Abstract
Background:
After surgery it is often recommended that patients should refrain from strenuous physical activity for 4–6 weeks. This recommendation is based on the time course of wound healing. Here, we present an overview of incisional wound healing with a focus on 2 principles that guide our postoperative recommendations: the gain of tensile strength of a wound over time and the effect of mechanical stress on wound healing.
Methods:
A systematic search of the English literature was conducted using OVID, Cochrane databases, and PubMed. Inclusion criteria consisted of articles discussing the dynamics of incisional wound healing, and exclusion criteria consisted of articles discussing nonincisional wounds.
Results:
Experiments as early as 1929 laid the groundwork for our postoperative activity recommendations. Research using animal models has shown that the gain in tensile strength of a surgical wound is sigmoidal in trajectory, reaching maximal strength approximately 6 weeks postoperatively. Although human and clinical data are limited, the principles gained from laboratory investigation have provided important insights into the relationship among mechanical stress, collagen dynamics, and the time course of wound healing.
Conclusion:
Our postoperative activity recommendations are based on a series of animal studies. Clinical research supporting these recommendations is minimal, with the most relevant clinical data stemming from early motion protocols in the orthopedic literature. We must seek to establish clinical data to support our postoperative activity recommendations so that we can maximize the physiologic relationships between wound healing and mechanical stress.
Wound healing consists of three phases: inflammation, proliferation, and remodeling.1 Each of these phases interacts through complex cellular and molecular processes, with the purpose of establishing and maintaining wound closure. An incisional wound is initially held together with suture material, but it must gain enough inherent strength to maintain closure.2 Patients with surgical wounds are discharged home with instructions to refrain from strenuous physical activity for 4–6 weeks. This postoperative regimen is outlined in almost all textbooks of surgery.3–5 What is the basis on which this recommendation was developed? Are there valid scientific data to support the rationale for not resuming strenuous physical activities for at least 4 weeks postoperatively?
Two principles of wound healing have provided the foundation for our postoperative recommendations. The first principle involves the gain of tensile strength of wounds over time. Understanding the process of strength gain is fundamental to assessing the safety of patient activity. The second principle involves the effect of mechanical stress on tensile strength gain. Here, we review the literature behind these principles so that we may use this information to inform our postoperative recommendations.
All surgical wounds are not created equal, as there are a wide range of wound types encountered depending on the surgery performed. It is important to note, therefore, that the information obtained from our literature search may not apply to all wound types and will need to be interpreted as such. Our goal is to review what literature is available on the topic of incisional wounds where data have been published. We believe that this will provide important insights into what is known and unknown to better guide us in forming evidence-based recommendations.
METHODS
A systematic search of the English literature was conducted using OVID, Cochrane databases, and PubMed. Article selection was limited to those published between January 1, 1900, and August 1, 2012. Inclusion criteria included articles discussing the dynamics of wound healing over time, factors influencing wound healing, and the molecular biology of wound healing. Our initial search resulted in a total of 14,678 articles, and filtering with inclusion criteria resulted in a total of 6708 published articles. Inclusion criteria required articles to relate to wound-healing biology, wound-healing dynamics, patient activity and wound healing, and wound healing of surgical incisions. Exclusion criteria included articles discussing nonincisional wounds. Title screen yielded a total of 255 potentially relevant articles. Abstract review resulted in a total of 120 articles, all of which were reviewed in their entirety for content and relevance to the current study. A total of 24 articles not in the original search were cited and reviewed for content. In total, 109 articles were found to meet all criteria for review and are presented here (Fig. 1).
Fig. 1.
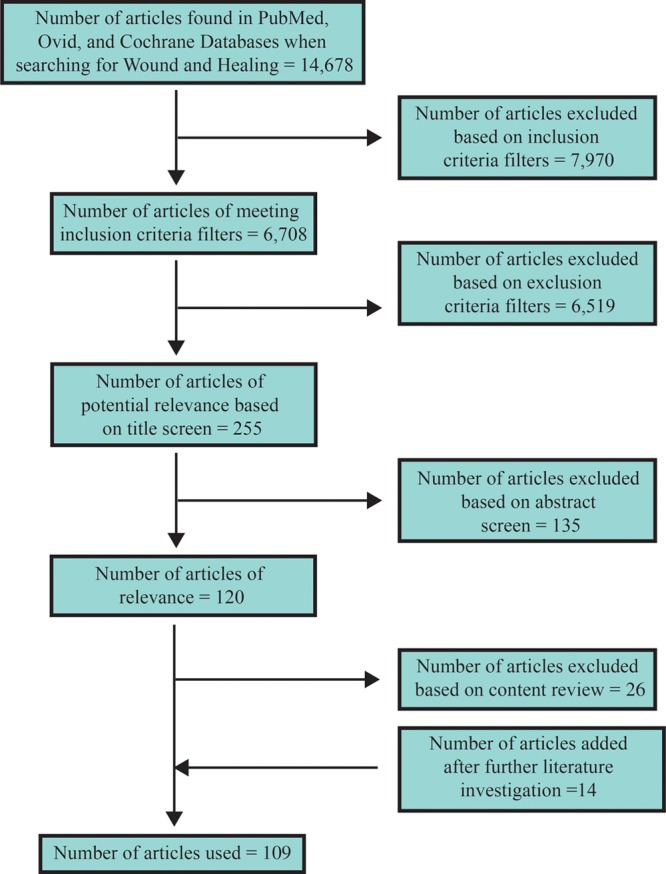
Flow diagram of article selection.
TENSILE STRENGTH
Animal Studies
Reliable analysis of the quality of wound healing via visual inspection is inaccurate due to the inability to see beneath the epithelium. Therefore, progress must be measured using physiologic parameters. Tensile strength is defined as the breaking strength of a material divided by its cross-sectional area and is therefore the most accurate physiologic measure for assessing a wound’s ability to withstand tension.6,7 A series of classic articles using tensile strength as a measure of wound healing in animal models has led to the recommendation that patients should abstain from full physical activity for 4–6 weeks after surgery.8–12
One of the earliest publications regarding wound tensile strength was written by Howes et al13 in 1929. In a rat model, it was found that tensile strength of a surgical wound was almost negligible until postoperative day 5, after which it increased to a maximum at 2 weeks. It was also concluded that over time the final strength of the wound became equal to that of intact skin based on extrapolation of their results (Fig. 2A).9,12
Fig. 2.
