Abstract
Background:
Smoke inhalation is a major source of morbidity and mortality. Heparin and N-acetylcysteine treatment has potential efficacy in inhalation injury. We investigated the impact of a heparin/N-acetylcysteine/albuterol nebulization protocol in adult patients with inhalation injury.
Methods:
A retrospective review was performed of adult inhalation injury patients, admitted to a regional burn center between January 2011 and July 2012, who underwent a protocol of alternating treatments of heparin and N-acetylcysteine/albuterol nebulization every 4 hours. The study cohort was matched 1:1 by age, sex, and burn size to a control cohort admitted within 5 years before protocol implementation.
Results:
The study (n = 20) and control cohorts (n = 20) were well matched, with nearly identical age (50 vs 49 years), sex distribution (70% male), burn size (total body surface area, 22% vs 21%), and inhalation injury, except grade I injuries (79% vs 47%, P = 0.01). The protocol did not change mortality (30% vs 25%, P = 0.72) or duration of mechanical ventilation (8.5 vs 8.8 days, P = 0.9). There was no difference in development of sepsis (40% vs 33%, P = 0.7) or acute respiratory distress syndrome (15% vs 10%, P = 1); however, those who received the protocol were more likely to develop pneumonia (45% vs 11%, P = 0.03).
Conclusions:
The implementation of a heparin/N-acetylcysteine/albuterol protocol did not reduce mortality or duration of mechanical ventilation in this cohort of adults with inhalation injury and resulted in a significant increase in pneumonia rates. Larger prospective studies are necessary, with close attention paid to minimizing the infection risk incurred from frequent administration of nebulized medications.
Smoke inhalation continues to be a major source of morbidity and mortality in burn patients, increasing the likelihood of mortality by as much as 25% despite the many advances in burn care over the past decades.1 Although inhalation injury is not uncommon among patients with burns, there remains no standard for its diagnosis, scoring, and subsequent treatment, which makes it difficult to evaluate the results of studies and treatments.2 For these reasons, inhalation injury was designated a top 10 research priority at the 2007 American Burn Association (ABA) Consensus Conference.3
Smoke inhalation occurs through a variety of mechanisms, including thermal injury to the respiratory tract mucosa; however, the exact mechanism of what occurs following smoke inhalation injury is not clearly understood. Studies have shown that airway edema combined with obstructive casts produced from cellular debris, fibrin clots, polymorphonuclear leukocytes, mucus, and mucin B5 cause the airway obstruction contributing to pulmonary failure.4 Inhalation injury leads to the destruction of the ciliated epithelium that lines the tracheobronchial tree and impairs surfactant production, ultimately leading to a proinflammatory cytokine cascade, altering capillary membrane integrity and exacerbating pulmonary edema.5 Neutrophils and mucus casts can cause upper-airway obstruction, contributing to pulmonary failure. Hollingsed et al6 found that patients with inhalation injury had a 73% incidence of respiratory failure (hypoxemia, multiple pulmonary infections, or prolonged ventilator support) and a 20% incidence of acute respiratory distress syndrome (ARDS).
Heparin and N-acetylcysteine nebulization treatment in combination with albuterol has potential efficacy in inhalation injury based initially on results from animal models and clinical studies in children. The primary mechanism is thought to be a combination of mucolysis by the N-acetylcysteine component, bronchodilation by the albuterol, and inhibition of fibrin clot formation within the airways by the anticoagulant heparin. Herndon and Traber’s7 laboratory reported the effects of aerosolized recombinant human antithrombin and heparin in an ovine model of acute lung injury induced by smoke inhalation and cutaneous flame burn. The results of this study strongly suggest that aerosolized recombinant human antithrombin and heparin in combination may be a novel therapeutic approach for airway management of burn victims with smoke inhalation injury. Desai et al8 have previously demonstrated that nebulized heparin/N-acetylcysteine is associated with reduced mortality in burned children with inhalation injury (n = 47), without significant side effects. The Holt9 group found no clinical benefit using a similar protocol in a treated group consisting of both children and adults (n = 62). Miller et al4 described the effect of a protocol with alternating nebulized N-acetylcysteine and 10,000 U of heparin as attenuating lung injury and the progression of ARDS in ventilated adult patients (n = 30) with acute lung injury. We therefore hypothesized that aerosolized anticoagulant may benefit adult burn patients with inhalation injury and implemented a nebulized heparin/N-acetylcysteine/albuterol (HNA) protocol at our burn center in 2011; this study details the clinical results of this protocol.
