Abstract
Background:
Magnetic resonance imaging (MRI) has not yet been established systematically to detect structural muscular changes after facial nerve lesion. The purpose of this pilot study was to investigate quantitative assessment of MRI muscle volume data for facial muscles.
Methods:
Ten healthy subjects and 5 patients with facial palsy were recruited. Using manual or semiautomatic segmentation of 3T MRI, volume measurements were performed for the frontal, procerus, risorius, corrugator supercilii, orbicularis oculi, nasalis, zygomaticus major, zygomaticus minor, levator labii superioris, orbicularis oris, depressor anguli oris, depressor labii inferioris, and mentalis, as well as for the masseter and temporalis as masticatory muscles for control.
Results:
All muscles except the frontal (identification in 4/10 volunteers), procerus (4/10), risorius (6/10), and zygomaticus minor (8/10) were identified in all volunteers. Sex or age effects were not seen (all P > 0.05). There was no facial asymmetry with exception of the zygomaticus major (larger on the left side; P = 0.012). The exploratory examination of 5 patients revealed considerably smaller muscle volumes on the palsy side 2 months after facial injury. One patient with chronic palsy showed substantial muscle volume decrease, which also occurred in another patient with incomplete chronic palsy restricted to the involved facial area. Facial nerve reconstruction led to mixed results of decreased but also increased muscle volumes on the palsy side compared with the healthy side.
Conclusions:
First systematic quantitative MRI volume measures of 5 different clinical presentations of facial paralysis are provided.
Lower motor cranial nerve dysfunction has an effect on the target muscles, typically leading to immediate flaccid paresis or paralysis and atrophy with fatty infiltration if the innervating cranial nerve is damaged.1 This effect is permanent if no reinnervation takes place or may lead to defective healing if the nerve damage is degenerative with spontaneous nerve regeneration or after nerve reconstruction surgery.2 Morphologically, the result of cranial nerve dysfunction is an alteration of the bulk of the target muscles, a decreased or increased muscle tone, and a change of the muscle position. Involvement of adjacent and functionally associated muscles with innervation by other nerves may occur. The magnetic resonance imaging (MRI) signal, computed tomography (CT) attenuation, and contrast medium enhancement are also affected.1
Facial palsy is the most frequent cranial nerve lesion.2,3 Case reports or small case series focusing on some exemplary facial muscles have demonstrated that CT can show qualitatively the consequences of end-stage appearance of denervation, like severe atrophy, asymmetrical muscle size, or fatty infiltration.4 On the other hand, MRI seems to facilitate depiction of the progressive evolution from an acute phase to a subacute and then to a chronic phase of facial nerve damage by delineating asymmetrical decreases in affected muscle volume, fatty infiltration of the involved muscle group, and variable signal intensity changes, including both T2 prolongation and postcontrast enhancement.5 Detection of pronounced preoperative MRI facial muscle asymmetry has been shown to predict a poorer functional outcome of facial nerve reconstruction.6 So far, quantitative assessment of facial musculature in patients has been restricted to selected muscles like the orbicularis oculi, orbicularis oris, and the buccinator muscle in myasthenia gravis patients,7,8 facial muscles around the lips to support craniofacial surgery,9 and the procerus after botulinum toxin injection.10 Furthermore, 3-dimensional (3D) reconstructions have not been performed in patients and have been restricted in healthy volunteers to only a few facial muscles.9,11,12
In this pilot study, we report on first quantitative MRI data of facial muscle volumes after 3D reconstruction for 13 mimic muscles and additionally for 2 chewing muscles as controls innervated by the motor trigeminal nerve in 10 healthy volunteers. Additionally, first qualitative data are presented for 5 patients with different types of acute and chronic peripheral facial palsy.
