Abstract
Study Design Systematic review.
Clinical Questions Compared with no stimulation, does electrical stimulation promote bone fusion after lumbar spinal fusion procedures? Does the effect differ based on the type of electrical stimulation used?
Methods Electronic databases and reference lists of key articles were searched up to October 15, 2013, to identify randomized controlled trials (RCTs) comparing the effect of electrical stimulation to no electrical stimulation on fusion rates after lumbar spinal fusion for the treatment of degenerative disease. Two independent reviewers assessed the strength of evidence using the Grades of Recommendation Assessment, Development and Evaluation (GRADE) criteria.
Results Six RCTs met the inclusion criteria. The following types of electrical stimulation were investigated: direct current (three studies), pulsed electromagnetic field (three studies), and capacitive coupling (one study). The control groups consisted of no stimulation (two studies) or placebo (four studies). Marked heterogeneity in study populations, characteristics, and design prevented a meta-analysis. Regardless of the type of electrical stimulation used, cumulative incidences of fusion varied widely across the RCTs, ranging from 35.4 to 90.6% in the intervention groups and from 33.3 to 81.9% in the control groups across 9 to 24 months of follow-up. Similarly, when stratified by the type of electrical stimulation used, fusion outcomes from individual studies varied, leading to inconsistent and conflicting results.
Conclusion Given the inconsistency in study results, possibly due to heterogeneity in study populations/characteristics and quality, we are unable to conclude that electrical stimulation results in better fusion outcomes compared with no stimulation. The overall strength of evidence for the conclusions is low.
Keywords: electrical stimulation, direct current, pulsed electromagnetic field, capacitive coupling, fusion, lumbar spine
Study Rationale and Context
Degenerative spinal conditions can lead to pain and neurologic symptoms. Patients who do not respond to nonoperative treatment often undergo spinal fusion. The lack of significant bone formation resulting in nonunion of the treated spinal segments, known as a pseudoarthrosis, is a potential long-term complication of a spinal fusion procedure. Although application of rigid instrumentation, such as the pedicle screw-rod construct, has increased fusion rates, pseudoarthrosis still occurs and has been shown to be the cause of persistent or recurrent pain and disability.1 2 Revision surgery is often recommended for these symptomatic cases of nonfusion and incidence is not insignificant as pseudoarthrosis is one of the most common indications for repeat surgery.3 Consequently, other measures including the use of biologics such as bone morphogenetic proteins or mesenchymal stem cell enriched allograft has been used to further increase the rate of bony union. Electrical stimulation has been suggested as an alternative means for increasing the fusion rate. However, the mechanism and efficacy of electrical stimulation remain unclear. The purpose of this systematic review is to evaluate the various types of stimulation and determine whether electrical stimulation induces bone fusion.
Clinical Question
Compared with no stimulation, does electrical stimulation promote bone fusion after lumbar spinal fusion procedures? Does the effect differ based on the type of electrical stimulation used (direct current [DC], pulsed electromagnetic field [PEMF], capacitive coupling [CC])?
Materials and Methods
Study design: Systematic review.
Search: The databases included PubMed, Cochrane collaboration database, and National Guideline Clearinghouse databases; bibliographies of key articles.
Dates searched: The data were searched from January 1980 to October 15, 2013.
Inclusion criteria: (1) Adults, (2) degenerative disease of the lumbar spine, (3) lumbar spinal fusion (any type/approach) with or without instrumentation, (4) comparison of electrical stimulation (including DC, PEMF, and CC) as an adjunctive treatment versus no stimulation, (5) randomized controlled trials (RCTs) published in English in peer-reviewed journals.
Exclusion criteria: (1) Pediatric patients, (2) cancer, trauma, inflammatory arthritis, or osteoporosis as indication for fusion procedure, (3) treatment of the cervical or thoracic spine, (4) use of biologics, (5) animal studies, (6) noncomparative studies (i.e., case series, case reports).
Outcomes: Proportion of patients achieving bony fusion.
Analysis: Descriptive statistics. Due to heterogeneity in study populations (including differences in the use of a placebo device, method of fusion assessment, definition of fusion, follow-up length, treatment indications, patient demographics, fusion procedure type/approach, and fusion graft materials), a meta-analysis was not performed.