METHODS
Study Design
The Texas Tech University Health Sciences Center Institutional Review Board approved this study, which was a single-center retrospective review of adult inhalation injury patients. The study cohort consisted of patients who were admitted to an ABA-verified regional burn center within an academic teaching hospital between January 2011 and July 2012 and treated with the HNA protocol. The control cohort was patients admitted within 5 years before implementation of the protocol, matched 1:1 for sex, burn severity, and age within the decade, in that order. Demographic, clinical, and outcome variables were acquired from the institutional Trauma Registry, which included duration of mechanical ventilation and mortality and secondary outcome measures consisting of the development of pneumonia, ARDS, sepsis, and length of stay (LOS). Patients were identified in the database as having pneumonia based on physician documentation; the authors used the definition of pneumonia based on Centers for Disease Control and Prevention criteria to verify these findings. Sepsis was determined based on the ABA sepsis criteria, with 3 or more of the following: temperature >39°C or <36.5°C, progressive tachycardia (>110 beats per minute), progressive tachypnea (>25 breaths per minute if not ventilated and minute ventilation >12 L/min if ventilated), thrombocytopenia (not applied until 3 days after initial resuscitation <100,000/μl), hyperglycemia, inability to continue enteral feedings >24 hours [abdominal distension, high gastric residuals, and uncontrollable diarrhea (>2500 ml/d)].3 ARDS was determined based on Berlin Criteria.10 Fisher’s exact text for proportions, Student’s t test for parametric variables, and Mann-Whitney U test for nonparametric variables were used to compare outcome measures. Kaplan-Meier survival analysis was performed for in-hospital survival. All analyses were performed using SPSS v.20 (Chicago, IL).
Clinical Management
All patients admitted with clinical suspicion of inhalation injury due to the mechanism of injury or findings on history or physical examination underwent measurement of carboxyhemoglobin levels and diagnostic bronchoscopy on admission to the burn center. Both the study and control cohorts received further therapeutic bronchoscopy, lung-protective ventilation, and the use of respiratory adjuncts such as chest physiotherapy, rotation therapy, and inhaled bronchodilators as needed, as determined by attending physician. All intubated patients had daily chest radiograph.
Treatment Protocol
The treatment protocol consisted of nebulization with 2.5 mg of albuterol/3 ml of 20% N-acetylcysteine (Mucomyst) and 5000 U of aerosolized heparin/3 ml of normal saline alternating every 4 hours for the first 7 days postadmission (Fig. 1). Syringes containing 5000 U of heparin were mixed by central pharmacy and sent to the burn center daily, where they were stored in the medication room until scheduled; the N-acetylcysteine was delivered in the same manner. The albuterol was available in the medication room as a premixed sterile vial. All medications were drawn by the respiratory therapist and administered into a new nebulizer each time; nebulizers were disconnected from the circuit at the end of each treatment. Only the study cohort received this protocol. The control group received albuterol or N-acetylcysteine on an “as-needed” basis if ordered by the physician; nobody in the control group received heparin.
Fig. 1.

Inhalation injury protocol. APRV indicates airway pressure release ventilation; ARDS, acute respiratory distress syndrome; CPT, chest physiotherapy; PRVC, pressure-regulated volume control.
Classification of Inhalation Injury
All recorded bronchoscopy examinations were retrospectively reviewed and categorized according to the published Abbreviated Injury Score (AIS) grading system (AIS 0: no injury, 1: mild, 2: moderate, 3: severe, and 4: massive injury; Fig. 2).11 This entailed the subjective interpretation of bronchoscopy reports without the use of bronchoscopy images or photographs of the subject’s inhalation injury.
Fig. 2.
