Supplemental Digital Content is available in the text.
Abstract
Background:
Preoperative anemia is independently associated with adverse outcomes after general and cardiac surgery. Outcomes after breast reconstruction are not established. We assessed the effect of preoperative anemia on 30-day postoperative morbidity and length of hospital stay (LOS) in patients undergoing immediate breast reconstruction.
Methods:
We identified patients undergoing immediate breast reconstruction from 2008 to 2010 from the American College of Surgeons’ National Surgical Quality Improvement Program database (a prospective outcomes-based registry from hospitals worldwide). De-identified data were obtained for demographics, preoperative risk factors, 30-day morbidity, and LOS. Morbidity variables included flap/graft/prosthesis, cardiac, respiratory, neurological, urinary, wound, and venous thromboembolism outcomes. Logistic regression assessed the crude and adjusted effect of anemia (hematocrit <36%) on postoperative 30-day morbidity. Measures of central tendency of LOS were compared across increasing severities of anemia in patients developing adverse events versus controls.
Results:
The study population included 10,958 patients; 1556 (16.74%) had preoperative anemia. Crude odds ratio for 30-day morbidity was significantly higher in anemic patients, unadjusted odds ratio = 1.33 (P < 0.008). This prevailed after extensive adjustment for confounding, yielding an adjusted odds ratio = 1.38 (P < 0.03). Patients who experienced adverse effects had protracted LOS, and the presence of anemia significantly amplified this effect.
Conclusions:
These data provide new insight into the effect of anemia in immediate breast reconstruction, demonstrating an independent association between preoperative anemia and 30-day morbidity. These findings suggest treating anemia when possible; however, prospective studies should explore the efficacy, safety, and cost-effectiveness of such treatments.
Hematocrit concentrations are almost always measured before elective surgeries such as breast reconstruction,1 yet few studies have explored the implications of preoperative low hematocrit on postoperative outcomes. The impact of preoperative anemia on postoperative outcomes has been analyzed in other surgeries, both cardiac and major noncardiac. Kulier et al,2 using the Multicenter Study of Perioperative Ischemia Epidemiology II database to study data from 5065 patients undergoing elective coronary artery bypass graft, found that preoperative anemia is an independent predictor of postoperative morbidity, and the level of morbidity amplifies with the extent of baseline risk factors. Moreover, Wu et al,3 analyzing a cohort of 310,311 elderly veterans using the VA National Surgical Quality Improvement Program database, found that even mild degrees of preoperative anemia are associated with increased 30-day mortality and adverse cardiac events. Furthermore, Musallam et al,4 analyzing data of 227,425 patients of all ages undergoing major noncardiac surgery using the American College of Surgeons’ National Surgical Quality Improvement Program (ACS-NSQIP) database, found that postoperative mortality and 30-day morbidity was higher in patients with preoperative anemia.
The major subspecialties analyzed in these studies were general, vascular, and orthopedic. Although a subgroup named “plastic surgery” was included, none of these focused specifically on breast reconstruction. Available data on the effect of preoperative anemia on breast reconstruction outcomes are limited.5–8 Such articles had either biased study designs, small number of patients, failed to adjust for major confounders, or were restricted to specific subtypes of breast reconstruction. None described in detail the exact relationship between anemia and adverse outcomes or quantified the impact of preexisting comorbidities on this association. It is unknown whether the effects of anemia on outcomes in breast reconstruction are caused by a low hematocrit level per se or by an association with other risk factors frequently prevalent in anemic patients.
Therefore, the main goal of the present study is to examine the impact of preoperative anemia on postoperative adverse outcomes in patients undergoing immediate breast reconstruction using a large prospective multicenter database (ACS-NSQIP, Appendix, Supplemental Digital Content 1, http://links.lww.com/PRSGO/A3). Specifically, our primary aim is to determine whether subnormal preoperative hematocrit before immediate reconstruction is an independent predictor of 30-day morbidity. We evaluate a potential dose-response relationship between decreasing hematocrit levels and increasing postoperative morbidity. Furthermore, we examine the impact of comorbidities and other risk factors on the relationship between preoperative anemia and postoperative morbidity. Finally, we analyze the effect modification of different severity levels of preoperative anemia on length of hospital stay (LOS) in patients who developed postoperative morbidity (complicated cases) as compared to those who did not (uncomplicated cases).
