Abstract
Background:
Distal radius fractures (DRFs) are one of the most common injuries among the elderly, resulting in significant expense and disability. The specific aims of this study are (1) to examine rates of therapy following DRFs and (2) to identify those factors that influence utilization of therapy and time span between DRF treatment and therapy among a national cohort of elderly patients.
Methods:
We examined national use of physical and occupational therapy among all Medicare beneficiaries who suffered DRFs between January 1, 2007, and October 1, 2007, and assessed the effect of treatment, patient-related, and surgeon-related factors on utilization of therapy.
Results:
Overall, 20.6% of patients received either physical or occupational therapy following DRF. Use of therapy varied by DRF treatment, and patients who underwent open reduction and internal fixation were more likely to receive therapy compared with patients who received closed reduction. Patients who received open reduction and internal fixation were also referred to therapy earlier compared with patients who received external fixation, percutaneous pinning, and closed reduction. Surgeon specialization is associated with greater use of postoperative therapy. Patient predictors of therapy use include younger age, female sex, higher socioeconomic status, and fewer comorbidity conditions.
Conclusion:
Use of therapy following DRF varies significantly by both patient- and surgeon-related factors. Identifying patients who benefit from postinjury therapy can allow for better resource utilization following these common injuries.
Distal radius fractures (DRFs) are the second most common fracture related to osteoporosis.1,2 They result in substantial disability and expense among individuals older than 60 years of age, with Medicare expenditures approaching nearly $170 million annually.3–4 Following DRF, many patients are referred for either occupational or physical therapy to expedite recovery.5,6 Occupational therapy is focused on modifying an individual’s environment, providing techniques and devices to increase independence with activities of daily living. Physical therapy is directed toward improving quality of life and well-being following an injury, with a specific focus on mobility and function. Although both have distinct goals, they are complementary when providing the approach to recovery.7,8
Although therapy is associated with improved outcomes following many acute and chronic conditions, its role following DRF is not clear.9,10 DRF therapy protocols vary widely and can include massage, soft-tissue compression, manual therapy techniques, heat/cold modalities, electrical simulation, ultrasound, whirlpool, and training (eg, self-care/home management training and community/work reintegration training).4,11–23 In some studies, participation in formal occupational therapy following DRF is correlated with improved wrist motion and grip strength.14,24 Yet, others do not show a clear benefit of therapist-directed therapy, and a recent randomized trial failed to demonstrate any advantage of a formal occupational therapy program for patients who underwent internal fixation following DRF.16,18,25 Nonetheless, many DRF patients undergo a 6-week program of hand therapy fully supervised by certified hand therapists (CHTs) with visits as many as 3 times a week. Strengthening exercises are usually introduced at 8–12 weeks, once sufficient motion has been restored, and therapy can last as long as 4 months.26 Therapy following DRF is both time intensive and financially expensive and comprises up to 20% of the total expense of caring for these common injuries.3,27 These expenses may not be obvious to surgeons, as the majority of therapy-related expenses are borne by hospitals rather than physician-owned facilities.28–31 This study will (1) examine rates of therapy following DRFs and (2) identify those factors that influence utilization of therapy and time span between DRF treatment and therapy among a national cohort of elderly patients.
MATERIALS AND METHODS
Data Source and Study Sample
Following international review board approval, we obtained Medicare claims data for all beneficiaries who suffered from lower forearm fracture during the year of 2007 from the Center for Medicare and Medicaid Services. Each claim contained at least one International Classification of Diseases, Ninth Revision, Clinical Modification code for fracture of the radius, with or without ulna fracture (813.00–813.93). All beneficiary claims were extracted from Medicare Carrier file, Outpatient file, and Provider Analysis and Review file. From this initial cohort, we identified DRF patients (n = 122,246) who underwent treatment within 14 days of diagnosis using relevant Current Procedural Terminology (CPT) codes and International Classification of Diseases, Ninth Revision (ICD-9) codes (Table 1). A 2-week period following injury was selected for analysis because the majority of DRF patients with unstable fractures would likely be candidates for a variety of fixation techniques within 2 weeks after the diagnosis. Patients who were not continuously enrolled in Medicare Part A and B during 2007 and patients who were enrolled in Medicare Part C plans (Part C claims are not required to be submitted to Medicare database) were excluded from the study to ensure the completeness of data. We also excluded patients with malignancy-related fractures. Figure 1 displays the creation of the study cohort.
Table 1.