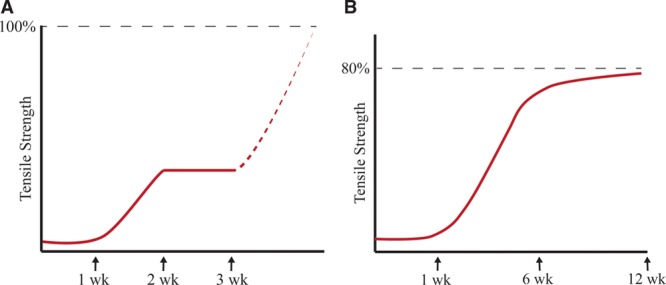
A, Adaptation of Howes et al’s original experiments. B, Adaptation of Levenson et al’s tensile strength versus time curve.
In 1965, Levenson et al found that rather than 2 discrete phases, there was 1 prolonged phase of tensile strength gain with a sigmoidal trajectory (Fig. 2B).12,14 Tensile strength continued to increase rapidly until week 6, after which it slowly reached a maximum at 3 months (Fig. 2B).12,14 In addition, Levenson et al found that healed skin only reached 80% of the tensile strength of unwounded skin.12
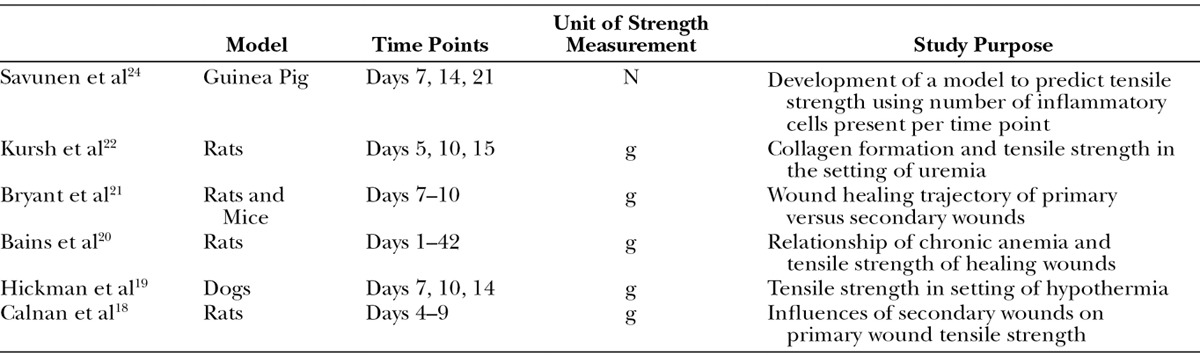
These findings are the foundation for our modern understanding of the time course of wound healing. Since the original results of Levenson et al, numerous investigations using animal models support the conclusion that 1 week postoperatively, there is a rapid gain in tensile strength lasting approximately 6 weeks.15–25 To demonstrate this, we developed a curve reflecting the findings of individual studies (Fig. 3A) and one with averaged data (Fig. 3B) to explore the trend (Table 1). Studies reporting breaking strength rather than tensile strength were excluded (Table 2). These results first show that the time course of wound healing is consistent among species.11 It is also evident from these graphs that the trend of tensile strength gain over time of a healing wound in animal models has remained consistent since the original studies by Howes and Levenson.
Fig. 3.
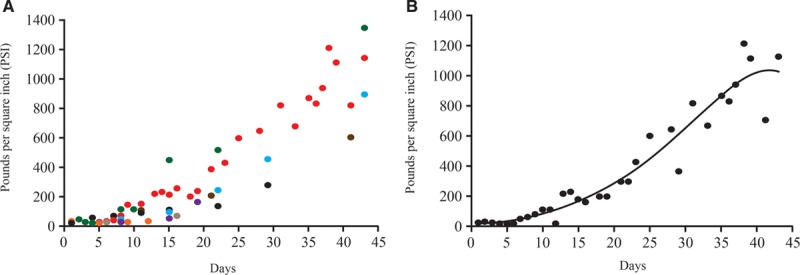
Combined results of studies listed in Table 1 with units converted to PSI. A,  Howes et al13;
Howes et al13;  Levenson et al12;
Levenson et al12;  Forrester et al14;
Forrester et al14;  Garden et al21;
Garden et al21;  Wickens et al23;
Wickens et al23;  Fried et al, 200035;
Fried et al, 200035;  Kaplan et al47;
Kaplan et al47;  Cornacoff et al24. B, Average PSI across all studies. PSI = pounds per square inch.
Cornacoff et al24. B, Average PSI across all studies. PSI = pounds per square inch.
Table 1.
Summary of Studies Reporting Tensile Strength Included in Figure 3
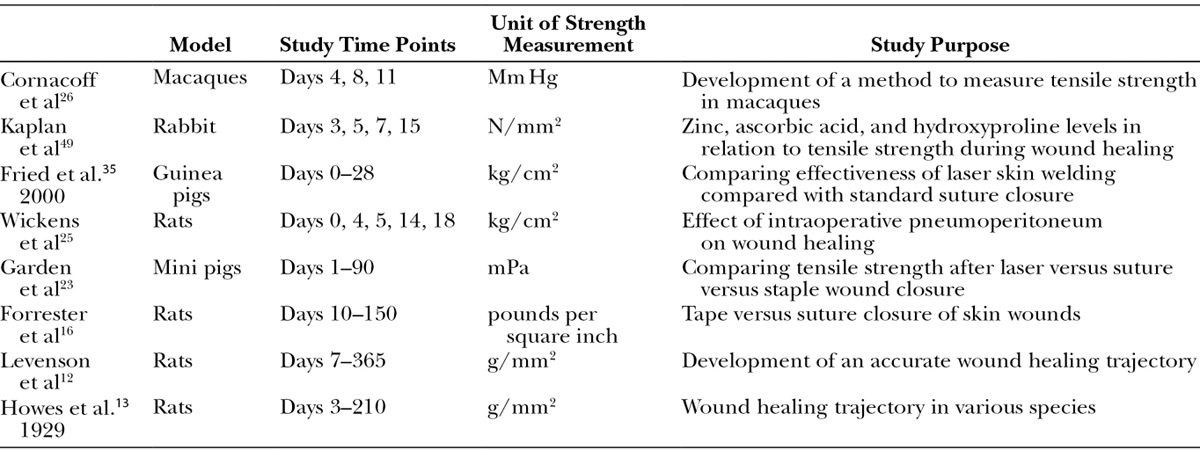
Table 2.
