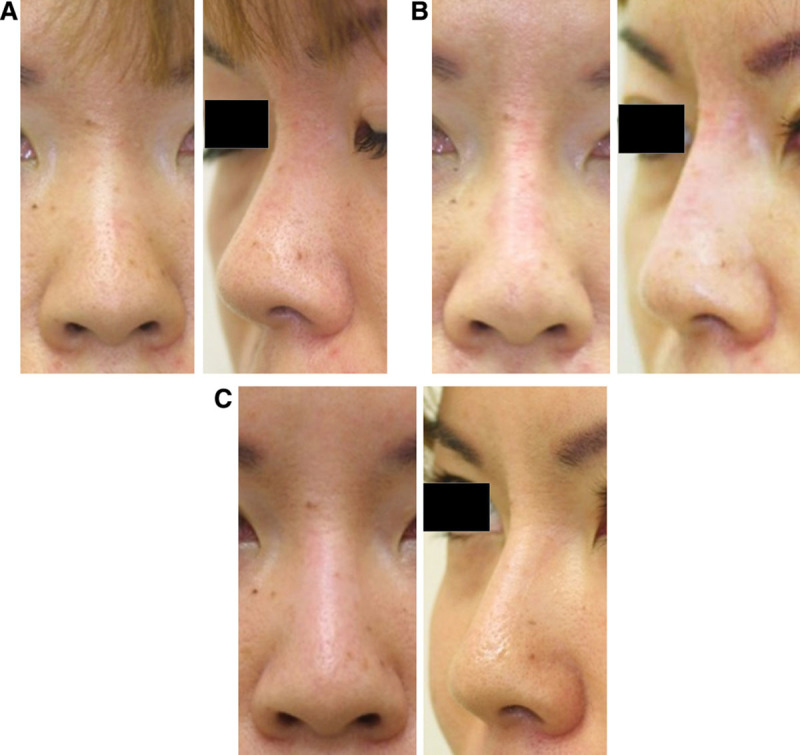
Case 3. A 38-year-old Japanese woman who underwent nasal augmentation via onlay injection to the nasal bone (left panels: frontal views; right panels: oblique views). HA (0.7 ml Restylane) was injected onto the nasal bone with a sharp 30-gauge needle. (A) Before, (B) immediately after treatment. (C) After 12 months, the projection of the nasal root was well preserved, and approximately half of the injected volume seemed to remain.
Figure 9.
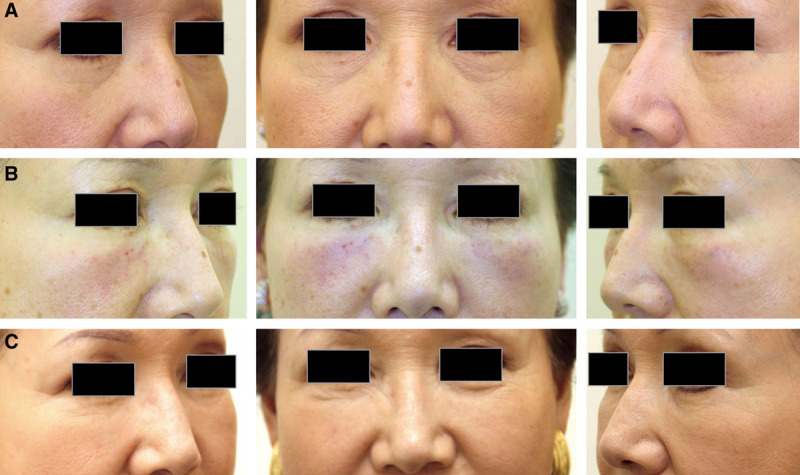
Case 6. A 66-year-old Japanese woman who underwent lid-cheek junction and tear trough augmentation via onlay injection to the maxilla and zygomatic bone (center panels: frontal views; left and right panels: oblique views). HA (1.5 ml Restylane per side) was injected onto the bone with a sharp 27-gauge needle at upright position under local anesthesia with infraorbital nerve block. (A) Before, (B) immediately after treatment. (C) After 18 months, the volumizing effect was well preserved, and approximately half of the injected volume seemed to sustain.
Figure 7.
