Abstract
Summary:
The Venous Thromboembolism Prevention study concludes that anticoagulation is effective in reducing the risk of thromboembolism in patients who are identified as higher risk by Caprini scores. This report critically assesses the statistics used in the Venous Thromboembolism Prevention study, its method of data presentation, and its conclusions. The usefulness of risk stratification and the value of anticoagulation—both prevailing concepts in risk reduction today—are challenged. Actual data show that chemoprophylaxis has no proven benefit in plastic surgery. Complications of anticoagulation predictably include excessive bleeding and hematomas, which may be serious and life-threatening. Several large published series of patients undergoing elective plastic surgery under total intravenous anesthesia have shown a much reduced risk of thromboembolism. A SAFE (Spontaneous breathing, Avoid gas, Face up, Extremities mobile) anesthesia method is discussed as a safer and more effective alternative to traditional general endotracheal anesthesia and anticoagulation. The choice for plastic surgeons is not between a venous thromboembolism and a hematoma. The choice is between a thromboembolism and adjusting our anesthesia and surgery habits to reduce the risk to a baseline level.
THE CONTROVERSY
Chemoprophylaxis is a controversial topic in plastic surgery today. The 2013 meeting of the American Society of Plastic Surgeons featured a debate1 on this subject, moderated by Dr. Simeon Wall Jr. Dr. Edwin Wilkins presented the case for chemoprophylaxis and I presented the case against it. The seriousness of thromboembolism and the need to reduce risk are not in dispute. Recent articles published in Plastic and Reconstructive Surgery uniformly support chemoprophylaxis to reduce thromboembolism rates in plastic surgery patients deemed to be at greater risk.2–6 Nevertheless, an informal poll of the audience, taken at the debate,1 revealed that roughly 70% of the attendees had not adopted anticoagulation as a preventative measure in their practices.
Excessive bleeding with chemoprophylaxis has been reported.7,8 A recent randomized study from Brazil8 documented an alarming number of hematomas (8 hematomas in 8 patients) in patients treated with the oral anticoagulant rivaroxaban. Proponents ask rhetorically, which is the lesser of 2 evils, a hematoma or a thromboembolism?1,9–13 A comment from Davison and Massoumi13 is frequently referenced1,10,12,14: “A hematoma is a medical stress, an inconvenience, an embarrassment, or an additional procedure, but rarely does it kill a patient.”
Unfortunately, advocates for chemoprophylaxis characterize “noncompliant” plastic surgeons as uninformed and their practices “inadequate.”3,14 Increasingly, plastic surgeons are willing to state (and testify) that chemoprophylaxis represents the standard of care for patients deemed to be at higher risk and are making themselves available as expert witnesses, even advertising their services online.12
Plastic surgeons who do not use anticoagulation in their practice are not insisting that proponents do the same or face serious professional consequences; the opposite is not true. The issue is not simply a debate of the merits but a question of standard of practice. Today, many hospitals and surgery centers have protocols for chemoprophylaxis, which may be the default option. The surgeon signs a form if he or she does not wish to comply. By not going along with this intervention, the surgeon may be (unfairly) perceived as deviating from the standard of practice and regarded negatively by nurses and colleagues. Plastic surgeons may be inclined to order anticoagulation simply for legal reasons (a problem endemic in medicine today), especially in view of the lack of literature in Plastic and Reconstructive Surgery supporting a decision not to order anticoagulation. This article seeks to remedy this imbalance by presenting the case against chemoprophylaxis on behalf of the majority of plastic surgeons who are not uninformed but unpersuaded of the benefit and safety of this intervention.
CAPRINI SCORES
The widely used Caprini scores,15,16 which Caprini admits are based on intuition, logic, emotion, and experience (hardly a sound scientific basis), are not universally accepted. Geerts (personal communication, April 16, 2013), the lead author of the American College of Chest Physicians (ACCP) 2004 and 2008 Guidelines on Antithrombotic and Thrombolytic Therapy, recommends against using Caprini scores to risk-stratify plastic surgery patients. He also cautions against extrapolating the ACCP guidelines to elective plastic surgery patients (Geerts WH, personal communication, April 16, 2013).
It is not difficult for a patient undergoing an abdominoplasty to acquire a moderate Caprini score. Using the 2010 Caprini model,16 a healthy 60-year-old woman (2 points) undergoing an abdominoplasty lasting 2–3 hours (3 points), at ideal weight, with no medical problems, on no medications, and with no history of thromboembolism would be assigned a Caprini score of 5 and would be eligible for anticoagulation.6,17
DATA PRESENTATION IN THE VENOUS THROMBOEMBOLISM PREVENTION STUDY
Only one large controlled study investigates the use of anticoagulation in plastic surgery patients, the Venous Thromboembolism Prevention (VTEP) study, published in Plastic and Reconstructive Surgery in 2011.3 Its title leaves little room for doubt about the conclusions: “Postoperative enoxaparin prevents symptomatic venous thromboembolism in high-risk plastic surgery patients.” My concerns about the ethics of chemoprophylaxis, and the reliability of the study conclusions, were recently published in Plastic and Reconstructive Surgery Global Open.18 I had expected to see a Letter to the Editor from these investigators defending their statistics and conclusions. I had also expected a vigorous defense at the recent debate.1 However, there was none, other than Dr. Wilkins’ general response that one cannot always trust the raw data. After the debate, my impression that the emperor wore no clothes was not diminished.
The VTEP study data are summarized in the authors’ illustrations, reproduced here in Figure 1. The actual numbers of thromboembolisms in the control and treatment groups were not disclosed in the article. To discover this information, the reader must multiply the percentages in the upper histogram by the known group sizes and then multiply these numbers by the percentages in the lower graph. The results are presented in Figure 2. The data reveal that thromboembolisms occur in patients across a range of Caprini scores; almost as many (47.6%) occur in patients with scores < 7 as in patients with scores ≥ 7. Figures 3 and 4 reveal that the incidence of this complication in treatment and control groups is the same, 1.2%. Figure 5 is a true histogram (percentages contained within the bars add up to 100%) that accounts for the difference in sample sizes; there is no evidence of a treatment benefit for patients with the highest Caprini scores.