Summary of Studies Reporting Breaking Strength
Human Studies
There are limited human data on the wound-healing trajectory. Given the obvious ethical barriers to studying tensile strength in humans, few studies have been published on the topic. Sandblom and later Lindstedt et al measured tensile strength at a single time point in human patients but did not measure strength gain over time.26,27 Other studies have sought to measure wound-healing progression in humans through subcutaneous polytetrafluoroethylene tubes that measure hydroxyproline content and other markers of collagen maturation.28–31 For example, a recent randomized trial investigated this concept by measuring hydroxyproline concentration and mRNA levels for type I and III procollagen.32 This was correlated with wound strength by measuring breaking strength of biopsies taken from the same wounds. If we could reliably correlate molecular findings such as these to a tensile strength curve, we may be able to indirectly assess the effect of patient activities on tensile strength.
PHYSIOLOGY OF THE WOUND-HEALING TRAJECTORY
The laboratory data elaborating the gain of tensile strength of incisional wounds over time are supported by the physiology of wound healing during the postoperative period. As with the biomechanical studies, much of the data regarding the physiology and histology of surgical wound healing come from animal models. The information presented below serves as a model for understanding how a simple incisional wound gains strength over time.
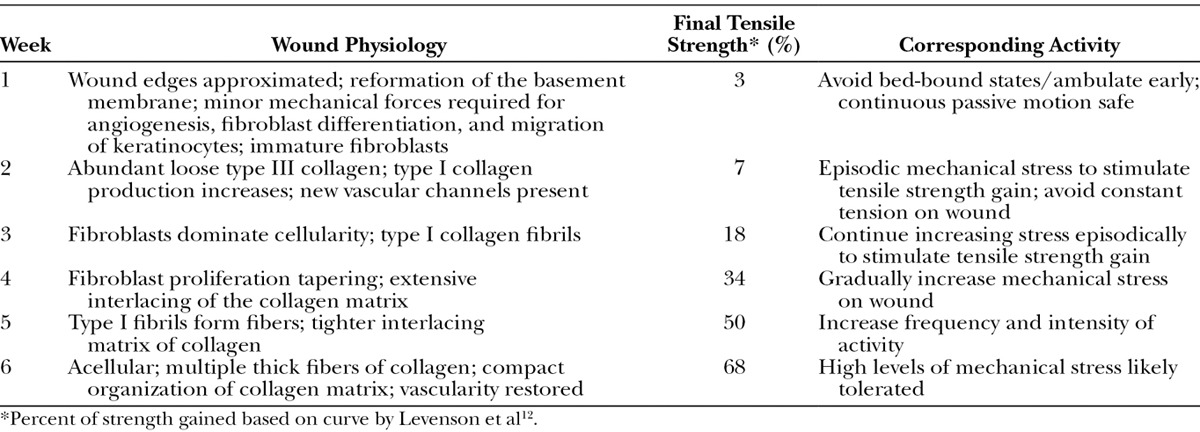
Week 1
Within the first 4 days after surgery, a surgical wound has minimal inherent strength because the dermal edges are held together solely by a hemostatic plug and sutures.12,33,34 Cytokines and growth factors released by local platelets, notably platelet-derived growth factor, stimulate the migration of the inflammatory cells to the wound site.36 This phase correlates to the latency period of the wound-healing trajectory (Fig. 2B). The latency period allows for proper wound healing by giving inflammatory cells, such as neutrophils and macrophages, time to proliferate and clean the wound of necrotic debris and bacteria before complete closure.37,38
In addition, throughout the latency period, inflammatory cells release transforming growth factor beta (TGF-β) and epidermal growth factor (EGF). These growth factors have been shown to stimulate immature fibroblasts to migrate into the wound and secrete type III collagen.35,39–42 Type III is a loose reticular form of collagen that interacts with the extracellular matrix, giving the wound its first degree of inherent strength.
Week 2
During the second postoperative week, fibroblasts proliferate rapidly and begin to produce type I collagen in addition to type III.35,39,43–45 Connective tissue growth factor (CTGF), a downstream mediator of TGF-β1, is believed to be the signal for increased production of type I collagen (Fig. 4).3,4,46,47 It is the accumulation of propeptide type I collagen in the extracellular space during this phase that leads to a significant increase in tensile strength.47,48 Procollagen is cleaved by peptidases to facilitate the formation of fibrils, which are strong, covalently linked collagen peptides (Fig. 5). Type III collagen does not undergo fibril formation due to its amino acid structure; therefore, type III collagen is weaker than type I.35 Although the amount of type I collagen increases during the second week, type III remains the dominant form. Thus, the strength of the wound is still limited to less than 10% of its final strength. Clinically, this is supported by the highest rate of wound dehiscence occurring during the second postoperative week.10,12,21,23,39,49 Disruption of the wound at this time can delay and reduce the slope of tensile strength gain.18
Fig. 4.
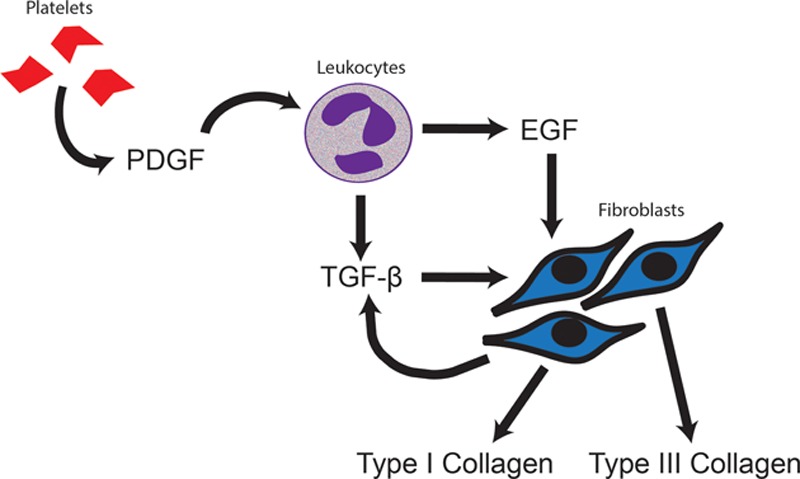
Platelet-derived growth factor (PDGF); epidermal growth factor (EGF); transforming growth factor beta (TGF-β). PDGF released by activated platelets stimulates leukocytes to secrete growth factors, such as EGF and TGF-β. These growth factors stimulate the migration of fibroblasts to the wound site. TFG-β is the primary signal for type I and III collagen production and is released by both leukocytes and fibroblasts.
Fig. 5.