Overall strength of evidence: Risk of bias for individual studies was based on using criteria set by The Journal of Bone and Joint Surgery 4 modified to delineate criteria associated with methodological quality and risk of bias based on recommendation from the Agency for Healthcare Research and Quality.5 6 The overall strength of evidence across studies was based on precepts outlined by the Grades of Recommendation Assessment, Development and Evaluation (GRADE) Working Group7 and recommendations made by the Agency for Healthcare Research and Quality (AHRQ).5 6
Details about methods can be found in the online supplementary material.
Results
We identified six RCTs, all rated level of evidence (LoE) II, which met the inclusion criteria and form the basis for this report (Fig. 1). Further details on the LoE rating for these studies as well as a list of excluded studies can be found in the online supplementary material.
Three studies compared DC stimulation to no stimulation,8 9 10 with only one study using a placebo device (i.e., an inactive stimulator) in the control group; three compared PEMF stimulation to no stimulation,9 11 12 with two employing placebo devices, and one study compared CC to no stimulation using placebo devices.13 Marked heterogeneity was present across the studies (Table 1).
Fig. 1.
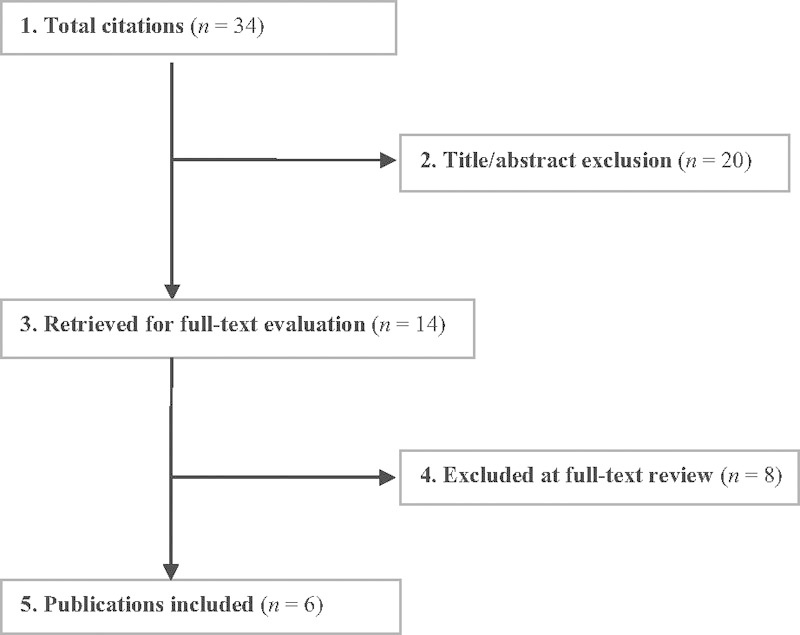
Flow chart showing results of literature search.
Table 1. Characteristics of included studies.
| Author (y) Study design (LoE) |
Demographics | Diagnosis | Fusion procedure | Definition of fusion outcomea | Follow-up | Funding/conflicts of interest |
|---|---|---|---|---|---|---|
| Anderson (2009) RCT (II) |
DC stimulation N = 53 Mean age: 69.3 y (range, 59–80) Male: 37.7% Control (placebo) N = 42 Mean age: 71.5 y (range, 59–84) Male: 31.0% |
• Spinal stenosis • Stenosis and degenerative spondylolisthesis • DDD • Stenosis and degenerative scoliosis |
• Posterolateral spinal fusion • Allograft • 1–4 levels |
• Fusion = continuous bony bridge either between the transverse process or at the lateral side of the facet joints on at least one side or a bilateral fusion of the facet joints (and fusion had to be achieved on all intended levels) • Doubtful fusion = unilateral facet joint fusion, questionable bilateral facet fusion, or possible presence of a cleft in the bony bridge • Nonunion = clearly definable cleft in the bony bridge, question fusion in one facet joint and none in the contralateral, or with resorption of most of the fusion mass |
24 mo DC: 90.6% (48/53) Placebo: 85.7% (36/42) |
Corporate/industry and federal funds were received in support of this work; no benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this article |
| Goodwin (1999) RCT (II) |
CC stimulation N = 85 Mean age: 45 y (range, 21–76) Male: 56.5% Control (placebo) N = 94 Mean age: 40 y (range, 22–73) Male: 52.1% |
• DDD • Herniated disc • Spondylolisthesis • Degenerative arthritis |
• Primary PLIF, ALIF, posterolateral fusion • Autograft, allograft, or autograft and allograft • Any type of internal fixation except interbody fusion cages • 1–2 levels |
