Abstract
Objective
To examine long-term outcomes of patients hospitalized with heart failure and atrial fibrillation.
Background
Atrial fibrillation is common among patients hospitalized with heart failure. Associations of preexisting and new-onset atrial fibrillation with long-term outcomes are unclear.
Methods
We analyzed 27,829 heart failure admissions between 2006 and 2008 at 281 hospitals in the American Heart Association’s Get With the Guidelines-Heart Failure program linked with Medicare claims. Patients were classified as having preexisting, new-onset, or no atrial fibrillation. We used Cox proportional hazards models to identify factors that were independently associated with all-cause mortality, all-cause readmission, and readmission for heart failure, stroke, and other cardiovascular disease at 1 and 3 years.
Results
After multivariable adjustment, preexisting atrial fibrillation was associated with greater 3-year risks of all-cause mortality (hazard ratio, 1.14; 99% CI, 1.08–1.20), all-cause readmission (1.09; 1.05–1.14), heart failure readmission (1.15; 1.08–1.21), and stroke readmission (1.20; 1.01–1.41), compared with no atrial fibrillation. There was also a greater hazard of mortality at 1 year among patients with new-onset atrial fibrillation (hazard ratio, 1.12; 99% CI, 1.01–1.24). New-onset atrial fibrillation was not associated with a greater risk of the readmission outcomes, compared with no atrial fibrillation. Stroke readmission rates at 1 year were just as high for patients with preserved ejection fraction as for patients with reduced ejection fraction.
Conclusions
Both preexisting and new-onset atrial fibrillation were associated with greater long-term mortality among older patients with heart failure. Preexisting atrial fibrillation was associated with greater risk of readmission.
Keywords: Atrial Fibrillation, Heart Failure, Medicare, Mortality, Outcome Assessment (Health Care), Patient Readmission
Introduction
Although atrial fibrillation (AF) is common among patients hospitalized with heart failure (HF), it is unclear whether preexisting and new-onset AF confer similar risks. In-hospital mortality and length of stay are greater among patients with HF and AF1; however, long-term prognosis is less clear. In some studies, concurrent HF and AF were associated with higher rates of all-cause mortality and other cardiovascular events.2–4 Other studies have shown no higher risk of adverse outcomes.5–7 Conflicting outcomes in patients with HF and AF may reflect prognostic differences between preexisting and new-onset AF or differences between HF with preserved ejection fraction (EF) and AF.
To clarify the long-term prognosis of patients with HF and preexisting or new-onset AF, and AF-associated risk in patients with HF with reduced or preserved EF, we examined long-term outcomes of patients hospitalized with HF and AF in a clinical registry linked with Medicare claims.
Methods
Data Sources
Data were from the American Heart Association’s Get With the Guidelines-Heart Failure registry and Medicare claims. As described previously, the voluntary hospital-based registry includes patients with HF as the primary cause of admission or who developed significant HF symptoms during the hospitalization.8,9 Outcome Sciences, Inc, is the data collection coordination center for the American Heart Association/American Stroke Association Get With the Guidelines programs.
The Medicare data consisted of research-identifiable inpatient files and corresponding denominator files for 2006 through 2008. The inpatient files contain institutional claims for facility costs covered under Medicare Part A and include beneficiary, physician, and hospital identifiers, admission and discharge dates, and diagnosis and procedure codes. The denominator files include dates of birth, sex, race/ethnicity, dates of death, and information about program eligibility and enrollment. We linked registry data to claims data using the method described by Hammill et al.10
Study Population
We identified Medicare beneficiaries who were 65 years or older, were discharged from a registry hospitalization between January 1, 2006, and December 31, 2008, and were enrollees in fee-for-service Medicare at discharge. We restricted the initial data set to patients who had a history of heart failure and who required documentation in the registry (at least 1 admission vital sign, presence or absence of medical history of AF, and presence or absence of a diagnosis of AF at presentation or upon hospitalization), were discharged alive, did not leave against medical advice, and were not transferred to another short-term hospital or to hospice. For patients with multiple hospitalizations in the registry, we selected the first as the index hospitalization. We stratified the population by AF status as documented in the registry: no AF (no medical history of AF or diagnosis of AF at presentation or during hospitalization), new-onset AF (diagnosis at presentation or during hospitalization and no preexisting AF), and preexisting AF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 427.31 in any position on an inpatient claim or ≥2 outpatient or carrier claims in the year before the study period). This approach has 94% sensitivity, 99% specificity, and 97% positive predictive value for identifying new-onset AF in administrative data.11
Outcomes
The outcomes of interest were all-cause mortality and readmission for any cause, HF, stroke, and other cardiovascular reasons at 1 and 3 years. We identified deaths on the basis of death dates in the Medicare mortality files. We defined readmission on the basis of any new nonelective inpatient claim not including the index hospitalization claim, transfers to or from another hospital, and admissions for rehabilitation. Table 1 shows the codes used to identify outcomes in the claims. Heart failure readmissions were readmissions with a primary diagnosis of HF. Stroke readmissions were those with a primary diagnosis of subarachnoid hemorrhage, intracerebral hemorrhage, ischemic stroke, or transient ischemic attack. Other cardiovascular readmissions were those with a diagnosis-related group of cardiovascular causes that did not also meet the criteria for a stroke or HF readmission and were not for a primary diagnosis of AF. In previous analyses, the positive predictive values for these outcomes were 97% for HF, 96% for stroke, and almost 100% for death and all-cause readmission.12,13
Table 1.
