Abstract
Background:
Abdominal wall, one of the most commonly transplanted composite tissues, is less researched and lacking animal models. Its clinical necessities were emphasized in multiple case series to reconstruct large abdominal defects. Previous animal models have only studied components of the abdominal wall transplant. We describe findings from a new model that more likely reflect clinical transplantation.
Methods:
Full-thickness hemiabdominal wall flap was procured from Brown Norway (BN) rats and transplanted to an orthotopic defect on Lewis rats. Three groups were studied: group 1: Lewis to Lewis syngeneic; group 2: BN to Lewis control; and group 3: BN to Lewis with postoperative cyclosporine. Vascular imaging and cross vessel section were performed along with full-thickness abdominal wall. Immune cell profiling with flow cytometry at different time points was studied in all groups.
Results:
Syngeneic group had no rejection. Control group consistently showed rejection around postoperative day 6. With cyclosporine treatment, however, transplant and recipient tissue integration was observed. Flow cytometry revealed that innate immunity is responsible for the initial inflammatory events following abdominal wall engraftment. Adaptive immunity cells, specifically interferon-γ-producing T helper (Th) 1 and interleukin-17-producing Th17 cells, dramatically and positively correlate with rejection progression of abdominal wall transplants.
Conclusions:
Technical, histological, and immunological aspects of a new rat model are described. These results give clues to what occurs in human abdominal wall transplantation. In addition, Th1, a proinflammatory cell, was found to be a potential biomarker for allograft rejection.
Multivisceral transplantation has become a clinical reality with the advance of surgical technique and understanding of transplant immunology.1 A major issue stems from this type of transplantation is the lack of soft-tissue coverage with the sudden increase in abdominal content. Most of these patients already have a decreased abdominal domain due to scarring from prior surgeries or tumor resection.2
To overcome this obstacle, in 2001, Levi et al3 performed the first abdominal wall transplantation along with small bowel. Since then, it has become one of the most common composite tissues transplanted clinically with hand and face; at least 15 cases were reported in the literature.2,4
Many unknown sequelae for this procedure, however, still exist, including the concern for lifelong immunosuppression and associated drug toxicities, uncertain degree of muscle atrophy, and questionable subsequent hernia in the long run. Nonetheless, there have not been any appropriate animal models described in the literature to study this specific type of vascularized composite allotransplantation.
Published animal models so far do not include all layers of the abdominal wall.5–7 Although skin is the most immunogenic component of a composite tissue, from previous study, it is known that combinations of different tissue types alter the level of immune reaction.8 An ideal animal model thus should include layers from the skin to the peritoneum to simulate the clinical scenario, otherwise no accurate immune data or functional studies can be generated. Therefore, the aim of this study is to establish an inexpensive and reproducible rat orthotopic model. We will examine its feasibility, postoperative course, pathology, and immune profile and describe the technical aspects of a full-thickness hemiabdominal wall transplantation.
MATERIALS AND METHODS
Animals
All animal experiments were performed in accordance with guidance from the Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital. Inbred Lewis (RT11) and Brown Norway (BN) (RT1n) male rats, weighing 250–330 g and 8–12 weeks old, were used as recipients and donors, respectively. Anesthesia was induced and maintained with inhaled isoflurane. They were maintained on rodent food ad libitum. Postoperative care included ketoprofen (5 mg/kg/d, subcutaneous) for 5 days and intramuscular cephazolin (50 mg/d) for 3 days.
Experimental Design
Eighteen rats were divided into the following groups:
Group 1 (Syngeneic): Lewis to Lewis (N = 5).
Group 2 (Allo-Control): BN to Lewis (N = 8).
Group 3 (Allo-CsA): BN to Lewis (N = 5); one dose of preoperative intraperitoneal antilymphocyte serum (ALS) 2.5 mg with 30 days of tapering subcutaneous cyclosporine (16 mg/kg/d × 10 days, 10 mg/kg/d × 10 days, and 5 mg/kg/d × 10 days).
Surgical Procedure
Donor Surgery
The full-thickness hemiabdominal wall flap was based on the common iliac vessels, preserving the deep inferior epigastric vessels, the deep circumflex iliac vessels, and the superficial inferior epigastric vessels (SIEVs).
A rectangular skin area of dimension 5 × 4 cm was drawn on the unilateral hemiabdomen of donor. The dissection starts with an oblique incision in the groin 1 cm inferiolateral and parallel to the inguinal ligament to preserve the SIEVs. The femoral artery and vein branches distal to the take off of the SIEVs were ligated. Incision along the previously drawn skin area was made full thickness through the underlying muscle (Fig. 1A). Medial incision lied in the exact midline of bilateral rectus abdominus muscles. Inguinal ligament was detached laterally from anterior superior iliac spine and medially from public tubercle. With the full-thickness abdominal flap flipped inferiorly, deep inferior epigastric vessels and deep circumflex iliac vessels could be seen and traced to their origin from the external iliac vessels (Fig. 1B). All other branches of the external iliac were tied off, eventually the internal iliac vessels as well to provide a longer pedicle. The flap was only connected by the common iliac vessels and ready to be harvested upon recipient site preparation; the donor rat was moved to the isoflurane infused anesthetic chamber temporarily.