Fig. 1.
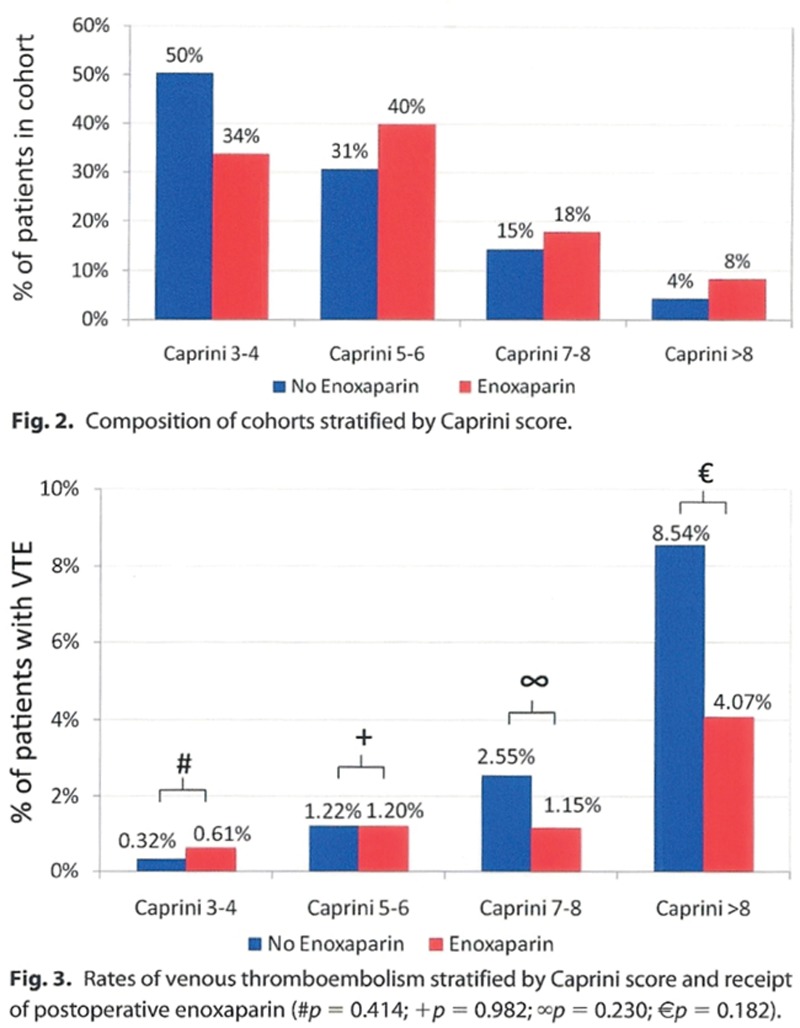
Figures 2 and 3 from the authors’ publication3 may be used to determine the actual number of patients who experienced thromboembolism. The percentages in the top histogram are multiplied by the known number of patients in the treatment (n = 1458) and control groups (n = 1876). These numbers are then multiplied by the percentages in the bottom graph to arrive at actual patient numbers depicted in Figure 2. The bottom graph appears to show an overall greater incidence of thromboembolism among control (blue) patients and greater treatment effectiveness in high-risk patients. VTE indicates venous thromboembolism. Illustration reprinted from Pannucci CJ, Dreszer G, Fisher Wachtman C, et al. Postoperative enoxaparin prevents symptomatic venous thromboembolism in high-risk plastic surgery patients. Plast Reconstr Surg. 2011;128:1093–1103.
Fig. 2.
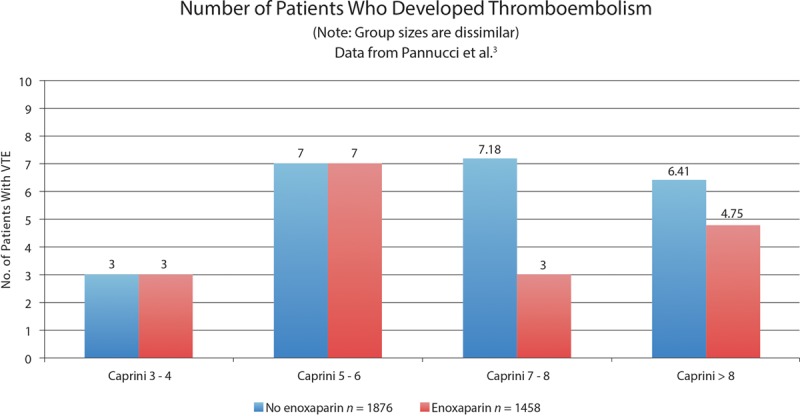
Histogram showing the distribution of patients as derived from the authors’ data. The number of control patients in the highest Caprini subgroup was 6.41 compared with 4.75 patients in the treatment group. It is unclear why the numbers are not whole. Almost half of the patients have scores < 7. This illustration shows actual patient numbers. It does not account for the 29% difference in sample sizes. VTE indicates venous thromboembolism.
Fig. 3.