PATIENTS AND METHODS
We analyzed data from the 2008 to 2010 ACS-NSQIP databases. This database is a prospective, validated, risk-adjusted, outcomes-based program to measure and improve the quality of surgical care.9 It provides feedback to member hospitals about 30-day risk-adjusted surgical mortality and morbidity10,11 and includes de-identified data for patients’ demographics, functional statuses, admission sources, preoperative risk factors, laboratory data, perioperative variables, and 30-day postoperative outcomes. For the purpose of our study, data were retrieved for all surgeries undertaken at participating medical centers (211 centers in 2008, 237 in 2009, and 258 in 2010). These centers are located in the United States of America, Canada, Lebanon, the United Arab Emirates, and the United Kingdom.
We identified 2957 female patients who underwent immediate breast reconstruction (our index surgery) in 2008, 3852 in 2009, and 4149 in 2010. These amounted to 10,958 patients. We excluded patients with missing preoperative hematocrit values (1665 patients) because this variable is used to define the exposure of interest. We performed the main analysis for 9293 patients.
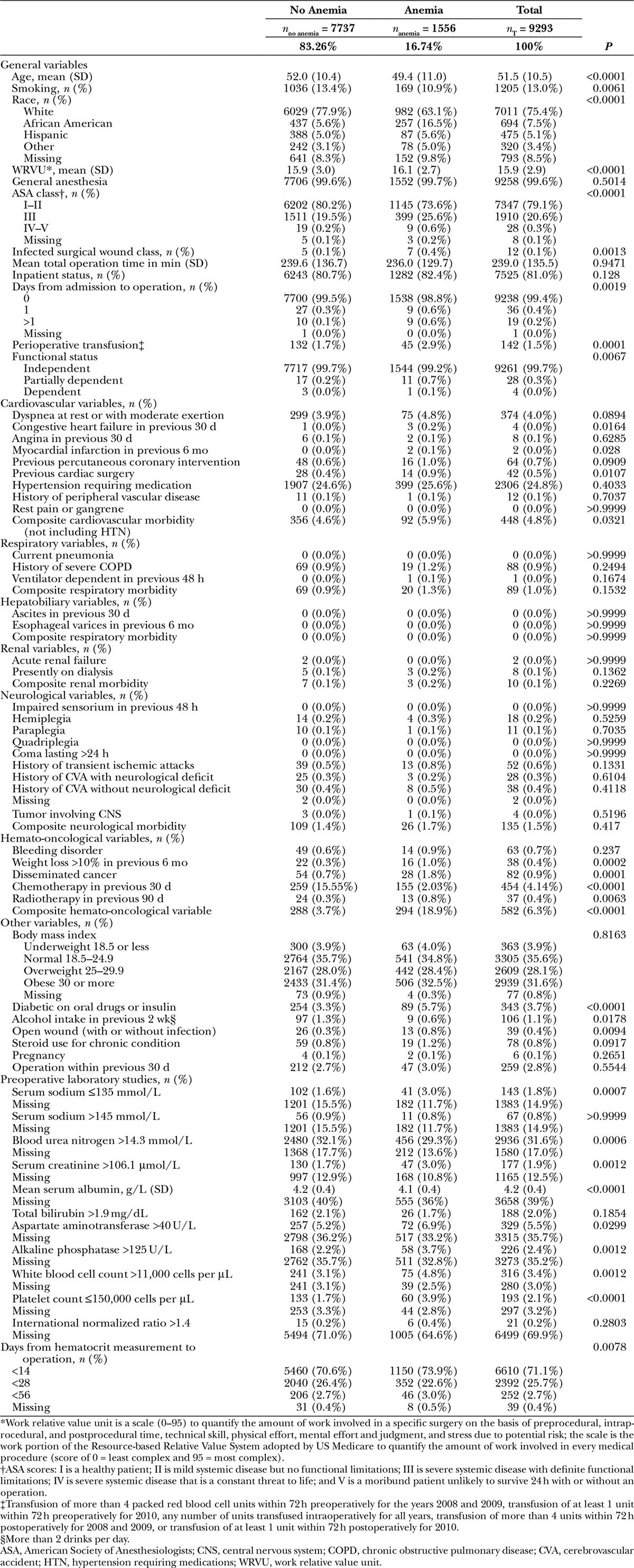
We compared demographics, preoperative and intraoperative variables between patients without anemia and those with anemia (Table 1).