PATIENTS AND METHODS
Ten healthy adult volunteers and 5 patients with facial palsy were recruited. Only volunteers without history of facial trauma, head trauma, facial palsy, or any other neurological disorder were included. None of the volunteers had a history of hereditary neuromuscular disorder or any other congenital disorder. After the examination of the volunteers, the established MRI protocol was applied in 5 patients (#11–15). In 1 patient (#11) with postoperative facial palsy, it was also possible to evaluate the preoperative MRI data. Preoperatively, this patient had no facial palsy. The study was approved by the local ethics committee, and informed consent was obtained from all participants. Age, sex, and handedness of all volunteers were recorded. The charts of the patients were reviewed for details of the facial palsy. The palsy was graded according to the House-Brackmann 6-point facial grading system13 and also according to the Stennert Index.14 Results of the needle electromyography tests were evaluated with regard to defective healing in the patients with chronic facial palsy.3,15
MRI Procedures
All healthy volunteers and patients underwent a 3T MRI examination (Magnetom Tim Trio; Siemens, Erlangen, Germany) by using a 12-channel head coil provided by the manufacturer. All participants were examined in supine position. The imaging protocol included a sagittal acquired T1-weighted 3D sequence [repetition time 2300 millisecond, echo time 3.03 millisecond, flip angle 9 degree, voxel size 1 × 1 × 1 mm (=1 mm3), in-plane matrix 256 × 256, time to acquisition 5:21 minutes) covering the whole head including the face. Each slice had 256 × 256 pixels, and each pixel had a specific value within the 4096 gray-scale values, that is, it was represented with a 12-bit value. One hundred ninety-two slices were acquired with a slice thickness of 1.0 mm. A total data set from a single 3D volume scan of 192 slices consisted therefore of (256 × 256 × 12 × 192)/8 = 1.9 × 107 bytes; considering that 2 bytes were needed for the 12-bit representation, the data set needed approximately 26-MB storage, representing a complete 3D description of the tissue within the head for each subject.
Quantitative Measurements
Segmentation of facial muscles was performed using the Avizo Fire 7.1 software package (Visage Imaging Inc., Carlsbad, Calif.). The facial muscles and 2 muscles of mastication, the masseter muscle and temporal muscle, were identified in 3D (coronal, sagittal, and axial) in the MRI data. The 2 chewing muscles were chosen as control muscles as both are innervated by the trigeminal nerve and not by the facial nerve. About 400–500 images were analyzed per subject. Principally, the software provides 3 different segmentation modes: fully automatic, semiautomatic, or interactive (manual), depending on the input images and desired results. Fully automatic segmentation was not feasible. The following facial muscles were segmented in the interactive (manual) mode: frontalis, corrugator supercilii, depressor anguli oris, depressor labii inferioris, and mentalis. Other examined facial muscles revealed better image contrast to the surrounding structures, particularly if the muscles were encircled by fat. Therefore, semiautomatic segmentation was feasible for the following muscles: procerus, risorius, orbicularis oculi, nasalis, zygomaticus major, zygomaticus minor, levator labii superior, orbicularis oris, and the 2 chewing muscles (Fig. 1). The interactive (manual) segmentation for the thin muscles was performed using the “brush-tools” option of the software, which is the most basic segmentation tool offered by the software. Each segmentation procedure started with the coronal MRI images. The target muscle was manually marked voxel by voxel in all layers of the selected MRI orientation until the muscle was completely encircled. In the next step, the procedure was repeated in the sagittal and axial MRI images, respectively, to verify the muscle borders. In the semiautomatic segmentation mode, areas of similar gray values were automatically selected in each MRI image layer by the software. By using the “limit line”-editing tool, it was possible to adjust manually incorrect automatic segmentations to the correct anatomic borders of the muscle. After this, the new contour was projected into the next MRI image of the same orientation and the procedure was repeated. In analogy to the interactive (manual) mode, the semiautomatic segmentation was also performed in all 3 image orientations (coronal, sagittal, and axial). The complete segmentation of a face required about 3 hours. The duration to segment a single muscle depended on its volume (eg, segmentation of the zygomaticus major took about 7 minutes for each side, whereas about 15 minutes were needed for the orbicularis oculi).
Fig. 1.
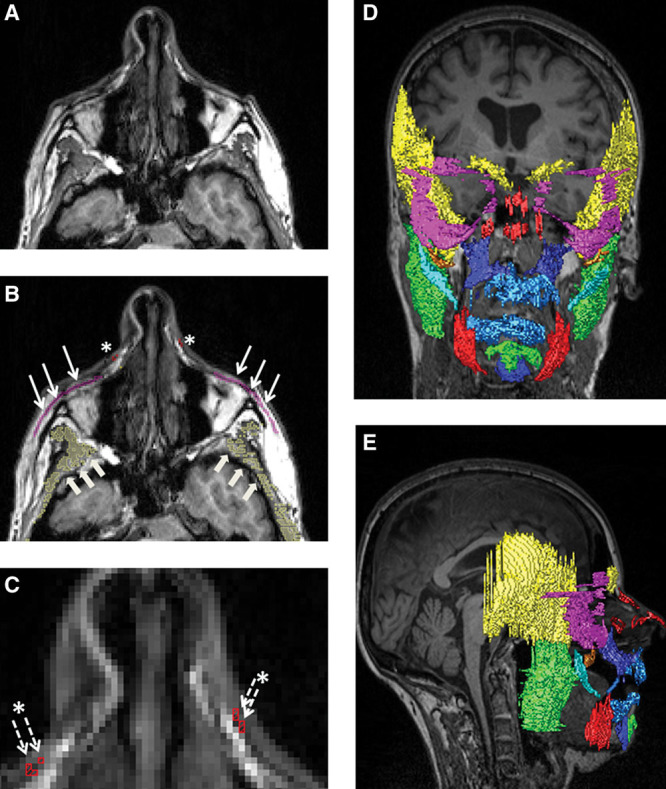
Example for semiautomatic muscle segmentation of the orbicularis oculi, nasalis, and the temporalis (patient D in Fig. 2). A, Original axial MRI image. B, Semiautomatic segmentation of the muscles: pink, orbicularis oculi (thin arrows); red, nasalis muscle (asterisks); yellow, temporal muscle (big arrows). C, Magnification of B, better visualizing the nasalis (asterisks and thin-dotted arrows). D, 3D reconstruction of the complete face of the same patient, frontal view. E, 3D reconstruction of the complete face of the same patient, lateral view; * indicates nasalis muscle.