Diagnosis Codes of DRF and Procedure Codes for Fracture Treatment among the Study Cohort
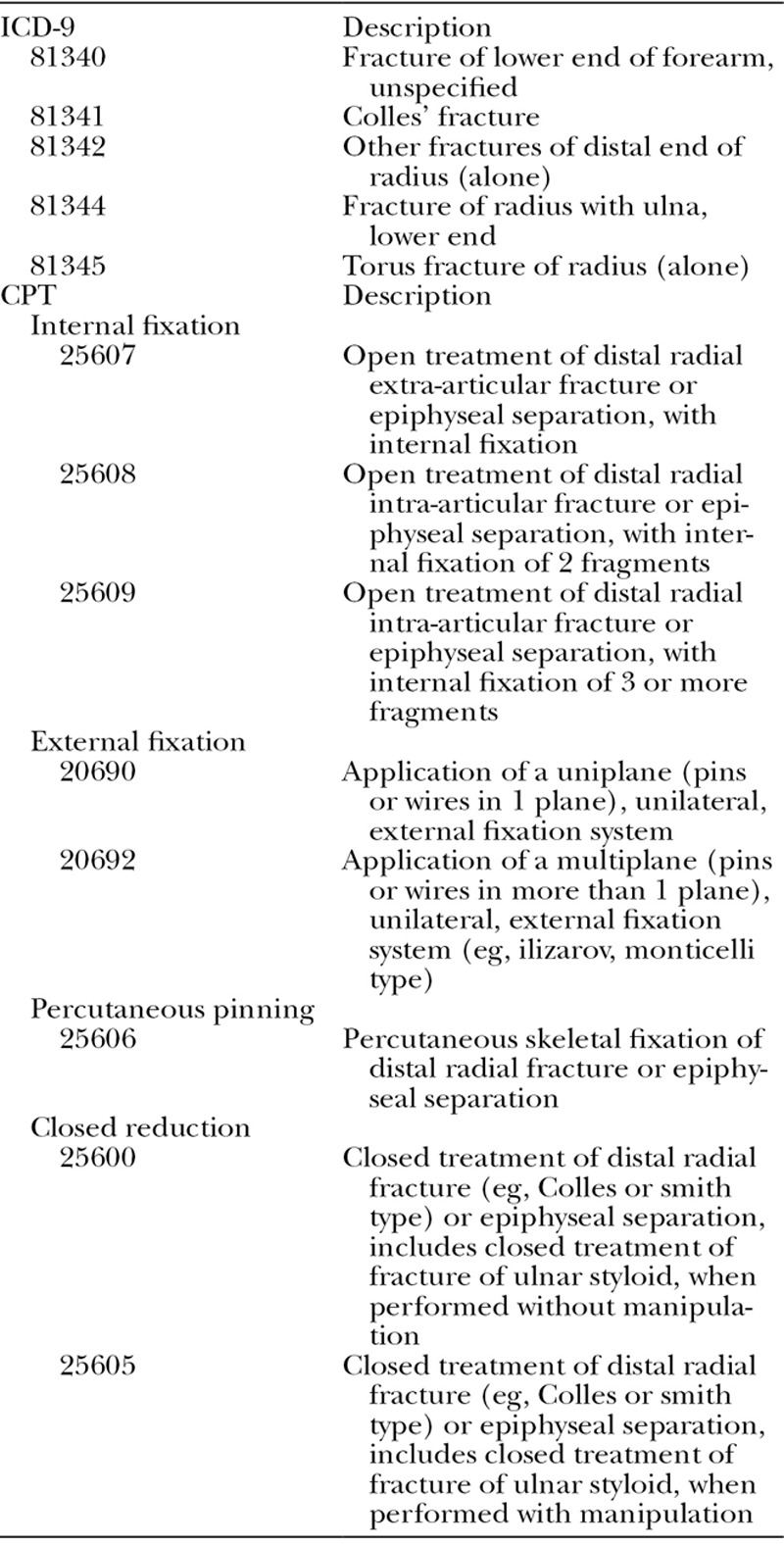
Fig. 1.
Inclusion criteria for study cohort.
VARIABLES
Dependent Variables
To follow each patient for 3 months for any use of physical or occupational therapy, we only include the patients (n = 46,754) who were diagnosed and treated from January 1st to October 1st, 2007, into the analysis. We defined the receipt of physical or occupational therapy as at least one Medicare claim of a therapy visit (CPT code 97001 and 97003) filed within 3 months following DRF with an associated diagnosis code of DRF to ensure that therapy was related to the injury. Additionally, we measured the time span from DRF treatment to initial therapy visit in days. The initiation of therapy is the first claim containing both diagnosis code of DRF and procedure code of physical or occupational therapy.
Independent Variables
DRF Treatment.
DRF fracture treatment was defined by CPT codes as the most invasive treatment within 14 days of DRF diagnosis and then categorized into 4 groups: open reduction and internal fixation (ORIF), external fixation ± pinning, percutaneous pinning, and closed reduction ± casting. Patients whose most invasive treatment was casting or splinting alone without reduction were excluded from the study due to potential differences in fracture severity.
Surgeon Characteristics.
We identified surgeons for each patient in cohort using Unique Physician Identification Numbers. Primary surgeon for each patient was defined as the surgeon who performed the most invasive treatment on patient within 14 days of DRF diagnosis. Patients for whom a primary treating surgeon could not be identified were excluded from further analysis. We included American Society for Surgery of the Hand (ASSH) membership during 2007 as a measure of surgeon specialization. Membership in ASSH requires a Subspecialty Certificate in Surgery of the Hand, providing a uniform measure of training qualifications. To measure the overall experience of each surgeon, we obtained number of years from graduation (to 2007) from American Medical Association Physician Masterfile. Finally, we included a measure of surgeon volume to examine surgeons’ experience on treating DRF patients as volume of surgeons can influence both the treatment outcomes and practice patterns.32–34 We defined surgeon volume as the number of DRF Medicare beneficiaries treated during the year of 2007. This was then grouped into 5, evenly distributed quintiles.
Patient Characteristics.
Using the Medicare denominator file, we obtained patient age, race, sex, and socioeconomic status (SES). Race was categorized as white, black, and other. To identify SES for each patient, residence zip code was used to construct socioeconomic measures according to 2000 US Census data on income, education, and occupation based on previously described methods.35 Clinical characteristics of patients included the presence of concurrent injuries with DRF and comorbid conditions. Using ICD-9 codes, we defined concurrent injury as any diagnosis of other injury on the same day of DRF diagnosis, including other fractures, neurologic condition (traumatic brain injury, subdural hematoma, and epidural hematoma), solid-organ injury (liver laceration/contusion, spleen laceration/contusion, bowel injury, diaphragmatic rupture, and kidney laceration/contusion), and other injuries, such as pneumothorax and hemothorax. For comorbid conditions, we assessed overall medical condition using the approach defined by Elixhauser et al.36 Additionally, we recorded complications and any second surgery for DRF within 3 months after the fracture. Complications of DRF identified in this study include nerve injury (ICD-9: 955.0–955.9), nerve compression/neuropathy of nerve (ICD-9: 354.2–354.9), carpal tunnel syndrome (CPT 354.0), and complex regional pain syndrome (ICD-9: 337.2, 337.21). The identification of DRF complication requires the presence of both diagnosis code of DRF and that complication in the same claim. Reoperation was defined as any second surgery under the diagnosis of DRF performed after the first surgical procedure. After the first treatment of DRF, each patient was followed for 3 months for complications and reoperation.