• Posterolateral: ○ Fusion = presence of mature appearing, uninterrupted bony masses bilaterally at the fusion levels, ideally on both anteroposterior and lateral radiographs. If orthopedic hardware present, there could not be any lucency or motion around the screws ○ Incomplete fusion = immature-appearing bone mass on either side, lack of a bone mass on one side, or lack on continuity in the bone mass on either side. Any evidence of motion or lucency around internal fixation hardware was also a sign of incomplete fusion ○ Nonunion (absence of fusion mass) = complete resorption of the graft • Interbody: ○ Success = > 75% assimilation of graft and vertebrae or 50–75% assimilation of graft and vertebrae - Failure = 25–50% assimilation of graft and vertebrae or < 25% assimilation of graft and vertebrae |
12 mo Overall: 53.1% (179/337) |
Biolectron assisted in study design and analysis support |
| Jenis (2000) RCT (II) |
PEMF stimulation N = 22 Mean age: 53.0 ± 11.1 y Male: 50.0% DC stimulation N = 17 Mean age: 51.0 ± 15.1 y Male: 41.2% Control (no placebo) N = 22 Mean age: 47.1 ± 13.5 y Male: 63.6% |
• NR | • Primary or revision lumbar or lumbosacral posterolateral fusion • Iliac crest autograft • Pedicle screw-rod instrumentation • 1, 2, or > 2 levels |
• Solid fusion = trabecular bridging bone • Possible pseudoarthrosis = lucencies within the fusion mass • Obvious pseudoarthrosis = clefts within the fusion mass and discontinuity between the transverse processes |
12 mo (% NR) |
NR |
| Kane (1988) RCT (II) |
DC stimulation N = 31 Mean age: NR Male: NR Control (no placebo) N = 28 Mean age: NR Male: NR |
Difficult spinal fusions: • 1 + previous failed spinal fusion(s) • Grade II or worse spondylolisthesis • Extensive bone grafting necessary for a multiple level fusion • Other high risk factors for failure of fusion, including gross obesity |
• Posterolateral fusion | • NR | 18 mo Overall: 93.7% (59/63) |
NR |
| Linovitz (2002) RCT (II) |
PEMF stimulation N = 125 Mean age: 56.8 ± 15.5 y Male: 40.8% Control (placebo) N = 118 Mean age: 56.6 ± 15.0 y Male: 36.4% |
• DDD • Instability • Spondylolisthesis • Spinal stenosis • Miscellaneous |
• Primary posterolateral fusion • Autograft ± allograft • No instrumentation • 1–2 levels |
• Grades: ○ Three, solid fusion = extensive continuity (≥ 75–100%) of the fusion mass without motion ○ Two, moderate fusion = continuity (≥ 50 to < 75%) of the fusion mass without motion ○ One, minimal fusion = a narrow band of continuity (≥ 25 to < 50%) in the fusion mass with motion ○ Zero, no fusion = discontinuity (0 to < 25%) of the fusion mass with motion • Definitions (when two levels involved, the lowest grade at either level used) ○ Fusion = grades 2 and 3 (continuity of ≥ 50% of the fusion mass without motion) ○ Nonunion = grades 0 and 1 (continuity of 0 to < 50% of the fusion mass with motion) |
9 mo PEMF: 83.2% (104/125) Placebo: 82.2% (97/118) |
Corporate/industry funds were received to support this work. One or more of the author(s) has/have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article |
| Mooney (1990) RCT (II) |
PEMF stimulation N = 107 Mean age: 37.9 y Male: 55.1% Control (placebo) N = 99 Mean age: 37.6 y Male: 52.5% |
• Internal disc disruption • HNP • DDD • Spondylolisthesis • Stenosis • Failed fusion • Other |
• Primary ALIF or PLIF • Autograft, allograft, or autograft and allograft • With or without internal fixation • 1–2 levels |
• Fusion = > 50% assimilated (in two-segment fusion, both levels had be graded as solidly fused) • Nonunion = NR |
12 mo PEMF: 91.6% (98/107) Placebo: 98.0% (97/99) |
NR |
Abbreviations: ALIF, anterior lumbar interbody fusion; CC, capacitive coupling; DC, direct current; DDD, degenerative disc disease; HNP, herniated nucleus pulpous; LoE, level of evidence; NR, not reported; PEMF, pulsed electromagnetic field; PLIF, posterior lumbar interbody fusion; RCT, randomized controlled trial.