Analyzed Facial Muscles
All images were evaluated by 3 investigators. Definite identification of a muscle was only considered successful if all investigators identified the muscle. Identification of the following mimic muscles was attempted: occipitalis, temporoparietalis, procerus, nasalis, depressor septi nasi, orbicularis oculi, corrugator supercilii, depressor supercilii, the 3 auricularis muscles (anterior, superior, and posterior), orbicularis oris, depressor anguli oris, risorius, zygomaticus major, zygomaticus minor, levator labii superior, levator labii superioris alaeque nasi, depressor labii inferioris, levator anguli oris, buccinator, mentalis, and transversus mentis. For the following muscles, a distinct identification was in general not possible for any of the participants: occipitalis, temporoparietalis muscle, depressor septi nasi, depressor supercilii, auricularis muscles, levator labii superioris alaeque nasi, levator anguli oris, and transversus mentis. All other muscles were identified by all investigators and included for further evaluation in the study: frontalis, procerus, risorius, corrugator supercilii, orbicularis oculi, nasalis, zygomaticus major, zygomaticus minor, levator labii superior, orbicularis oris, depressor anguli oris, depressor labii inferioris, mentalis, and additionally the masseter and the temporalis as chewing muscles. It should be mentioned that some muscles could not be identified in all volunteers: frontal (identification in 4/10 volunteers), procerus (4/10), risorius (6/10), and zygomaticus minor (8/10). These muscles were also not identified in all patients. The following muscles were identified in all 10 healthy controls and all patients: corrugator supercilii, orbicularis oculi, nasalis, zygomaticus major, levator labii superior, orbicularis oris, depressor anguli oris, depressor labii inferioris, mentalis, and the 2 chewing muscles, masseter and temporalis.
Statistics
All statistical analyses were performed using Microsoft Excel and IBM SPSS, version 21.0. The data of the healthy volunteers are presented as mean, SD, and 95% confidence intervals, if not indicated otherwise. Statistical significance was defined as P < 0.05. Volumes of each healthy muscle on the left and right side and the sum of all volumes on the healthy versus the palsy side in each patient were compared using the Wilcoxon signed rank test for paired data. Additionally, the data of the left and right side of each muscle were pooled for further analysis. The difference between sides was calculated by dividing the larger volume by the smaller volume and expressing it as a percentage value (% difference LR = [(largest/smallest volume) × 100] – 100). The Mann-Whitney U test for independent data was used to analyze for sex differences and the influence of age in the volunteers. The data of the patients are presented separately for each case. The difference between the healthy and the paralyzed facial side was calculated by dividing the volume from the palsy side by the volume of the healthy side and expressing it as a percentage (% difference PH = [(palsy side/healthy side) × 100] – 100), that is, a negative value indicates a smaller muscle volume on the palsy side compared with the healthy side and, vice versa, a positive value indicates a larger muscle volume on the paralyzed or reinnervated side, respectively.