Fig. 1.
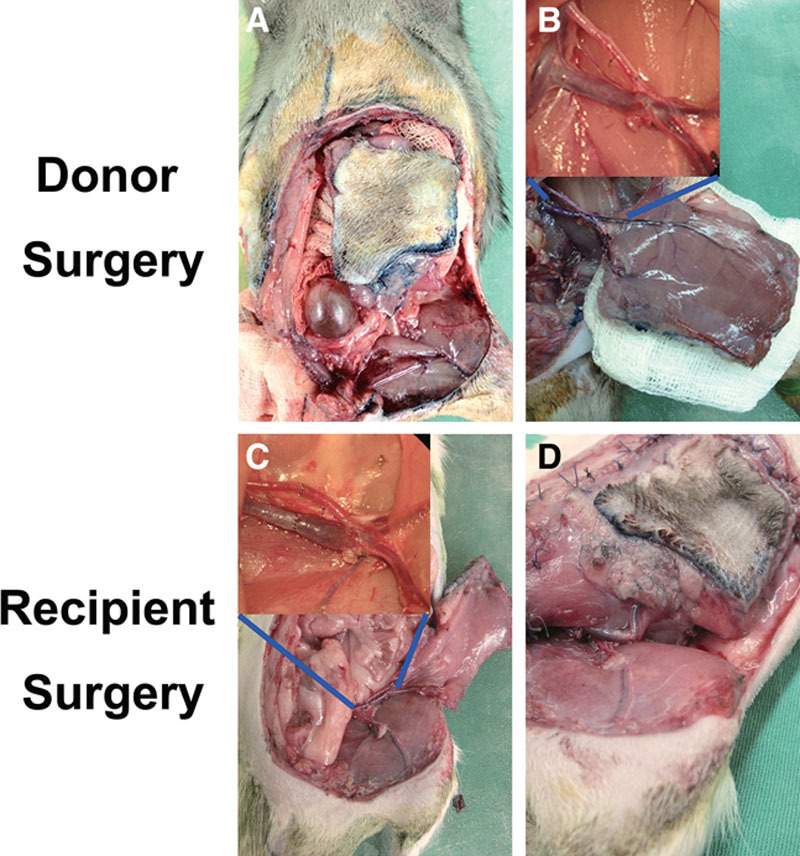
Transplant procedure. Donor surgery. A, Full-thickness incision made along planned dimensions, inguinal ligament detached from its origin and insertion. B, Abdominal flap connected to donor by common iliac vessels only awaits recipient preparation. Recipient surgery. C, End-to-end anastomosis with interrupted 10-0 Nylon. D, Transplanted abdominal inguinal ligament fixated to recipient free ends and full-thickness muscle closed in 1 layer with interrupted 4-0 Vicryl.
Recipient Surgery
Recipient vessels were the common femoral artery and vein in the groin area distal to the inguinal ligament.
A 5 × 4 cm area similar to the donor was drawn on the ipsilateral hemiabdomen. Different from the donor surgery, SIEVs were ligated. All branches of the femoral vessels distal to inguinal ligament were ligated to allow pedicle mobility. Full-thickness recipient defect was then created, leaving a short stump of inguinal ligament attached to their origin medially and laterally. The dissected abdominal flap was divided from the donor and irrigated with heparinized saline (50 U/ml) until venous outflow turned clear. Microanastomosis was performed between recipient femoral vessels and common iliac vessels of the transplanted abdominal wall with 10-0 Nylon (Fig. 1C). The inguinal ligament of the transplanted abdominal wall was fixed to the free ends of the recipient inguinal ligament stumps medially and laterally. The muscle layer is sutured to the defect using absorbable suture and the skin layer closed with 4-0 Nylon (Fig. 1D). Attention was made to ensure that transplant and recipient rectus muscle ends are reapproximated.
Postoperative Course and Rejection Timing
Daily weight, photograph, and visual inspection were done on each rat. Rejection period was defined as the time frame from start of epidermolysis/diffuse erythema to complete extrusion of the transplanted flap from the recipient site.
Histology Evaluation
Full-thickness section was performed on day 7, 14, 21, and 60 in Allo-Control group, day 30 and 60 in Allo-CsA group, and day 60 in Syngeneic group.