Codes for Outcomes and Comorbid Conditions Used in the Analysis
| Variable | ICD-9-CM Diagnosis Codes | Diagnosis Related Groups | Hierarchical Condition Categories |
|---|---|---|---|
| Outcome | |||
| Atrial fibrillation | 427.31 | ||
| Heart failure | 428.x, 402.x1, 404.x1, or 404.x3 |
||
| Stroke or transient ischemic attack | |||
| Subarachnoid hemorrhage | 430.x | ||
| Intracerebral hemorrhage | 431.x | ||
| Ischemic stroke | 433.x1, 434.x1, or 436 | ||
| Transient ischemic attack | 435.x | ||
| Readmission | |||
| Cardiovascular causes | 104–112, 115–118, 121–145, 479, 514–518, 525–527, 535, 536, and 547–558 (before October 1, 2007); 215–238, 242–254, 258–262, 280–316 (on or after October 1, 2007) |
||
| Covariates | |||
| Comorbid conditions | |||
| Protein-calorie malnutrition | 21 | ||
| Dementia | 49–50 | ||
| Disability | 100, 101, 102, 68, 69, 177, and 178 |
||
| Major psychiatric disorders | 54, 55, and 56 | ||
| Chronic liver disease | 25, 26, and 27 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
We identified the index hospitalization discharge dates from the registry. We analyzed outcomes using survival methods (time-to-event) and calculated days to death and first readmission. For patients who did not experience a particular outcome, we defined a censoring date as 1 or 3 years after discharge (depending on the outcome), the end of Medicare claims data availability, or the date the patient enrolled in a Medicare managed care plan, whichever occurred first. We treated death as a competing risk for the readmission outcomes.
Covariates
Baseline covariates included demographic characteristics, vital signs, medical history, comorbid conditions, and medical tests at admission from the registry. Demographic characteristics included age, sex, and race. Vital signs at admission included systolic blood pressure, respiratory rate, and heart rate. Tests at admission included blood urea nitrogen, serum creatinine, left ventricular EF, and serum sodium. Renal function was assessed using the Modification of Diet in Renal Disease formula for estimated glomerular filtration rate.14 From the registry, we identified medical history of anemia, implantable cardioverter-defibrillator, chronic obstructive pulmonary disease, depression, diabetes mellitus, hyperlipidemia, hypertension, ischemic etiology of HF, pacemaker, peripheral vascular disease, cerebrovascular accident or transient ischemic attack, renal insufficiency, and being a smoker in the past year. From the Medicare claims data, we identified comorbid conditions based on Hierarchical Condition Category codes on the index hospitalization claim (Table 1). Comorbid conditions included protein-calorie malnutrition, dementia,, major psychiatric disorders, and chronic liver disease. These variables have independent prognostic value for modeling all-cause hospital readmission and mortality after hospitalization for HF.15,16
Subgroups
The subgroups of interest were patients with preserved EF and patients with reduced EF (determined from the registry). Reduced EF included (a) quantitative EF <40%, (b) moderate or severe qualitative left ventricular systolic dysfunction, or (c) documented left ventricular systolic dysfunction. Preserved EF was EF ≥40% and an absence of (b) or (c).
Statistical Analysis
We describe baseline characteristics of the study population using frequencies with percentages for categorical variables and medians with interquartile ranges for continuous variables. We used χ2 tests and Wilcoxon rank-sum tests for differences in categorical variables and continuous variables, respectively. We used Kaplan-Meier methods to estimate unadjusted mortality and HF readmission rates at 1 and 3 years stratified by AF status. We included a single variable with 3 levels of AF status (none, preexisting, and new-onset) and conducted pairwise comparisons to test for differences between patients with preexisting AF and no AF, and between patients with new-onset and no AF. We tested for differences in mortality using log-rank tests. We estimated unadjusted readmission rates at 1 and 3 years using the cumulative incidence function, which accounts for the competing risk of mortality.17 We tested for differences in the readmission outcomes using Gray tests.18 Finally, we estimated multivariable relationships between patient characteristics and each outcome of interest using Cox proportional hazards models. If a variable had <5% missing values, we replaced the missing value with the median value for continuous variables and with the dominant category for categorical variables.19 If a variable had >5% missing values, we treated the missing values as a separate category; therefore, missing data for these variables could be included in the analysis.
For subgroup analyses, we estimated stroke readmission rates by HF subgroup (ie, preserved and reduced EF) and AF status (ie, preexisting and new-onset). We tested the differences between HF subgroups using Gray tests. We used a Cox proportional hazards model to examine whether associations between HF subgroup and stroke readmission differed by AF status. Specifically, in addition to demographic characteristics, medical history, and other clinical factors, we included an interaction between HF subgroup and AF status.