Analysis
To examine national variation in use of therapy following DRF, we calculated rates of therapy among DRF patients for each hospital referral region (HRR) in United States by dividing the number of patients received therapy after DRF treatment in that region by the number of cohort members resided in the region during 2007. HRRs are regions defined in The Dartmouth Atlas of Musculoskeletal Health Care to determining where Medicare beneficiaries were referred for major cardiovascular or neurosurgery by their residence address.37 The rates were plotted in a US map to reflect national use of therapy.
We used descriptive statistics to describe the characteristics of the patients and surgeons in our cohort. We then examined the receipt of therapy and the average number of days from treatment to therapy using hierarchical linear and logistic modeling (HLM) to assess the effect of patient- and surgeon-related factors and DRF treatment on these outcomes. HLM is a regression-based statistical technique that can be used for repeated-measures data and can accommodate both continuous and categorical variables.38 Compared with classic regression models, HLM provides a corrected standard error for nested data and can provide the proportion of the variation in the outcome accounted for by specific predictor variables. In our data, multiple patients could be treated by a single surgeon for a DRF, and HLM is advantageous in that it can account for clustered data in which predictor variables, such as surgeon characteristics, are nonindependent.38,39
RESULTS
Utilization of Physical or Occupational Therapy
A total of 9645 of 46,754 patients received either physical or occupational therapy within 3 months following DRF. Tables 2 and 3 display the characteristics of the patient and surgeon cohort of this study. The total payment (Medicare payment, copay/coinsurance, and primary payer payment) of outpatient therapy visit for our cohort was $12.3 million and $1284 per patient. Although average rate of therapy following DRF treatment was 20.6%, the percentage varied substantially among HRRs, from 2.2% in north New Mexico and south Alabama to 55.9% in North Dakota, north California, and east coast area (Fig. 2).
Table 2.
Demographic and Clinical Characteristics of Study Sample
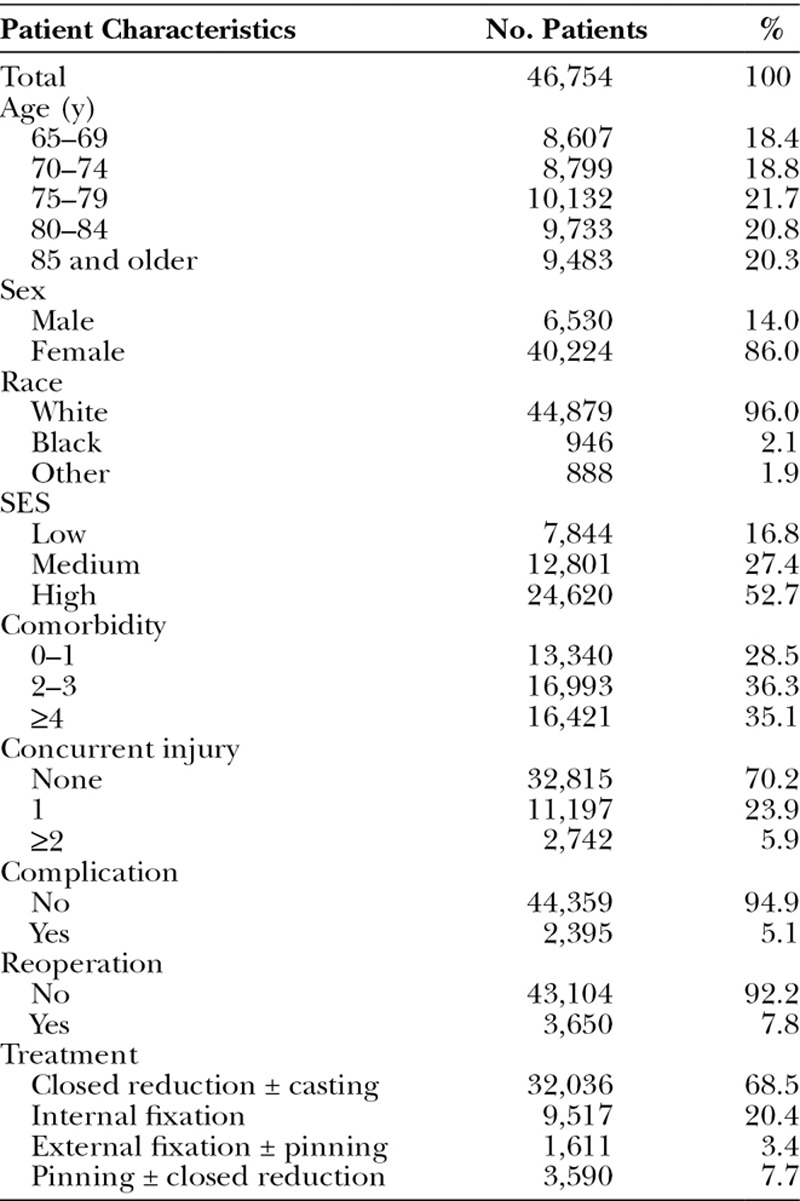
Table 3.
