Abstract
Summary:
A successful bilateral ear composite graft nonmicrosurgical reattachment is presented. In cases where suitable vessels are unavailable for microsurgical revascularization, the reconstructive challenge can be formidable for salvaging the unique anatomic and aesthetic structure of the ear. The case is presented of an 18-year-old woman who was a victim of an assault wherein both of her ears were intentionally amputated by her attacker. She underwent successful surgical reattachment followed by a postoperative regimen of hyperbaric oxygen, cooling, and meticulous wound care. The patient achieved 100% survival of her left ear graft and 95% survival of her right ear graft. Clinical photographs at 18 months are presented, along with a discussion of the possible implications for other reconstructive applications.
Complex composite anatomic amputations or deglovings pose a reconstructive challenge that has been largely answered by microsurgical revascularization whenever feasible. In the case of ear amputations, several reports appear in the literature documenting the success of such replantations,1–5 and the challenges in these difficult cases are nicely reviewed and summarized by Steffen et al6 In the absence of suitable vessels for microvascular reattachment, few options have proven to be predictably reliable as far as ensuring survival of a composite graft larger than 2 cm in maximal dimension. McDowell7 reported on the successful reattachment of a 2.5-cm fragment, and Larsen and Pless8 reported on the posterior shaving technique for salvage of ear cartilage for future reconstruction. Banking ear cartilage has also been described by creating a biologic subcutaneous pocket for future reconstruction at a later stage.9–12
We report the successful simultaneous composite reattachment of bilateral amputated pinnas in the same patient with nearly 100% survival of both ears.
CASE REPORT
An 18-year-old female of mixed ethnicity presented to the emergency department with a history of having had both ears intentionally amputated by her boyfriend with whom she was breaking up. Emergency medical technicians had salvaged one of the ear parts and placed it on ice. After transporting the patient and 1 of the amputated ears to the hospital, they were directed to return to the scene of the crime and diligently look for the other missing ear, which they subsequently found in a pool of blood on the kitchen floor.
On physical examination upon presentation, the patient was hemodynamically stable and cooperative but in acute distress due to anxiety. She was pleading to save her ears. A subtotal amputation of both ears was noted (Fig. 1), the left tangentially from the attachment of the superior helix which included the entire scapha and the lateral part of the concha to the caudal one third of the helical rim. A portion of the left superior helical rim skin remained dangling by a narrow skin attachment to the scalp (Fig. 2). The right ear was severed sagittally through the mid portion of the long axis of the pinna, including most of the scapha and virtually the entire concha (Fig. 3).
Fig. 1.
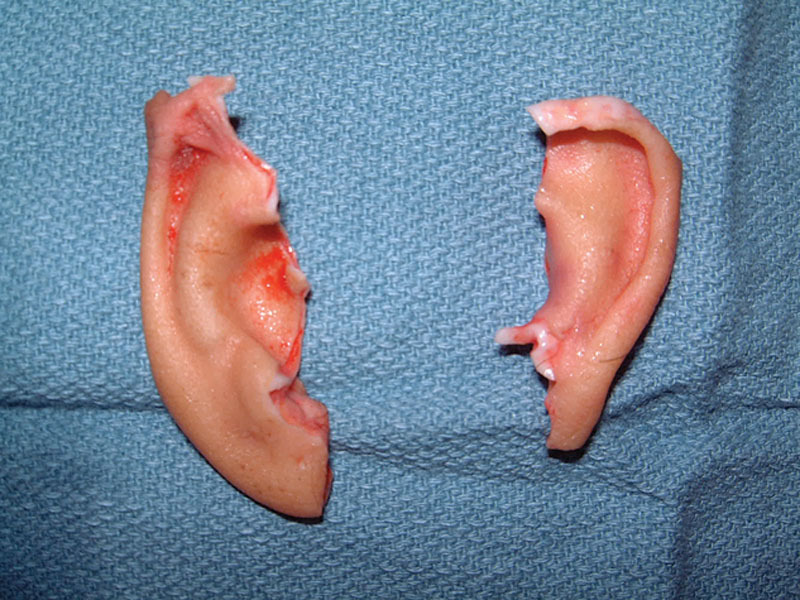
Bilateral severed ears.