Because of the number of comparisons in the analysis, we report 99% confidence intervals and used α=0.01 to establish statistical significance. All P values are based on 2-sided tests. We used R version 2.6 (R Foundation for Statistical Computing, Vienna, Austria) for the cumulative incidence analyses. For all other analyses, we used SAS version 9.2 (SAS Institute Inc, Cary, North Carolina). The institutional review board of the Duke University Health System approved the study.
Results
Among 27,829 patients admitted for HF at 281 hospitals, 9509 (34.2%) had preexisting AF, 2026 (7.3%) had new-onset AF, and 16,294 (58.5%) had no AF (Table 2). Patients with preexisting AF were more likely to have a history of stroke or transient ischemic attack (17.3% vs 14.5% for patients with no AF), but this difference was not observed for patients with new-onset AF. Patients with either preexisting or new-onset AF were more likely than patients with no AF to have preserved EF (64.0% and 65.4%, respectively, vs 59.8%).
Table 2.
Baseline Characteristics of the Study Population by Atrial Fibrillation Status at Hospital Admission
| Variable | Atrial Fibrillation Status | ||||
|---|---|---|---|---|---|
| None (n = 16,294) |
Preexisting (n = 9509) |
P Value* | New-Onset (n = 2026) |
P Value† | |
| Age, median (IQR), y | 79 (72–85) | 81 (75–87) | < .001 | 81 (75–87) | < .001 |
| Age group, No. (%) | |||||
| 65–79 y | 8410 (51.6) | 3875 (40.8) | < .001 | 858 (42.4) | < .001 |
| ≥ 80 y | 7884 (48.4) | 5634 (59.3) | 1168 (57.7) | ||
| Sex, No. (%) | < .001 | .13 | |||
| Female | 9084 (55.7) | 4997 (52.6) | 1094 (54.0) | ||
| Male | 7210 (44.3) | 4512 (47.5) | 932 (46.0) | ||
| Race, No. (%) | < .001 | < .001 | |||
| Black | 2317 (14.2) | 642 (6.8) | 137 (6.8) | ||
| White | 12307 (75.5) | 8258 (86.8) | 1684 (83.1) | ||
| Other/unknown | 1670 (10.2) | 609 (6.4) | 205 (10.1) | ||
| Medical history, No. (%) | |||||
| Anemia | 2867 (17.6) | 1795 (18.9) | .01 | 260 (12.8) | < .001 |
| COPD | 4422 (27.1) | 2707 (28.5) | .02 | 487 (24.0) | .003 |
| Cerebrovascular accident/TIA | 2356 (14.5) | 1646 (17.3) | < .001 | 265 (13.1) | .09 |
| Depression | 1588 (9.8) | 955 (10.0) | .44 | 154 (7.6) | .002 |
| Diabetes mellitus | 6747 (41.4) | 3277 (34.5) | < .001 | 646 (31.9) | < .001 |
| Hypertension | 12,243 (75.1) | 7037 (74.0) | .04 | 1369 (67.6) | < .001 |
| Hyperlipidemia | 6892 (42.3) | 3898 (41.0) | .04 | 730 (36.0) | < .001 |
| Ischemic heart failure etiology | 10,206 (62.6) | 5740 (60.4) | < .001 | 1106 (54.6) | < .001 |
| Pacemaker | 1679 (10.3) | 1689 (17.8) | < .001 | 229 (11.3) | .17 |
| Peripheral vascular disease | 2112 (13.0) | 1258 (13.2) | .54 | 185 (9.1) | < .001 |
| Renal insufficiency | 3066 (18.8) | 1688 (17.8) | .03 | 274 (13.5) | < .001 |
| Smoking in the past year | 1705 (10.5) | 632 (6.7) | < .001 | 158 (7.8) | < .001 |
| Comorbid conditions, No. (%) | |||||
| Chronic liver disease | 147 (0.9) | 77 (0.8) | .44 | 12 (0.6) | .16 |
| Dementia | 1183 (7.3) | 690 (7.3) | .99 | 156 (7.7) | .47 |
| Disability | 344 (2.1) | 199 (2.1) | .92 | 37 (1.8) | .40 |
| Malnutrition | 396 (2.4) | 230 (2.4) | .95 | 52 (2.6) | .71 |
| Psychiatric disorder | 201 (1.2) | 86 (0.9) | .02 | 15 (0.7) | .05 |
| Vital signs, No. (%) | |||||
| Systolic blood pressure, mm Hg | < .001 | < .001 | |||
| < 110 | 1880 (11.5) | 1432 (15.1) | 255 (12.6) | ||
| 110–150 | 8293 (50.9) | 5399 (56.8) | 1178 (58.1) | ||
| > 150 | 5963 (36.6) | 2605 (27.4) | 570 (28.1) | ||
| Missing | 158 (1.0) | 73 (0.8) | 23 (1.1) | ||
| Respiratory rate | .11 | .11 | |||