Characteristics of Surgeons Who Cared for Medicare Beneficiaries with DRF in 2007
Fig. 2.
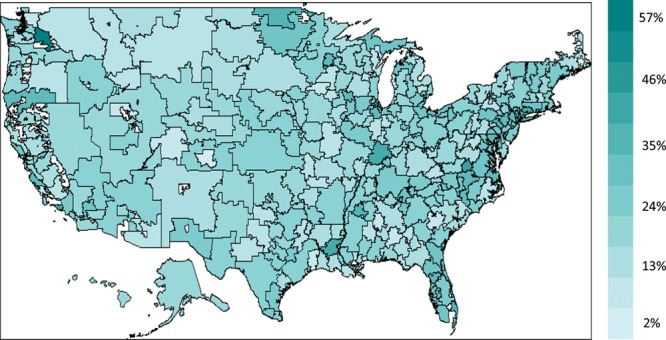
Regional distribution of physical and occupational therapy following DRF in the United States among Medicare beneficiaries by hospital referral region.
We examined the influence of fracture treatment on the receipt of therapy controlling for patient and surgeon characteristics (Table 4). Compared with patients who were treated by closed reduction and casting, patients who underwent ORIF were more likely to receive physical or occupational therapy [odds ratios (OR) = 1.66; 95% confidence interval (CI), 1.55–1.77]. Other surgical treatments such as external fixation and percutaneous pinning also predicted higher rate of therapy utilization.
Table 4.
Correlates of Receipt of Occupational or Physical Therapy following DRF among Medicare Beneficiaries in 2007
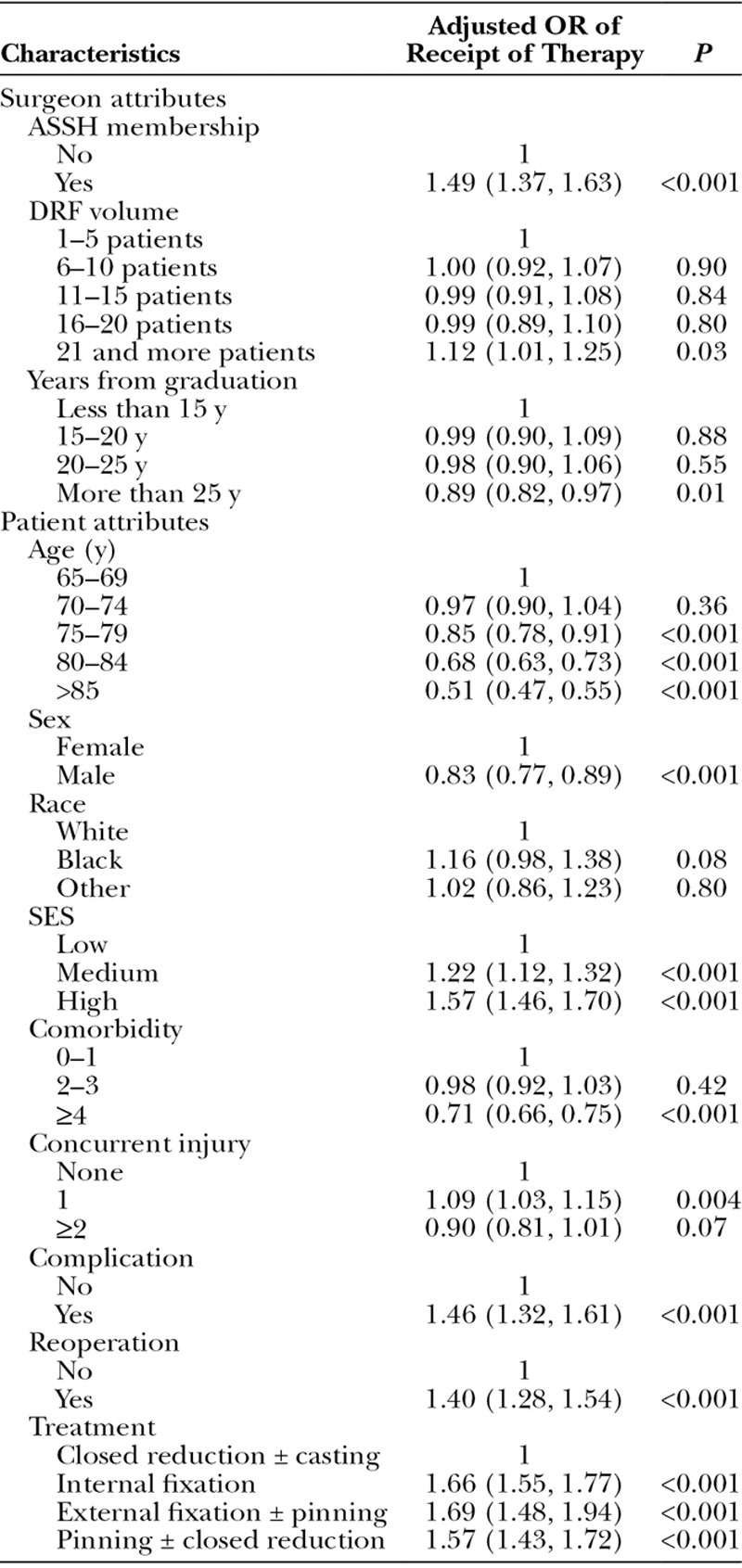
Patient and surgeon attributes were also significantly correlated with receipt of therapy (Table 3). For example, elderly patients were less likely to receive therapy compared with younger patients (OR = 0.51; 95% CI, 0.47–0.55). Furthermore, patients of high (OR = 1.57; 95% CI, 1.46–1.70) or medium (OR = 1.22; 95% CI, 1.12–1.32) SES were more likely to receive therapy compared with patients of lower SES. Patients with a greater number of comorbid conditions were less likely to receive therapy compared with healthier patients (OR = 0.71; 95% CI, 0.66–0.75). Finally, male patients were less likely to receive therapy compared with female patients (OR = 0.83; 95% CI, 0.77–0.89).