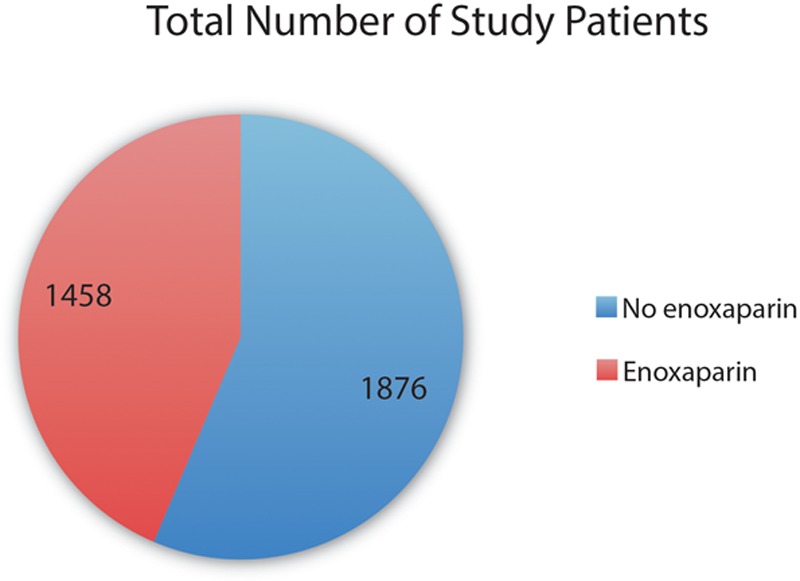
Numbers of patients in the control group (blue) and patients who received postoperative enoxaparin (red) in the Venous Thromboembolism Prevention study. The number of patients in the control group was 29% larger (1876 vs 1458) than the number of patients in the treatment group.
Fig. 4.

Calculated numbers of patients who developed thromboembolism among control patients (blue) and anticoagulated patients (red). The proportion of patients in each group (24 vs 18) was very similar to the percentage difference in sample sizes, making the incidence of this complication the same, 1.2% in both control and treatment groups. VTE indicates venous thromboembolism.
Fig. 5.
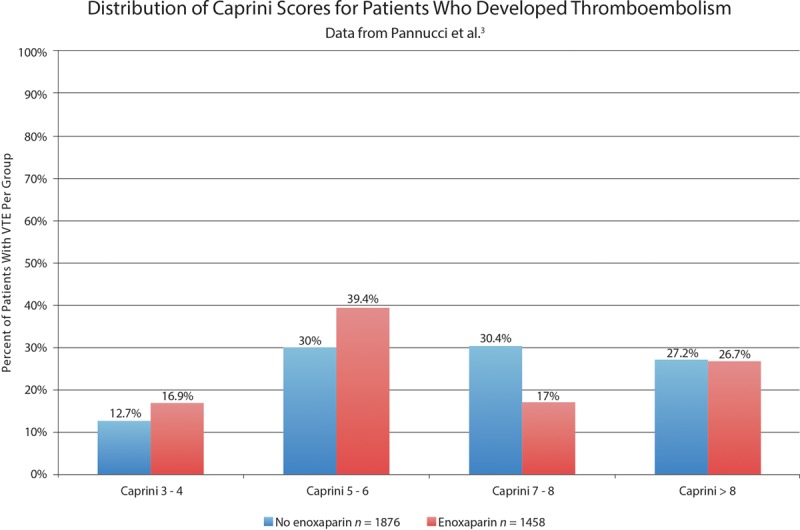
Histogram showing the distribution of affected patients by Caprini group. Unlike the authors’ graph (bottom graph, Fig. 1), this is a true histogram. The percentages add up to 100%. The graph accounts for the 29% difference in sample sizes. There is a very similar amount of blue and red, reflecting the equal overall incidence of this complication. There is no evidence of a treatment benefit in patients with the highest Caprini scores.
DATA ADJUSTMENT
Despite the VTEP study’s title, the actual data do not support its conclusions. To find a significant treatment benefit, the authors used logistic regression. The authors determined that the mean Caprini score was higher for the treatment group than for the historical control group. Controlling for this difference seems reasonable. However, the authors also adjusted their data to account for a disparity in mean length of hospital stay (3.8 days for treated patients vs 3.1 days for historical controls), a much more questionable statistical maneuver for several reasons. First, the length of hospitalization is not a known factor increasing the risk of thromboembolism. In fact, Caprini15 believes that patients after discharge may be just as sedentary as they were in hospital, remarking, “these individuals spend most of the time in a recliner, which is not early ambulation but rather early angulation.” Second, from a statistical perspective, the sample sizes in the hospital stay subgroups are much too small to allow a reliable statistical analysis. Third, anticoagulation was continued for the duration of the hospitalization,3 so that patients with longer admissions would have also received longer periods of anticoagulation.
Even with the authors’ adjustments, the data are too evenly distributed to skew sufficiently to find a significant treatment advantage for patients with higher Caprini scores. Nevertheless, nonsignificant differences (P = 0.230 and P = 0.182) are used to support the authors’ conclusions. These investigators also report a significant (P = 0.042) overall treatment benefit. It is difficult to imagine how an identical 1.2% incidence of this complication for treatment and control patients (Figs. 3 and 4) could be adjusted to show a significant overall treatment benefit. Statistical modeling requires prudence so that it does not become a form of statistical photoshopping. This method should not be used to adapt the data to conform to the investigators’ favored outcome.1
PREVAILING WISDOM
Twelve authors were listed on the VTEP article3 including some well-known researchers. The study was funded by the Plastic Surgery Foundation.3 Pannucci3 has a grant from the National Institute of Health, and of course, the University of Michigan is a respected academic institution. Do these considerations impart authority to the conclusions? Sackett,19 one of the founders of evidence-based medicine, once commented: “The first sin committed by experts consists in adding their prestige and their position to their opinions, which give the latter far greater persuasive power than they deserve on scientific grounds alone.” No degree of personal or institutional authority can take precedence over the facts. Pannucci et al2–5 have written extensively in favor of anticoagulation, including 4 articles published in Plastic and Reconstructive Surgery in the last 2 years. None of us is immune to our own prejudices.