| < 30 | 14,534 (89.2) | 8551 (89.9) | 1829 (90.3) | ||
| ≥ 30 | 1072 (6.6) | 603 (6.3) | 131 (6.5) | ||
| Missing | 688 (4.2) | 355 (3.7) | 66 (3.3) | ||
| Heart rate | < .001 | < .001 | |||
| < 80 | 7699 (47.3) | 4288 (45.1) | 750 (37.0) | ||
| 80–100 | 5675 (34.8) | 3099 (32.6) | 671 (33.1) | ||
| > 100 | 2353 (14.4) | 1813 (19.1) | 568 (28.0) | ||
| Missing | 567 (3.5) | 309 (3.3) | 37 (1.8) | ||
| Test results, No. (%) | |||||
| Left ventricular ejection fraction | < .001 | < .001 | |||
| < 40% | 6502 (39.9) | 3410 (35.9) | 697 (34.4) | ||
| ≥ 40% | 9749 (59.8) | 6083 (64.0) | 1325 (65.4) | ||
| Missing | 43 (0.3) | 16 (0.2) | 4 (0.2) | ||
| Blood urea nitrogen | .02 | < .001 | |||
| < 20 | 4619 (28.4 ) | 2573 (27.1) | 639 (31.5) | ||
| 20–50 | 8579 (52.7) | 5202 (54.7) | 1047 (51.7) | ||
| > 50 | 1813 (11.1) | 1026 (10.8) | 176 (8.7) | ||
| Missing | 1283 (7.9) | 708 (7.5) | 164 (8.1) | ||
| Estimated glomerular filtration rate | < .001 | < .001 | |||
| < 30 | 3013 (18.5) | 1386 (14.6) | 274 (13.5) | ||
| 30–59 | 7041 (43.2) | 4489 (47.2) | 898 (44.3) | ||
| ≥ 60 | 4961 (30.5) | 2915 (30.7) | 651 (32.1) | ||
| Missing | 1279 (7.9) | 719 (7.6) | 203 (10.0) | ||
| Serum sodium | .32 | .34 | |||
| < 135 | 2684 (16.5) | 1628 (17.1) | 361 (17.8) | ||
| 135–145 | 11,642 (71.5) | 6779 (71.3) | 1434 (70.8) | ||
| > 145 | 293 (1.8) | 177 (1.9) | 30 (1.5) | ||
| Missing | 1675 (10.3) | 925 (9.7) | 201 (9.9) | ||
Abbreviations: COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack.
For the comparison between patients with no atrial fibrillation and patients with preexisting atrial fibrillation.
For the comparison between patients with no atrial fibrillation and patients with new-onset atrial fibrillation.
Compared with patients with no AF, patients with preexisting or new-onset AF had higher observed cumulative incidence of all-cause mortality at 1 and 3 years; patients with new-onset AF had higher mortality at 1 year (P=.001) and a nonsignificant trend toward higher mortality at 3 years (P=.03; Table 3). Patients with preexisting or new-onset AF had fewer other cardiovascular readmissions at both 1 and 3 years. Stroke readmission rates were similar for patients with preexisting and new-onset AF compared with no AF at both 1 and 3 years.
Table 3.
Observed Cumulative Incidence of Mortality and Readmission by Atrial Fibrillation Status at Hospital Admission (N = 27,829)
| Outcome | Atrial Fibrillation Status | ||||
|---|---|---|---|---|---|
| None (n = 16,294) |
Preexisting (n = 9509) |
P Value† | New-Onset (n = 2026) |
P Value‡ | |
| Cumulative Incidence at 1 Year* | |||||
| Mortality | 4788 (30.1) | 3392 (36.4) | < .001 | 663 (33.5) | .001 |
| All-cause readmission | 9900 (61.8) | 5892 (62.9) | .02 | 1187 (59.7) | .23 |
| Heart failure readmission | 4375 (27.4) | 2668 (28.5) | .04 | 513 (25.8) | .21 |
| Stroke readmission | 447 (2.8) | 288 (3.1) | .20 | 63 (3.2) | .33 |
| Other cardiovascular readmission | 3020 (19.0) | 1443 (15.5) | < .001 | 306 (15.4) | < .001 |
| Cumulative Incidence at 3 Years* | |||||
| Mortality | 7707 (56.7) | 5156 (63.4) | < .001 | 1000 (57.9) | .03 |
| All-cause readmission | 11,919 (79.0) | 7032 (79.3) | .06 | 1442 (76.5) | .12 |
| Heart failure readmission | 5750 (39.3) | 3476 (40.4) | .05 | 665 (36.5) | .05 |
| Stroke readmission | 766 (5.7) | 481 (6.0) | .22 | 112 (6.7) | .11 |
| Other cardiovascular readmission | 4343 (30.4) | 2098 (25.1) | < .001 | 441 (24.9) | < .001 |
Values are presented as number of events (cumulative incidence per 100 patients).