With respect to surgeon characteristics, ASSH members (OR = 1.49; 95% CI, 1.37–1.63) were more likely to refer patients to physical or occupational therapy than non-ASSH members.
Time from Diagnosis to Therapy Consultation
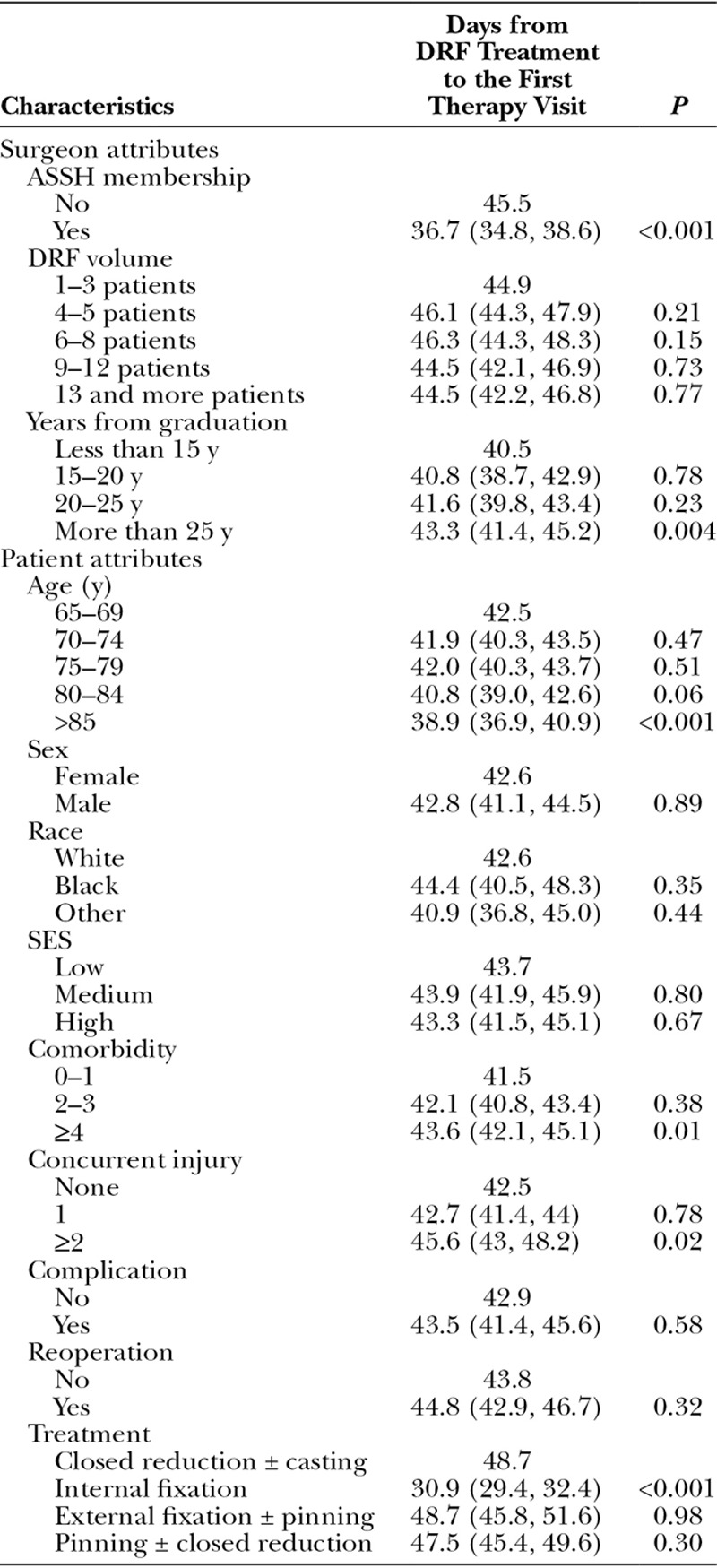
We examined the time from fracture treatment to therapy consultation in days (Table 5). In multivariate analysis, fracture treatment and surgeon ASSH membership were significantly correlated with the time to therapy. Interestingly, the majority of patients do not receive therapy for at least a month following treatment. For example, patients who underwent ORIF received therapy within 30.9 days compared with 48.7 days for patients treated by external fixation and 47.5 days for patients treated by percutaneous pinning. Furthermore, ASSH members referred patients to therapy earlier compared with nonmembers (36.7 d vs 45.5 d, P < 0.001).
Table 5.
Time from Treatment to Initiation of Physical or Therapy following DRFs among Medicare Beneficiaries in 2007
DISCUSSION
In this cohort of Medicare beneficiaries in 2007, we identified significant differences in the utilization of occupational or physical therapy by provider, patient, and treatment-related factors. Following DRFs, approximately 21% of patients received therapy, with expenditures approaching $12.3 million in this cohort. Patients who were younger, female, with higher SES, and fewer comorbid conditions were more likely to receive therapy following DRF. Surgeons who commonly manage patients with DRFs are more likely to refer patients for therapy and surgeons who are ASSH members. Although the average time to therapy was approximately 1 month following injury, patients who undergo ORIF following DRF receive therapy 15–20 days sooner compared with other treatment strategies. In summary, referral to therapy following DRF varies widely, with important implications for patient outcomes and the cost of care of these common injuries.