Fig. 2.
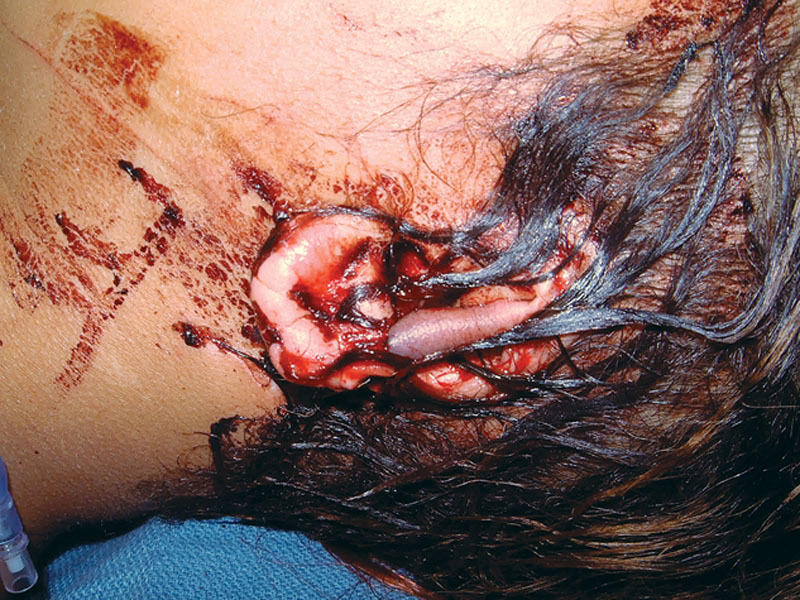
Left ear defect.
Fig. 3.
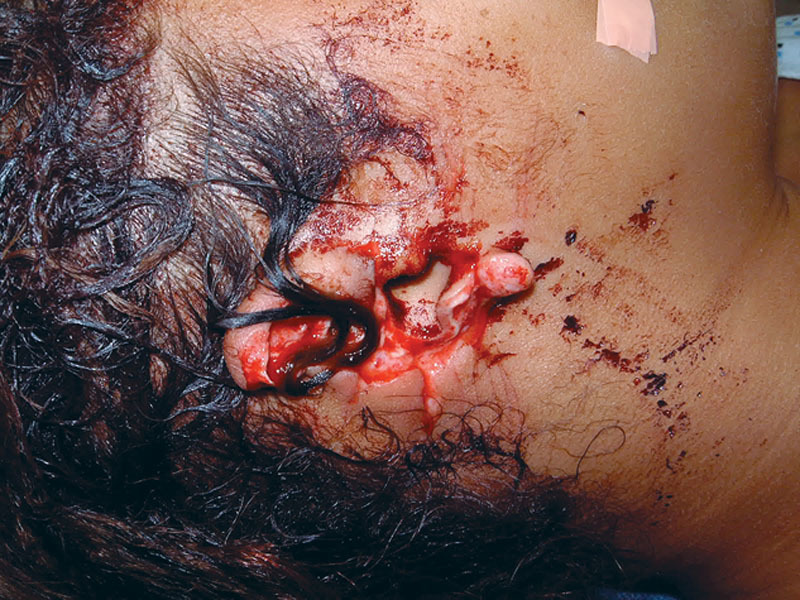
Right ear defect.
OPERATIVE COURSE
Although the patient was being stabilized in the emergency department, the amputated parts were taken to the operating room for microsurgical inspection and evaluation. No suitable vessels larger than 0.3 mm were found. After informed consent was obtained, it was decided to attempt bilateral ear reattachment with a planned postoperative course of hyperbaric oxygen treatments. The amputated ears were carefully and precisely anatomically reattached (Figs. 4, 5). The total cold ischemia time for the amputated ears was 7 hours 37 minutes. The operative time was 2 hours 35 minutes.
Fig. 4.
Left ear reattached.
Fig. 5.