RESULTS
MRI of Facial Muscle Volumes in Healthy Volunteers
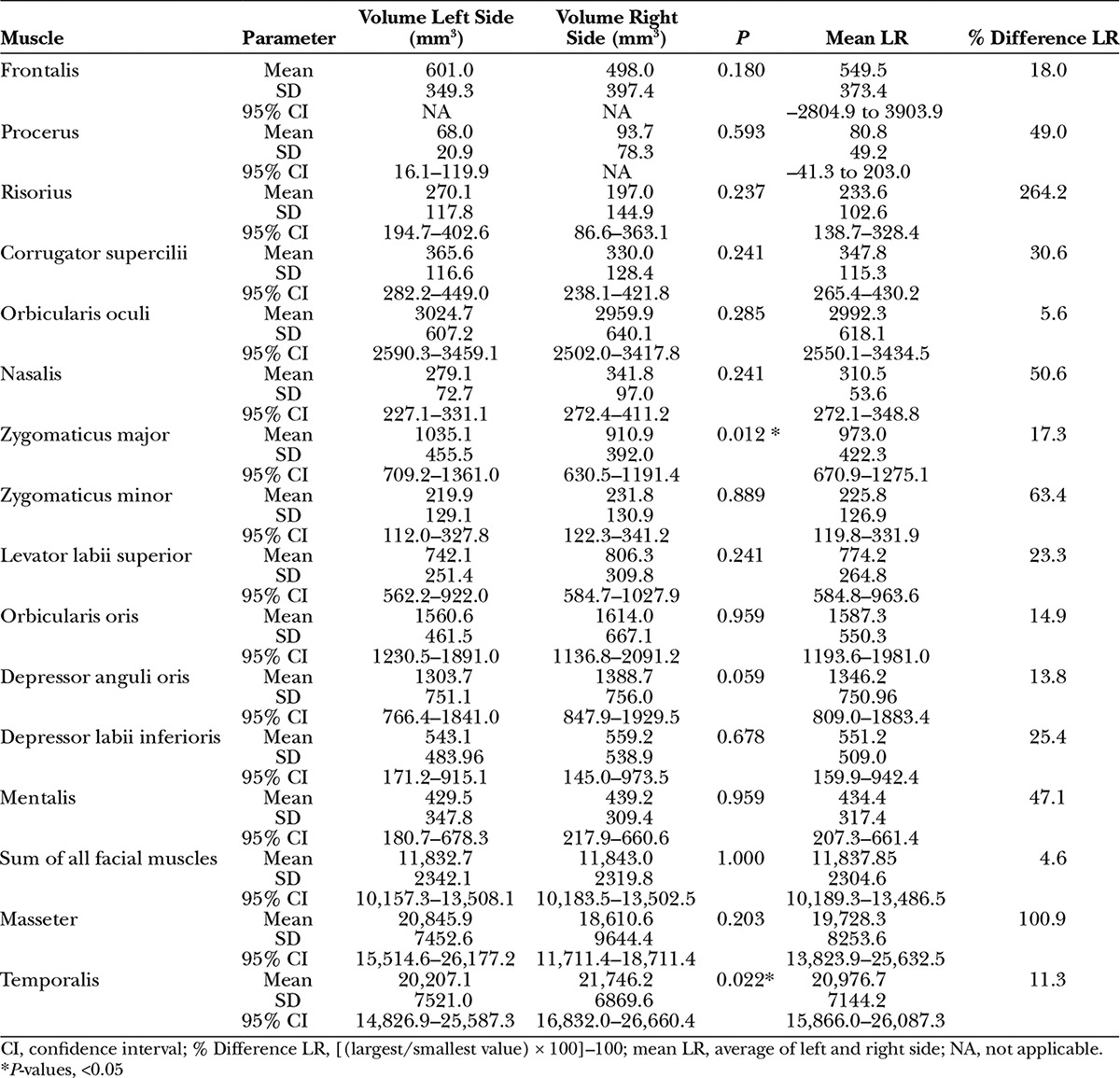
Five women and 5 men were analyzed. The mean age was 33 ± 14 years (95% confidence interval, 23–43 years). An overview about the volumes of the facial and the 2 chewing muscles is presented in Table 1. There was no sex difference (all P > 0.05) or influence of age (all P > 0.05) on muscle volume for both sides of the face. A significant side difference was only seen for the zygomaticus major muscle (larger on the left side; P = 0.012) and the temporal muscle (larger on the right side; P = 0.022). Of the facial muscles, the orbicularis oculi (5.6%), the depressor anguli oris (13.8%), and the orbicularis oris muscle (14.9%) had the smallest but insignificant mean side asymmetry. The largest and smallest volume for the mimic muscles was observed for the orbicularis oculi (mean of both sides: 2992 mm3) and the procerus muscle (80.8 mm3), respectively. Summing the volumes of all measured facial muscles, there was no significant side difference. The overall side asymmetry was 4.6%.
Table 1.