Synthesizing the data regarding therapy following DRF is difficult owing to the heterogeneity of techniques available, variation in timing of therapy, and the level of therapist involvement. Although clinical trials have suggested that specific therapeutic modalities may be effective, the evidence is largely derived from small, single-center studies. Furthermore, in recent years, the surgical treatment for DRFs has changed dramatically. Before the introduction of volar locking plates, casting, percutaneous pinning, external fixation, and fragment-specific-fixation techniques were commonly used to achieve stable fixation.40 For many patients, active therapy to regain wrist motion begins after external fixators or pins are removed, either because of the physical constraints of the immobilization devices or because the fixation is not rigid enough to withstand motion at the wrist. However, patients may still benefit from range of motion and edema control for digits before the removal of pins and fixators to overcome debilitating finger stiffness at the metacarpophalangeal and interphalangeal joints.41,42 The advent of volar plates with locking screws has heightened popularity for internal fixation.43–45 The volar approach provides biomechanically stable fixation, and locking screws prevent hardware loosening in weak, osteoporotic bone.17,45 Their increased stability also allows patients to begin earlier wrist motion after surgery and has prompted some surgeons to forego formal therapy protocols in lieu of minimally supervised self-directed hand therapy.43,45,46 Interestingly, our data suggest that patients who undergo internal fixation are more likely to receive occupational therapy compared with patients treated by other methods. Although volar plate fixation affords a stable construct for wrist fixation that may obviate therapy, many surgeons still refer routinely to occupational therapists without the supporting evidence that this ancillary treatment is needed to regain full function.
This study has several notable limitations. First, this study is an analysis of claims data, so we cannot identify those patients with a therapy referral but did not attend the therapy. Claims data do not comprehensively assess therapy duration and intensity, and we cannot assess the possibility of that patients may receive home-based therapy through other avenues not captured by the Centers for Medicare and Medicaid Services. Accuracy of administrative data is also a common concern; however, previous examination of Medicare claims indicates data accuracy greater than 80%.47,48 Another important limitation of claims data is designation as a CHT is not available. As our data are only for Medicare beneficiaries in the year of 2007, the measure of surgeon volume based on these data could only partially reflect surgeons’ DRF volume and it is not possible to capture all the DRF patients for each surgeon. Additionally, fracture patterns, influencing the receipt, frequency, and duration of therapy sessions, are not recorded in Medicare claims. Although information regarding comorbid conditions and concurrent injuries can be obtained from claims data, it is impossible to discern fracture severity and patterns, and we are not able to discern the reason for therapy referral, such as digit stiffness or wrist stiffness. Furthermore, we identified complications by a 3-month follow-up after DRF treatment, but it is not possible to capture the complications not managed or to differentiate complications such as stiffness of fingers or wrist that may influence the initiation of therapy. In this DRF cohort, the majority of patients underwent closed reduction and the place where the procedure was provided (operating room or physician’s office) might indicate different patterns of the fracture. However, due to the limitation of claim data, such information was not available. Finally, our study was drawn from a single year and may not reflect practice patterns over time.
Despite these limitations, our findings have important implications for surgeons caring for DRF patients. Our results reflect common barriers to care in the United States for vulnerable individuals.49 In our cohort, older patients with more comorbid conditions and of lower SES were less likely to go to therapy, regardless of the type DRF treatment they received. In the United States, variation in care and poor access to services among disadvantaged individuals is common for many acute and chronic conditions. For example, mortality rates are higher among individuals of lower SES, largely due to differences in health behaviors, such as smoking and alcohol consumption and access to care.40–42 Differences in care may also be attributable to physician training, and we observed that specialized hand surgeons were more likely to refer patients for occupational therapy rather than physical therapy compared with nonspecialized surgeons. Similar phenomena have been described for other clinical settings, and specialist physicians and surgeons are more likely to choose aggressive treatment regimens, refer patients for reconstructive procedures, and perform a greater number of diagnostic and therapeutic procedures.50–52 Streamlining the referral of patients for therapy may improve access to postacute health care, such as services provided by CHTs practice, in all patient populations. As an important part of postoperation care to keep edema and pain in minimum and to reduce arm stiffness, there is no accepted strategy for rehabilitation following DRFs to date, and future efforts should be directed toward comparing specific modalities and techniques in a systematic and rigorous way. Large, comparative, multicenter studies that examine discrete therapy regimens in a diverse sample will provide higher level evidence than small, single-center reviews of a specific technique without a comparison group. Additionally, more research is needed to identify those individuals who will derive the greatest benefit from therapy and those who will recover without a formal therapy program.
CONCLUSIONS
Our study showed that the usage of therapy following DRF varies by patient and surgeon factors. Future efforts directed toward identifying the most effective therapy regimens and those patients who most benefit from therapy will provide an opportunity for improving the care of these common injuries.