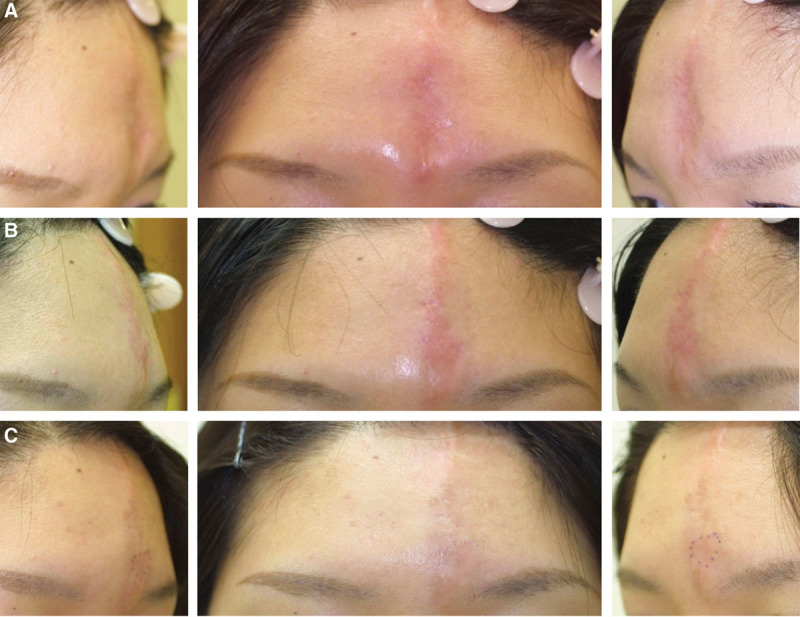
Case 4. A 29-year-old Japanese woman with localized scleroderma (morphea; en coup de sabre) sought to correct the concave deformity on the forehead. (A) Before treatment. An onlay injection of HA (1.0 ml Restylane) to the frontal bone was performed with a sharp 30-gauge needle to elevate the overlying tissue and skin of the defect until the filler resulted in overcorrection by 10–20%. (B) Immediately after treatment. (C) After 12 months, this volumization was well preserved.
Figure 8.
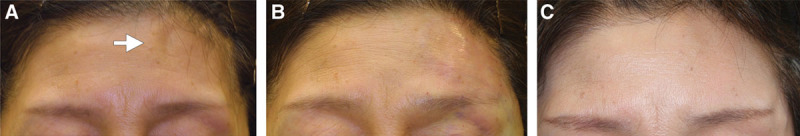
Case 5. A 62-year-old Japanese woman who underwent surgery for a cerebral aneurysm 6 years before consultation had a concave bony deformity on the left forehead and temple area. (A) Before treatment. After performing a CT scan to confirm the underlying bony structure, an onlay injection of HA to the bone was performed until this concave deformity was partially overcorrected. (B) Immediately after injection. The forehead area was injected with 3.0 ml Restylane via a sharp 30-gauge needle, and the temple area was injected with 2.0 ml Macrolane via a sharp 27-gauge needle. (C) After 12 months, the volumization was well preserved to the same level as the surrounding surface.
Case 1
A 59-year-old Japanese woman who underwent a surgery for a meningioma 7 years before consultation exhibited a concave bony deformity on the forehead (Fig. 2A, before treatment). After confirming the existence of the bony structure at the site of depression by CT and MRI, an onlay injection of HA (1.0 ml Restylane) was performed with a sharp 30-gauge needle onto the depressed frontal bone (Fig. 2B, immediately after injection). At 12 months, the skin remained elevated to the same level as the surrounding area (Fig. 2C). The volumization effect was also confirmed by direct comparison of MRIs taken before treatment and 12 months after treatment (Fig. 3). MRIs taken 12 months after treatment showed that the injected space under the galea was filled with heterogeneous material. Compared with MRIs of an HA syringe, the augmented space showed similar but not identical intensity to HA and the content looked heterogeneous, suggesting that HA partially, but not completely, remained in the space. In addition, the space seemed to be surrounded by a material with low intensity in both T1- and T2-weighted images, suggesting that this material may be a fibrotic capsule.
Figure 3.