Immune Profiling with Flow Cytometry
Peripheral blood was taken for cytometry analysis for cell surface markers (CD4, CD8, and CD25) and cytokines [interferon (IFN)-γ, interleukin (IL)-17, IL-4, IL-10, and FoxP3] 1 week before surgery as baseline and then on a weekly basis. For intracellular cytokine staining, cells were stimulated with phorbol 12-myristate 13-acetate (50 ng/ml), ionomycin (1 μg/ml), and monesine (4 μM) for 4 hours.
Optical Imaging for Vascular Pattern
The in vivo fluorescence imaging for blood flow network was at a resolution of 85 μm with Pearl Impulse (LI-COR, Lincoln, NE). Blood flow to the transplant was imaged with the fluorescence probe NiraWave C (Miltenyi, Auburn, CA). Baseline optical imaging was performed on postoperative day (POD) 1, then on POD 60 and 120 in group 1, and 2 days after the start of rejection in groups 2 and 3.
Statistical Analysis
The statistical significance of the allograft survival rate and weight change was calculated using the Kaplan-Meier method and Mann-Whitney test, respectively. The significance of flow cytometry was evaluated by the 2-tailed Student’s t test. P < 0.05 was considered statistically significant.
RESULTS
Postoperative Course and Rejection Timing
Procedure had 100% success rate. Average surgical time for 1 rat was 4 hours. Total ischemia time was 60 minutes and average flap weighed 10 grams.
Group 1 showed integration of the transplanted tissues (Fig. 2) with no observable muscle atrophy and hernia on POD 60 (Fig. 4). Rejection occurred in group 2 starting around POD 6. Group 3 showed temporary tolerance of the transplant with proper healing until withdrawal of immunosuppressants; rejection initiated 2 weeks after the stop of cyclosporine around POD 45 (Fig. 3). The progression of rejection had a much slower course than in Allo-Control group. In both groups 2 and 3, transplanted flaps were routinely spitted out from the recipients; the abdominal defect all healed by secondary intention with recipient skin and peritoneum (Fig. 2). The transplant vessels underwent autoamputation distal to the anastomoses.
Fig. 2.
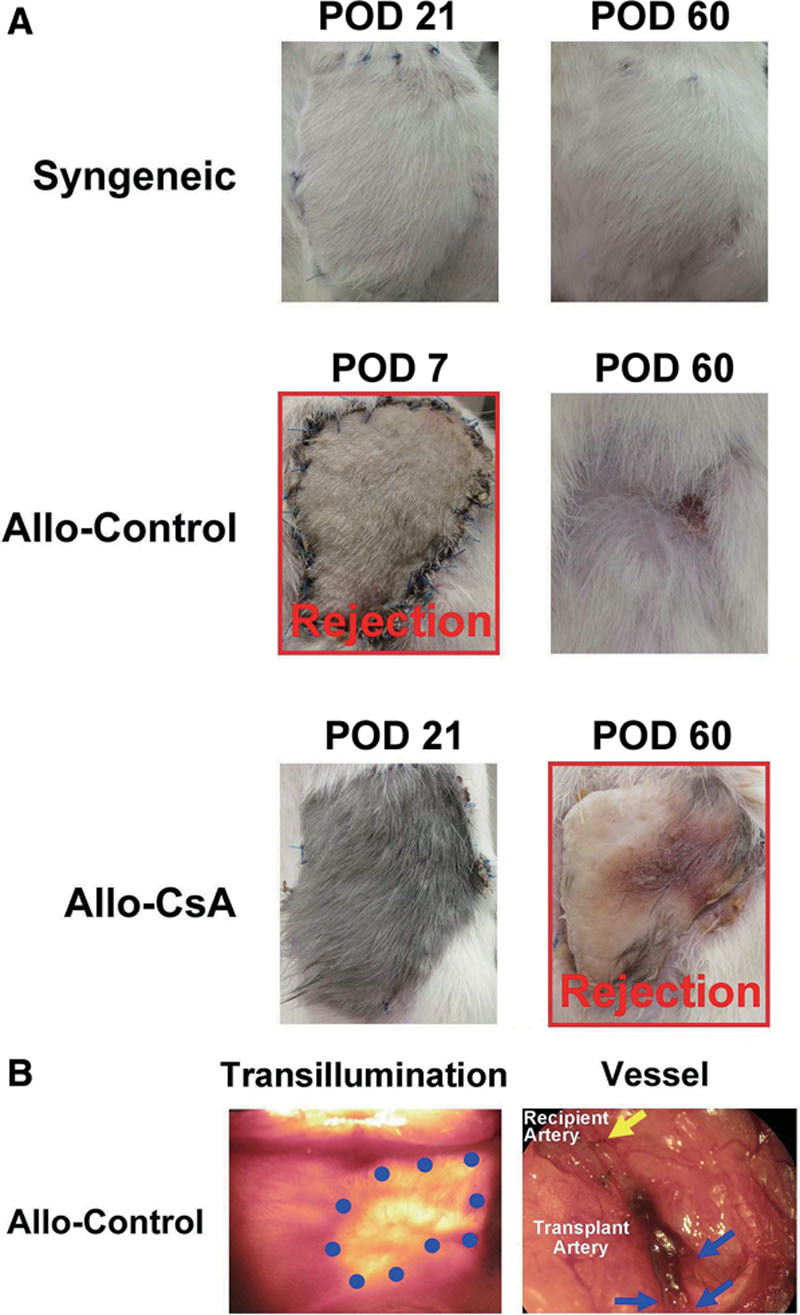
Posttransplantation course. A, Rejection time. Syngeneic group showed integration of the transplanted tissue with hair growing out. Rejection occurred in Allo-Control starting around POD 6 and proceeded from ischemia, necrosis, to spitting out of the transplant. Cyclosporine-treated group (Allo-CsA) showed temporary tolerance of the tissue until 2 weeks after withdrawal of immunosuppressant around POD 45. By POD 60, flap was necrotic. B, In Allo-Control group, the transplants were eventually extruded from the recipients, prior abdominal defects all healed secondarily with the original space covered with skin and peritoneum only. Blue dots outline the border of the previous abdominal defect under transillumination. Vessel thrombosis and autoamputation occurred distal to anastomosis on transplant side only when the allograft was extruded. Blue arrows point to sites of vessel breakage while yellow arrow points to locations of microanastomosis.