Magnetic Resonance of Facial Muscle and Chewing Muscle Volumes in Healthy Adult Volunteers
Facial Muscles Volumes in Patients with Facial Palsy
The data of the 5 patients are summarized in Table 2 and Figure 2. Two patients had an acute palsy originating postoperatively after vestibular schwannoma surgery (patients #11 and #14). Taking into account the physiological side asymmetry, the acute facial palsy resulted in both cases in a decrease of muscle volume on the palsy side for some facial muscles already after 2 weeks (patient #11) and even more pronounced after 2 months without regeneration (patient #14). When looking on the sum of all measured facial muscles, side asymmetry was not present 2 weeks after facial nerve lesion but significantly 2 months after acute nerve lesion with a summary difference of –21.5%. In patient #11, the volume of the temporal muscle was larger 2 weeks after lesion onset compared with the situation before. In all other cases, the temporal muscle was smaller on the palsy side. With the exception of patient #15, the masseter muscle was also smaller on the palsy side compared with the normal side. Two patients had chronic palsy after facial nerve transection due to meningioma and petrosal neurofibroma surgery, respectively (patients #12 and #13). In 1 case (patient #13), MRI was performed before the planned facial nerve reconstruction, and in the other case (patient #12), the facial nerve was already reconstructed and the MRI was performed 7 months later, that is, during the phase of facial nerve regeneration. Needle electromyography in patient #12 showed already distinct synkinesis between the upper and lower face. Eighteen months after the facial nerve lesion and chronic palsy, no signs of muscle reinnervation were seen clinically and also electrophysiologically in patient #13, with most facial muscles being smaller on the palsy side than on the healthy side. On the other hand, 7 months after facial nerve reconstruction and occurrence of clinical and electrophysiological signs of first reinnervation, the MRI-based muscle measurements yielded mixed results in patient #12: some facial muscles showed a higher and others a smaller volume on the facial palsy side. Looking at the summed muscle volumes, the volume decrease on the palsy side was evident before the planned reconstruction (–24%, patient #13) but disappeared after functional recovery (7%, patient #12). One case of chronic congenital palsy (patient #15) without any facial reconstruction was also included in our study. The circumscribed palsy in the lower face was reflected by a combination of smaller facial muscles with function to pull up the corner of the mouth, but a higher volume of the orbicularis oris muscle and muscles to pull down the corner of the mouth on the palsy side. In summary, if at all, only a slight summary volume decrease was seen on the palsy side compared with the healthy side (–9%, patient #15).
Table 2.
Magnetic Resonance of Facial Muscle Asymmetry in 5 Patients with Acute and Chronic Facial Palsy
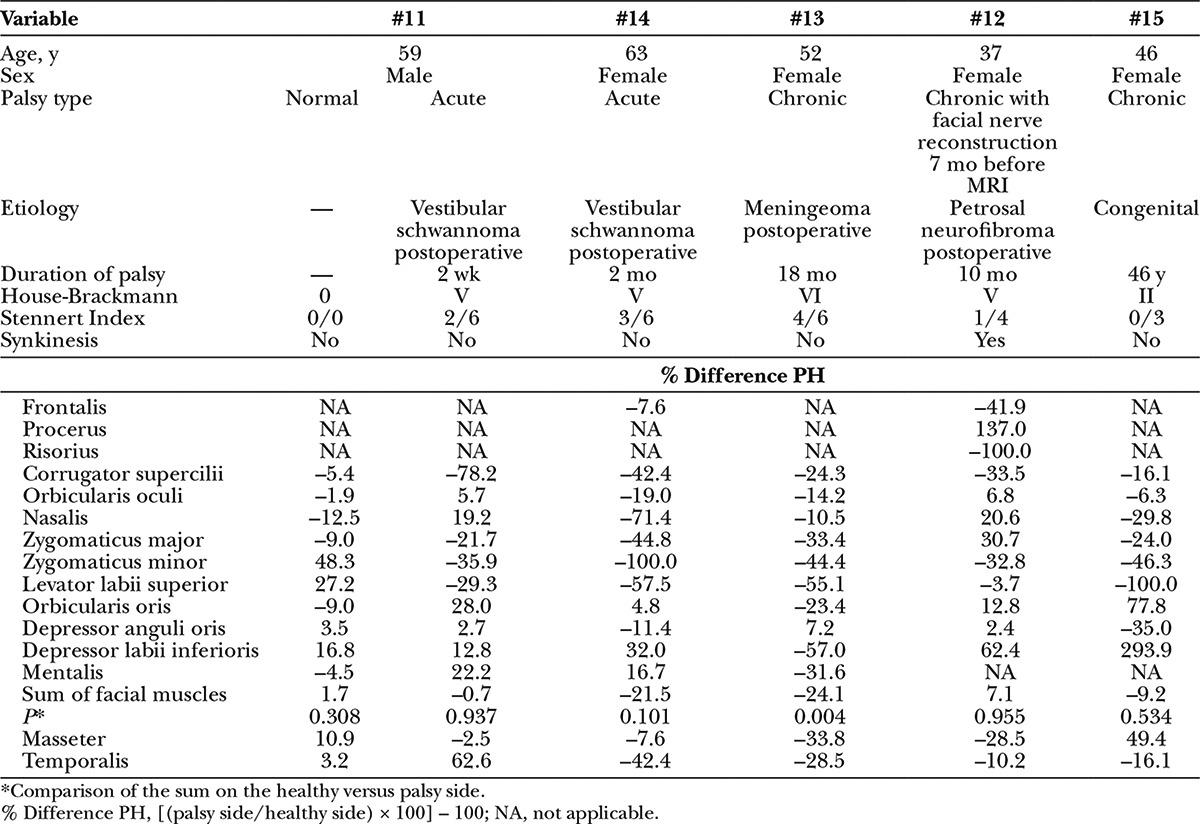
Fig. 2.