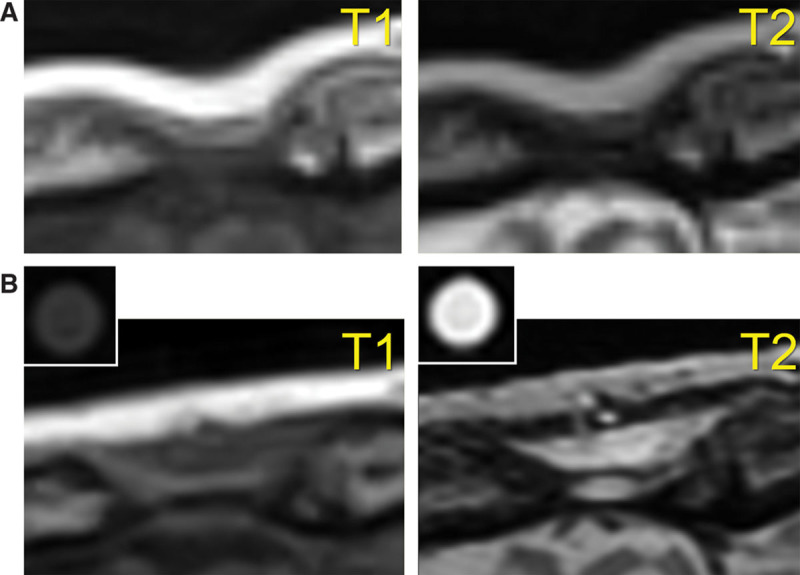
MRIs of case 1. Left panels are T1-weighted MRIs, and right panels are T2-weighted MRIs. An HA syringe was also analyzed as a control. (A) Before treatment and (B) 12 months after treatment.
Case 2
A 51-year-old Japanese woman suffering from cleidocranial dysostosis presented a congenital bony deformity on the forehead and nasal root (Fig. 4A, before treatment). After confirming the existence of a bony structure at the site of depression by CT scan, an onlay injection of HA (2.5 ml Restylane) was performed with a sharp 30-gauge needle onto the depressed frontal bone and the nasal bone until the deformity was overcorrected by 20% at the forehead (Fig. 4B, immediately after injection). Twelve months after treatment, the forehead skin remained overcorrected, although the skin level was lower than immediately after the injection (Fig. 4C). The volumization was also confirmed by MRI 12 months after treatment. To perform more detailed qualitative analysis on the MRIs, quantitative T2 maps were calculated from 8 spin-echo images, using linear least squares curve fitting on a pixel-by-pixel basis with image software (Fig. 5). On T2 mapping images, the injected space on the forehead generally appeared yellow, which is approximately 500–700 ms, although the control HA syringe appeared white, showing a little higher intensity. The treated space on the nasal bone was yellow to white, suggesting that HA partly remains in the space. Although any further qualification of the yellow space is not possible, a major portion of the injected space seemed not to be filled with HA of the same condition, which strongly suggested tissue neogenesis such as fibrosis and/or calcification/ossification and capsular formation.
Figure 4.
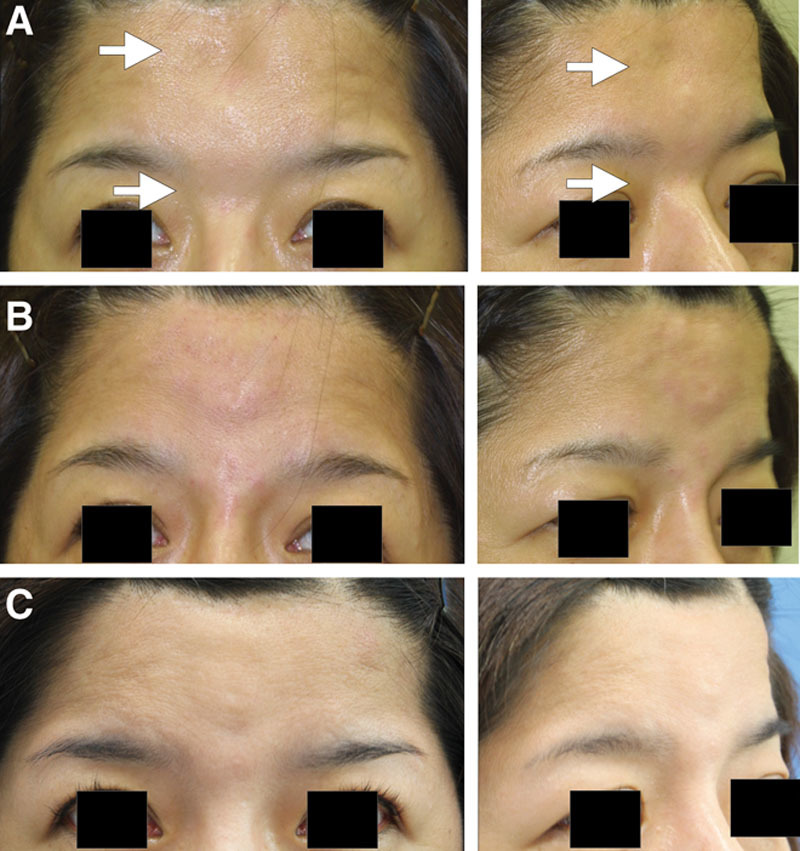
Case 2. HA was injected to correct a congenital bony deformity on the forehead and nasal root. (A) Before treatment, (B) immediately after injection, and (C) 12 months after treatment.