For the comparison between patients with no atrial fibrillation and patients with preexisting atrial fibrillation.
For the comparison between patients with no atrial fibrillation and patients with new-onset atrial fibrillation.
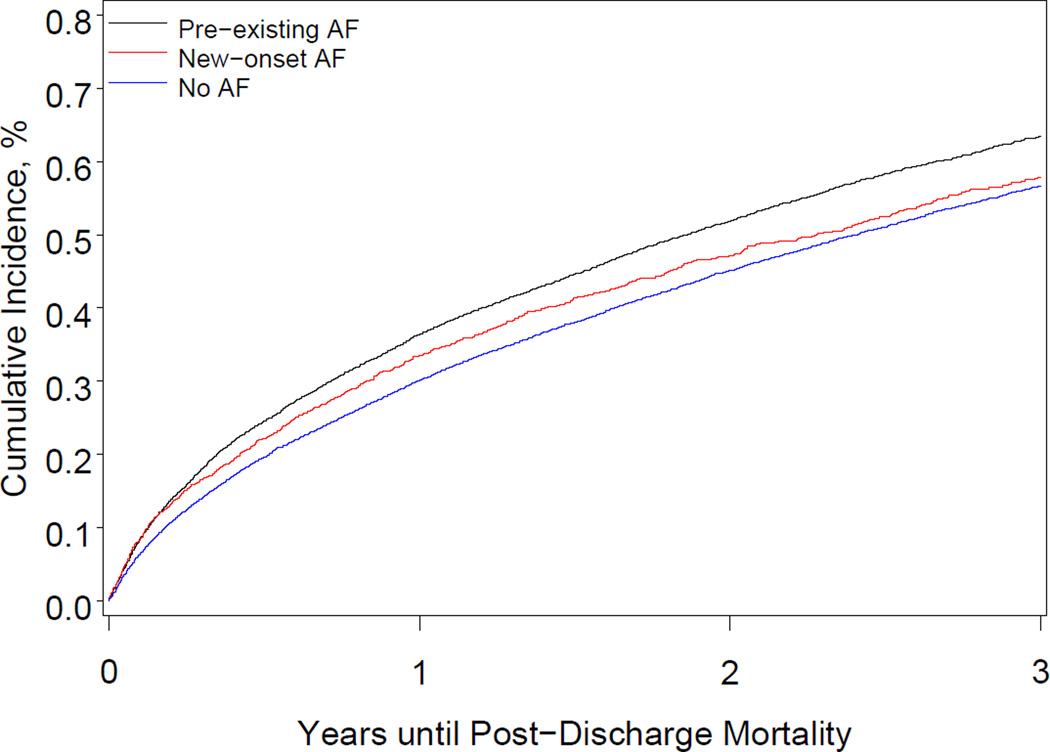
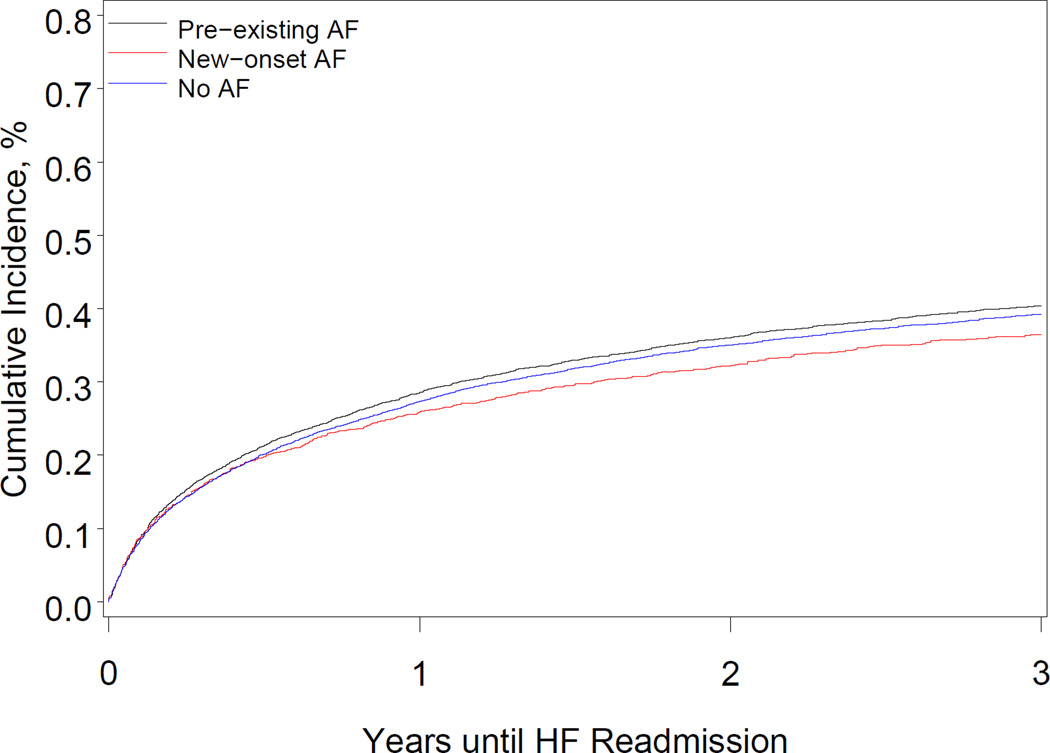
After multivariable adjustment, preexisting AF was associated with a higher risk of all-cause mortality, all-cause readmission, and AF readmission, compared with no AF (Table 4). The Figure shows the cumulative incidence of all-cause mortality and HF readmission. Preexisting AF was associated with a higher risk of stroke readmission at 3 years. After multivariable adjustment for significant covariates, the hazard of all-cause mortality among patients with new-onset AF increased modestly, though it was not statistically significant at 3 years (P=.05). New-onset AF was not associated with higher risks of all-cause readmission, HF readmission, stroke readmission, or other cardiovascular readmission.