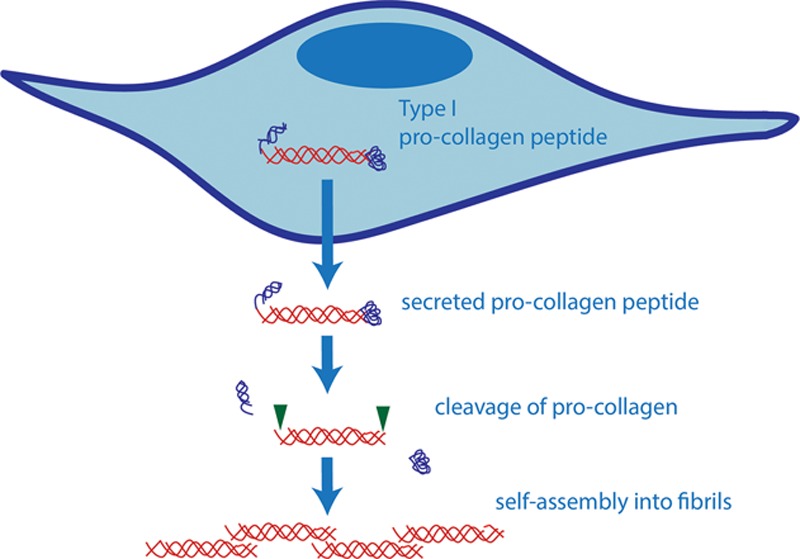
Collagen synthesis pathway. Procollagen with N and C terminal cleavage domains is secreted into the extracellular space by fibroblasts. The N and C terminal chains are cleaved by peptidases, resulting in a mature collagen peptide. The peptides then self-assemble into fibrils.
Weeks 3–5
The wound’s strength grows rapidly as type I fibrils cross-link and aggregate into large fibers.35,40,42,45,50 This step improves the tensile strength of incisional wounds because the fibers bind to cell-membrane proteins across the wound interface.51,52 The fibers, therefore, act as a bridge between the 2 wound edges and provide significant resistance to tensile force (Fig. 6).
Fig. 6.
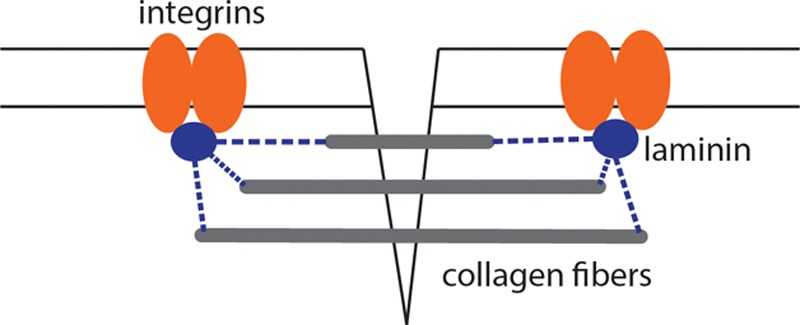
Collagen fibrils aggregate into fibers, which have the ability to bind cell-membrane proteins such as integrins. The collagen, therefore, serves as a bridge among the wound edges and provides significant resistance to tensile force across the incision.
As the healing process continues, the fibers further organize by forming a dense three-dimensional matrix that is stronger than the individual fibers alone.12,53 Matrix formation is mediated by interactions between the collagen fibers and the surrounding extracellular matrix components, including heparin sulfate and other proteoglycans.54,55 Growth factors released by fibroblasts and other late inflammatory mediators are also believed to play a role in matrix organization.40,56 This period corresponds to the continued steep slope of the wound-healing trajectory, and by 4 weeks postoperatively, the gain in tensile strength is over 50% complete.12,16,23
Week 6 to 1 Year
By week 6, the concentration of strong collagen fibers begins to resemble that of intact skin. The collagen matrix matures to become denser and more uniformly aligned.12,35,47 Wound disruption is unlikely because the tensile strength of the wound is approaching 80% of intact skin.12 This is why nearly all surgery textbooks recommend advising patients against resuming strenuous physical activity until 6 weeks postoperatively.1,4,57 Over the next 2 months, a small gain in strength will result from further aggregation of type I collagen fibrils into fibers.43–45,47 The alignment of those fibers into an organized scar will continue throughout 1 year.10,12,35 This process correlates with the plateau of the wound-healing curve.
MECHANICAL STRESS AND WOUND HEALING
We have discussed in detail the first guiding principle behind postoperative activity recommendations: the gain in tensile strength of a wound over time. The second key principle is the effect that mechanical stress has on the strength curve. Although excess mechanical stress through patient activity can lead to wound disruption, we do not keep our patients bedbound for 4–6 weeks postoperatively. Aside from the risks of venous thromboembolism, pneumonia, and pressure ulcers, there are reasons related to wound healing as well that encourage us to recommend limited activity. Tensile strength gain depends on collagen production and organization, which is intimately related to mechanical stress.12,18,58 This is a principle that has significant implications in clinical practice, as it formed the basis for Ilizarov’s bony distraction experiments.59–61
Laboratory Studies
Mechanical stress is required for the gain of tensile strength of a wound over time.62 This has been demonstrated in studies using space environments and limb offloading models, where the strength gain of healing wounds is significantly impaired in the absence of mechanical stimuli.62–66 Davidson et al found that wounds from rats in space had 62% less collagen after 10 days compared with controls in a normal gravity environment.63 The mechanism by which mechanical stress stimulates wound healing is not entirely understood but is believed to be due to the stimulation of TGF-β and other growth factor signaling pathways by mechanoreceptors in the skin.62,67 This theory is supported by investigations showing that mechanical force on a wound is required for angiogenesis and the migration of fibroblasts, all of which have been associated with TGF-β activity.65,68–71
Mechanical stress is not only required for normal wound healing, but it also accelerates the gain in tensile strength. This is likely due to the upregulation of the aforementioned growth factors, enhancing collagen production and angiogenesis.65,71–73 This principle was demonstrated by Langrana et al, who found that episodic and sequential tissue expansion of a surgical incision beginning 1 week after surgery was not only tolerated but resulted in significantly greater tensile strength at each time point compared with controls.74 Similarly, van Royen et al found that joint rotation initiated 1 week after surgery in rabbits significantly increased the tensile strength of healing wounds compared with immobilized controls.66
Clinical Studies
The clinical literature directly supporting our postoperative recommendations that patients avoid strenuous activity for 4–6 weeks postoperatively is minimal. However, the use of controlled mechanical stress is supported by the orthopedic literature, where the safety of early postoperative movement has been well documented. Joint rotation protocols beginning early in the postoperative period after orthopedic procedures are common and do not confer additional wound-healing complications.75–80 A study by Wasilewski et al even showed a reduced incidence of surgical wound-healing complications in those undergoing early movement protocols.81 These studies challenge us to consider what role early controlled movement may have in surgical wound healing within the realm of plastic surgery. Incision-directed exercises, in addition to simple ambulation, may stimulate particular wound types and accelerate tensile strength gain.66,76–78,80,81
MECHANICAL STRESS AND SCARRING
Because it is uncommon for elective surgical wounds to come apart in the postoperative period, the most practical concern regarding patient activity in the postoperative period is the possibility of abnormal scarring.71,80–84 The relationship between mechanical stress and collagen dynamics can have negative implications for scar formation.21,50,58,87,88 This is supported by the increased incidence of abnormal scar formation in areas subject to the most movement, such as the scapular region and chest.69,89,90 Minimization of movement over these areas has been shown to reduce the degree of abnormal scarring.73,83,91,92 A number of investigations have found that mechanical force on wounds, particularly cyclical force, stimulates the release of growth factors such as TGF-β.56,93–97 The upregulation of these factors and their receptors is associated with keloid and hypertrophic scar formation.97–100 These studies suggest that although mechanical force has the potential to accelerate tensile strength gain, the trade-off may be an increase in abnormal scarring.