DISCUSSION
Semipermanent Volumization after Onlay Injection of HA
Volumizing effects by HA injections are typically temporary, and repeated injections with an interval of 6–12 months are usually required for maintaining the cosmetic effects.6–8 Absorption of HA is generally faster when injected subcutaneously than intradermally. In this preliminary trial, however, we noted that long-lasting (1 to several years) results were achieved at a much higher rate when HA was injected onto the bone than into the dermis, subcutaneous adipose tissue, or muscle. In 86.6% of injected sites, more than half of the augmented volume was maintained for more than 1 year. Almost all patients in this study showed a high level of satisfaction, and the long-lasting results were from a single treatment session accompanied by minimal downtime.
Previously, some clinicians have reported occasional long-lasting results following HA injections,8 although the conditions and reasons for the longevity remain unclear. Our results suggest that onlay HA injections to the bone lead to semipermanent volumizing effects and that the final retention rate was high and relatively predictable. Our qualitative assessment of the augmented tissue using MRIs indicated that injected HA did remain only partly in the injected condition, as most of the HA exhibited altered intensity on MRIs, suggesting that injected HA was degraded and partly replaced with other materials as discussed below.
Possible Mechanism of Long-lasting Effects
Although HA injection to the breast for cosmetic augmentation is controversial,8 HA has also been used for large-volume (20–120 ml) volumization in breast or any other sites.9–11 Capsular wall formation after HA injection was observed frequently after a bolus and a large-volume injection.9 It is suspected that the minimization of HA nodule surface area by a bolus large-volume injection delays the absorption process and may lead to HA cyst formation before complete absorption. Our results showed no significant correlation between longevity of volumizing effects and injection volume, suggesting that another mechanism is involved in the longevity.
The capsular formation after HA injection has also been shown by an experimental study.12 Injected HA is surrounded by macrophages, but the resultant inflammatory changes are much less than those that accompany injection of other materials, such as synthetic materials or necrotized fat tissue.12 Thus, an HA cyst may be a relatively acceptable condition compared to a foreign body granuloma or an oil cyst after fat grafting, both of which are known to be problematic with sustained inflammation.
In our study, HA was injected to produce small nodules (0.02–0.15 ml each), although some of the nodules may have been overlapped during the injection procedure. A previous clinical study lends some support to our results, as relatively long-lasting volumizing effects were observed following submuscular injection of HA immediately above the periosteum with an 18-gauge blunt cannula, although the reason for the observed longevity remained unclear.13 In our study, the radiographic images at 12 months demonstrated heterogeneous intensity of the injected space and surrounding structure with low intensity on both T1- and T2-weighted images, strongly suggesting that the space was surrounded by a fibrotic capsule and fibrous and/or calcified tissue was partially formed within the capsule. This phenomenon of fibrosis and/or calcification/ossification was also confirmed by our clinical inspection at 12 months with a small needle inserted into the tissue, as we clearly detected an abnormally hard tissue similar to a fibrous and calcified sponge on the bone. The tissue neogenesis may result from persistent activation of resident stem cells located in the periosteum rather than from formation of an HA cyst. The periosteum is known to contain stem cells multilayered in its most inner cambial zone14,15; the periosteal stem cells perform essential functions for bone generation.16 Continuous inflammatory reactions accompanying phagocytosis of HA may stimulate the periosteum and induce gradual replacement of injected HA with newly formed fibrotic tissue and/or calcification/ossification. Although we did not use in our series, hyaluronidase may be useful to discriminate the volumes between the remaining HA and newly formed tissue. The underlying mechanism of the long-lasting effects is to be elucidated, and further studies are needed.