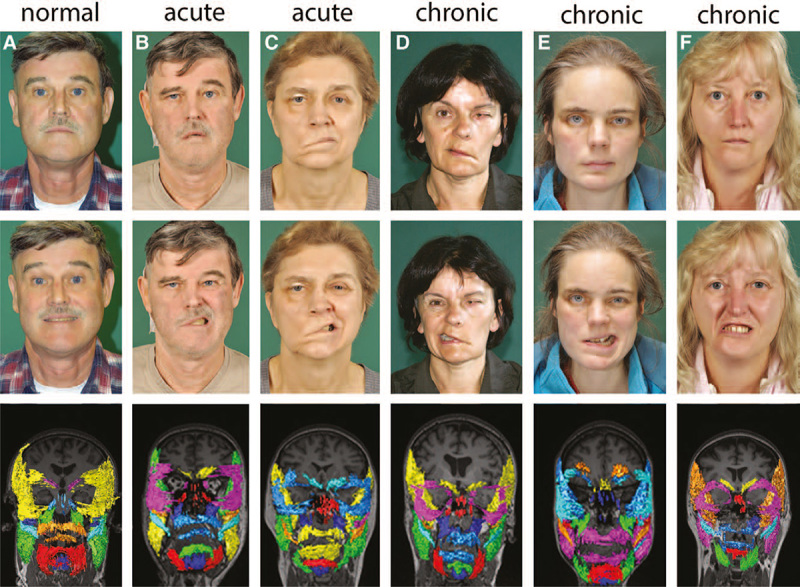
Patient before and after onset of acute complete peripheral facial palsy (A, B), acute palsy 2 months after surgery (C), chronic complete facial palsy before reconstruction (D), after facial nerve reconstruction (E), and distinct congenital chronic palsy of the lower face (F). The faces are shown at rest (upper row) and while showing the teeth (middle row). A coronal view of the 3D reconstructions of the evaluated facial muscles and chewing muscles is shown in the lower row. Color coding of the facial muscles is varying from patient to patient.
DISCUSSION
This study demonstrated the feasibility of quantitative magnetic resonance facial muscle measurements in a sample of 10 healthy volunteers aged 23–43 years and in 5 patients with facial palsy. Despite the small sample size, this is to our knowledge the largest and, concerning the number of different mimic muscles, the most comprehensive study on data of facial muscle volumes so far. Since the group of healthy adults was small and limited to a certain age range, the presented data are certainly preliminary and cannot be regarded as normative reference values. A recent study using ultrasonography to measure facial muscles did not reveal a stringent left-right side difference.16 Therefore, the side difference revealed for the zygomatic minor muscle and also the observation of no sex differences should be considered in light of the above-mentioned limitations. It is difficult to estimate the accuracy of the calculated muscle volumes because literature on MRI and CT volumetry in the face is sparse. Often only diameters or thicknesses are calculated. Furthermore, the measurements depend on the applied MRI technique. Goto et al17 measured the MRI volume of the masseter muscle during jaw opening and closing and calculated manually a volume of 29,000–30,000 mm3 (present study: volume at rest about 20,000 mm3). CT data for the masseter ranges from 22.000 to 38.000 mm3).18 Some MRI studies have described facial muscle differences between both sides only qualitatively,6,19 whereas other studies on semiautomatic and automatic 3D reconstructions of facial or chewing muscles only present impressive pictures but no quantitative volume data.9,12 Recently, manual volumetry of the procerus muscles was used to quantify the effect of muscle atrophy after botulinum toxin injection.10 Unfortunately, the authors only present percent volume changes but no absolute values. Ultimately, the data presented here allow for the first time at least a quantitative comparison of all regions of both facial sides.