Some might argue that we had better accept chemoprophylaxis because it is the trend in medicine and surgery—“everyone else is doing it.”1 Interestingly, orthopedic surgeons may be having second thoughts; the 2012 recommendations of the American College of Chest Physicians allow for the use of aspirin instead of low-molecular-weight heparin in orthopedic surgery.20 Perhaps surprisingly, a large randomized study among hospitalized patients, reported recently in the New England Journal of Medicine,21 found no benefit in thromboembolism risk for patients who were given enoxaparin. This finding was made all the more remarkable by the fact that the study was funded by the manufacturer of enoxaparin. If plastic surgeons are not careful, they may be jumping on the anticoagulation bandwagon just as our colleagues are jumping off.
HEMATOMAS
This controversy would be less important if anticoagulation did not add to the complication rate of surgery. Pannucci et al4 conclude that the hematoma rate is not increased by enoxaparin. At the same time, these investigators ask plastic surgeons to choose between a thromboembolism and a hematoma.1,10–12 Enoxaparin is known to cause wound hematomas in approximately 11% of patients and drug-induced thrombocytopenia in 1.5% of patients.22 Figure 6 compares hematoma rates in recent publications.8,23–25 Two of these studies23,24 report hematoma rates of 7.3% and 12.5% in anticoagulated patients, in the expected range. These figures contrast with rates of < 1% among untreated and control patients.8,23,25
Fig. 6.
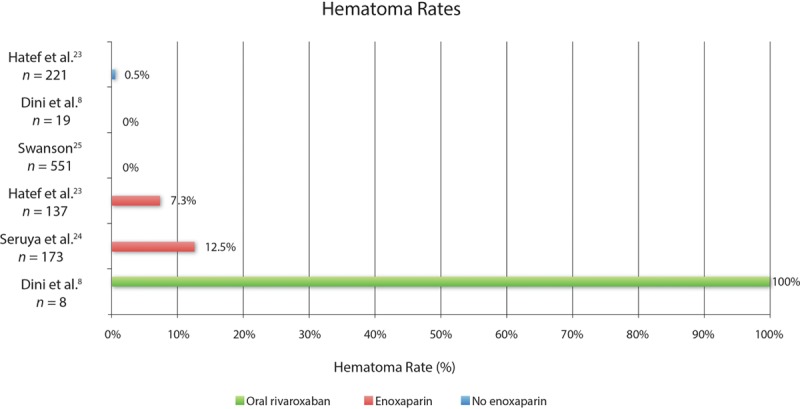
Hematoma rates in recent published series of plastic surgery patients treated without anticoagulation (blue), with enoxaparin injections (red), and with oral rivaroxaban (green). Patients in series reporting a 0% hematoma rate did not receive anticoagulation.
As any plastic surgeon will attest, hematomas are not just an inconvenience. A seroma is an inconvenience; hematomas have serious consequences. They frequently cause skin necrosis and wound dehiscences.8 Hematomas are likely to cause anemia, adding to patient morbidity, especially after combined liposuction and abdominoplasty procedures that involve substantial blood loss.26 Bleeding may lead to unplanned blood transfusions and hospitalizations.23 Hematomas are not conducive to a successful elective cosmetic surgery practice. With widespread implementation of chemoprophylaxis, some patient deaths will inevitably result from exsanguination, iatrogenic deaths in patients who were unlikely to develop a thrombosis in the first place. Even one such death is unacceptable if the benefit of anticoagulation is unproven.18
A compensatory benefit is unclear; thromboembolisms still occur despite anticoagulation (Fig. 7).13,23,24 “Chemoprophylaxis” may not live up to its billing; it does not prevent venous stasis, hypercoagulability, or vessel injury—the Virchow triad of factors implicated in the formation of a thrombosis.27
Fig. 7.
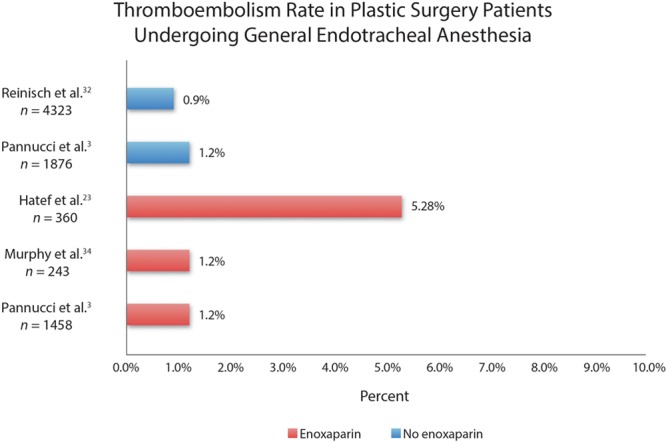
Examples of thromboembolism rates in plastic surgery patients undergoing general endotracheal anesthesia. Patients treated without enoxaparin are indicated in blue and patients treated with enoxaparin are indicated in red.
RISK STRATIFICATION
Risk stratification aims to determine the risk of an individual suffering a particular condition. The VTEP study reveals that affected patients are spread across all Caprini groups (Fig. 2). The finding that there were almost equal numbers of patients affected by thromboembolism in patients with Caprini scores < 7 (20 patients) as in patients with scores ≥ 7 (22 patients) casts doubt on the value of risk stratification. Approximately half (52.4%) of the affected patients will be identified and receive treatment. Patients selected for treatment by risk stratification have a 3.0% thromboembolism risk (22 of 735) instead of a 1.2% thromboembolism risk, a difference of < 2%. If used as a screening test, risk stratification (Caprini score ≥ 7) would have a sensitivity of 52.4% and a specificity of 3.0%, or a false-positive rate of 97.0% and a false-negative rate of 47.6%, dismal numbers indeed.
Alternatively, one could treat patients with Caprini scores < 7 and capture almost as many cases of thromboembolism (20 patients). Admittedly, the number of treated patients would be greater. However, those patients selected would be more robust (ie, younger and healthier) and better able to tolerate bleeding. In fact, one might argue that risk stratification in effect assigns a greater bleeding risk (11% instead of < 1%)8,22–25 to patients who are least able to tolerate the hemodynamic effects of blood loss, an example of the law of unintended consequences and a challenge to the principle of primum non nocere. It makes more sense to adopt a treatment strategy that benefits all patients, making risk stratification unnecessary.