Perspective for Treatment Applications
We hypothesize that the volumizing effects of the treatment proposed in this study result from tissue induction through activation of periosteum-resident stem cells. Plastic surgeons occasionally encounter similar phenomena in clinical practice. One example is cauliflower ear, and this hypertrophied tissue is seen after repeated traumatic hematoma in the auricle17 (Fig. 10). The perichondrium has the potential for chondrogenesis,18 and after hematoma formation (perichondrium detachment from the cartilage), activated perichondrium-resident stem cells may fill the dead space with chondrogenesis, fibrosis, and/or ossification. Secondly, new bone formation at the edge of a tissue expander was experimentally demonstrated in rabbits,19 and this bone formation was induced by activated periosteal stem cells in the elevated periosteum. We also confirmed the presence of osteogenesis at the edge of a tissue expander after human scalp expansion (Fig. 11).
Figure 10.
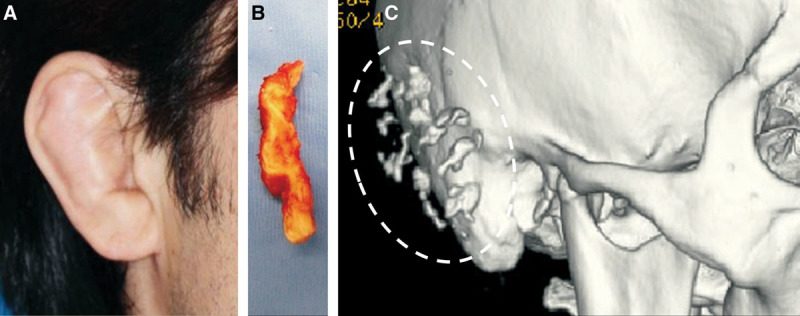
Chondrogenesis and ossification induced by repeated trauma/hematoma to the auricle. A 38-year-old Japanese male presented with auricular hypertrophy (cauliflower ear) on both sides due to long-term judo training (A). An otoplasty involving resection of the hypertrophied tissue was performed, and chondrogenesis containing many ossified areas was observed in the surgical specimen (B). The preoperative CT scan revealed multiple ossifications in the auricle (C).
Figure 11.
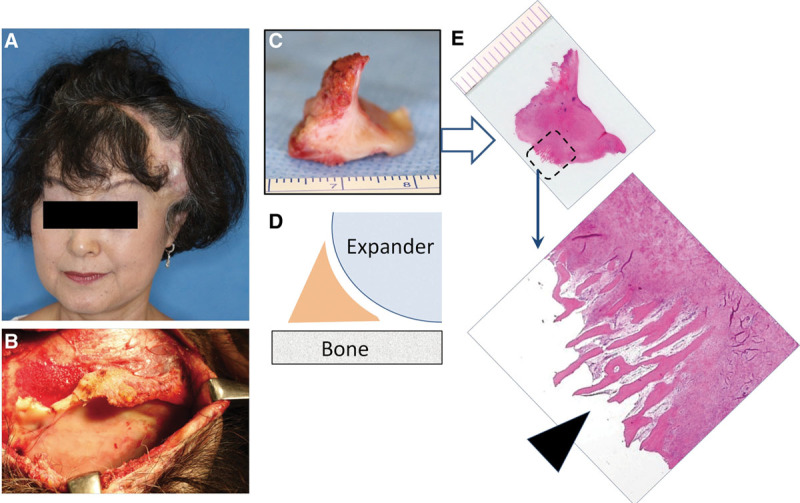
Ossification induced by sustained periosteum suspension at the edge of tissue expander (TE) capsule. A 58-year-old Japanese woman underwent scalp expansion with a TE for treatment of traumatic alopecia in temporal-frontal region (A). When removing the TE (B), we obtained the newly formed tissue specimen at the edge of TE (C, D). Histology (H&E staining; E) confirmed the presence of ossification (osteogenesis) at the lateral bottom edge of the TE capsule (arrows). It is considered that the sustained mechanical force suspended the periosteum from the bone and induced the ossification.