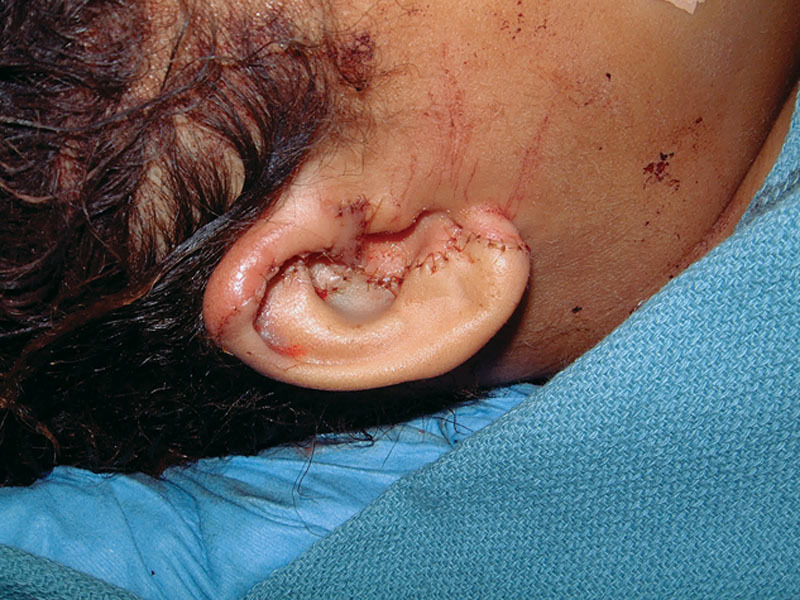
Right ear reattached.
POSTOPERATIVE COURSE
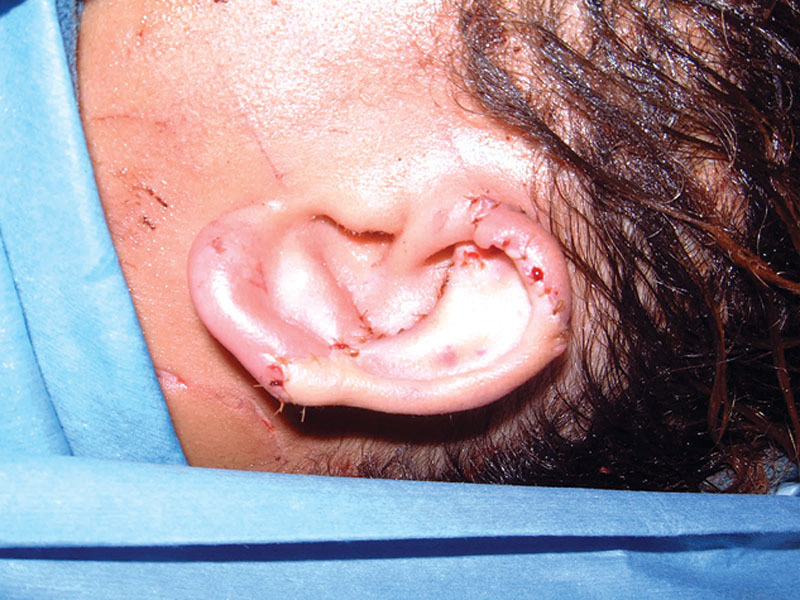
Hyperbaric oxygen treatments were begun immediately postoperatively from recovery room. A standard protocol of twice daily 90-minute dives at 2 atmospheres was followed for 14 days. She was maintained on intravenous antibiotics and intravenous ascorbic acid 1000 mg every 12 hours to minimize ischemic injury. The ears were generously lubricated with Bacitracin ointment to avoid desiccation, and ice-cold compresses were applied for the first 48 hours postoperatively and reapplied every 15 minutes by nursing staff and family who were educated on doing the same. The patient was discharged on postoperative day 5 with arrangements made for completing her 2-week course of hyperbaric oxygen therapy therapy on an outpatient basis. At 2 weeks postoperatively, the patient demonstrated 100% survival of the left ear graft and 95% survival of the right ear graft, with minor loss of a portion of the lobule which responded to topical wound care. At 4 weeks, she had entirely healed. A subsequent z-plasty several months later was performed to correct the small contour deformity of the right lobule. At 2 years, she had a stable result with no complaints except for mild hypesthesia (Figs. 6–8).
Fig. 6.