“SAFE” ANESTHESIA
SAFE (Spontaneous breathing, Avoid gas, Face up, Extremities mobile) anesthesia is offered as an alternative method to reduce thromboembolism risk and improve safety. As in traditional general anesthesia, it requires the assistance of an anesthesiologist or certified nurse anesthetist.25,26
The Caprini model does not include the type of anesthesia as a risk factor.15,16 However, there have been multiple reports of reduced thromboembolism risk in patients who are administered intravenous anesthesia without muscle relaxation (Fig. 8).25,28–33 The theory is that avoiding muscle paralysis prevents blood pooling in the lower extremities,7,30,31 reducing the opportunity for venous stasis—a known factor implicated in thromboembolism.27
Fig. 8.
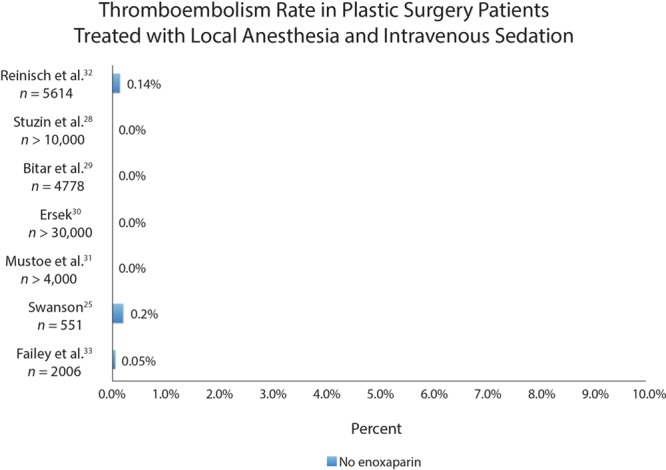
Examples of thromboembolism rates in plastic surgery patients treated with local anesthesia and intravenous sedation. Patients were not treated with anticoagulation. This empirical evidence supports the use of total intravenous anesthesia to reduce the incidence of thromboembolism. The same horizontal scale is used in Figure 7. The difference in complication rates is profound.
Figure 7 depicts the incidence of this complication in series of plastic surgery patients treated with general endotracheal anesthesia.3,23,32,34 A German study published in Plastic and Reconstructive Surgery in 200835 assessing serious complications after liposuction found that all 8 liposuction fatalities occurred in patients administered general anesthesia and none occurred in patients treated with intravenous sedation and local anesthesia. A survey conducted by Reinisch et al32 found a significant reduction in risk of thromboembolism among face-lift patients treated with intravenous sedation and local anesthetic compared with patients who received traditional general endotracheal anesthesia. Although there is no prospective controlled study, this empirical evidence is compelling and should not be dismissed (Geerts WH, personal communication, April 16, 2013). My own experience includes only one case of deep venous thrombosis during the last 10 years, occurring in 2005 in an abdominoplasty patient with a score of 3 using the 2010 Caprini model. This case was encountered during a prospective 5-year clinical study of 551 consecutive patients treated with liposuction and abdominoplasty.25 Fortunately, she made a full recovery (Fig. 9). No cases of thromboembolism occurred in a 10-year prospective clinical study of cosmetic breast surgery patients.36 Details of the anesthetic sequence including medications and dosing are beyond the scope of this article but are published separately.26 My experience is by no means unique.28–31
Fig. 9.
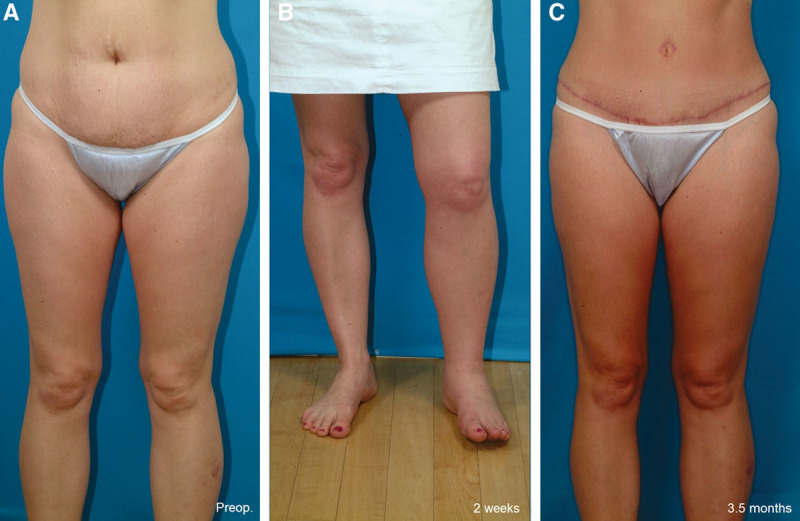
This 39-year-old woman is seen before surgery (A), 2 weeks after an abdominoplasty and liposuction of her lower body (B), and 3.5 months after surgery (C). She developed swelling of the left lower extremity 9 days after surgery. A Doppler ultrasound scan revealed a thrombosis extending from the left popliteal vein to the common femoral vein. She did not develop pulmonary emboli. She was hospitalized and treated with intravenous heparin, followed by oral warfarin. She made a full recovery. She had no risk factors for a deep venous thrombosis other than a 3-hour operation (Caprini score 3 using the 2010 scoring system).
There is little prospect of a prospective controlled study. Such a study would not be feasible considering the low incidence of this complication.18 Moreover, ethical considerations may not permit such a study because of the profound empirical treatment difference (Figs. 7 and 8 use the same horizontal scale). In view of the many other advantages of SAFE anesthesia (eg, eliminating the risk of malignant hyperthermia),1,18,26 it may be difficult to justify a traditional general endotracheal anesthetic if a safer alternative is available.