Conversely, some investigations have shown reduced scar formation in the setting of controlled mechanical stress. Langrana et al found that in episodically expanded surgical incisions, scar formation was significantly reduced on both gross and microscopic levels.74 Other histologic studies have found earlier and more uniform alignment of collagen in wounds subject to controlled mechanical stress.16,87,101–109 On a molecular level, a recent study found that CTGF was significantly downregulated after 24 hours of cyclically stretching fibroblasts in culture.87 The exact mechanism by which earlier collagen organization reduces later scar formation is unknown. However, one theory is that improved tensile strength early in the wound-healing process may protect the wound from later excessive shear stress that would otherwise cause excessive scarring.70
DISCUSSION
The work of Howes and Levenson laid the groundwork for our clinical recommendation to have patients abstain from strenuous physical activity for 4–6 weeks after surgery. From the scientific data available and our understanding of wound-healing physiology, we can indirectly make informed recommendations for patients after surgery (Table 3). It is important to note, however, that we do not yet have the scientific basis to directly apply the data to human patient activity. Further research is needed to understand how these biomechanical results can be extrapolated to human patients. We must also investigate how the time course of wound healing varies in different cutaneous regions and from different types of incisions.81 This knowledge would allow more accurate and region-specific postoperative activity planning, which would have a direct effect on patient care.
Table 3.
Summary of Physiology and Activity Recommendations
Another area of research that has shown great progress is the use of molecular markers for collagen maturation, such as hydroxyproline content and mRNA levels of collagen subtypes. This potential area of research is more feasible in humans than studying tensile strength directly as it would be much less invasive. Further research is needed to generate a reproducible correlation between subcutaneous markers and the tensile strength curve in humans. Understanding how these molecular markers correspond with patient activities in the postoperative period would provide us with a greater scientific basis for our recommendations.
Early motion research has highlighted potential applications for controlled movement protocols beyond basic patient activities for accelerating wound healing. We need to understand how specific activities or incision-directed exercises fit into the wound-healing curve. It is especially important to understand how specific patient activities in the postoperative period affect scar formation. Although the literature favors a negative impact of mechanical stress on scarring, the available research suggests that the timing and character of mechanical stress are important factors dictating the fibrotic response. There may be a role for controlled mechanical stress early in the postoperative period for certain wound types. Further research is needed to understand how best we can take advantage of the stress-collagen relationship without increasing scar formation.
CONCLUSION
Two principles are fundamental to understanding our postoperative activity recommendations: the process by which wounds gain strength and the effect of mechanical stress on wound healing. Although many studies have explored the time course of wound healing in animal models, further research needs to be done in humans to allow for more accurate and patient-specific protocols. This information will help us translate wound-healing dynamics into clinical practice and improved patient outcomes.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Thorne C, Grabb WC, Smith JW. Grabb and Smith’s Plastic Surgery. 6th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Lindblad WJ. Tensile strength of a wound: its use in human wound healing studies. Wound Repair Regen. 1998;6:178–179. [PubMed] [Google Scholar]
- 3.Souba WW, Fink MP American College of Physicians (2003) ACS Surgery: Principles & Practice. 6th ed. Hamilton, Ont., London: BC Decker; 2008. [Google Scholar]
- 4.Jarrell BE, Carabasi RA. Surgery. 5th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Wiliams & Wilkins; 2008. [Google Scholar]
- 5.Henry MM, Thompson JN. Clinical Surgery. 3rd ed. Edinburgh, New York: Elsevier Saunders; 2012. [Google Scholar]
- 6.Ridge MD, Wright V. The directional effects of skin. A bio-engineering study of skin with particular reference to Langer’s lines. J Invest Dermatol. 1966;46:341–346. [PubMed] [Google Scholar]
- 7.Williams DF, Harrison ID. The variation of mechanical properties in different areas of a healing wound. J Biomech. 1977;10:633–642. doi: 10.1016/0021-9290(77)90063-x. [DOI] [PubMed] [Google Scholar]
- 8.Armitage CM, Howes EL, Mandl I. Enzymes in the healing wound. Surg Forum. 1956;6:54–58. [PubMed] [Google Scholar]
- 9.Howes EL, Harvey SC. The clinical significance of experimental studies in wound healing. Ann Surg. 1935;102:941–946. doi: 10.1097/00000658-193511000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howes EL, Harvey SC. The age factor in the velocity of the growth of fibroblasts in the healing wound. J Exp Med. 1932;55:577–590. doi: 10.1084/jem.55.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howes EL, Harvey SC. Recent studies in wound healing. Yale J Biol Med. 1930;2:285–294. [PMC free article] [PubMed] [Google Scholar]
- 12.Levenson SM, Geever EF, Crowley LV, et al. The healing of rat skin wounds. Ann Surg. 1965;161:293–308. doi: 10.1097/00000658-196502000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howes EL, Sooy JW, Harvey SC. The healing of wounds as determined by their tensile strength. JAMA. 1929;92:42–48. [Google Scholar]
- 14.Nimini ME, de Guia E, Bavetta LA. Collagen, hexosamine and tensile strength of rabbit skin during aging. J Invest Dermatol. 1966;47:156–158. doi: 10.1038/jid.1966.120. [DOI] [PubMed] [Google Scholar]