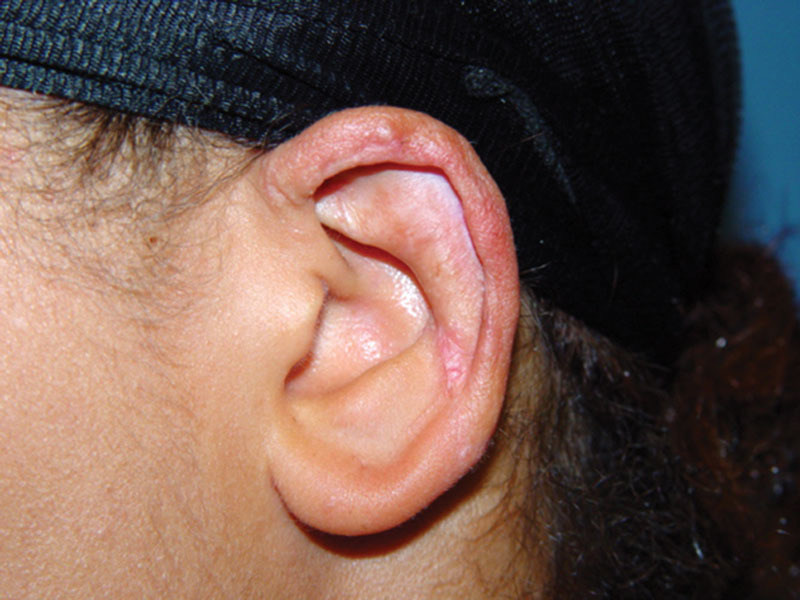
Left ear 18 months postoperatively.
Fig. 8.

Posterior view of the patient 18 months postoperatively.
Fig. 7.
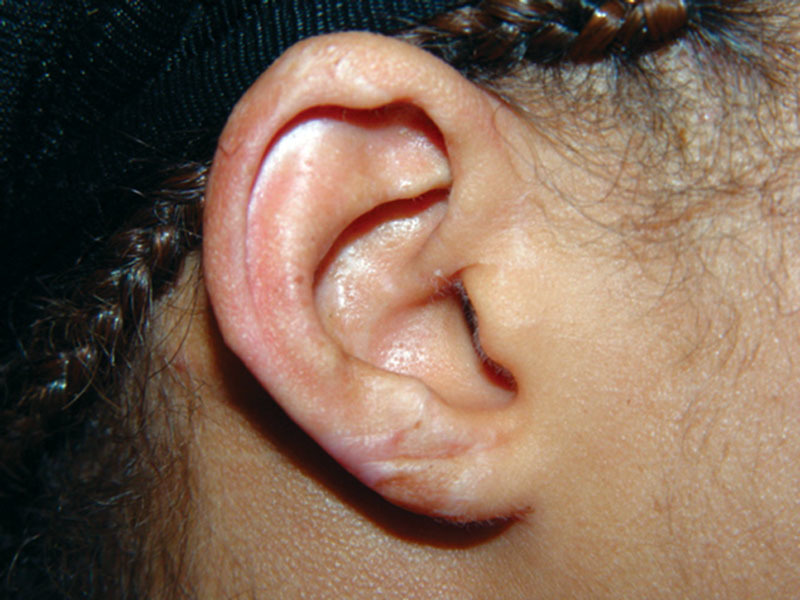
Right ear 18 months postoperatively.
CLINICAL CORRELATION
If we extrapolate from our knowledge of skin graft physiology, the initial appearance of the reattached ears was noted to be pale consistent with the imbibition phase of initial graft survival wherein the reattached part is entirely avascular. Over the ensuing 48 hours, the grafts were noted to begin demonstrating venous congestion with a bluish hue, consistent with inosculation, and by 72–96 hours, the bluish discoloration began to get replaced with the pink blush of revascularization. Keeping the reattached ears well lubricated prevented desiccation, desquamation, and de-epithelialization.