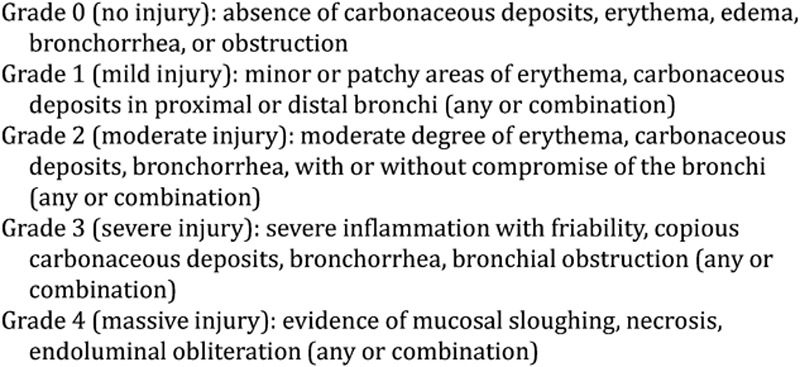
Bronchoscopic criteria used to grade inhalation injury.
Severity of Pneumonia
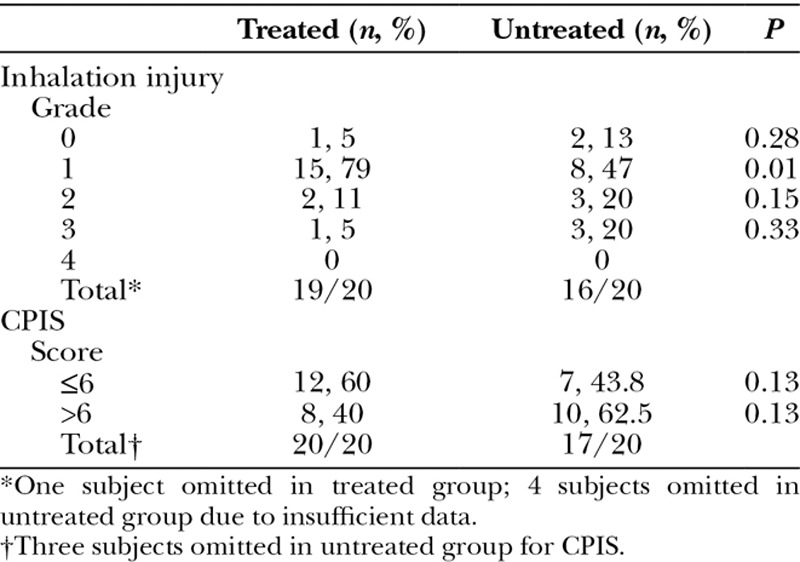
Although there is no validated tool to assess the severity of hospital-acquired pneumonia in burn patients, we retrospectively designated a Clinical Pulmonary Infection Score (CPIS), as defined by Fartoukh et al12 (Fig. 3), to provide an objective measure of the severity of pneumonia and to further explore the degree of severity in the treated group when compared with the untreated group. A total score greater than 6 out of a maximum of 12 correlates with higher bacterial counts, enhancing the likelihood ratio for hospital- acquired pneumonia.
Fig. 3.
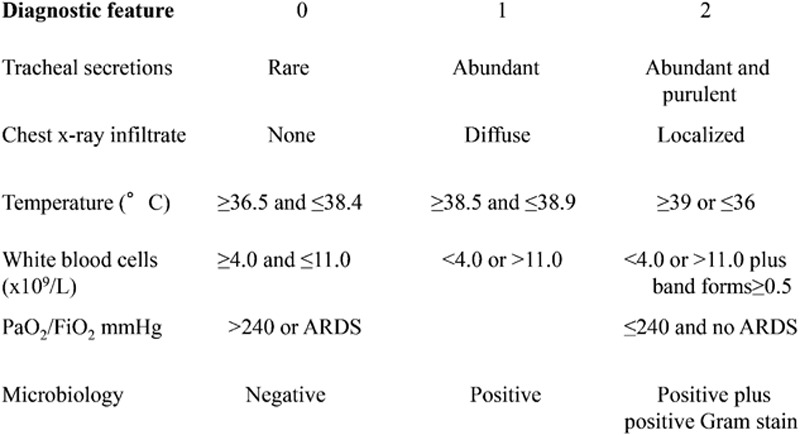
Clinical pulmonary infection score. ARDS indicates acute respiratory distress syndrome.