- 15.Forrester JC, Zederfeldt BH, Hayes TL, et al. Wolff’s law in relation to the healing skin wound. J Trauma. 1970;10:770–779. doi: 10.1097/00005373-197009000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Watts GT, Baddeley MR, Wellings R, et al. The nature of wound healing: experimental tensile strength studies with Deca Durabolin and S35. Ann Surg. 1965;162:109–112. doi: 10.1097/00000658-196507000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calnan J, Fry HJ, Saad N. Wound healing and wound hormones. A study of tensile strength in rats. Br J Surg. 1964;51:448–456. doi: 10.1002/bjs.1800510614. [DOI] [PubMed] [Google Scholar]
- 18.Hickman GA, Paletta FX. Effect of hypothermia on wound healing in the canine hind limb: tensile strength study. Plast Reconstr Surg. 1965;35:424–427. doi: 10.1097/00006534-196504000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Bains JW, Crawford DT, Ketcham AS. Effect of chronic anemia on wound tensile strength: correlation with blood volume, total red blood cell volume and proteins. Ann Surg. 1966;164:243–246. doi: 10.1097/00000658-196608000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryant WM, Weeks PM. Secondary wound tensile strength gain: a function of collagen and mucopolysaccharide interaction. Plast Reconstr Surg. 1967;39:84–91. doi: 10.1097/00006534-196701000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Kursh ED, Klein L, Schmitt J, et al. The effect of uremia on wound tensile strength and collagen formation. J Surg Res. 1977;23:37–42. doi: 10.1016/0022-4804(77)90188-3. [DOI] [PubMed] [Google Scholar]
- 22.Garden JM, Robinson JK, Taute PM, et al. The low-output carbon dioxide laser for cutaneous wound closure of scalpel incisions: comparative tensile strength studies of the laser to the suture and staple for wound closure. Lasers Surg Med. 1986;6:67–71. doi: 10.1002/lsm.1900060114. [DOI] [PubMed] [Google Scholar]
- 23.Savunen TJ, Viljanto JA. Prediction of wound tensile strength: an experimental study. Br J Surg. 1992;79:401–403. doi: 10.1002/bjs.1800790508. [DOI] [PubMed] [Google Scholar]
- 24.Wickens JC, Whelan RL, Allendorf JD, et al. Wound tensile strength and contraction rate are not affected by laparotomy or pneumoperitoneum. Surg Endosc. 1998;12:1166–1170. doi: 10.1007/s004649900808. [DOI] [PubMed] [Google Scholar]
- 25.Cornacoff JB, Howk K, Pikounis B, et al. Development of a method for the evaluation of wound tensile strength in cynomolgus macaques. J Pharmacol Toxicol Methods. 2008;57:74–79. doi: 10.1016/j.vascn.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Lindstedt E, Sandblom P. Wound healing in man: tensile strength of healing wounds in some patient groups. Ann Surg. 1975;181:842–846. doi: 10.1097/00000658-197506000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandblom P. Determination of the tensile strength of the healing wound as a clinical test. J Int Chir. 1953;13:Extra pages, 1–Extra pages, 4. [PubMed] [Google Scholar]
- 28.Goodson WH, III, Hunt TK. Development of a new miniature method for the study of wound healing in human subjects. J Surg Res. 1982;33:394–401. doi: 10.1016/0022-4804(82)90054-3. [DOI] [PubMed] [Google Scholar]
- 29.Diegelmann RF, Lindblad WJ, Cohen IK. A subcutaneous implant for wound healing studies in humans. J Surg Res. 1986;40:229–237. doi: 10.1016/0022-4804(86)90156-3. [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen LN. Collagen deposition in the subcutaneous tissue during wound healing in humans: a model evaluation. APMIS Suppl. 2003:1–56. [PubMed] [Google Scholar]
- 31.Williams JZ, Abumrad N, Barbul A. Effect of a specialized amino acid mixture on human collagen deposition. Ann Surg. 2002;236:369–74; discussion 374. doi: 10.1097/00000658-200209000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danielsen PL, Agren MS, Jorgensen LN. Platelet-rich fibrin versus albumin in surgical wound repair: a randomized trial with paired design. Ann Surg. 2010;251:825–831. doi: 10.1097/SLA.0b013e3181d3548c. [DOI] [PubMed] [Google Scholar]
- 33.Cohen IK, McCoy BJ, Diegelmann RF. An update on wound healing. Ann Plast Surg. 1979;3:264–272. doi: 10.1097/00000637-197909000-00011. [DOI] [PubMed] [Google Scholar]
- 34.White JF, Werkmeister JA, Darby IA, et al. Collagen fibril formation in a wound healing model. J Struct Biol. 2002;137:23–30. doi: 10.1006/jsbi.2002.4460. [DOI] [PubMed] [Google Scholar]
- 35.Fried NM, Walsh JT., Jr Laser skin welding: in vivo tensile strength and wound healing results. Las. Surg. Med. 2000;27:55–65. doi: 10.1002/1096-9101(2000)27:1<55::aid-lsm8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 37.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 38.Casey WJ, Peacock EE, Jr, Chvapil M. Induction of collagen synthesis in rats by transplantation of allogenic macrophages. Surg Forum. 1976;27:53–55. [PubMed] [Google Scholar]
- 39.Lenaers A, Lapiere CM. Type III procollagen and collagen in skin. Biochim Biophys Acta. 1975;400:121–131. doi: 10.1016/0005-2795(75)90132-4. [DOI] [PubMed] [Google Scholar]
- 40.Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 41.Brown GL, Curtsinger LJ, White M, et al. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg. 1988;208:788–794. doi: 10.1097/00000658-198812000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 43.Croft CB, Tarin D. Ultrastructural studies of wound healing in mouse skin. I. Epithelial behaviour. J Anat. 1970;106(Pt 1):63–77. [PMC free article] [PubMed] [Google Scholar]
- 44.Tarin D, Croft CB. Ultrastructural studies of wound healing in mouse skin. II. Dermo-epidermal interrelationships. J Anat. 1970;106(Pt 1):79–91. [PMC free article] [PubMed] [Google Scholar]
- 45.Tarin D, Croft CB. Ultrastructural features of wound healing in mouse skin. J Anat. 1969;105(Pt 1):189–190. [PubMed] [Google Scholar]