DISCUSSION
Reducing metabolic demand of nonvascularized tissues with cooling is well established and was in fact described by Conley and Vonfraenkel13 in 1956. The use of HBO in assisting both ischemic flaps, traumatized tissues, osteoradionecrosis, and even composite grafts is well established in the literature. Unfortunately, with few exceptions, most of the HBO literature consists of poorly controlled, primarily retrospective studies with an array of clinical situations from which meaningful useful clinical information is at best difficult to interpret. The experimental evidence supporting the use of HBO in composite grafts was shown in the rat model by several authors.14,15 Survival advantages in the rabbit model have also been reported by multiple authors.16–19 The anecdotal clinical evidence supporting the use of HBO in composite grafts has also been reported by multiple authors.20–22 In a single ear near-complete amputation and reattachment reported by Komorowska-Timek and Hardesty,22 their patient’s injury included 2 small skin bridges and their clinical protocol included keeping the patient extra warm in a “hot” room for the first 72 hours postoperatively, presumably to maximize vasodilation in the small soft-tissue bridge attachments that remained, unlike our use of cooling the reattached ear parts which had been completely severed. In addition, our patient’s amputations were transcartilagenous with the entire surface area contact consisting exclusively of the 3–5 mm thickness of the severed cartilaginous-cutaneous composite unit.
The known clinical effects of HBO that are virtually undisputed include the stimulation of angiogenesis and neovascularization, reduction of superoxide formation, and reduction of venous congestion.23–25 All of these properties were clearly demonstrated in our patient’s clinical postoperative course.
CONCLUSIONS
The justification for the clinical decision making in the care of this patient was partially patient driven, but also by the fact that few options exist in such a case wherein the loss of such vitally important aesthetic anatomic structures can only be replaced by multiple subsequent staged complex and costly reconstructive procedures. The unexpected success of the outcome in this patient suggests that there may be a more expanded clinical role for HBO therapy in a prospective fashion in our armamentarium for complex reconstructions, both in the setting of trauma and even in the setting of elective reconstructive surgery, including the reconstruction of complex composite defects of the nose and ear. Incorporating HBO therapy into our clinical protocols may allow us to potentially redefine our traditional concepts of the limits of predictable composite graft survival. With improved access to hyperbaric oxygen chambers, this may become more feasible with time.
Although controlled prospective clinical trials may be difficult to design, it is this author’s opinion that ample evidence in the literature from both animal studies and clinical case reports such as the one reported here strongly suggests that there is a potential adjunctive role for HBO therapy in ensuring enhanced composite graft survival in the clinical setting. Precise protocols in such settings remain to be established, but established guidelines exist and sound clinical decision making on an individual basis remains paramount.
Footnotes
Presented at the North Carolina Society of Plastic Surgeons Meeting, October 2013, Kiawah Island, S.C., and the Aesthetic Facial Reconstruction Meeting directed by Dr. Fred Menick, 2002, Nashville, Tenn.
Disclosure: The author has no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the author.
REFERENCES
- 1.Pennington DG, Lai MF, Pelly AD. Successful replantation of a completely avulsed ear by microvascular anastomosis. Plast Reconstr Surg. 1980;65:820–823. doi: 10.1097/00006534-198006000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Mutimer KL, Banis JC, Upton J. Microsurgical reattachment of totally amputated ears. Plast Reconstr Surg. 1987;79:535–541. doi: 10.1097/00006534-198704000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y, Tajima S. Completely successful replantation of an amputated ear by microvascular anastomosis. Plast Reconstr Surg. 1989;84:665–668. [PubMed] [Google Scholar]