RESULTS
Demographics
During the study period, 35 patients were admitted with inhalation injury, of whom 20 received the HNA treatment. The remaining patients either died within 48 hours of admission or had very mild injury with anticipated discharge within 48 hours. The study and control groups were well matched (Table 1), with nearly identical age (50 vs 49 years), sex distribution (70% male), and burn size (total body surface area, 22% vs 21%).
Table 1.
Demographics and Clinical Outcomes of Treated and Untreated Groups
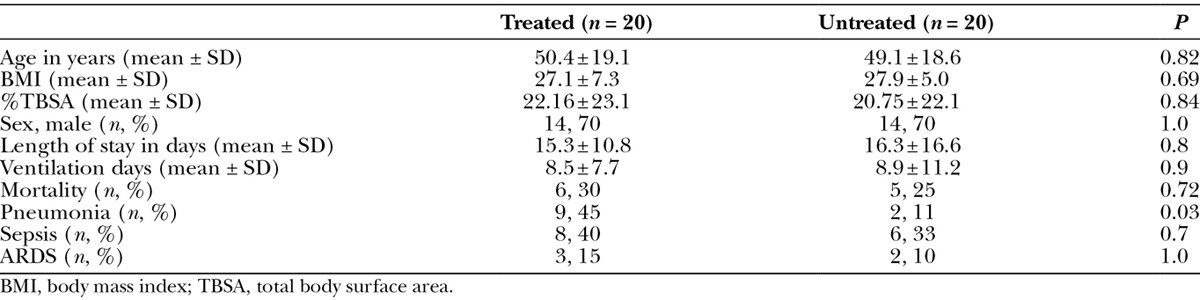
Clinical Outcomes
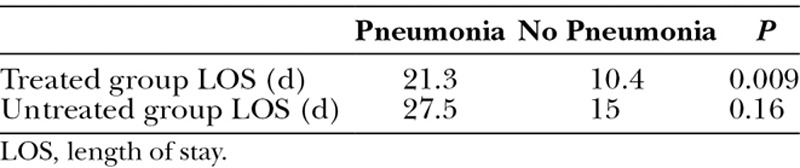
There were no documented complications directly attributed to administering the protocol, such as airway occlusion, inadvertent extubation, or bleeding. No difference was found in duration of mechanical ventilation in patients treated with HNA and those who were not (8.5 vs 8.8 days, P = 0.9). The development of sepsis (40% vs 33%, P = 0.7) or ARDS (15% vs 10%, P = 1) did not differ between study and control groups. The LOS for subjects with pneumonia in both the treated and untreated groups (Table 2) was prolonged (21.3 days vs 27.5 days, P = 0.23) in comparison with subjects without pneumonia (10.4 days vs 15 days, P = 0.20). Furthermore, within the treated cohort, there was a statistically significant difference in the LOS between those with and without pneumonia (21.3 days vs 10.4 days, P = 0.009). There was 30% mortality in the study group versus 25% mortality in the control group (P = 0.72), with no significant difference on survival analysis (data not shown).
Table 2.
Influence of Pneumonia on Length of Stay
Inhalation Injury
The 2 groups were similar and well matched when retrospectively evaluating inhalation injury with the AIS grading system (Table 3). With the exception of grade I injuries (79% vs 47%, P = 0.01), there was not a statistically significant difference in the overall degree of inhalation injury between the 2 groups: grade 0 (5% vs 13%, P = 0.28), grade 2 (11% vs 20%, P = 0.15), and grade 3 (5% vs 20%, P = 0.33). There were no subjects who were classified as grade 4 in either group.
Table 3.