Based on our hypothesis, the onlay HA injection to the bone can be applied to any site that is supported by underlying bone, and therefore, this treatment will be useful for a wide range of cosmetic and reconstructive purposes (Fig. 12). For facial rejuvenation, this semipermanent volumization can be applied for volume restoration in the temple, lid-cheek junction, tear trough, midcheek (nasojugal) groove, nasolabial fold, marionette line, and jaw line, although it cannot be applied to the buccal region and the upper and lower palpebral area. Cosmetic facial contouring procedures such as nasal and chin enhancements are also good candidates. We also found that using a larger volume (10–20 ml per session) of HA enabled reconstruction of major facial defects such as Parry–Romberg syndrome, lupus erythematosus profundus, localized scleroderma, hemifacial microsomia, and posttraumatic or postsurgical craniomaxillofacial deformity, though it usually needs multiple sessions of this procedure. In addition, defects of the palate and nasal base in cleft lip and palate patients may also be corrected with the HA treatment. Thus, we can use this method to treat any tissue defects (not only the face) as an alternative to fat grafting or bone grafting, as long as the target area has a healthy bony floor. Similarly, it is likely that in addition to HA, any type of injectable material will work similarly if the substance remains in the space for weeks or months and can be injected through a small needle.
Figure 12.
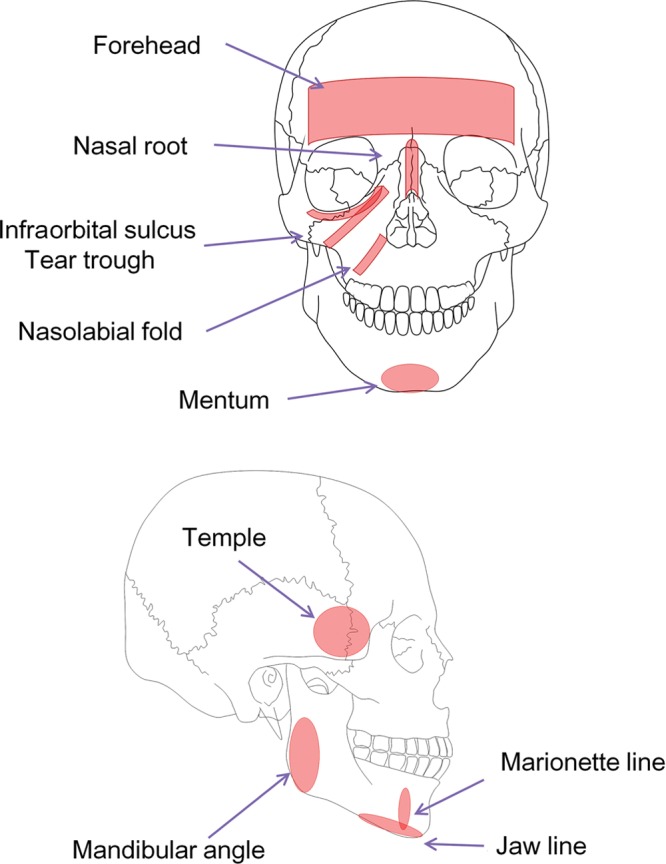
Applicable sites on the face. Onlay injection of HA can be applied to any site supported by underlying bony structures. Therefore, the buccal region and the upper and lower palpebral area cannot be treated due to the absence of a bony floor.
CONCLUSIONS
We proposed the onlay injecting method of an absorbable HA filler onto the bone instead of conventional intradermal or subcutaneous injection methods. Our clinical results showed excellent semipermanent volumizing effects without any major complications. The injected HA was slightly absorbed during the 12-month follow-up period. The injected space was considered to be preserved by a combination of capsular formation, fibrous/calcified tissue formation within the capsule, and persistent HA deposits. In addition to the high efficacy and the longevity of volumization, this treatment has many advantages, including safety, minimal invasiveness, and short downtime. Onlay injection of HA to the bone, which likely activates periosteum-resident stem cells, is a novel therapeutic concept and can be applied to a variety of cosmetic and reconstructive needs as a much less invasive alternative to fat or bone grafting. Further studies are needed to examine the perpetuity of the effects, elucidate the tissue induction mechanism, and optimize the treatment protocol.
ACKNOWLEDGMENT
We thank Mr. Yuichi Suzuki and Mr. Yasushi Watanabe for technical assistance in collecting and analyzing data from MRIs.