Fig. 4.
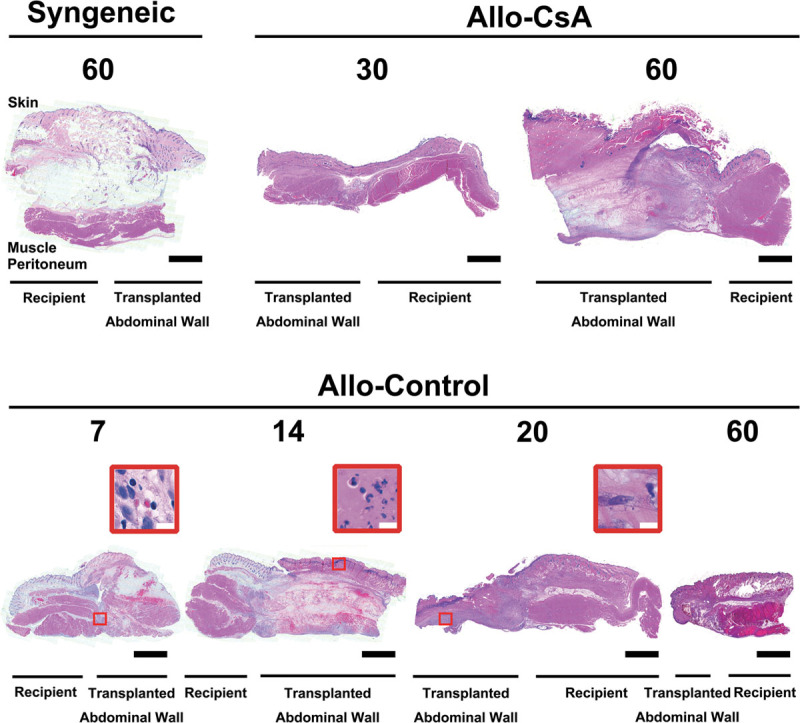
Cross-sectional histology of full-thickness abdominal wall at the suture junction between transplanted and recipient tissues. No obvious muscle atrophy or inflammatory cells were found on either side in syngeneic group on POD 60 and in Allo-CsA group on POD 30. After withdrawal of immunosuppressant, full necrotic changes are seen on POD 60 in Allo-CsA group. In the Allo-control group, however, destruction of the transplant tissue was a progressive continuum. Subcutaneous edema and hemorrhage were observed on POD 7 with lymphocytes in the muscle layer (red box), progressively worsened on POD 14 with dense neutrophils at the epidermal-dermal junction (red box), and with fibroblasts along the peritoneal surface seen on POD 20 (red box). Muscle destruction to frank necrosis and fibrosis resulted by POD 20 and POD 60. All changes were limited to the transplant tissue. The white bars and black bars are 10 μm and 1 mm, respectively.
Fig. 3.
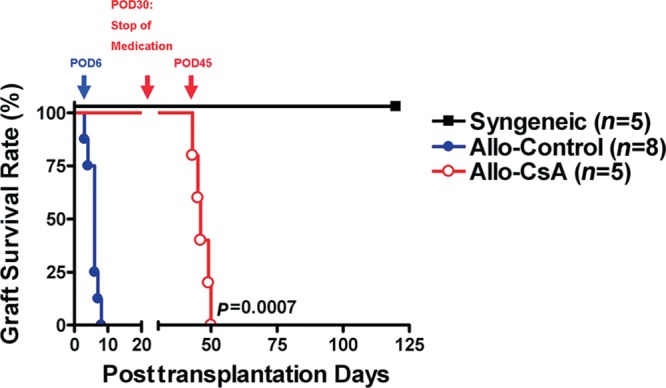
Abdominal flap survival. Difference in length of flap survival in Allo-CsA group vs Allo-Control group was statistically significant.