Table 1.
Baseline Characteristics of Patients
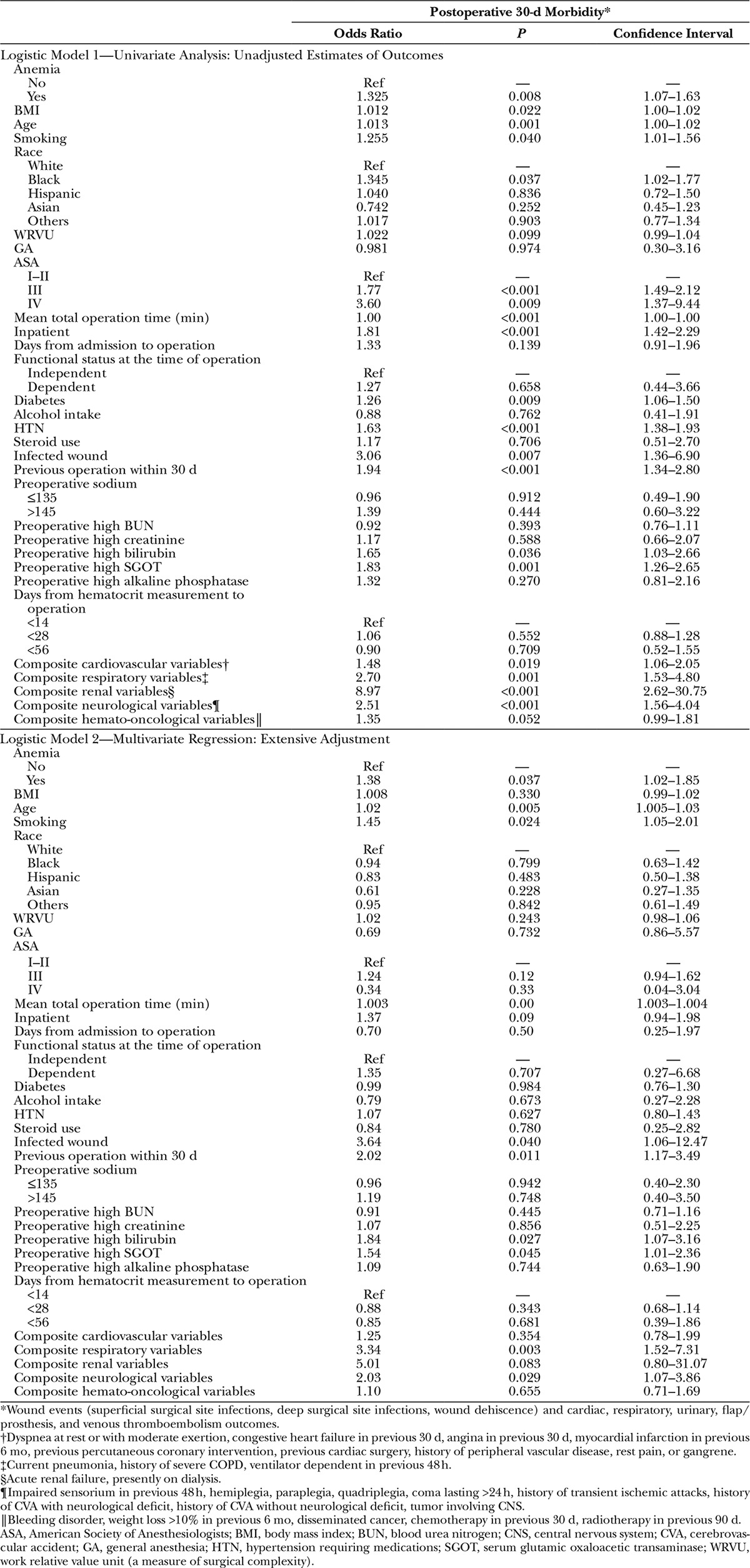
We used a modelwise approach and adjusted extensively for all parameters found to be statistically significant in our univariate analysis and for additional clinically relevant confounders (Table 2).
Table 2.