Fusion was assessed via radiograph in four studies (Goodwin 1999, Jenis 2000, Kane 1988, and Mooney 1990) and via computed tomography in two studies (Andersen 2009 and Linovitz 2002).
Fusion: Any Electrical Stimulation
• Regardless of the type of electrical stimulation used, the proportion of patients achieving bony fusion across all six RCTs varied, ranging from 35.4 to 90.6% compared with 33.3 to 81.9% in the control groups across 9 to 24 months of follow-up8 9 10 11 12 13 (Fig. 2).
Fig. 2.
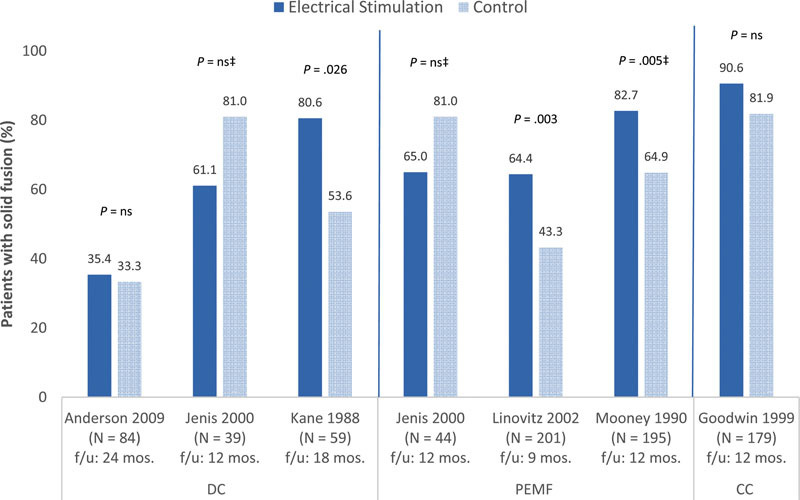
Proportion of patients that achieved solid fusion in the intervention (electrical stimulation) and the control (placebo/no stimulation)* groups following lumbar spinal fusion procedures†. CC, capacitive coupling; DC, direct current; PEMF, pulsed electromagnetic field. *The following RCTs used a placebo device in the control group: Anderson 2009, Goodwin 1999, Linovitz 2002, and Mooney 1990. †Marked heterogeneity in study population and design was present across the six RCTs. Anderson 2009: Elderly population (mean age 70 years); industry sponsored/funded; random sequence generation not reported; no intention-to-treat analysis; did not control for possible confounding factors. Goodwin 1999: Industry sponsored/funded; random sequence generation and statement of concealed allocation not reported; no intention-to treat analysis; unclear if cointerventions were applied equally; < 80% of patients followed. Jenis 2000: patient diagnoses not reported; funding/conflicts of interest not reported; statement of concealed allocation not reported; no intention-to-treat analysis; < 80% of patients followed; did not control for possible confounding factors. Kane 1988: Age and % male not reported; difficult spinal fusions; no definition of fusion outcome provided; funding/conflicts of interest not reported; statement of concealed allocation not reported; no intention-to-treat analysis; unclear if cointervention were applied equally. Linovitz 2002: Industry sponsored/funded; statement of concealed allocation not reported. Mooney 1990: Younger population (mean age 38 years); random sequence generation and statement of concealed allocation not reported; no intention-to-treat analysis; unclear if cointervention were applied equally. ‡These studies did not report p values; p values were calculated by this article's authors using the STATA software program.
Fusion: Type of Electrical Stimulation
Direct Current Stimulation
Pulsed Electromagnetic Field Stimulation
Capacitive Coupling Stimulation
Clinical Guidelines
One clinical guideline, produced by the American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/CNS) Joint Section on Disorders of the Spine and Peripheral Nerves, was found that reviewed the evidence for the efficacy of bone growth stimulators as adjuncts for bone fusion following fusion surgery for degenerative disease of the lumbar spine.14 The authors concluded that there is insufficient evidence to recommend a treatment standard. These guidelines were based on evidence from four RCTs, three cohort studies, and two case series.