Footnotes
Disclosure: Dr. Waljee was supported by 2012 Clinical Research Grant funded by the American Foundation for Surgery of the Hand. Dr. Chung received NIH Research Grant by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institute on Aging, R01-AR062066, 2011–2016, and received Midcareer Investigator Award in Patient-Oriented Research by National Institute of Arthritis and Musculoskeletal and Skin Diseases, AR053120, 2008–2013. Neither of the other authors has any financial disclosures. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Cummings SR, Black DM, Rubin SM. Lifetime risks of hip, Colles’, or vertebral fracture and coronary heart disease among white postmenopausal women. Arch Intern Med. 1989;149:2445–2448. [PubMed] [Google Scholar]
- 2.Cummings SR, Kelsey JL, Nevitt MC, et al. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178–208. doi: 10.1093/oxfordjournals.epirev.a036281. [DOI] [PubMed] [Google Scholar]
- 3.Shauver MJ, Yin H, Banerjee M, et al. Current and future national costs to Medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36:1282–1287. doi: 10.1016/j.jhsa.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Handoll HH, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;3:CD003324. doi: 10.1002/14651858.CD003324.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Stucki G, Stier-Jarmer M, Grill E, et al. Rationale and principles of early rehabilitation care after an acute injury or illness. Disabil Rehabil. 2005;27:353–359. doi: 10.1080/09638280400014105. [DOI] [PubMed] [Google Scholar]
- 6.Gitlin LN, Hauck WW, Winter L, et al. Effect of an in-home occupational and physical therapy intervention on reducing mortality in functionally vulnerable older people: preliminary findings. J Am Geriatr Soc. 2006;54:950–955. doi: 10.1111/j.1532-5415.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 7.Penrod JD, Boockvar KS, Litke A, et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120. doi: 10.1111/j.1532-5415.2004.52309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halbert J, Crotty M, Whitehead C, et al. Hip Fracture Rehabilitation Trial Collaborative Group. Multi-disciplinary rehabilitation after hip fracture is associated with improved outcome: a systematic review. J Rehabil Med. 2007;39:507–512. doi: 10.2340/16501977-0102. [DOI] [PubMed] [Google Scholar]
- 9.Amini D. Occupational therapy interventions for work-related injuries and conditions of the forearm, wrist, and hand: a systematic review. Am J Occup Ther. 2011;65:29–36. doi: 10.5014/ajot.2011.09186. [DOI] [PubMed] [Google Scholar]
- 10.Hagsten B, Svensson O, Gardulf A. Early individualized postoperative occupational therapy training in 100 patients improves ADL after hip fracture: a randomized trial. Acta Orthop Scand. 2004;75:177–183. doi: 10.1080/00016470412331294435. [DOI] [PubMed] [Google Scholar]
- 11.Coyle JA, Robertson VJ. Comparison of two passive mobilizing techniques following Colles’ fracture: a multi-element design. Man Ther. 1998;3:34–41. doi: 10.1054/math.1998.0314. [DOI] [PubMed] [Google Scholar]
- 12.Souer JS, Buijze G, Ring D. A prospective randomized controlled trial comparing occupational therapy with independent exercises after volar plate fixation of a fracture of the distal part of the radius. J Bone Joint Surg Am. 2011;93:1761–1766. doi: 10.2106/JBJS.J.01452. [DOI] [PubMed] [Google Scholar]
- 13.Milica L, Mirjana K, Lidija D, et al. Pulsed electromagnetic field during cast immobilization in postmenopausal women with Colles’ fracture. Srp Arh Celok Lek. 2012;140:619–624. [PubMed] [Google Scholar]
- 14.Watt CF, Taylor NF, Baskus K. Do Colles’ fracture patients benefit from routine referral to physiotherapy following cast removal? Arch Orthop Trauma Surg. 2000;120:413–415. doi: 10.1007/pl00013772. [DOI] [PubMed] [Google Scholar]
- 15.Lyngcoln A, Taylor N, Pizzari T, et al. The relationship between adherence to hand therapy and short-term outcome after distal radius fracture. J Hand Ther. 2005;18:2–8; quiz 9. doi: 10.1197/j.jht.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Oskarsson GV, Hjall A, Aaser P. Physiotherapy: an overestimated factor in after-treatment of fractures in the distal radius? Arch Orthop Trauma Surg. 1997;116:373–375. doi: 10.1007/BF00433993. [DOI] [PubMed] [Google Scholar]
- 17.Maciel JS, Taylor NF, McIlveen C. A randomised clinical trial of activity-focussed physiotherapy on patients with distal radius fractures. Arch Orthop Trauma Surg. 2005;125:515–520. doi: 10.1007/s00402-005-0037-x. [DOI] [PubMed] [Google Scholar]
- 18.Krischak GD, Krasteva A, Schneider F, et al. Physiotherapy after volar plating of wrist fractures is effective using a home exercise program. Arch Phys Med Rehabil. 2009;90:537–544. doi: 10.1016/j.apmr.2008.09.575. [DOI] [PubMed] [Google Scholar]
- 19.Bruder A, Taylor NF, Dodd KJ, et al. Exercise reduces impairment and improves activity in people after some upper limb fractures: a systematic review. J Physiother. 2011;57:71–82. doi: 10.1016/S1836-9553(11)70017-0. [DOI] [PubMed] [Google Scholar]
- 20.Basso O, Pike JM. The effect of low frequency, long-wave ultrasound therapy on joint mobility and rehabilitation after wrist fracture. J Hand Surg Br. 1998;23:136–139. doi: 10.1016/s0266-7681(98)80248-9. [DOI] [PubMed] [Google Scholar]
- 21.Toomey R, Grief-Schwartz R, Piper MC. Clinical evaluation of the effects of whirlpool on patients with Colle’s fracture. Physiotherapy Canada. 1986;38:280–284. [Google Scholar]
- 22.Challis MJ, Jull GJ, Stanton WR, et al. Cyclic pneumatic soft-tissue compression enhances recovery following fracture of the distal radius: a randomised controlled trial. Aust J Physiother. 2007;53:247–252. doi: 10.1016/s0004-9514(07)70005-3. [DOI] [PubMed] [Google Scholar]