Logistic Models: Univariate and Multivariate Analyses
We then evaluated a potential dose-response relationship between decreasing preoperative hematocrit and increasing 30-day morbidity. Point estimates reflecting the incidence of 30-day morbidity with decreasing hematocrit levels were calculated for all patients and plotted in Figure 1. We assessed furthermore the influence of baseline risk factors (comorbid conditions) on the association between preoperative anemia and morbidity. For this, we categorized patients into high- and low-risk groups based on relevant risk factors and calculated point estimates of their 30-day morbidity incidence across different hematocrit levels (Fig. 1). Risk factors defining high-risk group include age ≥65 years, smoking, infected/dirty wound, perioperative transfusions, previous operation within 30 days, respiratory comorbidity (current pneumonia, history of severe chronic obstructive pulmonary disease, ventilator dependent in previous 48 h), renal comorbidity (acute renal failure, presently on dialysis), neurological comorbidity (impaired sensorium in previous 48 h, hemiplegia, quadriplegia, coma lasting >24 h, history of transient ischemic attack or cerebrovascular accident, central nervous system tumor), and some abnormal preoperative laboratory values (high serum glutamic oxaloacetic transaminase, high bilirubin). Pairwise Fisher’s exact tests with Bonferroni corrections were used to assess whether the difference in incidence per hematocrit group was statistically significant.
Fig. 1.
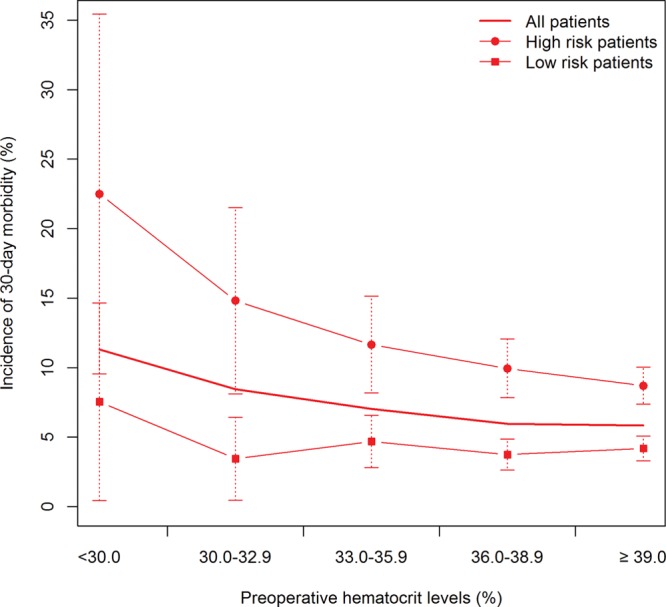
Dose-response curve of incidence of 30-d morbidity events for high- and low-risk groups vs preoperative hematocrit level. The incidence of 30-d morbidity with 95% CI is calculated for patients in 5 different categories of preoperative hematocrit level: <30.0, 30.0–32.9, 33–35.9, 36–38.9, and ≥39.
For the secondary outcome measure (increased LOS), we assessed the impact of postoperative adverse events on LOS and the effect modification of preoperative anemia on this association (Fig. 2). We tested differences in measures of central tendency (mean and median) and measures of variability (interquartile range and variance) between increasing levels of anemia after stratifying for the presence or absence of an adverse event. Kruskal-Wallis rank sum test with Bonferroni corrections was used to assess significance (Table 4).
Fig. 2.
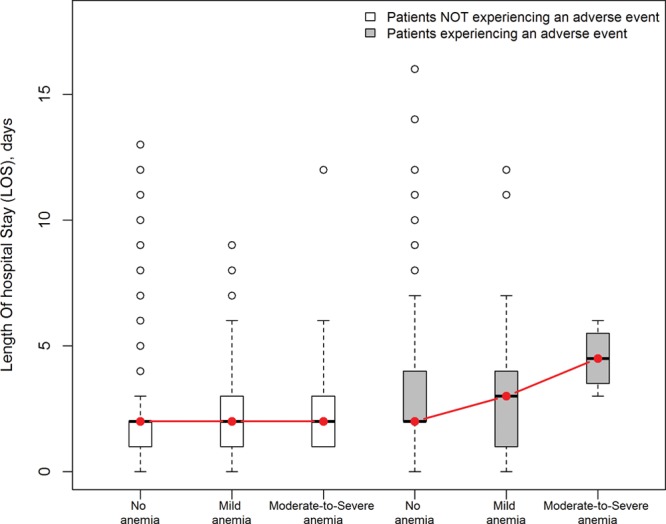
Effect modification of preoperative anemia levels on the association between postoperative adverse events and LOS. Anemia significantly amplified LOS in patients with low preoperative hematocrit experiencing an adverse event as compared to patients with low preoperative hematocrit not experiencing an adverse event.