Histological Evaluation
Group 1 (Syngeneic) POD 60
No pathology was seen on either side. There was no observable muscle atrophy comparing the transplanted and its adjacent recipient abdominal wall (Fig. 4). The recipient and transplant arterial structures were intact.
Group 2 (Allo-Control) POD 7, 14, 21, and 60
On POD 7, there was significant subcutaneous edema and hemorrhage on the transplant side (Fig. 4). Scattered inflammatory cells consisted of lymphocytes, and fibroblasts were seen at the transplant-recipient muscle junction. Compared with the recipient muscle, transplanted muscle began to lose its striation. The architecture of transplant artery was still intact compared with the recipient artery. On POD 14, full-thickness necrosis with worsening edema and diffuse hemorrhage was seen throughout the transplant. Epidermolysis was seen with an intense purple band of neutrophils in the dermal layer with vacuolization. Muscle architecture was lost, and a dense layer of fibroblast was seen in the peritoneum and at transplant-recipient junction. The transplant arterial structure began to break down with denser inflammatory infiltrate between media and intima; intima started to peel away and slough off. On POD 21, the entire transplant was necrotic, no muscular structure seen. There were lots of fibroblasts mixed with lymphocytes throughout the flap with a band of neutrophils in the remaining dermis. By POD 60, most transplanted abdominal walls were extruded or dry gangrenous. There was organized thrombosis in the transplant vessel leading to global ischemia and necrosis. Recipient artery stayed unchanged.
Group 3 (Allo-CsA) POD 30 and 60
On POD 30, there was no major difference between the recipient and transplant tissues. Scattered fibroblasts with some muscle fibrosis can be seen on the transplant side. Transplant artery remained intact. On POD 60, the transplanted abdominal flap appeared fibrotic and necrotic. Loss of all muscle architecture and dense neutrophils were seen as a dark purple line in dermis. Dense fibroblast mixed with lymphocytes at the transplant junction and peritoneum. Transplant vessel with intimal destruction and immune cell occlusion were seen (Fig. 4).
Vascular Pattern
In Syngeneic group, skin vessels followed a stable pattern with outgrowth beyond suture line from the flap on POD 60 and 120. In Allo-Control group, blood flow was not visualized even at 2 days after the start of rejection. However, image taken at 2 days after the start of rejection in Allo-CsA group showed retention of the blood flow in the skin, suggesting a much slower rejection process compared to Allo-Control group. Allo-CsA group at POD 45 also showed vasculature outgrowth from POD 1 (Fig. 5). Macroscopic evidence of vessel outgrowth from the transplanted flap was seen routinely in the Syngeneic and Allo-CsA groups (Fig. 5).
Fig. 5.
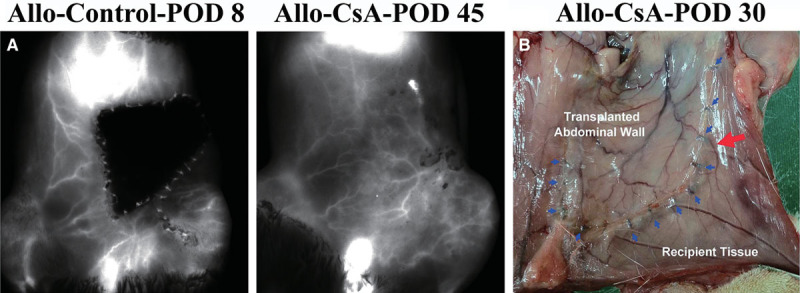
The in vivo fluorescence imaging for blood flow network at a resolution of 85 μm with Pearl Impulse. A, In Allo-control group, vascular network is not visualized possibly due to thickened tissue or thrombosis of blood vessels as early as 2 days after rejection started. The blood flow was preserved; however, 2 days after start of rejection in the Allo-CsA group, corresponded to the slower rejection course observed in this treated group even after withdrawal of immunosuppressant. B, Macroscopic evidence of vessel outgrowth from the transplanted abdominal wall to surrounding recipient abdominal tissue was seen. Blue arrows denote the suture line and red arrow points to the outgrown blood vessel.
Immune Profiles
In the Syngeneic group, after transplantation, there was an initial increase in the granulocyte ratio, with no increase in monocyte and lymphocyte compared to the baseline. On the contrary, in the Allo-Control and Allo-CsA groups, monocytes increased significantly to at least 2× baseline value during the rejection process peaking at the beginning of the rejection period. Lymphocyte percentage, however, underwent minimal increase throughout the rejection compared to baseline values in Allo-Control group. Allo-CsA group displayed further decrease in lymphocyte ratio after transplantation as a result of ALS administration (data not shown).