Inhalation Injury Severity and Clinical Pulmonary Infection Score
Pneumonia Severity and Microbiology
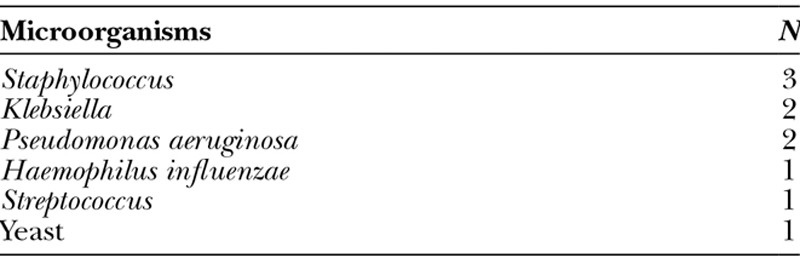
The incidence of pneumonia diagnosed during inpatient stay was 45% for the study group when compared with 11% for the controls (P = 0.03). There was no difference in the CPIS between the study and control cohorts that scored ≤6 (60% vs 43.8%, P = 0.13) or >6 (40% vs 62.5%, P = 0.13). The bacteria most frequently isolated in patients with microbiologic evidence of pneumonia were Staphylococcus species followed by Pseudomonas, Klebsiella, Streptococcus species, Haemophilus influenzae, and yeast (Table 4).
Table 4.
Microorganisms Responsible for Pneumonia
DISCUSSION
This study failed to demonstrate a clinical benefit of nebulized HNA in a cohort of adult inhalation injury patients; rather, it showed a significant increase in pneumonia in patients undergoing this treatment. This is similar to the results of Holt et al9 who in 2008 reported that a nebulized heparin/N-acetylcysteine–treated group (combined pediatric and adult population) showed no clinical benefit but had a higher incidence of pneumonia compared with an untreated group (63% vs 50%), although this finding was not statistically significant.
These results contrast with multiple animal studies, mainly in sheep, which showed promise. Cox et al13 demonstrated that nebulized heparin administration in an ovine model of smoke inhalation injury led to a decrease in tracheobronchial cast formation, minimized barotrauma, improved oxygenation, and reduced pulmonary edema. Enkhbaatar et al14 reported beneficial effects of anticoagulants administered through different routes in the ovine model of ARDS induced by smoke inhalation and cutaneous flame burn. Their study showed that the pathological changes included a severe fall in plasma antithrombin concentration, lung tissue accumulation of leukocytes, and excessive production of nitric oxide. Treatment of injured sheep with anticoagulants attenuated all of the pulmonary pathophysiology observed.
There is some clinical evidence for the benefit of HNA, which formed the basis for initiating our protocol. The largest study was performed by Desai et al8 in 1998, who found that in pediatric patients with bronchoscopically confirmed inhalation injury necessitating ventilatory support, there was a significant decrease in the incidence of atelectasis, reintubation, and mortality for the 47 children treated with the regimen of nebulized heparin/N-acetylcysteine when compared with controls. Miller et al4 showed significant improvement in lung injury scores, respiratory resistance, compliance measurements, hypoxia scores, and a survival benefit using a similar protocol in 30 adults. The reduction in mortality in their cohort was 38%, with a number needed to treat of only 3; however, we were unable to replicate these findings.