Procedures
All female patients who underwent immediate breast reconstruction from 2008 to 2010 were identified using the following Current Procedural Terminology (CPT) codes: 19160, 19301, 19162, 19302, 19180, 19303, 19182, 19304, 19200, 19305, 19220, 19306, 19307, 19240 for mastectomy and 19340, 19342, 19357, 19361, 19364, 19366, 19367, 19368, 19369 for breast reconstruction. Patients coded simultaneously 2 CPTs (one from the mastectomy list and another from the breast reconstruction list) define the immediate breast reconstruction population.
We defined preoperative hematocrit concentration as the last hematocrit measurement before the index surgery and preoperative anemia as a hematocrit concentration <36% (World Health Organization criteria).12 Hematocrit concentration >29% to <36% was defined as mild anemia and ≤29% as moderate-to-severe anemia.
Postoperative outcomes were morbidity within 30 days and increased LOS. Morbidity variables included events affecting the graft (prosthesis or flap failure), heart (acute myocardial infarction), respiratory tract (pneumonia), central nervous system (cerebrovascular accident), urinary tract (infection), wound (superficial and deep incisional surgical site infection and wound dehiscence), and venous thromboembolism events (deep venous thrombosis requiring therapy and pulmonary embolism). LOS was defined as the date of discharge minus the date of surgery.
Statistical Analysis
Continuous variables, such as age, are presented with their mean and standard deviation as “mean (SD),” whereas categorical variables, such as gender, are presented as the number of patients and its corresponding proportion with respect to the exposure group, “n (%).” P-values for continuous variables correspond to a Kruskal-Wallis rank sum test of the null assuming the location parameters of the distribution of the variable are the same in each exposure group. P-values for categorical variables correspond to a Fisher’s exact test for testing the null of independence of rows and columns in a contingency table with fixed marginal (Table 1).
For the primary study outcome (occurrence of morbidity within 30 d of surgery), adjusted odds ratio reflecting 30-day morbidity was estimated using a multivariable logistic regression model comparing patients in the preoperative anemia group to those with no anemia.
Data management and analyses were done with STATA/SE 11, and graphs were plotted using R 2.15.1. All tests excluded missing values; their frequencies are reported in the Appendix (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A3). Authors had full access to and take full responsibility for the integrity of the data.
Ethical Approval
De-identified patient information is freely available to all institutional members who comply with the ACS-NSQIP Data Use Agreement. The Data Use Agreement implements the protections afforded by the Health Insurance Portability and Accountability Act of 1996 and the ACS-NSQIP Hospital Participation Agreement. This study conforms to the Helsinki Declaration.
RESULTS
Study Population
We included data for 9293 patients. We obtained hematocrit concentrations for 9042 (97.3%) patients within approximately 2 months of surgery [6644 (71.5%) were obtained within 14 d]. Preoperative anemia was present in 16.7% of our cohort. Data were almost complete, apart from some missing laboratory variables, such as serum sodium (17.9%), blood urea nitrogen (17.0%), serum creatinine (12.5%), serum albumin (39%), aspartate aminotransferase (35.7%), alkaline phosphatase (35.2%), and international normalized ratio (69.9%).
Demographics and medical history are summarized in Table 1. Patients had a mean age of 51.5 years (SD, 10.5; range, 18–95). Compared with patients without anemia, patients with anemia were more likely to have a higher body mass index and a race other than white. Patients with anemia also had a higher prevalence of diabetes and cardiovascular disorders; they were more likely to be at a high American Society of Anesthesiologists class and nonindependent in functional status. Furthermore, they were more likely to have disseminated cancer, have received chemo or radiotherapy, present with abnormal preoperative laboratory studies, and undergo perioperative transfusions. However, they had lower age and prevalence of smoking.
Univariate analysis revealed that several preoperative factors (Table 2) were associated with significantly higher odds of 30-day morbidity. The cumulative incidence of 30-day morbidity was 5.9% for patients without anemia compared with 7.6% for patients with anemia, with an unadjusted odds ratio of 1.33 and 95% confidence interval (CI) of 1.07–1.63.