Specifically looking at the proinflammatory IFN-γ- and IL-17-producing T cells in these groups, they positively correlated with rejection of abdominal wall allotransplant (Fig. 6). It appeared that CD4+IFN-γ+ and CD8+IFN-γ+ cells increased specifically before rejection. Especially evident in the CD4+IFN-γ+ subpopulation, even with preoperative ALS treatment in the Allo-CsA group, its relative percentage was unchanged initially and increased significantly before rejection process in both Allo-Control and Allo-CsA groups. It did not react to the initial stress from surgery as seen by the initial peaks in other cell populations. By contrast, CD4+IL-17+ and CD8+IL-17+ cells not only increased during time of rejection but also highly reactive to the initial surgical stress after transplantation. As for the anti-inflammatory T cells, both CD4+IL-10+ and CD8+IL-10+ cells increased during both initial surgical stress and rejection period regardless of preoperative ALS, especially obvious in the CD4+IL-10+ population. CD4+IL-4+ and CD8+IL-4+ cells had the similar profile (data not shown).
Fig. 6.
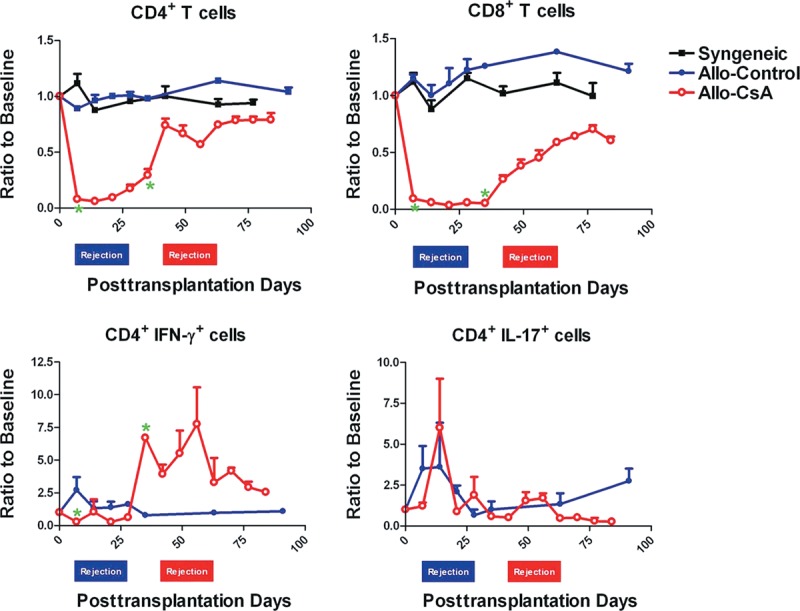
Immune profiles by flow cytometry. Initial drops of total CD4+ and CD8+ T cells in Allo-CsA group reflect preoperative ALS administration. The composition of IFN-γ- or IL-17-producing inflammatory T cells in the peripheral blood of Allo-Control and Allo-CsA groups was obtained from at least 2 independent experiments. Specifically, POD 7, the usual rejection start time for Allo-Control group, and POD 30, the end day of cyclosporine administration for Allo-CsA group, were scrutinized and marked with asterisk when the difference between the 2 groups is statistically significant.
DISCUSSION
The need for abdominal wall transplantation is often seen with intestine/multivisceral organ transplantation; there are estimated more than 1500 intestines with or without visceral organs transplanted worldwide since 1985.1 Due to the sudden increase in volume in an often retracted abdominal domain, it is estimated that 20% of these patients are not amenable to conventional surgical methods for immediate closure.3 With an already suppressed immune system from organ transplantation, leaving the abdomen open renders further complications.
Rat is an ideal animal model because of its low cost and the feasibility of orthotopic allograft placement. It has been shown that there are enormous similarities between human and rat abdominal wall structures in terms of their layered muscle pattern and orientations.9
In this study, we transplant only one side of the abdomen and use the contralateral side as self-control to monitor how allograft affects its surrounding recipient tissues. From optical imaging and macroscopic findings, the skin on each hemiabdomen was predominantly supplied by its ipsilateral pedicle, but perforator vessels could cross over to supply neighboring areas of the contralateral abdomen (Fig. 5). This finding echoes deep inferior epigastric perforator flaps in human and was not seen with the previous total abdominal skin model by Nasir et al5 where muscular perforators were excluded in the flap.