Table 4.
Associations Between Preexisting or New-Onset Atrial Fibrillation and Mortality and Readmission After Adjustment for Baseline Characteristics*
| Outcome | Preexisting Atrial Fibrillation | New-Onset Atrial Fibrillation | ||
|---|---|---|---|---|
| Adjusted HR (99% CI) |
P Value | Adjusted HR (99% CI) |
P Value | |
| Outcomes at 1 Year | ||||
| Mortality | 1.15 (1.08–1.22) | < .001 | 1.12 (1.01–1.24) | .005 |
| All-cause readmission | 1.08 (1.03–1.13) | < .001 | 1.05 (0.96–1.16) | .15 |
| Heart failure readmission | 1.13 (1.06–1.21) | < .001 | 1.08 (0.95–1.23) | .11 |
| Stroke readmission | 1.17 (0.95–1.44) | .05 | 1.19 (0.84–1.68) | .19 |
| Other cardiovascular readmission | 0.89 (0.81–0.98) | .002 | 0.92 (0.79–1.07) | .15 |
| Outcomes at 3 Years | ||||
| Mortality | 1.14 (1.08–1.20) | < .001 | 1.08 (0.98–1.18) | .05 |
| All-cause readmission | 1.09 (1.05–1.14) | < .001 | 1.06 (0.97–1.15) | .08 |
| Heart failure readmission | 1.15 (1.08–1.21) | < .001 | 1.07 (0.95–1.20) | .16 |
| Stroke readmission | 1.20 (1.01–1.41) | .005 | 1.27 (0.98–1.64) | .02 |
| Other cardiovascular readmission | 0.91 (0.85–0.99) | .003 | 0.92 (0.81–1.05) | .09 |
Abbreviations: CI, confidence interval; HR; hazard ratio.
The reference group was the cohort of patients with no atrial fibrillation. The multivariable models adjusted for all variables listed in Table 1.
Figure.
Cumulative Incidence of All-Cause Death and Heart Failure Readmission According to Atrial Fibrillation Status
Panel A shows the cumulative incidence of all-cause death among patients with preexisting atrial fibrillation, patients with new-onset atrial fibrillation, and patients with no atrial fibrillation. Panel B shows the cumulative incidence of readmission for heart failure among patients with preexisting atrial fibrillation, patients with new-onset atrial fibrillation, and patients with no atrial fibrillation.
The percentage of patients who had HF with reduced EF was 35.9% among patients with preexisting AF and 34.4% among patients with new-onset AF. Among patients with preexisting or new-onset AF, unadjusted 3-year stroke readmission rates were higher among patients with preserved EF than among patients with reduced EF despite similar rates of oral anticoagulation (53% vs 57%, respectively; Table 5). There was an interaction between EF and AF for stroke readmission. After multivariable adjustment, the risk of stroke readmission at 1 year was similar for HF with preserved EF and HF with reduced EF. The risk of stroke readmission for new-onset AF at 3 years was lower with reduced EF than with preserved EF (hazard ratio, 0.56; 95% CI, 0.32–0.98; P=.008).
Table 5.
Unadjusted Cumulative Incidence and Adjusted Hazards of Stroke Readmission in Patients With Preexisting or New-Onset Atrial Fibrillation and Heart Failure With Either Preserved or Reduced Ejection Fraction*
| Stroke Readmission |
Preexisting Atrial Fibrillation | New-Onset Atrial Fibrillation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative Incidence† | Adjusted Risk | Cumulative Incidence† | Adjusted Risk | |||||||
| Reduced EF (n = 3410) |
Preserved EF (n = 6099) |
P Value |
HR (99% CI) |
P Value |
Reduced EF (n = 697) |
Preserved EF (n = 1329) |
P Value |
HR (99% CI) |
P Value |
|
| 1 year | 15 (2.2) | 48 (3.7) | .08 | 0.91 (0.63–1.30) | .48 | 86 (2.6) | 202 (3.4) | .03 | 0.67 (0.32–1.37) | .15 |
| 3 years | 23 (3.7) | 89 (8.2) | .002 | 0.87 (0.66–1.16) | .22 | 142 (5.0) | 339 (6.5) | .005 | 0.56 (0.32–0.98) | .008 |
Abbreviation: EF, ejection fraction; HR, hazard ratio.