PATIENT CONSENT
Patients provided written consent for the use of their images.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Fagien S. Facial soft-tissue augmentation with injectable autologous and allogeneic human tissue collagen matrix (autologen and dermalogen). Plast Reconstr Surg. 2000;105:362–373; discussion 374. doi: 10.1097/00006534-200001000-00057. [DOI] [PubMed] [Google Scholar]
- 2.Goa KL, Benfield P. Hyaluronic acid. A review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs. 1994;47:536–566. doi: 10.2165/00003495-199447030-00009. [DOI] [PubMed] [Google Scholar]
- 3.Lupo MP. Hyaluronic acid fillers in facial rejuvenation. Semin Cutan Med Surg. 2006;25:122–126. doi: 10.1016/j.sder.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Carruthers JD, Glogau RG, Blitzer A Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type a, hyaluronic acid dermal fillers, and combination therapies—consensus recommendations. Plast Reconstr Surg. 2008;121(5 Suppl):5S–30S; quiz 31S. doi: 10.1097/PRS.0b013e31816de8d0. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev M, Anantheswar Y, Ashok B, et al. Facial granulomas secondary to injection of semi-permanent cosmetic dermal filler containing acrylic hydrogel particles. J Cutan Aesthet Surg. 2010;3:162–166. doi: 10.4103/0974-2077.74493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olenius M. The first clinical study using a new biodegradable implant for the treatment of lips, wrinkles, and folds. Aesthetic Plast Surg. 1998;22:97–101. doi: 10.1007/s002669900172. [DOI] [PubMed] [Google Scholar]
- 7.Gensanne D, Josse G, Schmitt AM, et al. In vivo visualization of hyaluronic acid injection by high spatial resolution T2 parametric magnetic resonance images. Skin Res Technol. 2007;13:385–389. doi: 10.1111/j.1600-0846.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 8.Micheal JM. Is breast augmentation using hyaluronic acid safe? Aesthetic Plast Surg. 2010;34:65–68. doi: 10.1007/s00266-009-9450-9. [DOI] [PubMed] [Google Scholar]
- 9.Heden P, Olenius M, Tengvar M. Macrolane for breast enhancement: 12-month follow-up. Plast Reconstr Surg. 2011;127:850–860. doi: 10.1097/PRS.0b013e318200ae57. [DOI] [PubMed] [Google Scholar]
- 10.McCleave MJ, Grover R, Jones BM. Breast enhancement using Macrolane™: a report of complications in three patients and a review of this new product. J Plast Reconstr Aesthet Surg. 2010;63:2108–2111. doi: 10.1016/j.bjps.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Inglefield C. Early clinical experience of hyaluronic acid gel for breast enhancement. J Plast Reconstr Aesthet Surg. 2011;64:722–729. doi: 10.1016/j.bjps.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Cossío S, Castaño-Oreja MT. Biocompatibility of two novel dermal fillers: histological evaluation of implants of a hyaluronic acid filler and a polyacrylamide filler. Plast Reconstr Surg. 2006;117:1789–1796. doi: 10.1097/01.prs.0000214656.07273.b0. [DOI] [PubMed] [Google Scholar]
- 13.Lowe NJ, Grover R. Injectable hyaluronic acid implant for malar and mental enhancement. Dermatol Surg. 2006;32:881–885; discussion 885. doi: 10.1111/j.1524-4725.2006.32190.x. [DOI] [PubMed] [Google Scholar]
- 14.Malizos KN, Papatheodorou LK. The healing potential of the periosteum molecular aspects. Injury. 2005;36 Suppl 3:S13–S19. doi: 10.1016/j.injury.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Chang H, Knothe Tate ML. Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl Med. 2012;1:480–491. doi: 10.5966/sctm.2011-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Naik A, Xie C, et al. Periosteal stem cells are essential for bone revitalization and repair. J Musculoskelet Neuronal Interact. 2005;5:360–362. [PubMed] [Google Scholar]
- 17.Ohlsén L, Skoog T, Sohn SA. The pathogenesis of cauliflower ear. An experimental study in rabbits. Scand J Plast Reconstr Surg. 1975;9:34–39. doi: 10.3109/02844317509022854. [DOI] [PubMed] [Google Scholar]
- 18.Benecke JE, Jr, Gadre AK, Linthicum FH., Jr Chondrogenic potential of tragal perichondrium: a cause of hearing loss following stapedectomy. Laryngoscope. 1990;100:1292–1293. doi: 10.1288/00005537-199012000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Abrahamsson P, Isaksson S, Gordh M, et al. Periosteal expansion of rabbit mandible with an osmotic self-inflatable expander. Scand J Plast Reconstr Surg Hand Surg. 2009;43:121–125. doi: 10.1080/02844310902771798. [DOI] [PubMed] [Google Scholar]