Transplanted abdomen had a predictable pattern of rejection. The rats receiving no treatment routinely produced clinical signs of rejection around POD 6 following the sequence of erythema, epidermolysis, ischemia, and necrosis. This progression mirrors other rat transplant models for the face and hindlimb.10,11 The immune response was highly specific to the transplanted abdomen sparing the recipient tissues (Fig. 4). The dense inflammatory infiltrate in the skin confirmed the concept of skin being the most immunogenic component histologically.8 Interestingly, in the mesothelium-lined peritoneum, a monolayer of specialized lubricating epithelial cells, there were minimal inflammatory cells but mostly fibroblasts alone.
All flaps undergoing rejection developed ischemia at the end. This was reflected in the intimal destruction seen on cross vessel section; inflammatory cells and tissue destruction were specific to the transplant vessels with eventual thrombosis and autoamputation distal to the anastomosis (Figs. 2B and 4).
The phenomenon that recipient defects all healed by secondary intention concomitantly as the rejected allografts being extruded could be a favorable finding if clinical transplants ever became unsalvageable from rejection (Fig. 2B). The grown-in skin and peritoneum over the defect lacked the muscular layer but provided these rats a stable coverage for visceral organs.
During the period of temporary tolerance in Allo-CsA group, tissue healing and integration was comparable to that of syngeneic tissue. There was macroscopic evidence of vessel outgrowth from the allograft (Fig. 5B). No nerve coaptation was performed on purpose to simulate the published clinical transplants.2–4 Occasional intercostal nerve fibers could be seen across the suture line; but it was difficult to distinguish whether this was due to incidental approximation of recipient and transplant nerve endings or a neurotization effect from the recipient nerve endings growing into transplanted muscles. There was no gross muscle atrophy in the syngeneic group as far as POD 120, but this was relatively a short-term result. One could at least conclude from these findings that tissue healing and neovascularization occur in a proper manner in abdominal wall transplantation as long as tolerance was induced.
Different combinations of tissue types generate different immune profiles in composite tissue allotransplantation.8 Scrutinizing all cell population changes at different time points, T helper (Th) 1 and Th17 cells underwent dramatic increase during the period of acute rejection. Our data also suggested that granulocyte acted as a nonspecific inflammatory marker, whereas monocyte played a key role in mounting the process of acute rejection of abdominal wall allograft. In addition, the relative magnitude of monocyte increase could suggest the intensity of rejection since rejection progression was much faster and intense in the Allo-Control group vs the Allo-CsA group.
Currently, there are no biomarkers available in the field of composite allotransplants. Routine monitoring methodologies for hand recipients have been skin biopsies, vascular imaging, or by graft appearance.12,13 These methods recognize clinical rejection after the fact but not before its onset. IFN-γ-producing CD4+ Th1 cells in our study did not react to the initial surgical stress but specifically increase to 7× the baseline levels before rejection occurrence. Its instant change in magnitude with the timing of change before clinical rejection makes it a potential biomarker for prediction and early diagnosis of transplant rejection. Th1 cells are also simple to detect by intracellular cytokine staining because of its substantial and constant percentages in the peripheral blood (Fig. 6). Screening for Th1 changes can potentially fine-tune patient therapy after transplantation by minimizing rejection episodes and improving long-term survival of allograft.
IL-17-secreting Th17 cells, these recently discovered proinflammatory T cells, are found to highly associate with solid organ rejection.14–16 Its role in vascularized composite allotransplantation, however, has not been elucidated. (Fig. 6) Th17 reacts to initial surgical stress despite ALS administration and increases sharply during the rejection period, suggesting having a key role in acute inflammation associated with rejection in abdominal transplant.
Th1 cells peaking before and Th17 cells peaking during the process of acute rejection propose a temporal relationship and the possible dominant roles of each proinflammatory T cells at different stages to mount or sustain a clinical rejection in abdominal wall transplantation. Designing neutralizing antibody specifically targeting these effector T cells, that is, anti-p35 to Th1 or anti-p19 to Th17, can potentially unlock new therapeutic means at different time points of acute rejection. Antagonism of Th1 and Th17 cells with IL-12p40 antibody has already been shown to prolong cardiac allograft survival via a decrease in both T helper cell-specific transcription factors.17
The next phase is to study the long-term functional outcome and muscular atrophic changes using EMG electromyography and biopsy to compare allografts with and without intercostal neurorrhaphy. In addition, based on the immune data, various suppressive regimens targeting at Th1 and Th17 cells should be experimented while paying close attention to the temporal relationship and the validity of using IFN-γ-producing CD4+ Th1 cell as a biomarker for rejection.
CONCLUSIONS
We established the first anatomical rat model to reflect human abdominal wall transplantation and defined its postoperative course, immune profiles, and histological changes. This model is easily reproducible and provides the foundation for further functional and immunological studies for further clinical application.
ACKNOWLEDGMENTS
We thank Chuen Hsueh for pathology reading, Ling-Yi Shih and Chih-Fan Lin for flow cytometry, and Hsien-Tang Lin for vessel staining. We also thank Chang Gung Molecular Imaging Center for TissueFAXS Plus (TissueGnostics, Wien, Austria).