After adjustment for statistically significant and clinically relevant confounders (Table 3), preoperative anemia remained independently and significantly associated with increased 30-day morbidity (adjusted odds ratio, 1.38; 95% CI, 1.02–1.85).
Table 3.
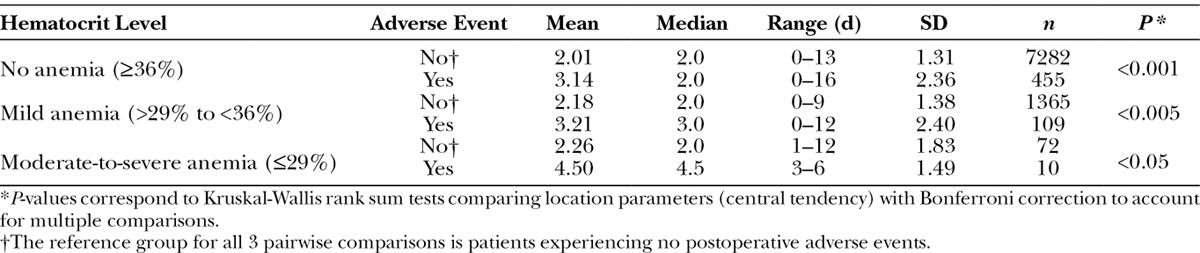
Pairwise Comparisons of Length of Hospital Stay Within the Different Hematocrit Levels Between Patients Who Experienced an Adverse Event and Those Who Did Not
Furthermore, univariate analysis established a primary association between decreased preoperative hematocrit and increased postoperative morbidity. Figure 1 illustrates the dose-dependent nature of this effect. We also observed a dose-response effect when patients were categorized into high/low risk, with a significantly higher incidence of 30-day morbidity in high-risk patients for hematocrit groups of 30% or higher.
We further analyzed the implication of postoperative morbidity on LOS and examined whether the occurrence of morbidity reached synergy with different severities of preoperative anemia. As expected, LOS was increased in the presence of postoperative adverse events (mean, 3.23; SD, 2.62) compared to patients who experienced no complications (mean, 2.06; SD, 1.61). However, for the subpopulation of patients having preoperative low hematocrit and developing a postoperative adverse event, anemia significantly amplified LOS as compared to the subpopulation with low preoperative hematocrit and not experiencing an adverse event (Fig. 2). Figure 2 illustrates the effect modification of anemia on the association between LOS and adverse events.
Table 3 complements Figure 2 and provides all 3 pairwise comparisons.
Finally, anemia may have served as an indication to receive a transfusion in this cohort. In fact, because anemia can potentially lead to both the occurrence of transfusions and adverse events, we expected an association between anemia and transfusions. Indeed, while 1.7% of patients without anemia required transfusions, 2.9% of those with anemia underwent transfusions (Pearson’s chi-square(1), 11.9; P < 0.001). In our analysis, transfusions predicted the occurrence of 30-day morbidity (unadjusted odds ratio, 38.48; 95% CI, 26.68–55.51). In other words, transfusions seem to represent a surrogate outcome of morbidity (or an alternative way of measuring it).
DISCUSSION
This large epidemiological study is the first to investigate the nature and extent of preoperative anemia on postoperative outcomes in patients undergoing immediate breast reconstruction. Using a comprehensive worldwide multicenter prospective database, we showed that preoperative anemia is independently associated with an increased risk of 30-day morbidity. In addition, when anemia was present concomitantly with a known preoperative risk factor, it led to a significant increase in the effect of this risk factor on 30-day morbidity. Furthermore, anemia amplified the LOS for patients who experienced postoperative adverse events. This suggests that patients having baseline risk factors predisposing them to a postoperative adverse event (Table 3) may have significantly higher LOSs if preoperative anemia is present. This risk may be further increased when anemia is moderate to severe.
The observed effects of anemia on 30-day morbidity and LOS were evident across all types of immediate reconstruction and aspects of medical history (age, race, body mass index, smoking, diabetes, hypertension requiring medications, previous chemo or radiotherapy, etc.). They were also observed irrespective of the duration from hematocrit measurement to operation.