Spontaneous Breathing
Elective outpatient plastic surgery may be performed using intravenous infusions of propofol (provided it remains available) in combination with a laryngeal mask airway.26 Muscle paralysis is unnecessary, even when performing abdominoplasties with rectus plication. Infusion of the abdomen with an anesthetic solution provides adequate anesthesia of the abdominal wall.25,26 Spontaneous breathing allows the anesthetist to use the patient’s respiratory rate to guide intraoperative dosing of analgesics, expediting recovery. Respiratory alkalosis and secondary hypokalemia from mechanical ventilation are avoided.26
Avoid Gas
Inhalational agents have side effects.26 These include cardiovascular and respiratory depression, bronchial irritation, malignant hyperthermia, increased nausea, and possible exposure to operating room personnel.
Face Up
The patient may be turned from side to side to access all areas for liposuction.25,26 Supine and lateral positioning avoid the need for a hip bolster and pelvic pressure that might impair venous return from the lower extremities.23 Avoiding prone positioning makes intubation and mechanical ventilation unnecessary, avoids facial pressure, allows simultaneous breast surgery (best performed first to optimize sterility), and eliminates an unnecessary delay in surgery for patient repositioning.
Extremities Mobile
By turning the patient from the supine position to each side to infuse the areas with anesthetic solution and then repeating the process for liposuction, the lower extremities are kept moving, reducing the opportunity for venous stasis.26
CHOOSING AN ANESTHESIA METHOD
Figure 10 demonstrates the 4 commonly used anesthetic methods. Local anesthesia is impractical for large cases or combination surgery. Conscious sedation provides a reduced risk of deep venous thrombosis.31 However, these patients typically receive higher doses of benzodiazepines and fentanyl, prolonging recovery times.37 General endotracheal anesthesia provides adequate anesthesia but carries additional risks, as discussed.26 Total intravenous anesthesia offers an ideal “goldilocks” anesthetic,26 combining patient comfort and safety. Surgical decisions typically rest on an assessment of the anticipated benefit versus risk. The same analysis applies to administration of a medication or anesthetic (Figs. 11 and 12).
Fig. 10.
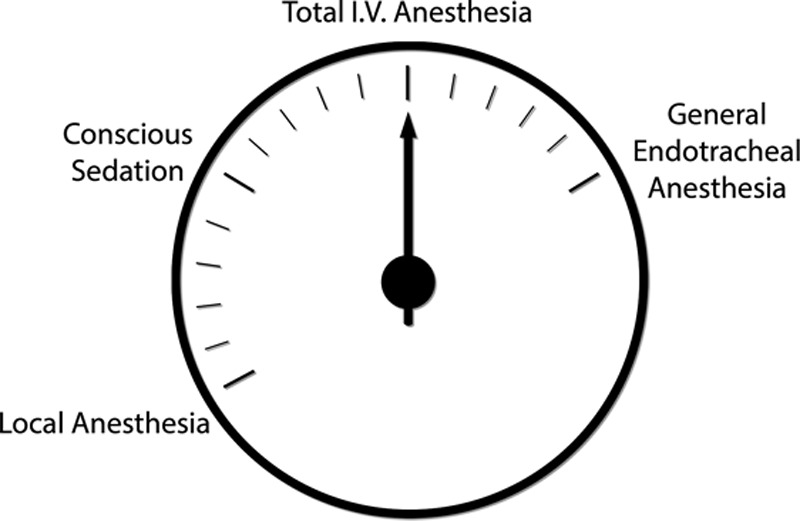
Four commonly used methods of anesthesia in elective plastic surgery. Total intravenous anesthesia offers a favorable balance between local and general endotracheal anesthesia, with optimal safety and improved patient recovery.
Fig. 11.
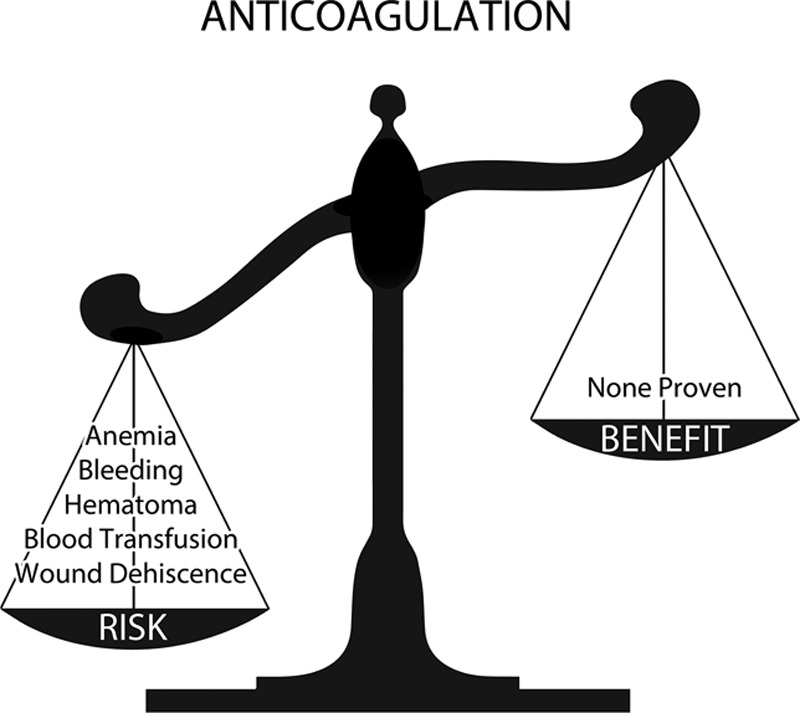
Risk versus benefit analysis for anticoagulation. Chemoprophylaxis introduces new risks without a proven compensatory benefit.
Fig. 12.