Evidence Summary
The overall strength of evidence evaluating the efficacy of electrical stimulation as an adjunctive treatment to promote bone fusion after lumbar spinal fusion procedures compared with no stimulation is low (Table 2); that is, we have low confidence that evidence reflects the true effect and further research is likely to change the confidence in the estimate of effect and likely to change the estimate. With respect to DC stimulation, PEMF stimulation, and CC stimulation considered separately, the overall strength of evidence for each remains low.
Table 2. Evidence summary.
| Outcomes | Strength of evidence | Conclusions/comments |
|---|---|---|
| Compared with no stimulation, does electrical stimulation promote bone fusion after lumbar spinal fusion procedures? | ||
| Fusion: Any electrical stimulation |

|
• Cumulative incidences of fusion varied across six RCTs, ranging from 35.4 to 90.6% in the electrical stimulation groups compared with 33.3 to 81.9% in the control groups across 9 to 24 mo of follow-up. |
| Does the effect on fusion differ based on the type of electrical stimulation used (direct current, pulsed electromagnetic field, capacitive coupling)? | ||
| Fusion: DC stimulation |

|
• Individual study results varied (three RCTs). Compared with controls, DC stimulation resulted in better fusion outcomes in one study, worse fusion outcomes in a second study, and similar fusion outcomes in the third study. |
| Fusion: PEMF stimulation |

|
• Individual study results varied (three RCTs). Two trials reported better fusion results following PEMF stimulation compared with control, while the third reported poorer fusion results in the intervention group. |
| Fusion: CC stimulation |

|
• One RCT investigated CC stimulation and reported a similar proportion of patients achieving fusion at 12 mo between the intervention and control groups. |
Abbreviations: CC, capacitive coupling; DC, direct current; PEMF, pulse electromagnetic field; RCT, randomized controlled trial.
Discussion
-
Strengths
○ The question was reviewed systematically.
-
Limitations
○ Few studies available to address the impact of different types of electrical stimulation.
○ Heterogeneity among the individual studies precluded application of a meta-analysis.
○ Random sequence generation, statement of concealed allocation, and intention-to-treat were reported very infrequently across the RCTs.
○ Loss to follow-up and controlling for possible confounding factors were not reported in two studies each, possibly biasing results.
○ The use of a placebo device in the control group was not consistent across studies.
Wide ranges of reported fusion rates (33.3 to 90.6%) among the six RCTs are unexpected. Most notably, Anderson reported alarmingly low fusion rates of 33.3 and 35.4% in nonstimulated and stimulated groups at 24 months, respectively. This follow-up is longer than the other studies reviewed. However, it is important to note that their study was done in the elderly. Fusion is influenced by a multitude of factors such as age, sex, smoking status, surgical technique, grafts, and type of implants. In addition, the timing of fusion assessment and the criteria to determine fusion can vary. These factors likely explain the heterogeneity of outcomes reported.
The implications in clinical practice of the use of electrical stimulation to promote bone fusion following lumbar spine surgery cannot be determined from the available evidence. However, it appears complications associated with its use are low.
Additional large RCTs are warranted. Future RCTs need to focus on a single pathological process, encompass similar surgical procedures, and standardize electrical stimulation protocols. Clear a priori definitions of bone fusion need to be established and assessed by blinded reviewers.
Acknowledgments
Analytic support for this work was provided by Spectrum Research, Inc. with funding from AOSpine.
Footnotes
Disclosures Paul Park, Consultant: Globus, Medtronic, Biomet; Royalties: Globus Darryl Lau, none Erika D. Brodt, none Joseph R. Dettori, none
Editorial Perspective
This systematic review regarding the efficacy of electrical stimulation for lumbar fusion surgery goes straight to the heart of one of the great unanswered questions of spinal reconstruction surgery: When has an arthrodesis surgery resulted in a “successful” fusion? After over six decades of performing fusion surgery, our spine community still remains less than clear on questions relating to the correlation of patient-related outcomes to fusion and radiographic confirmation of fusion. Efforts to predictably increase rates of fusion are aplenty and have left virtually no type of modulation affecting bone healing go unused.