To do so, the muscles were segmented manually or semiautomatically. Although a fully automatic segmentation procedure would be worthwhile and less time-consuming, it was not feasible with the commercial software we used. A manual or semiautomatic analysis required about 3 hours, that is, to use MRI quantification in clinical routine, and it will be mandatory to develop an automatic procedure. Manual segmentation involves the investigator subjectively assessing an image, leading to intrarater and interrater variability. If a clear, objective, delineation of facial muscles is present in the MRI data sets, theoretically the automatic algorithm could detect it without any variability. To date, only the masseter and the temporal muscle have been segmented automatically as a proof-of-principle applying a no-commercial algorithm.12 An application in larger patient groups is pending.
Not all facial muscles could be segmented because some facial muscles seemed to be thinner than the MRI slice thickness used. Others were interwoven with each other so that a separation from each other or from the surrounding tissue was not possible. Nevertheless, the analyzed muscles were distributed over the whole face. Therefore, the method seems able to provide an overview about side differences in patients with unilateral facial palsy and to evaluate regional differences in relation to different mimic functions. Although facial palsy is the most frequent cranial nerve palsy, so far no method has been established to objectively and quantitatively evaluate the status of the facial muscles. At the moment, the muscles are judged and graded clinically when the patient is asked to perform typical mimic movements. Furthermore, needle electromyography is used to evaluate the severity of the lesion.3,15 Electroneurography using surface electromyography to evaluate the summary action potential is normally only performed on a single muscle (typically the nasalis muscle) to predict the probability of recovery.3 It took a learning curve of about 1-week training to detect and delineate reliably the analyzed muscles. It is entirely conceivable to teach any radiologist in this method to give the plastic surgeon or head and neck surgeon information on the mimic muscle status in the individual patient.
The casuistic evaluation of a first series of 5 patients using quantitative MRI muscle volumetry demonstrated that the nonuse of muscles in acute palsy seems to cause decreases in facial muscle volume within several weeks (patient #11). In accordance to these findings, it has also been recently shown by MRI volumetry that the procerus muscle volume starts to decrease 1 month after botulinum toxin injection and that the effect holds up for about a year.10 A much more severe de-efferentation than botulinum toxin injection takes place after facial nerve transaction with muscle atrophy being a secondary effect of this transection.20 So far, the denervation and reinnervation processes of the human mimic muscles have not been studied quantitatively in detail. Most of the knowledge we have stems from human limb muscles. Here, histology typically reveals no degenerative changes during the first 2 months after nerve lesion but considerably reduced muscle fiber diameters 4 months to 1 year after denervation.21,22 Taking into account that we only had cross-sectional measurements but no longitudinal time course of quantitative MRI (exception: patient #11), the results (Table 2) suggest that significant mimic muscle atrophy already starts early (patient #11), is significantly present after 2 months following denervation (patients #13, #14, and #15), and seems to be at least partially reversible approximately 10 months after facial nerve reconstruction (patient #12). Furthermore, synkinesis (patient #12) or compensatory activity of nonaffected facial muscles in patients with chronic palsy (patient #15) seems to cause muscle hypertrophy compared with the healthy side.
CONCLUSIONS
For patients considered as candidates for facial nerve reconstruction surgery after longer denervation time and for monitoring these patients after surgery, the presented quantitative MRI method will be very helpful as an additional tool to evaluate the functional status of the mimic muscles. As a next step, future studies should establish normative MRI muscle volume data in a large sample size. MRI is often performed to assess the underlying disease of a patient with facial palsy. In these situations, MRI should be used to measure facial muscle volumes and to learn more about its value in monitoring facial disease progression or evaluating treatment effects as reflected by the facial muscles as our functional end organ of mimics and nonverbal communication.
PATIENT CONSENT
Patients provided written consent for the use of their images.