- 4.Safak T, Ozcan G, Keçik A, et al. Microvascular ear replantation with no vein anastomosis. Plast Reconstr Surg. 1993;92:945–948; discussion 949. [PubMed] [Google Scholar]
- 5.de Chalain T, Jones G. Replantation of the avulsed pinna: 100 percent survival with a single arterial anastomosis and substitution of leeches for a venous anastomosis. Plast Reconstr Surg. 1995;95:1275–1279. doi: 10.1097/00006534-199506000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Steffen A, Katzbach R, Klaiber S. A comparison of ear reattachment methods: a review of 25 years since Pennington. Plast Reconstr Surg. 2006;118:1358–1364. doi: 10.1097/01.prs.0000239539.98956.b0. [DOI] [PubMed] [Google Scholar]
- 7.McDowell F. Successful replantation of a severed half ear. Plast Reconstr Surg. 1971;48:281–283. [Google Scholar]
- 8.Larsen J, Pless J. Replantation of severed ear parts. Plast Reconstr Surg. 1976;57:176–179. doi: 10.1097/00006534-197602000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Mladick RA, Horton CE, Adamson JE, et al. The pocket principle: a new technique for the reattachment of a severed ear part. Plast Reconstr Surg. 1971;48:219–223. [PubMed] [Google Scholar]
- 10.Pribaz JJ, Crespo LD, Orgill DP, et al. Ear replantation without microsurgery. Plast Reconstr Surg. 1997;99:1868–1872. doi: 10.1097/00006534-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Konig F. On filling defects of the nostril wall. Berl Klin Woch. 1902;39:137. [Google Scholar]
- 12.Gillies HA. New free graft applied to the reconstruction of the nostril. Br J Surg. 1943;30:305. [Google Scholar]
- 13.Conley JJ, Vonfraenkel PH. The principle of cooling as applied to the composite graft in the nose. Plast Reconstr Surg (1946) 1956;17:444–451. doi: 10.1097/00006534-195606000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F, Cheng C, Gerlach T, et al. Effect of hyperbaric oxygen on survival of the composite ear graft in rats. Ann Plast Surg. 1998;41:530–534. doi: 10.1097/00000637-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Fodor L, Ramon Y, Meilik B, et al. Effect of hyperbaric oxygen on survival of composite grafts in rats. Scand J Plast Reconstr Surg Hand Surg. 2006;40:257–260. doi: 10.1080/02844310600907868. [DOI] [PubMed] [Google Scholar]
- 16.Rubin JS, Marzella L, Myers RA, et al. Effect of hyperbaric oxygen on the take of composite skin grafts in rabbit ears. J. Hyperbaric Med. 1988;3:79. [Google Scholar]
- 17.Renner G, McClane SD, Early E, et al. Enhancement of auricular composite graft survival with hyperbaric oxygen therapy. Arch Facial Plast Surg. 2002;4:102–104. doi: 10.1001/archfaci.4.2.102. [DOI] [PubMed] [Google Scholar]
- 18.Lewis D, Goldztein H, Deschler D. Use of hyperbaric oxygen to enhance auricular composite graft survival in the rabbit model. Arch Facial Plast Surg. 2006;8:310–313. doi: 10.1001/archfaci.8.5.310. [DOI] [PubMed] [Google Scholar]
- 19.Li EN, Menon NG, Rodriguez ED, et al. The effect of hyperbaric oxygen therapy on composite graft survival. Ann Plast Surg. 2004;53:141–145. doi: 10.1097/01.sap.0000112284.55035.aa. [DOI] [PubMed] [Google Scholar]
- 20.Nichter LS, Morwood DT, Williams GS, et al. Expanding the limits of composite grafting: a case report of successful nose replantation assisted by hyperbaric oxygen therapy. Plast Reconstr Surg. 1991;87:337–340. [PubMed] [Google Scholar]
- 21.Rapley JH, Lawrence WT, Witt PD. Composite grafting and hyperbaric oxygen therapy in pediatric nasal tip reconstruction after avulsive dog-bite injury. Ann Plast Surg. 2001;46:434–438. doi: 10.1097/00000637-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Komorowska-Timek E, Hardesty RA. Successful reattachment of a nearly amputated ear without microsurgery. Plast Reconstr Surg. 2008;121:165e–169e. doi: 10.1097/01.prs.0000304240.90298.eb. [DOI] [PubMed] [Google Scholar]
- 23.Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):131S–141S. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamboni WA, Browder LK, Martinez J. Hyperbaric oxygen and wound healing. Clin Plast Surg. 2003;30:67–75. doi: 10.1016/s0094-1298(02)00068-8. [DOI] [PubMed] [Google Scholar]
- 25.Friedman HI, Friedman HI, Fitzmaurice M, et al. An evidence-based appraisal of the use of hyperbaric oxygen on flaps and grafts. Plast Reconstr Surg. 2006;117(7 Suppl):175S–190S; discussion 191S. doi: 10.1097/01.prs.0000222555.84962.86. [DOI] [PubMed] [Google Scholar]