Footnotes
Drs. Lao and Wang contributed equally to this study.
Drs. Lao and Ramirez are former Microsurgery Fellows of Chang Gung Memorial Hospital.
Presented at American Society for Reconstructive Microsurgery, Annual Meeting, January 12–15, 2013, Naples, Fla.; 17th World Congress of the International Confederation for Plastic, Reconstructive, and Aesthetic Surgery, February 24–March 1, 2013, Santiago, Chile; and Midwestern Association of Plastic Surgeons, 52nd Annual Scientific Meeting, April 27–28, 2013, Chicago, Ill.
Disclosure: Drs. Ramirez and Lao were sponsored by Ministry of Foreign Affairs and Overseas Chinese Affairs Council, Republic of China, respectively. This work was supported by Chang Gung Memorial Hospital, Taiwan, ROC (CMRPG3B1041). The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Vianna RM, Mangus RS, Tector AJ. Current status of small bowel and multivisceral transplantation. Adv Surg. 2008;42:129–150. doi: 10.1016/j.yasu.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Selvaggi G, Levi DM, Cipriani R, et al. Abdominal wall transplantation: surgical and immunologic aspects. Transplant Proc. 2009;41:521–522. doi: 10.1016/j.transproceed.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Levi DM, Tzakis AG, Kato T, et al. Transplantation of the abdominal wall. Lancet. 2003;361:2173–2176. doi: 10.1016/S0140-6736(03)13769-5. [DOI] [PubMed] [Google Scholar]
- 4.Cipriani R, Contedini F, Santoli M, et al. Abdominal wall transplantation with microsurgical technique. Am J Transplant. 2007;7:1304–1307. doi: 10.1111/j.1600-6143.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 5.Nasir S, Bozkurt M, Klimczak A, et al. Large antigenic skin load in total abdominal wall transplants permits chimerism induction. Ann Plast Surg. 2008;61:572–579. doi: 10.1097/SAP.0b013e31816d8275. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Erdmann D, Chang JC, et al. A model of sequential heart and composite tissue allotransplant in rats. Plast Reconstr Surg. 2010;126:80–86. doi: 10.1097/PRS.0b013e3181dbbb64. [DOI] [PubMed] [Google Scholar]
- 7.Pradka SP, Ong YS, Zhang Y, et al. Increased signs of acute rejection with ischemic time in a rat musculocutaneous allotransplant model. Transplant Proc. 2009;41:531–536. doi: 10.1016/j.transproceed.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Lee WP, Yaremchuk MJ, Pan YC, et al. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg. 1991;87:401–411. doi: 10.1097/00006534-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Brown SH, Banuelos K, Ward SR, et al. Architectural and morphological assessment of rat abdominal wall muscles: comparison for use as a human model. J Anat. 2010;217:196–202. doi: 10.1111/j.1469-7580.2010.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demir Y, Ozmen S, Klimczak A, et al. Tolerance induction in composite facial allograft transplantation in the rat model. Plast Reconstr Surg. 2004;114:1790–1801. doi: 10.1097/01.prs.0000142414.92308.ab. [DOI] [PubMed] [Google Scholar]
- 11.Quatra F, Lowenberg DW, Buncke HJ, et al. Induction of tolerance to composite tissue allograft in a rat model. Microsurgery. 2006;26:573–578. doi: 10.1002/micr.20297. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman CL, Ouseph R, Blair B, et al. Graft vasculopathy in clinical hand transplantation. Am J Transplant. 2012;12:1004–1016. doi: 10.1111/j.1600-6143.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- 13.Hautz T, Zelger B, Grahammer J, et al. Molecular markers and targeted therapy of skin rejection in composite tissue allotransplantation. Am J Transplant. 2010;10:1200–1209. doi: 10.1111/j.1600-6143.2010.03075.x. [DOI] [PubMed] [Google Scholar]
- 14.Fábrega E, López-Hoyos M, San Segundo D, et al. Changes in the serum levels of interleukin-17/interleukin-23 during acute rejection in liver transplantation. Liver Transpl. 2009;15:629–633. doi: 10.1002/lt.21724. [DOI] [PubMed] [Google Scholar]
- 15.Vanaudenaerde BM, Dupont LJ, Wuyts WA, et al. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J. 2006;27:779–787. doi: 10.1183/09031936.06.00019405. [DOI] [PubMed] [Google Scholar]
- 16.Antonysamy MA, Fanslow WC, Fu F, et al. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- 17.Xie A, Wang S, Zhang K, et al. Treatment with interleukin-12/23p40 antibody attenuates acute cardiac allograft rejection. Transplantation. 2011;91:27–34. doi: 10.1097/tp.0b013e3181fdd948. [DOI] [PubMed] [Google Scholar]