Rates of oral anticoagulation were 53% for heart failure with preserved ejection fraction and 57% for heart failure with reduced ejection fraction.
Values are presented as number of events (cumulative incidence per 100 patients).
Discussion
Our analysis of long-term outcomes of more than 27,000 patients hospitalized with HF and AF had several important findings. First, AF was common and was associated with worse outcomes. Patients with AF had higher mortality, and patients with preexisting AF had higher rates of readmission, including readmission for HF. Finally, the risk of stroke was as high in patients with preserved EF as in those with reduced EF.
A previous analysis of short-term outcomes in the registry showed that AF was independently associated with higher mortality.1 Our study extends these observations. Patients with HF and AF had worse long-term outcomes than patients with HF alone. These data also suggest that outcomes are similarly poor for patients with new-onset AF. Although this finding is not novel, observational data continue to show that new-onset AF is undertreated compared with preexisting AF.17,18 Patients with new-onset AF are less likely to be treated with stroke prevention therapies regardless of stroke risk.20,21
To our knowledge, ours is the first study to report cause-specific readmission rates among patients with HF and AF. Preexisting AF was associated with higher rates of readmission for all causes, HF, and stroke. Higher rates of readmission in patients with preexisting vs new-onset AF likely reflects the cumulative risks of AF and subsequent adverse events. Consistent with findings from clinical trials,4,22 the risk of myocardial infarction in patients with HF and AF was low. Future studies should examine factors associated with cause-specific readmission to target potential interventions to reduce morbidity.
The risk of stroke in patients with HF and preserved EF and AF has not been thoroughly studied, and most recommendations for anticoagulation therapy in this population are based on expert consensus and small observational studies. Current guidelines recommend oral anticoagulation therapy for all patients with HF and AF.23 Post hoc analyses of the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) and the Rivaroxaban Versus Warfarin in Nonvalvular Atrial Fibrillation (ROCKET-AF) study found that patients with concomitant AF and preserved or reduced EF had similar rates of stroke, whereas an analysis of the Apixaban for the Prevention of Stroke in Subjects with Atrial Fibrillation (ARISTOTLE) study found that patients with reduced ejection fraction had higher rates of stroke.24–26 Given the limited data, equipoise remains with regard to whether HF with preserved EF should be considered a moderate risk factor for stroke and be considered as part of the “C” in the CHADS2 score. We found that patients with preserved and reduced EF had similar risk for stroke readmission after adjustment. These data suggest that patients with HF and AF should be treated with stroke prophylaxis regardless of EF. Our analyses were restricted to patients with a prior diagnosis of HF since patients with AF and rapid rates who develop new-onset HF theoretically have a different risk profile for stroke. We recognize that the stroke rate in the no AF population was higher than one would expect in a sinus rhythm population, but these higher rates may reflect other causes of stroke.
Our study has some limitations. First, the data were derived from a clinical registry linked with Medicare claims data, and our patient population was older than the average HF population. It is uncertain whether the outcome-specific event rates and hazards are generalizable. However, characteristics and outcomes of Medicare beneficiaries in previous HF registries were similar to the broader Medicare population with HF, suggesting that findings from these registries are generalizable.27,28 Second, we assumed the coding was accurate for preexisting and new-onset AF in the registry and for reasons for hospitalization in the Medicare data. The diagnosis of AF was not through electrographic confirmation. It is possible that errors in coding affected the analysis, but previous work suggests that the coding algorithms we used have high specificity.29,30 Third, data regarding medications taken after discharge and adherence to those medications were not available. Fourth, because we accessed an inpatient registry, we did not have outpatient data, such as New York Heart Association classification, and could not account for this in our analysis. Lastly, as with any retrospective analysis, unmeasured covariates likely influenced the outcomes.
In conclusion, in this nationwide cohort of more than 27,000 patients with both HF and AF, patients with preexisting and new-onset AF had higher mortality rates than patients with no AF. Moreover, preexisting AF was associated with a higher risk of all-cause and HF readmission rates. Whether AF is a marker of deterioration of HF or a mediator of adverse outcomes requires further study. The risk of stroke among patients with HF and AF is high, even among those with preserved EF. Given the morbidity, mortality, and economic burden associated with HF and AF, better treatment options and prevention measures are needed.
Acknowledgment
Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the manuscript.