This association between low hematocrit and increased adverse outcomes has been described for a variety of surgical patient populations: vascular, orthopedic, cardiac, and major noncardiac surgery patients.13–16 Few studies have attempted examining the role of preoperative anemia in patients undergoing breast reconstruction. Vega et al5 suggested that a relative preoperative anemia state caused by autologous blood donation leads to a greater frequency of surgical morbidity in free transverse rectus abdominus myocutaneous flap breast reconstruction. Furthermore, Hill et al6 demonstrated that preoperative anemia is a significant predictor of free flap failure and vascular thrombosis in general microvascular reconstruction. They added that flap failure had a statistically significant exposure-response relationship to anemia, with the probability of failure increasing incrementally with decreases in preoperative hematocrit. Moreover, Ting et al7 pointed out that preoperative anemia might negatively impact deep inferior epigastric perforators flap survival and patient morbidity in breast reconstruction because transfusion requirements are increased. Our study reinforces these findings and suggests that the detrimental effects of preoperative anemia occur across all types of immediate reconstruction. In addition, they are associated with a higher risk of major morbidity, including graft, wound, cardiac, respiratory, neurological, urinary, and thromboembolic complications.
It also demonstrated a dose-dependent significant increase in morbidity with decreasing hematocrit levels. This suggests that the effect of an underlying disease or risk factor on postoperative adverse events is amplified by the presence of anemia. Similarly, anemia seems to amplify LOS in patients having risk factors predicting the occurrence of adverse events (Table 2).
The key strengths of our study are the large number of patients and the reliable and comprehensive data collection of the ACS-NSQIP. This database provides more than 60 demographic, preoperative, and perioperative variables for adjustment. Our study adjusted for extensive confounding. This completeness, together with the good discrimination in our model, suggests that the effect of anemia is independent and cannot be straightforwardly explained through an association with other known risk factors.
Our findings should lead to a careful consideration of appropriate interventions to correct preoperative anemia in patients undergoing immediate breast reconstruction. Preoperative diagnosis and treatment of anemia (apart from transfusions of red blood cells) have almost never been undertaken routinely before surgery.17 Our study supports the present guidelines recommending measuring hematocrit concentration 28 days before surgery and subsequent investigations in anemic patients. At least in elective surgical cases (such as immediate breast reconstruction), it is strongly recommended to treat preoperative anemia; it is easy to detect and, in many situations, inexpensive to treat.1 Proactive treatment is likely less costly than postoperative transfusions and may improve patient outcomes and decrease LOS, which often translate into reduced health care costs, a growing national health care concern.
Our study had limitations. Approximately 3% of the preoperative hematocrit concentrations were obtained >7 weeks before surgery and might not accurately predict hematocrit concentrations at the time of surgery. Still, variations in hematocrit are usually low in the absence of an emergency operation or major bleeding, which in our database would have been identified by preoperative packed red blood cell transfusions; these occurred in 0.03% of the patients only. However, it is worth noting that NSQIP does not indicate the use of 4 or fewer transfusions in the preoperative period for the years 2008 and 2009, which means that some patients viewed in the analysis as not having received preoperative transfusions might have received 4 or fewer transfusions. Nevertheless, about 99.4% of patients without documented preoperative transfusions in these 2 years underwent same-day surgery, suggesting that preoperative transfusion was unlikely and they indeed had a significant association between anemia and postoperative adverse outcomes.
Additionally, the long-term effects of the multiple transfusions cancer patients often receive could not be accounted for in the analysis. This is due to the nature of the NSQIP database which does not provide the number of all previous transfusions.
Finally, given the retrospective nature of our study, we cannot determine the causal relationship between low hematocrit and risk of postoperative adverse events. Neither can we relate the etiology nor chronicity of abnormal hematocrit with outcomes.
Nevertheless, our study strongly suggests that implementation of anemia screening and treatment should be considered in patients undergoing immediate breast reconstruction. A future interesting study entails subdividing immediate breast reconstruction into autologous and implant based and comparing in these 2 cohorts the incidence of 30-day morbidity associated with preoperative anemia. This analysis is not possible in NSQIP; this database uses CPT codes to identify types of reconstruction, and some CPTs do not differentiate between autologous and implant-based reconstruction (eg, CPT 19361 refers to breast reconstruction with latissimus dorsi with or without implant, although that code is now modified so it should be possible in the future).
Additional prospective studies are needed to determine whether our findings are also reproducible in other breast surgery populations (namely, delayed breast reconstruction, mastectomy alone) and to assess the efficacy, safety, and cost-effectiveness of preoperative anemia management in immediate breast reconstruction.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Ruth Lucas for her kind assistance in part of the statistical analysis.