Footnotes
Disclosure: Dr. Volk was supported by a grant from the Interdisciplinary Center for Clinical Research (IZKF), Jena University Hospital, Jena. Dr. Karamyan was supported by a grant from the German Academic Exchange Service (DAAD). No benefits in any form have been or will be received. Neither of the other authors has any financial disclosures. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Connor SE, Chaudhary N, Fareedi S, et al. Imaging of muscular denervation secondary to motor cranial nerve dysfunction. Clin Radiol. 2006;61:659–669. doi: 10.1016/j.crad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Volk GF, Pantel M, Guntinas-Lichius O. Modern concepts in facial nerve reconstruction. Head Face Med. 2010;6:25. doi: 10.1186/1746-160X-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volk GF, Klingner C, Finkensieper M, et al. Prognostication of recovery time after acute peripheral facial palsy: a prospective cohort study. BMJ Open. 2013 Jun 20;3:e003007. doi: 10.1136/bmjopen-2013-003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harnsberger HR, Dillon WP. Major motor atrophic patterns in the face and neck: CT evaluation. Radiology. 1985;155:665–670. doi: 10.1148/radiology.155.3.4001368. [DOI] [PubMed] [Google Scholar]
- 5.Fischbein NJ, Kaplan MJ, Jackler RK, et al. MR imaging in two cases of subacute denervation change in the muscles of facial expression. AJNR. 2001;22:880–884. [PMC free article] [PubMed] [Google Scholar]
- 6.Kaylie DM, Wax MK, Weissman JL. Preoperative facial muscle imaging predicts final facial function after facial nerve grafting. AJNR. 2003;24:326–330. [PMC free article] [PubMed] [Google Scholar]
- 7.Farrugia ME, Robson MD, Clover L, et al. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain. 2006;129(Pt 6):1481–1492. doi: 10.1093/brain/awl095. [DOI] [PubMed] [Google Scholar]
- 8.Farrugia ME, Kennett RP, Hilton-Jones D, et al. Quantitative EMG of facial muscles in myasthenia patients with MuSK antibodies. Clin Neurophysiol. 2007;118:269–277. doi: 10.1016/j.clinph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Olszewski R, Liu Y, Duprez T, et al. Three-dimensional appearance of the lips muscles with three-dimensional isotropic MRI: in vivo study. Int J Comput Assist Radiol Surg. 2009;4:349–352. doi: 10.1007/s11548-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 10.Koerte IK, Schroeder AS, Fietzek UM, et al. Muscle atrophy beyond the clinical effect after a single dose of OnabotulinumtoxinA injected in the procerus muscle: a study with magnetic resonance imaging. Dermatol Surg. 2013;39:761–765. doi: 10.1111/dsu.12125. [DOI] [PubMed] [Google Scholar]
- 11.Kale EH, Mumcuoglu EU, Hamcan S. Automatic segmentation of human facial tissue by MRI-CT fusion: a feasibility study. Comput Methods Programs Biomed. 2012;108:1106–1120. doi: 10.1016/j.cmpb.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Rezaeitabar Y, Ulusoy I. Automatic 3D segmentation of individual facial muscles using unlabeled prior information. Int J Comput Assist Radiol Surg. 2012;7:35–41. doi: 10.1007/s11548-011-0567-3. [DOI] [PubMed] [Google Scholar]
- 13.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 14.Stennert E, Limberg CH, Frentrup KP. [An index for paresis and defective healing–an easily applied method for objectively determining therapeutic results in facial paresis (author’s transl)]. HNO. 1977;25:238–245. [PubMed] [Google Scholar]
- 15.Grosheva M, Wittekindt C, Guntinas-Lichius O. Prognostic value of electroneurography and electromyography in facial palsy. Laryngoscope. 2008;118:394–397. doi: 10.1097/MLG.0b013e31815d8e68. [DOI] [PubMed] [Google Scholar]
- 16.Volk GF, Wystub N, Pohlmann M, et al. Quantitative ultrasonography of facial muscles. Muscle Nerve. 2013;47:878–883. doi: 10.1002/mus.23693. [DOI] [PubMed] [Google Scholar]
- 17.Goto TK, Tokumori K, Nakamura Y, et al. Volume changes in human masticatory muscles between jaw closing and opening. J Dent Res. 2002;81:428–432. doi: 10.1177/154405910208100614. [DOI] [PubMed] [Google Scholar]
- 18.Gionhaku N, Lowe AA. Relationship between jaw muscle volume and craniofacial form. J Dent Res. 1989;68:805–809. doi: 10.1177/00220345890680051001. [DOI] [PubMed] [Google Scholar]
- 19.Kaylie DM, Jackson CG, Aulino JM, et al. Preoperative appearance of facial muscles on magnetic resonance predicts final facial function after acoustic neuroma surgery. Otol Neurotol. 2004;25:622–626. doi: 10.1097/00129492-200407000-00034. [DOI] [PubMed] [Google Scholar]
- 20.Gutmann E. The Denervated Nerve. Prague: Publishing House of the Czechoslovak Academy of Sciences; 1962. [Google Scholar]
- 21.Bowden RE, Gutmann E. Denervation and re-innervation of human voluntary muscle. Brain. 1944;67:273–313. [Google Scholar]
- 22.Bowden RE, Gutmann E. Clinical value of muscle biopsies. Lancet. 1945;2:768–771. doi: 10.1016/s0140-6736(45)91117-2. [DOI] [PubMed] [Google Scholar]