Electric stimulation with its proven effect on bone healing had seemingly faded out of popularity after receiving a lot of attention in the 1990s. It is frustrating that despite seven high-grade, level-2 PRCTs in the end, the results of this systematic review remain ambiguous and do not provide meaningful guidance for practitioners interested in providing scientifically guided counseling to their patients. In reviewing these studies in context with one another, the number of variables appears overwhelming.
Host factors (i.e., age, time from previous surgery, number of revisions, indication for index procedure, presence of complications, infection, diabetes, nicotine cessation), technique factors (quality of revision surgery, stability of construct stiffness, bone graft quality and quantity used, adjuvant bone growth factors employed, blood supply to host bone) all can play a significant role in bone healing. Establishing bone union itself remains unclear with computed tomography reformats, bone scans, dynamic radiographs, and surgical exploration with probing all remaining accepted forms of confirmation for fusion. Yet again, the resulting variables confound attempts at systematically evaluating the present day literature. The present study shows no conclusive evidence in support of using electric stimulation for routine lumbar fusion surgery.
Supplementary Material
References
- 1.Kornblum M B Fischgrund J S Herkowitz H N Abraham D A Berkower D L Ditkoff J S Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis Spine (Phila Pa 1976) 2004297726–733., discussion 733–734 [DOI] [PubMed] [Google Scholar]
- 2.Nandyala S V, Marquez-Lara A, Fineberg S J, Pelton M, Singh K. Prospective, randomized, controlled trial of silicate-substituted calcium phosphate versus rhBMP-2 in a minimally invasive transforaminal lumbar interbody fusion. Spine (Phila Pa 1976) 2014;39(3):185–191. doi: 10.1097/BRS.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 3.Martin B I, Mirza S K, Comstock B A, Gray D T, Kreuter W, Deyo R A. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine (Phila Pa 1976) 2007;32(3):382–387. doi: 10.1097/01.brs.0000254104.55716.46. [DOI] [PubMed] [Google Scholar]
- 4.Wright J G, Swiontkowski M F, Heckman J D. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85-A(1):1–3. [PubMed] [Google Scholar]
- 5.Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Publication No. 10(12)-EHC063-EF Rockville, MD: Agency for Healthcare Research and Quality; April 2012. Available at: www.effectivehealthcare.ahrq.gov [PubMed]
- 6.West S, King V, Carey T S, Rockville, MD: Agency for Healthcare Research and Quality; 2002. Systems to Rate the Strength of Scientific Evidence. Evidence Report/Technology Assessment No. 47 (Prepared by the Research Triangle Institute-University of North Carolina Evidence-based Practice Center, Contract No. 290–97–0011) [PMC free article] [PubMed] [Google Scholar]
- 7.Atkins D, Best D, Briss P A. et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen T, Christensen F B, Egund N. et al. The effect of electrical stimulation on lumbar spinal fusion in older patients: a randomized, controlled, multi-center trial: part 2: fusion rates. Spine (Phila Pa 1976) 2009;34(21):2248–2253. doi: 10.1097/BRS.0b013e3181b02c59. [DOI] [PubMed] [Google Scholar]
- 9.Jenis L G, An H S, Stein R, Young B. Prospective comparison of the effect of direct current electrical stimulation and pulsed electromagnetic fields on instrumented posterolateral lumbar arthrodesis. J Spinal Disord. 2000;13(4):290–296. doi: 10.1097/00002517-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kane W J. Direct current electrical bone growth stimulation for spinal fusion. Spine (Phila Pa 1976) 1988;13(3):363–365. doi: 10.1097/00007632-198803000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Linovitz R J Pathria M Bernhardt M et al. Combined magnetic fields accelerate and increase spine fusion: a double-blind, randomized, placebo controlled study Spine (Phila Pa 1976) 200227131383–1389., discussion 1389 [DOI] [PubMed] [Google Scholar]
- 12.Mooney V. A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine (Phila Pa 1976) 1990;15(7):708–712. doi: 10.1097/00007632-199007000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin C B Brighton C T Guyer R D Johnson J R Light K I Yuan H A A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions Spine (Phila Pa 1976) 199924131349–1356., discussion 1357 [DOI] [PubMed] [Google Scholar]
- 14.Resnick D K, Choudhri T F, Dailey A T. et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 17: bone growth stimulators and lumbar fusion. J Neurosurg Spine. 2005;2(6):737–740. doi: 10.3171/spi.2005.2.6.0737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