There are several potential explanations for the lack of demonstrated benefit in the current study. It is possible that because this is a small retrospective series, it is underpowered to detect a difference. Because mortality has decreased substantially among burn patients overall, it is less useful as a clinical outcome measure; the aggressive push toward ventilator liberation in hospitals nationwide has reduced overall ventilator days and made this a less sensitive indicator. These factors increase the difficulty of showing improvement in clinical outcomes in this population, thus requiring higher numbers of subjects to do so. It is also probable that the dosage, timing, and administration of medication were not optimized to this population and that larger doses, more frequent administration, or longer duration of treatment may have shown benefit. Miller et al,4 for example, used 10,000 U of heparin, whereas Holt et al9 and the current authors used 5000 U per dose; thus, it is possible that our subjects were simply underdosed. Further prospective investigation will be necessary to determine optimal dosing regimens. Finally, it is possible that there is no true benefit from HNA in adults with inhalation injury. Because adults are more likely to have concurrent pulmonary disease and a history of smoking, it is possible that the efficacy of the treatment is reduced compared with animal models and children. Medical history is replete with instances where treatments that are very effective in animal models do not, unfortunately, translate to clinical effectiveness, especially in critical illness; the substitution of pyruvate for lactate as a resuscitation fluid is a good example.15
In addition to not showing benefit, this study actually showed harm from the intervention in the form of increased pneumonia. There is a biologic basis for the predisposition of smoke-injured lungs to develop pneumonia. Tracheobronchial injury impairs normal mucociliary clearance of the lung, which can lead to distal atelectasis and a protein-rich exudate that serves as a medium for bacterial overgrowth.16,17 Brusselaers et al18 described the result of combustible products as causing “de-epithelialization in the tracheobronchial tree and lower respiratory tract lesions,” which results in an increase in extravascular lung water with decreased pulmonary compliance, inactivation of surfactant with microatelectasis, and pseudomembrane formation of mucus, cellular debris, fibrinous exudates, polymorphonuclear leukocytes, and clumps of bacteria. The incidence of pneumonia in patients with inhalation injury varies from 15% to 60%17–20 and is associated with increased mortality1–6 and significantly increased use of resources.20 We suspect that the reason for the increase in pneumonia over baseline in subjects treated with HNA in the current study is due to the frequent interruptions to the ventilator circuit and deficits in sterility in the preparation and administration of the nebulized medication rather than a direct effect of the medications themselves. The authors are unaware of any biologic basis to suggest that either heparin or N-acetylcysteine is a proinfectious or proinflammatory agent; therefore, we feel that direct causality is unlikely.
Future directions in evaluating HNA should include prospective data collection, correlating bronchoscopy findings with the AIS grading system, increasing the dose of heparin used in the protocol to 10,000 U, stringent monitoring of the sterile technique used to deliver medications, and maintaining a nebulizer in place in the circuit to minimize potential disruptions. Ideally, a multicenter randomized control trial under the auspices of the ABA would be conducted to provide a definitive answer to the question of clinical benefit of HNA. Given the paucity of effective interventions for inhalation injury, the authors feel that further investigation should be performed before determining its efficacy or futility.
This study has several limitations in addition to those inherent in a single-institution retrospective review with historical control. The overall number of subjects was small and could have resulted in type II statistical error. The dose of anticoagulant selected was at the lower end of what has previously been reported, as discussed above; given the unproven nature of the treatment, our primary goal was to minimize adverse events, and therefore, this dose was selected. We retrospectively used the AIS grading system and CPIS to stratify the severity of inhalation injury and pneumonia, respectively. Consequently, there were subjects omitted from both the treated and untreated cohorts due to insufficient data. In addition, due to the subjective interpretation of bronchoscopy reports, our classification of the degree of inhalation injury may not adequately represent the actual level of injury sustained by each subject. Nevertheless, the study and control groups were similar with regard to inhalation injury severity based on bronchoscopic scoring, with the exception of more grade I injury patients included in the study group. However, even if this difference were to affect pneumonia rates and clinical outcomes, it would be expected that the trend would be in the opposite direction to what was found, with the study cohort doing better and having fewer cases of pneumonia due to a higher number of less severe injuries. Therefore, we do not think that this minor difference accounts for the study findings. Because this protocol was used as part of clinical management and not in the context of a prospective trial, rigorous overview of protocol compliance was not undertaken, and it is likely that protocol deviations occurred, reducing the impact of the intervention.