Risk versus benefit analysis for SAFE anesthesia. Total intravenous anesthesia avoids risk factors associated with general endotracheal anesthesia and offers improved safety, with no downside. VTE indicates venous thromboembolism.
MEDICOLEGAL CONSEQUENCES
As discussed earlier, the data reveal that risk stratification using Caprini scores is ineffective. In reality, it is impossible to reliably predict which patients will be affected. Thromboembolisms cannot be considered “never events,”34 in that it is unreasonable to expect a surgeon to never encounter one. After all, pulmonary emboli can occur even without surgery. The best we can do is endeavor to lower the probability to a baseline risk. Blaming the surgeon for such an unpredictable event compounds the tragedy of a patient death caused by a pulmonary embolus. Unless we recognize the limitations of risk stratification and chemoprophylaxis, our colleagues may soon be held liable for: (1) not prescribing anticoagulation to a patient who develops a thromboembolism or (2) prescribing anticoagulation to a patient who suffers bleeding consequences that may include death. Do we really wish to needlessly expand our medicolegal liability?
CONCLUSIONS
Chemoprophylaxis has no proven benefit in plastic surgery. Risk stratification is ineffective. A SAFE alternative to chemoprophylaxis is available that not only avoids additional risk but also adds to patient safety. The choice for plastic surgeons is not between a venous thromboembolism and a hematoma. The choice is between a thromboembolism and adjusting our anesthesia and surgery habits to reduce the risk to a baseline level.
Footnotes
Presented at Plastic Surgery 2013: The 82nd Annual Scientific Meeting of the American Society of Plastic Surgeons, October 11–15, San Diego, Calif.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the author.
REFERENCES
- 1.Wilkins E, Swanson E, Wall S., Jr Point-counterpoint: what’s the evidence in DVT prophylaxis?. Presented at: Plastic Surgery 2013: 82nd Annual Scientific Meeting of the American Society of Plastic Surgeons; October 11–15; San Diego, Calif. [Google Scholar]
- 2.Pannucci CJ, Jaber RM, Zumsteg JM, et al. Changing practice: implementation of a venous thromboembolism prophylaxis protocol at an academic medical center. Plast Reconstr Surg. 2011;128:1085–1092. doi: 10.1097/PRS.0b013e31822b67ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pannucci CJ, Dreszer G, Wachtman CF, et al. Postoperative enoxaparin prevents symptomatic venous thromboembolism in high-risk plastic surgery patients. Plast Reconstr Surg. 2011;128:1093–1103. doi: 10.1097/PRS.0b013e31822b6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pannucci CJ, Wachtman CF, Dreszer G, et al. The effect of postoperative enoxaparin on risk for reoperative hematoma. Plast Reconstr Surg. 2012;129:160–168. doi: 10.1097/PRS.0b013e318236215c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pannucci CJ, Barta RJ, Portschy PR, et al. Assessment of postoperative venous thromboembolism risk in plastic surgery patients using the 2005 and 2010 Caprini Risk score. Plast Reconstr Surg. 2012;130:343–353. doi: 10.1097/PRS.0b013e3182589e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy RX, Jr, Alderman A, Gutowski K, et al. Evidence-based practices for thromboembolism prevention: summary of the ASPS Venous Thromboembolism Task Force Report. Plast Reconstr Surg. 2012;130:168e–175e. doi: 10.1097/PRS.0b013e318254b4ee. [DOI] [PubMed] [Google Scholar]
- 7.Durnig P, Jungwirth W. Low-molecular-weight heparin and postoperative bleeding in rhytidectomy. Plast Reconstr Surg. 2006;118:502–507; discussion 508–509. doi: 10.1097/01.prs.0000228180.78071.44. [DOI] [PubMed] [Google Scholar]
- 8.Dini GM, Ferreira MC, Albuquerque LG, et al. How safe is thromboprophylaxis in abdominoplasty? Plast Reconstr Surg. 2012;130:851e–857e. doi: 10.1097/PRS.0b013e31826d9fc0. [DOI] [PubMed] [Google Scholar]
- 9.Young VL, Watson ME. The need for venous thromboembolism (VTE) prophylaxis in plastic surgery. Aesthet Surg J. 2006;26:157–175. doi: 10.1016/j.asj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Pannucci CJ, Wilkins EG. Hematoma risk should not preclude the use of venous thromboembolism prophylaxis. Aesthet Surg J. 2009;29:338; author reply 339. doi: 10.1016/j.asj.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Pannucci CJ. Reply: reducing venous thromboembolism risk without chemoprophylaxis. Plast Reconstr Surg. 2013;131:451e–452e. doi: 10.1097/PRS.0b013e31827c72b7. [DOI] [PubMed] [Google Scholar]
- 12.Wilkins E. Preventing VTE: reviewing the evidence and implications for practice.. Presented at: Plastic Surgery 2013: 82nd Annual Scientific Meeting of the American Society of Plastic Surgeons; October 11–15, 2013; San Diego, Calif. [Google Scholar]
- 13.Davison SP, Massoumi W. Our complication, your problem. Plast Reconstr Surg. 2007;120:1428–1429. doi: 10.1097/01.prs.0000279376.12476.b4. [DOI] [PubMed] [Google Scholar]
- 14.Pannucci CJ, Oppenheimer AJ, Wilkins EG. Practice patterns in venous thromboembolism prophylaxis: a survey of 606 reconstructive breast surgeons. Ann Plast Surg. 2010;64:732–737. doi: 10.1097/SAP.0b013e3181ba57a0. [DOI] [PubMed] [Google Scholar]
- 15.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–78. doi: 10.1016/j.disamonth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Caprini JA. Risk assessment as a guide to thrombosis prophylaxis. Curr Opin Pulm Med. 2010;16:448–452. doi: 10.1097/MCP.0b013e32833c3d3e. [DOI] [PubMed] [Google Scholar]
- 17.Clavijo-Alvarez JA, Pannucci CJ, et al. Prevention of venous thromboembolism in body contouring surgery: a national survey of 596 ASPS surgeons. Ann Plast Surg. 2011;66:228–232. doi: 10.1097/SAP.0b013e3181e35c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanson E. Chemoprophylaxis for venous thromboembolism prevention: concerns regarding efficacy and ethics. Plast Reconstr Surg Glob Open. 2013;1:e23. doi: 10.1097/GOX.0b013e318299fa26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sackett DL. The sins of expertness and a proposal for redemption. BMJ. 2000;320:1283. [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Chest Physicians Antithrombotic Guidelines. 9th ed. Available at: http://www.chestnet.org/Guidelines-and-Resources/Guidelines-and-Consensus-Statements/Antithrombotic-Guidelines-9th-Ed. Accessed November 13, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Kakkar AK, Cimminiello C, Goldhaber SZ, et al. LIFENOX Investigators. Low-molecular-weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365:2463–2472. doi: 10.1056/NEJMoa1111288. [DOI] [PubMed] [Google Scholar]
- 22.Enoxaparin Side Effects. Available at: http://www.drugs.com/sfx/enoxaparin-side-effects.html. Accessed November 13, 2013.