- 46.Frazier K, Williams S, Kothapalli D, et al. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 47.Orgel JP, Irving TC, Miller A, et al. Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci USA. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran GN. Molecular structure of collagen. Int Rev Connect Tissue Res. 1963;1:127–182. doi: 10.1016/b978-1-4831-6755-8.50009-7. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan B, Gönül B, Dinçer S, et al. Relationships between tensile strength, ascorbic acid, hydroxyproline, and zinc levels of rabbit full-thickness incision wound healing. Surg Today. 2004;34:747–751. doi: 10.1007/s00595-004-2827-0. [DOI] [PubMed] [Google Scholar]
- 50.Chang PJ, Chen MY, Huang YS, et al. Morphine enhances tissue content of collagen and increases wound tensile strength. J Anesth. 2010;24:240–246. doi: 10.1007/s00540-009-0845-1. [DOI] [PubMed] [Google Scholar]
- 51.Parry DA. The molecular and fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys Chem. 1988;29:195–209. doi: 10.1016/0301-4622(88)87039-x. [DOI] [PubMed] [Google Scholar]
- 52.Jokinen J, Dadu E, Nykvist P, et al. Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem. 2004;279:31956–31963. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- 53.Madden JW, Peacock EE., Jr Studies on the biology of collagen during wound healing. I. Rate of collagen synthesis and deposition in cutaneous wounds of the rat. Surgery. 1968;64:288–294. [PubMed] [Google Scholar]
- 54.Osterholm C, Barczyk MM, Busse M, et al. Mutation in the heparan sulfate biosynthesis enzyme EXT1 influences growth factor signaling and fibroblast interactions with the extracellular matrix. J Biol Chem. 2009;284:34935–34943. doi: 10.1074/jbc.M109.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuart K, Paderi J, Snyder PW, et al. Collagen-binding peptidoglycans inhibit MMP mediated collagen degradation and reduce dermal scarring. PLoS ONE. 2011;6:e22139. doi: 10.1371/journal.pone.0022139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah M, Foreman DM, Ferguson MW. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994;107(Pt 5):1137–1157. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- 57.Janis JE. Essentials of Plastic Surgery Handbook: A UT Southwestern Medical Center Handbook. St. Louis, MO: Quality Medical Pub; 2007. [Google Scholar]
- 58.Madden JW, Peacock EE., Jr Studies on the biology of collagen during wound healing. 3. Dynamic metabolism of scar collagen and remodeling of dermal wounds. Ann Surg. 1971;174:511–520. doi: 10.1097/00000658-197109000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989:263–285. [PubMed] [Google Scholar]
- 60.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989:249–281. [PubMed] [Google Scholar]
- 61.Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990:8–26. [PubMed] [Google Scholar]
- 62.Farahani RM, DiPietro LA. Microgravity and the implications for wound healing. Int Wound J. 2008;5:552–561. doi: 10.1111/j.1742-481X.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davidson JM, Aquino AM, Woodward SC, et al. Sustained microgravity reduces intrinsic wound healing and growth factor responses in the rat. FASEB J. 1999;13:325–329. doi: 10.1096/fasebj.13.2.325. [DOI] [PubMed] [Google Scholar]
- 64.Delp MD. Unraveling the complex web of impaired wound healing with mechanical unloading and physical deconditioning. J Appl Physiol. 2008;104:1262–1263. doi: 10.1152/japplphysiol.90393.2008. [DOI] [PubMed] [Google Scholar]
- 65.Radek KA, Baer LA, Eckhardt J, et al. Mechanical unloading impairs keratinocyte migration and angiogenesis during cutaneous wound healing. J Appl Physiol. 2008;104:1295–1303. doi: 10.1152/japplphysiol.00977.2007. [DOI] [PubMed] [Google Scholar]
- 66.van Royen BJ, O’Driscoll SW, Dhert WJ, et al. A comparison of the effects of immobilization and continuous passive motion on surgical wound healing in mature rabbits. Plast Reconstr Surg. 1986;78:360–368. doi: 10.1097/00006534-198609000-00013. [DOI] [PubMed] [Google Scholar]
- 67.Agha R, Ogawa R, Pietramaggiori G, et al. A review of the role of mechanical forces in cutaneous wound healing. J Surg Res. 2011;171:700–708. doi: 10.1016/j.jss.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 68.Wipff PJ, Rifkin DB, Meister JJ, et al. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogawa R, Okai K, Tokumura F, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20:149–157. doi: 10.1111/j.1524-475X.2012.00766.x. [DOI] [PubMed] [Google Scholar]
- 70.Farahani RM. Wound healing in the context of mechanical strain: “Coupled pendulums” hypothesis. Med Hypotheses. 2007;69:711. doi: 10.1016/j.mehy.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 71.Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibson T, Kenedi RM, Craik JE. The mobile micro-architecture of dermal collagen: a bio-engineering study. Br J Surg. 1965;52:764–770. doi: 10.1002/bjs.1800521017. [DOI] [PubMed] [Google Scholar]
- 73.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254:217–225. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 74.Langrana NA, Alexander H, Strauchler I, et al. Effect of mechanical load in wound healing. Ann Plast Surg. 1983;10:200–208. doi: 10.1097/00000637-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Johnson DP, Eastwood DM. Beneficial effects of continuous passive motion after total condylar knee arthroplasty. Ann R Coll Surg Engl. 1992;74:412–416. [PMC free article] [PubMed] [Google Scholar]
- 76.Ververeli PA, Sutton DC, Hearn SL, et al. Continuous passive motion after total knee arthroplasty. Analysis of cost and benefits. Clin Orthop Relat Res. 1995:208–215. [PubMed] [Google Scholar]
- 77.Bennett LA, Brearley SC, Hart JA, et al. A comparison of 2 continuous passive motion protocols after total knee arthroplasty: a controlled and randomized study. J Arthroplasty. 2005;20:225–233. doi: 10.1016/j.arth.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Johnson DP. The effect of continuous passive motion on wound-healing and joint mobility after knee arthroplasty. J Bone Joint Surg Am. 1990;72:421–426. [PubMed] [Google Scholar]
- 79.Salter RB. The biologic concept of continuous passive motion of synovial joints. The first 18 years of basic research and its clinical application. Clin Orthop Relat Res. 1989:12–25. [PubMed] [Google Scholar]