Supplemental Digital Content
Footnotes
Dr. Sarhane and Mr. Flores contributed equally to this work. Poster presented at the American Association of Plastic Surgeons Annual Meeting, April 20–23, 2013, New Orleans, LA; a poster presented at the Plastic Surgery Research Council Annual Meeting, May 2–4, 2013, Santa Monica, CA; a podium presented at the WSRM 2013 Congress, July 11–14, 2013, Chicago, IL; and a podium presented at the ACS NSQIP National Conference, July 13–16, 2013, San Diego, CA.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was supported, in part, by the Open Access Promotion Fund of the Johns Hopkins University Libraries. The Article Processing Charge was paid for by the Open Access Promotion Fund of the Johns Hopkins University Libraries.
Disclaimer: The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Goodnough LT, Shander A, Spivak JL, et al. Detection, evaluation, and management of anemia in the elective surgical patient. Anesth Analg. 2005;101:1858–1861. doi: 10.1213/01.ANE.0000184124.29397.EB. [DOI] [PubMed] [Google Scholar]
- 2.Kulier A, Levin J, Moser R, et al. Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116:471–479. doi: 10.1161/CIRCULATIONAHA.106.653501. [DOI] [PubMed] [Google Scholar]
- 3.Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–2488. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 4.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378:1396–1407. doi: 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 5.Vega SJ, Nguyen TV, Forsberg C, et al. Efficacy of preoperative autologous blood donation in free TRAM flap breast reconstruction. Plast Reconstr Surg. 2008;121:241e–246e. doi: 10.1097/PRS.0b013e31816b1421. [DOI] [PubMed] [Google Scholar]
- 6.Hill JB, Patel A, Del Corral GA, et al. Preoperative anemia predicts thrombosis and free flap failure in microvascular reconstruction. Ann Plast Surg. 2012;69:364–367. doi: 10.1097/SAP.0b013e31823ed606. [DOI] [PubMed] [Google Scholar]
- 7.Ting J, Rozen WM, Le Roux CM, et al. Predictors of blood transfusion in deep inferior epigastric artery perforator flap breast reconstruction. J Reconstr Microsurg. 2011;27:233–238. doi: 10.1055/s-0031-1275486. [DOI] [PubMed] [Google Scholar]
- 8.Tzilinis A, Lofman AM, Tzarnas CD. Transfusion requirements for TRAM flap postmastectomy breast reconstruction. Ann Plast Surg. 2003;50:623–627. doi: 10.1097/01.SAP.0000054181.58934.1E. [DOI] [PubMed] [Google Scholar]
- 9.American College of Surgeons National Surgical Quality Improvement Program. ACS NSQIPSurgical Quality Improvement. Available at: http://site.acsnsqip.org. Accessed January 19, 2013.
- 10.Fink AS, Campbell DA, Jr, Mentzer RM, Jr, et al. The National Surgical Quality Improvement Program in non-veterans administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236:344–353. doi: 10.1097/00000658-200209000-00011. discussion 353–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khuri SF, Henderson WG, Daley J, et al. Principal Site Investigators of the Patient Safety in Surgery Study. The patient safety in surgery study: background, study design, and patient populations. J Am Coll Surg. 2007;204:1089–1102. doi: 10.1016/j.jamcollsurg.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 13.Spence RK, Carson JA, Poses R, et al. Elective surgery without transfusion: influence of preoperative hemoglobin level and blood loss on mortality. Am J Surg. 1990;159:320–324. doi: 10.1016/s0002-9610(05)81227-9. [DOI] [PubMed] [Google Scholar]
- 14.Nelson AH, Fleisher LA, Rosenbaum SH. Relationship between postoperative anemia and cardiac morbidity in high-risk vascular patients in the intensive care unit. Crit Care Med. 1993;21:860–866. doi: 10.1097/00003246-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Hogue CW, Jr, Goodnough LT, Monk TG. Perioperative myocardial ischemic episodes are related to hematocrit level in patients undergoing radical prostatectomy. Transfusion. 1998;38:924–931. doi: 10.1046/j.1537-2995.1998.381098440856.x. [DOI] [PubMed] [Google Scholar]
- 16.Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 17.Gombotz H, Rehak PH, Shander A, et al. Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47:1468–1480. doi: 10.1111/j.1537-2995.2007.01286.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