Funding/Support: This study was funded under contract #HHSA29020050032I (Duke University DEcIDE Center) from the Agency for Healthcare Research and Quality, US Department of Health and Human Services, as part of the Developing Evidence to Inform Decisions About Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
Financial Disclosures: Dr. Fonarow reported receiving grant funding and other research support from GlaxoSmithKline; receiving honoraria from Boston Scientific/Guidant, GlaxoSmithKline, Medtronic, Merck, Novartis, Pfizer, and St Jude Medical; and serving as a consultant for Amgen, Gambro, GlaxoSmithKline, Medtronic, Merck, Novartis, Pfizer, Relypsa, Scios, and St Jude Medical. Dr. Fonarow holds the Eliot Corday Chair of Cardiovascular Medicine at UCLA and is also supported by the Ahmanson Foundation. Dr. Masoudi reported receiving salary support through his institution from the American College of Cardiology and the Oklahoma Foundation for Medical Quality; and receiving payment for editorial board service from the American Heart Association and the Massachusetts Medical Society. Dr. Curtis reported receiving research grants from GlaxoSmithKline and Johnson & Johnson. Dr. Hernandez reported receiving grant funding from Amylin Pharmaceutical and Johnson & Johnson; and receiving honoraria from AstraZeneca, Corthera, and Sanofi-Aventis. Dr. Piccini reported serving as a consultant for Forest Laboratories; receiving grant funding from Janssen Pharmaceuticals and Boston Scientific; and participating on advisory boards for Sanofi-Aventis and Johnson & Johnson.
Abbreviations
- AF
atrial fibrillation
- DRG
diagnosis related group
- HF
heart failure
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mountantonakis SE, Grau-Sepulveda MV, Bhatt DL, Hernandez AF, Peterson ED, Fonarow GC. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of get with the guidelines-heart failure. Circ Heart Fail. 2012;5:191–201. doi: 10.1161/CIRCHEARTFAILURE.111.965681. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed A, Thornton P, Perry GJ, Allman RM, DeLong JF. Impact of atrial fibrillation on mortality and readmission in older adults hospitalized with heart failure. Eur J Heart Fail. 2004;6:421–426. doi: 10.1016/j.ejheart.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 4.Olsson LG, Swedberg K, Ducharme A, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 5.Rivero-Ayerza M, Scholte Op Reimer W, Lenzen M, et al. New-onset atrial fibrillation is an independent predictor of in-hospital mortality in hospitalized heart failure patients: results of the EuroHeart Failure Survey. Eur Heart J. 2008;29:1618–1624. doi: 10.1093/eurheartj/ehn217. [DOI] [PubMed] [Google Scholar]
- 6.Swedberg K, Olsson LG, Charlesworth A, et al. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET. Eur Heart J. 2005;26:1303–1308. doi: 10.1093/eurheartj/ehi166. [DOI] [PubMed] [Google Scholar]
- 7.Carson PE, Johnson GR, Dunkman WB, Fletcher RD, Farrell L, Cohn JN. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT Studies. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI102–VI110. [PubMed] [Google Scholar]
- 8.Hong Y, LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–186. doi: 10.1097/01.hpc.0000243588.00012.79. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow GC, Abraham WT, Albert NM, et al. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):141–147. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 13.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf. 2008;17:20–26. doi: 10.1002/pds.1518. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Keenan PS, Normand SL, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 16.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–1701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 17.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2nd ed. Hoboken, New Jersey: John Wiley & Sons; 2002. [Google Scholar]
- 18.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 19.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York, New York: Springer-Verlag New York, Inc; 2010. [Google Scholar]
- 20.Nieuwlaat R, Capucci A, Camm AJ, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26:2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 21.Nieuwlaat R, Prins MH, Le Heuzey JY, et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008;29:1181–1189. doi: 10.1093/eurheartj/ehn139. [DOI] [PubMed] [Google Scholar]
- 22.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 23.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 24.Badheka AO, Rathod A, Kizilbash MA, et al. Comparison of mortality and morbidity in patients with atrial fibrillation and heart failure with preserved versus decreased left ventricular ejection fraction. Am J Cardiol. 2011;108:1283–1288. doi: 10.1016/j.amjcard.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 25.van Diepen S, Hellkamp AS, Patel MR, et al. Efficacy and safety of rivaroxaban in patients with heart failure and nonvalvular atrial fibrillation: insights from ROCKET AF. Circ Heart Fail. 2013;6:740–747. doi: 10.1161/CIRCHEARTFAILURE.113.000212. [DOI] [PubMed] [Google Scholar]
- 26.McMurray JJ, Ezekowitz JA, Lewis BS, et al. Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circ Heart Fail. 2013;6:451–460. doi: 10.1161/CIRCHEARTFAILURE.112.000143. [DOI] [PubMed] [Google Scholar]
- 27.Heidenreich PA, Fonarow GC. Are registry hospitals different? A comparison of patients admitted to hospitals of a commercial heart failure registry with those from national and community cohorts. Am Heart J. 2006;152:935–939. doi: 10.1016/j.ahj.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 28.Curtis L, Hernandez A, Greiner M, Fonarow G, Schulman K. Validity of a national heart failure quality of care registry: comparison of Medicare patients in OPTIMIZE-HF versus non-OPTIMIZE-HF hospitals. Circulation. 2007;115:e595. doi: 10.1161/CIRCOUTCOMES.108.822692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quach S, Blais C, Quan H. Administrative data have high variation in validity for recording heart failure. Can J Cardiol. 2010;26:306–312. doi: 10.1016/s0828-282x(10)70438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]