- 23.Hatef DA, Kenkel JM, Nguyen MQ, et al. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast Reconstr Surg. 2008;122:269–279. doi: 10.1097/PRS.0b013e3181773d4a. [DOI] [PubMed] [Google Scholar]
- 24.Seruya M, Venturi ML, Iorio ML, et al. Efficacy and safety of venous thromboembolism prophylaxis in highest risk plastic surgery patients. Plast Reconstr Surg. 2008;122:1701–1708. doi: 10.1097/PRS.0b013e31818dbffd. [DOI] [PubMed] [Google Scholar]
- 25.Swanson E. Prospective clinical study of 551 cases of liposuction and abdominoplasty performed individually and in combination. Plast Reconstr Surg Glob Open. 2013;1:e32. doi: 10.1097/GOX.0b013e3182a333d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson E. Prospective study of lidocaine, bupivacaine and epinephrine levels and blood loss in patients undergoing liposuction and abdominoplasty. Plast Reconstr Surg. 2012;130:702–722; discussion 723–725. doi: 10.1097/PRS.0b013e31825dc408. [DOI] [PubMed] [Google Scholar]
- 27.Lowe GD. Virchow’s triad revisited: abnormal flow. Pathophysiol Haemost Thromb. 2003;33:455–457. doi: 10.1159/000083845. [DOI] [PubMed] [Google Scholar]
- 28.Stuzin JM, Baker TJ, Baker TM. Deep venous thrombosis and pulmonary embolus after face lift: a study of incidence and prophylaxis (Discussion). Plast Reconstr Surg. 2001:107:1576–1577. doi: 10.1097/00006534-200105000-00044. [DOI] [PubMed] [Google Scholar]
- 29.Bitar G, Mullis W, Jacobs W, et al. Safety and efficacy of office-based surgery with monitored anesthesia care/sedation in 4778 consecutive plastic surgery procedures. Plast Reconstr Surg. 2003;111:150–156; discussion 157–158. doi: 10.1097/01.PRS.0000037756.88297.BC. [DOI] [PubMed] [Google Scholar]
- 30.Ersek RA. Dissociative anesthesia for safety’s sake: ketamine and diazepam—a 35-year personal experience. Plast Reconstr Surg. 2004;113:1955–1959. doi: 10.1097/01.prs.0000122402.52595.10. [DOI] [PubMed] [Google Scholar]
- 31.Mustoe TA, Buck DW, II, Lalonde DH. The safe management of anesthesia, sedation, and pain in plastic surgery. Plast Reconstr Surg. 2010;126:165e–176e. doi: 10.1097/PRS.0b013e3181ebe5e9. [DOI] [PubMed] [Google Scholar]
- 32.Reinisch JF, Bresnick SD, Walker JW, et al. Deep venous thrombosis and pulmonary embolus after face lift: a study of incidence and prophylaxis. Plast Reconstr Surg. 2001;107:1570–1575; discussion 1576–1577. doi: 10.1097/00006534-200105000-00044. [DOI] [PubMed] [Google Scholar]
- 33.Failey C, Aburto J, de la Portilla HG, et al. Office-based outpatient plastic surgery utilizing total intravenous anesthesia. Aesthet Surg J. 2013;33:270–274. doi: 10.1177/1090820X12472694. [DOI] [PubMed] [Google Scholar]
- 34.Murphy RX, Jr, Peterson EA, Adkinson JM, et al. Plastic surgeon compliance with national safety initiatives: clinical outcomes and “never events.”. Plast Reconstr Surg. 2010;126:653–656. doi: 10.1097/PRS.0b013e3181de1929. [DOI] [PubMed] [Google Scholar]
- 35.Lehnhardt M, Homann HH, Daigeler A, et al. Major and lethal complications of liposuction: a review of 72 cases in Germany between 1998 and 2002. Plast Reconstr Surg. 2008;121:396e–403e. doi: 10.1097/PRS.0b013e318170817a. [DOI] [PubMed] [Google Scholar]
- 36.Swanson E. Prospective comparative clinical evaluation of 784 consecutive cases of breast augmentation and vertical mammaplasty, performed individually and in combination. Plast Reconstr Surg. 2013;132:30e–45e. doi: 10.1097/PRS.0b013e3182910b2e. discussion 46e–47e. [DOI] [PubMed] [Google Scholar]
- 37.Byun MY, Fine NA, Lee JY, et al. The clinical outcome of abdominoplasty performed under conscious sedation: increased use of fentanyl correlated with longer stay in outpatient unit. Plast Reconstr Surg. 1999;103:1260–1266. doi: 10.1097/00006534-199904040-00026. [DOI] [PubMed] [Google Scholar]