- 23.Härén K, Wiberg M. A prospective randomized controlled trial of manual lymph drainage (mld) for the reduction of hand oedema after distal radius fracture. Hand Ther. 2006;11:41–47. [Google Scholar]
- 24.Kay S, McMahon M, Stiller K. An advice and exercise program has some benefits over natural recovery after distal radius fracture: a randomised trial. Aust J Physiother. 2008;54:253–259. doi: 10.1016/s0004-9514(08)70004-7. [DOI] [PubMed] [Google Scholar]
- 25.Wakefield AE, McQueen MM. The role of physiotherapy and clinical predictors of outcome after fracture of the distal radius. J Bone Joint Surg Br. 2000;82:972–976. doi: 10.1302/0301-620x.82b7.10377. [DOI] [PubMed] [Google Scholar]
- 26.Christensen OM, Kunov A, Hansen FF, et al. Occupational therapy and Colles’ fractures. Int Orthop. 2001;25:43–45. doi: 10.1007/s002640000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker DJ, Yun H, Kilgore ML, et al. Health services utilization after fractures: evidence from Medicare. J Gerontol A Biol Sci Med Sci. 2010;65:1012–1020. doi: 10.1093/gerona/glq093. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JM, Sass TR. Physician ownership of ancillary services: indirect demand inducement or quality assurance? J Health Econ. 1995;14:263–289. doi: 10.1016/0167-6296(95)00003-z. [DOI] [PubMed] [Google Scholar]
- 29.Muenzen PM, Kasch MC, Greenberg S, et al. A new practice analysis of hand therapy. J Hand Ther. 2002;15:215–225. doi: 10.1016/s0894-1130(02)70004-5. [DOI] [PubMed] [Google Scholar]
- 30.Baser O, Fan Z, Dimick JB, et al. Outlier payments for cardiac surgery and hospital quality. Health Aff (Millwood) 2009;28:1154–1160. doi: 10.1377/hlthaff.28.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swedlow A, Johnson G, Smithline N, et al. Increased costs and rates of use in the California workers’ compensation system as a result of self-referral by physicians. N Engl J Med. 1992;327:1502–1506. doi: 10.1056/NEJM199211193272107. [DOI] [PubMed] [Google Scholar]
- 32.Waljee JF, Hawley S, Alderman AK, et al. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25:3694–3698. doi: 10.1200/JCO.2007.10.9272. [DOI] [PubMed] [Google Scholar]
- 33.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 34.Rogers SO, Jr, Wolf RE, Zaslavsky AM, et al. Relation of surgeon and hospital volume to processes and outcomes of colorectal cancer surgery. Ann Surg. 2006;244:1003–1011. doi: 10.1097/01.sla.0000231759.10432.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 36.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Sledge CB. The Dartmouth atlas of musculoskeletal health care. J Bone Joint Surg Am. 2001;83:1129-a–1130. [Google Scholar]
- 38.Vittinghoff E, Glidden DV, Shiboski SC, et al. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures. 2nd ed. New York, New York: Springer; 2012. [Google Scholar]
- 39.Boudourakis LD, Wang TS, Roman SA, et al. Evolution of the surgeon-volume, patient-outcome relationship. Ann Surg. 2009;250:159–165. doi: 10.1097/SLA.0b013e3181a77cb3. [DOI] [PubMed] [Google Scholar]
- 40.Chung KC, Shauver MJ, Yin H, et al. Variations in the use of internal fixation for distal radial fracture in the United States Medicare population. J Bone Joint Surg Am. 2011;93:2154–2162. doi: 10.2106/JBJS.J.012802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CooneyIII WP, Dobyns JH, Linscheid RL. Complications of Colles’ fractures. J Bone Joint Surg Am. 1980;62:613–619. [PubMed] [Google Scholar]
- 42.Missakian ML, Cooney WP, Amadio PC, et al. Open reduction and internal fixation for distal radius fractures. J Hand Surg Am. 1992;17:745–755. doi: 10.1016/0363-5023(92)90327-l. [DOI] [PubMed] [Google Scholar]
- 43.Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29:96–102. doi: 10.1016/j.jhsa.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Drobetz H, Kutscha-Lissberg E. Osteosynthesis of distal radial fractures with a volar locking screw plate system. Int Orthop. 2003;27:1–6. doi: 10.1007/s00264-002-0393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orbay JL, Fernandez DL. Volar fixation for dorsally displaced fractures of the distal radius: a preliminary report. J Hand Surg Am. 2002;27:205–215. doi: 10.1053/jhsu.2002.32081. [DOI] [PubMed] [Google Scholar]
- 46.Smith DW, Brou KE, Henry MH. Early active rehabilitation for operatively stabilized distal radius fractures. J Hand Ther. 2004;17:43–49. doi: 10.1197/j.jht.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Losina E, Barrett J, Baron JA, et al. Accuracy of Medicare claims data for rheumatologic diagnoses in total hip replacement recipients. J Clin Epidemiol. 2003;56:515–519. doi: 10.1016/s0895-4356(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 48.Du X, Freeman JL, Warren JL, et al. Accuracy and completeness of Medicare claims data for surgical treatment of breast cancer. Med Care. 2000;38:719–727. doi: 10.1097/00005650-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Losina E, Wright EA, Kessler CL, et al. Neighborhoods matter: use of hospitals with worse outcomes following total knee replacement by patients from vulnerable populations. Arch Intern Med. 2007;167:182–187. doi: 10.1001/archinte.167.2.182. [DOI] [PubMed] [Google Scholar]
- 50.Shah BR, Hux JE, Laupacis A, et al. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28:600–606. doi: 10.2337/diacare.28.3.600. [DOI] [PubMed] [Google Scholar]
- 51.Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293:2609–2617. doi: 10.1001/jama.293.21.2609. [DOI] [PubMed] [Google Scholar]
- 52.Alderman AK, Hawley ST, Waljee J, et al. Correlates of referral practices of general surgeons to plastic surgeons for mastectomy reconstruction. Cancer. 2007;109:1715–1720. doi: 10.1002/cncr.22598. [DOI] [PubMed] [Google Scholar]