- 80.Maniar RN, Baviskar JV, Singhi T, et al. To use or not to use continuous passive motion post-total knee arthroplasty presenting functional assessment results in early recovery. J Arthroplasty. 2012;27:193–200, e191. doi: 10.1016/j.arth.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 81.Wasilewski SA, Woods LC, Torgerson WR, Jr, et al. Value of continuous passive motion in total knee arthroplasty. Orthopedics. 1990;13:291–295. doi: 10.3928/0147-7447-19900301-07. [DOI] [PubMed] [Google Scholar]
- 82.Wong VW, Levi K, Akaishi S, et al. Scar zones: region-specific differences in skin tension may determine incisional scar formation. Plast Reconstr Surg. 2012;129:1272–1276. doi: 10.1097/PRS.0b013e31824eca79. [DOI] [PubMed] [Google Scholar]
- 83.Atkinson JA, McKenna KT, Barnett AG, et al. A randomized, controlled trial to determine the efficacy of paper tape in preventing hypertrophic scar formation in surgical incisions that traverse Langer’s skin tension lines. Plast Reconstr Surg. 2005;116:1648–56; discussion 1657. doi: 10.1097/01.prs.0000187147.73963.a5. [DOI] [PubMed] [Google Scholar]
- 84.Meyer M, McGrouther DA. A study relating wound tension to scar morphology in the pre-sternal scar using Langers technique. Br J Plast Surg. 1991;44:291–294. doi: 10.1016/0007-1226(91)90074-t. [DOI] [PubMed] [Google Scholar]
- 85.Bux S, Madaree A. Involvement of upper torso stress amplification, tissue compression and distortion in the pathogenesis of keloids. Med Hypotheses. 2012;78:356–363. doi: 10.1016/j.mehy.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 86.Wong VW, Paterno J, Sorkin M, et al. Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. FASEB J. 2011;25:4498–4510. doi: 10.1096/fj.10-178087. [DOI] [PubMed] [Google Scholar]
- 87.Kanazawa Y, Nomura J, Yoshimoto S, et al. Cyclical cell stretching of skin-derived fibroblasts downregulates connective tissue growth factor (CTGF) production. Connect Tissue Res. 2009;50:323–329. [PubMed] [Google Scholar]
- 88.Peacock EE., Jr Production and polymerization of collagen in healing wounds of rats: some rate-regulating factors. Ann Surg. 1962;155:251–257. doi: 10.1097/00000658-196200000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akaishi S, Akimoto M, Ogawa R, et al. The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg. 2008;60:445–451. doi: 10.1097/SAP.0b013e3181238dd7. [DOI] [PubMed] [Google Scholar]
- 90.Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr. 2006;18:396–402. doi: 10.1097/01.mop.0000236389.41462.ef. [DOI] [PubMed] [Google Scholar]
- 91.Ogawa R, Akaishi S, Huang C, et al. Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med Sch. 2011;78:68–76. doi: 10.1272/jnms.78.68. [DOI] [PubMed] [Google Scholar]
- 92.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125:557–568. doi: 10.1097/PRS.0b013e3181c82dd5. [DOI] [PubMed] [Google Scholar]
- 93.Salgado RM, Alcántara L, Mendoza-Rodríguez CA, et al. Post-burn hypertrophic scars are characterized by high levels of IL-1β mRNA and protein and TNF-α type I receptors. Burns. 2012;38:668–676. doi: 10.1016/j.burns.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 94.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 95.Ogawa R. Mechanobiology of scarring. Wound Repair Regen. 2011;19(Suppl 1):s2–s9. doi: 10.1111/j.1524-475X.2011.00707.x. [DOI] [PubMed] [Google Scholar]
- 96.Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008;71:493–500. doi: 10.1016/j.mehy.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 97.Wang Z, Fong KD, Phan TT, et al. Increased transcriptional response to mechanical strain in keloid fibroblasts due to increased focal adhesion complex formation. J Cell Physiol. 2006;206:510–517. doi: 10.1002/jcp.20486. [DOI] [PubMed] [Google Scholar]
- 98.Suarez E, Syed F, Alonso-Rasgado T, et al. Up-regulation of tension-related proteins in keloids: knockdown of Hsp27, α2β1-integrin, and PAI-2 shows convincing reduction of extracellular matrix production. Plast Reconstr Surg. 2013;131:158e–173e. doi: 10.1097/PRS.0b013e3182789b2b. [DOI] [PubMed] [Google Scholar]
- 99.Tredget EE, Wang R, Shen Q, et al. Transforming growth factor-beta mRNA and protein in hypertrophic scar tissues and fibroblasts: antagonism by IFN-alpha and IFN-gamma in vitro and in vivo. J Interferon Cytokine Res. 2000;20:143–151. doi: 10.1089/107999000312540. [DOI] [PubMed] [Google Scholar]
- 100.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–426. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 101.Arem AJ, Madden JW. Effects of stress on healing wounds: I. Intermittent noncyclical tension. J Surg Res. 1976;20:93–102. doi: 10.1016/0022-4804(76)90104-9. [DOI] [PubMed] [Google Scholar]
- 102.Berard CW, Woodward SC, Herrmann JB, et al. Healing of incisional wounds in rats: the relationship of tensile strength and morphology to the normal skin wrinkle lines. Ann Surg. 1964;159:260–270. doi: 10.1097/00000658-196402000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bunting CH, Eades CC. The effect of mechanical tension upon the polarity of growing fibroblasts. J Exp Med. 1926;44:147–149. doi: 10.1084/jem.44.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brunius U, Ahrén C. Healing of skin incisions during reduced tension of the wound area. A tensiometric and histologic study in the rat. Acta Chir Scand. 1969;135:383–390. [PubMed] [Google Scholar]
- 105.Buck RC. Regeneration of tendon. J Pathol Bacteriol. 1953;66:1–18. [PubMed] [Google Scholar]
- 106.Sussman MD. Effect of increased tissue traction upon tensile strength of cutaneous incisions in rats. Proc Soc Exp Biol Med. 1966;123:38–41. doi: 10.3181/00379727-123-31396. [DOI] [PubMed] [Google Scholar]
- 107.Mason ML, Allen HS. The rate of healing of tendons: an experimental study of tensile strength. Ann Surg. 1941;113:424–459. doi: 10.1097/00000658-194103000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Milch RA. Tensile strength of surgical wounds. J Surg Res. 1965;5:377–380. doi: 10.1016/s0022-4804(65)80025-7. [DOI] [PubMed] [Google Scholar]
- 109.Peacock EE., Jr Dynamic aspects of collagen biology. I. Synthesis and assembly. J Surg Res. 1967;7:433–445. doi: 10.1016/0022-4804(67)90090-x. [DOI] [PubMed] [Google Scholar]