CONCLUSIONS
The use of a nebulized HNA protocol in a cohort of adults with smoke inhalation resulted in increased incidence of pneumonia with no clinical benefit. This conclusion held true after retrospectively matching the degree of inhalation injury and accounting for the severity of pneumonia between the study and control cohorts.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Latenser BA, Miller SF, Bessey PQ, et al. National Burn Repository 2006: a ten-year review. J Burn Care Res. 2007;28:635–658. doi: 10.1097/BCR.0B013E31814B25B1. [DOI] [PubMed] [Google Scholar]
- 2.Davis CS, Janus SE, Mosier MJ, et al. Inhalation injury severity and systemic immune perturbations in burned adults. Ann Surg. 2013;257:1137–1146. doi: 10.1097/SLA.0b013e318275f424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenhalgh DG, Saffle JR, Holmes JH, IV, et al. American Burn Association Consensus Conference on Burn Sepsis and Infection Group. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 4.Miller AC, Rivero A, Ziad S, et al. Influence of nebulized unfractionated heparin and N-acetylcysteine in acute lung injury after smoke inhalation injury. J Burn Care Res. 2009;30:249–256. doi: 10.1097/BCR.0b013e318198a268. [DOI] [PubMed] [Google Scholar]
- 5.Reper P, Dankaert R, van Hille F, et al. The usefulness of combined high-frequency percussive ventilation during acute respiratory failure after smoke inhalation. Burns. 1998;24:34–38. doi: 10.1016/s0305-4179(97)00037-5. [DOI] [PubMed] [Google Scholar]
- 6.Hollingsed TC, Saffle JR, Barton RG, et al. Etiology and consequences of respiratory failure in thermally injured patients. Am J Surg. 1993;166:592–596; discussion 596–597. doi: 10.1016/s0002-9610(05)80662-2. [DOI] [PubMed] [Google Scholar]
- 7.Enkhbaatar P, Cox RA, Traber LD, et al. Aerosolized anticoagulants ameliorate acute lung injury in sheep after exposure to burn and smoke inhalation. Crit Care Med. 2007;35:2805–2810. doi: 10.1097/01.ccm.0000291647.18329.83. [DOI] [PubMed] [Google Scholar]
- 8.Desai MH, Mlcak R, Richardson J, et al. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine [correction of acetylcystine] therapy. J Burn Care Rehabil. 1998;19:210–212. doi: 10.1097/00004630-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Holt J, Saffle JR, Morris SE, et al. Use of inhaled heparin/N-acetylcystine in inhalation injury: does it help? J Burn Care Res. 2008;29:192–195. doi: 10.1097/BCR.0b013e31815f596b. [DOI] [PubMed] [Google Scholar]
- 10.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Endorf FW, Gamelli RL. Inhalation injury, pulmonary perturbations, and fluid resuscitation. J Burn Care Res. 2007;28:80–83. doi: 10.1097/BCR.0B013E31802C889F. [DOI] [PubMed] [Google Scholar]
- 12.Fartoukh M, Maitre B, Honoré S, et al. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med. 2003;168:173–179. doi: 10.1164/rccm.200212-1449OC. [DOI] [PubMed] [Google Scholar]
- 13.Cox CS, Jr, Zwischenberger JB, Traber DL, et al. Heparin improves oxygenation and minimizes barotrauma after severe smoke inhalation in an ovine model. Surg Gynecol Obstet. 1993;176:339–349. [PubMed] [Google Scholar]
- 14.Enkhbaatar P, Esechie A, Wang J, et al. Combined anticoagulants ameliorate acute lung injury in sheep after burn and smoke inhalation. Clin Sci (Lond) 2008;114:321–329. doi: 10.1042/CS20070254. [DOI] [PubMed] [Google Scholar]
- 15.Fink MP. The therapeutic potential of pyruvate. J Surg Res. 2010;164:218–220. doi: 10.1016/j.jss.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 16.O’Sullivan ST, O’Connor TP. Immunosuppression following thermal injury: the pathogenesis of immunodysfunction. Br J Plast Surg. 1997;50:615–623. doi: 10.1016/s0007-1226(97)90507-5. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard GB, Langlinais PC, Shimazu T, et al. The morphology of smoke inhalation injury in sheep. J Trauma. 1991;31:1477–1486. doi: 10.1097/00005373-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Brusselaers N, Logie D, Vogelaers D, et al. Burns, inhalation injury and ventilator-associated pneumonia: value of routine surveillance cultures. Burns. 2012;38:364–370. doi: 10.1016/j.burns.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Edelman DA, Khan N, Kempf K, et al. Pneumonia after inhalation injury. J Burn Care Res. 2007;28:241–246. doi: 10.1097/BCR.0B013E318031D049. [DOI] [PubMed] [Google Scholar]
- 20.Dissanaike S, Cox S, Arrieta S. The association of pneumonia with clinical outcome in burned patients with inhalation injury. Surg Sci. 2013;4:7–14. [Google Scholar]


