Abstract
Objective
To examine rates of discordance in neonatal risk factors and neurodevelopmental outcomes within very low birth weight twin pairs and factors associated with discordant outcomes.
Study Design
Rates of neonatal risk factors and neurodevelopmental outcomes and discordance in outcomes were examined for 88 very low birth weight twin pairs born 1990–2005 and followed through 20 months corrected age.
Results
Discordance rates ranged from 17–42% for neonatal risk factors and 18–31% for neurodevelopmental outcomes. In regression analysis, affected co-twins were significantly more likely to have had an abnormal cerebral ultrasound than their unaffected co-twins in pairs discordant for cerebral palsy (OR: 13.00, 95% CI: 2.22–76.03) and in pairs discordant for neurodevelopmental impairment (OR: 4.00, 95% CI: 1.13–14.18). Outcomes and discordance in outcomes were similar for monochorionic and dichorionic pairs.
Conclusion
Despite shared genetics and risk factors, twins may have discordant outcomes. Information on discordant neonatal and neurodevelopmental outcomes is important for counseling families of twins.
Keywords: neonatal morbidity, neurodevelopmental impairment, chorionicity
INTRODUCTION
The rate of multiple births has increased dramatically since 19801 with twins accounting for a significant portion of preterm births.2–4 Approximately 8–10% of all twins are born of very low birth weight (VLBW, <1.5kg) 1 and 12.5% at less than 32 weeks gestation.4 These infants are at risk for both neonatal morbidities including periventricular hemorrhage and bronchopulmonary dysplasia (BPD) and later neurodevelopmental disabilities such as cerebral palsy and cognitive impairments.3, 5–6
Multiple factors influence the neurodevelopmental outcomes of VLBW infants. These include gestational age, birth weight, and intrauterine growth failure as well as neonatal morbidities and socioeconomic factors such as maternal education.3, 5–6 Chorionicity may be an additional risk factor for twins with some studies reporting higher rates of neonatal and neurodevelopmental morbidities in monochorionic than dichorionic pairs.7–10
Because individuals within a twin pair (co-twins) share a genetic background as well as many perinatal risk factors and the postnatal environment, they may be expected to have similar neurodevelopmental outcomes. However, differences in neonatal morbidities between co-twins may lead to discordant neurodevelopmental outcomes. Previous research has examined the effect of birth weight discordance on overall neonatal morbidity11–13 and neurodevelopmental outcomes9–10, 14 in populations spanning a wide range of gestational age with variable results. Although the relationship of discordant neonatal cerebral ultrasound findings to neurodevelopmental outcomes in co-twins has previously been reported,15 to our knowledge the rates of discordant neurodevelopmental outcomes within VLBW twin pairs or their relationship to discordance in other neonatal risk factors have not been examined. Information regarding the potential discordance in outcomes of VLBW co-twins is important to provide anticipatory guidance to families of twins and to focus intervention efforts. We thus sought to examine rates of discordance in neonatal risk factors and 20 month neurodevelopmental outcomes within VLBW twin pairs and factors associated with discordant neurodevelopmental outcomes. We hypothesized that discordance in neurodevelopmental outcomes within pairs would be associated with discordance in neonatal risk factors.
POPULATION AND METHODS
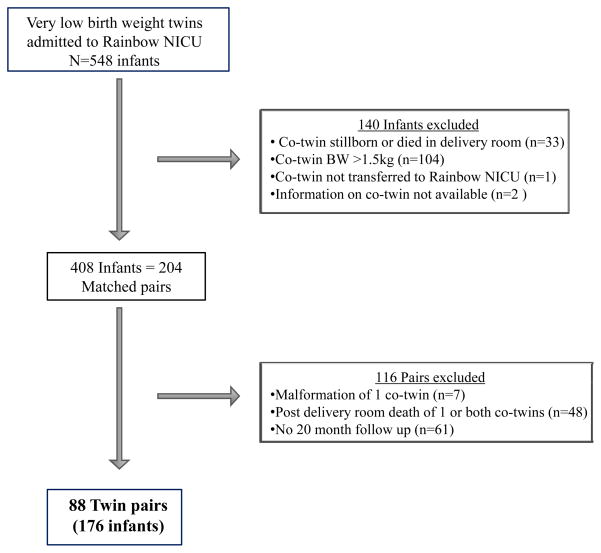
The population included 88 twin pairs (176 infants) admitted to the Rainbow Babies and Children’s Hospital neonatal intensive care unit between 1990 and 2005 in which both co-twins were live-born, had a birth weight ≤1.5 kg, were free of major congenital malformations, and who had developmental follow up at 20 months corrected age. Information on infants excluded or lost to follow up is shown in Figure 1.
Figure 1.
Population Flow Chart
Maternal, Perinatal, and Neonatal Data Collection[CWRU1]
Perinatal and neonatal data were extracted from the hospital chart at the time of neonatal discharge. Sociodemographic information included level of maternal education and race/ethnicity. Perinatal factors considered included antenatal steroid therapy, type of delivery, and low Apgar scores (≤5 at 5 minutes) as well as birth weight, gestational age, and intrauterine growth failure defined as small for gestational age (SGA) based on birth weight less than the10th percentile for gestational age16. Gestational age was determined from the date of the mother’s last menstrual period and was confirmed with obstetric measures, including ultrasonographic findings in the majority of cases[CWRU2]. Neonatal risk factors considered included a severely abnormal cerebral ultrasound (grade III–IV intraventricular hemorrhage, periventricular leukomalacia, or ventricular dilation at discharge), BPD (oxygen requirement at 36 weeks corrected age), and culture-proven sepsis and/or necrotizing enterocolitis (NEC).
For the present study, we also reviewed birth charts for information on infertility treatment and placental chorionicity. The chorionicity was obtained from the placental pathology report when available (n=65) or determined from maternal obstetric ultrasound report (n=4), gender difference between co-twins (n=6), blood type difference (n=6), or chart notes (n=2). We could not determine chorionicity in 5 twin pairs. Furthermore, we were unable to classify same gender, dichorionic pairs as monozygotic or dizygotic[CWRU3] as genetic markers were not examined.
Neurodevelopmental Outcome Measures
The infants were followed longitudinally until a corrected age of 18–20 months according to the protocol of the ongoing neonatal follow-up program at Rainbow Babies and Children’s Hospital. At 18–20 months, the follow-up assessment included [CWRU4]a neurologic examination of muscle tone17 and a developmental assessment. Cerebral palsy was defined as a persistent disorder of movement and posture attributable to a non-progressive disturbance of brain development18 and diagnosed on the basis of abnormal muscle tone in one or more extremities. This included spastic diplegia, hemiplegia, and quadriplegia. Developmental outcome was assessed using the Bayley Scales of Infant Development Mental Developmental Index (MDI) by a developmental specialist who was blind to the neonatal course of the child. Children born in 1990 and1991 (n= 16 pairs) were tested with the original Bayley Scales of Infant Development (Bayley I)19 and those born between 1992 and 2005 (n= 72 pairs) with the revised Bayley II Scales.20 The Bayley II was considered the primary outcome measure for this study. For group analysis, we used [CWRU5]a correction factor for infants tested with the Bayley I based on the published differences from a sample of 200 children given both versions in counter-balanced order.20 The correction factor ranged from 0 points for an MDI of 50 on the Bayley I to 13.5 points for an MDI of 123–127. Neurodevelopmental impairment was defined as one or more of the following: subnormal MDI (<70), cerebral palsy, deafness requiring amplification, and/or unilateral or bilateral blindness.
Parents provided written informed consent for participation in the follow-up study. The Institutional Review Board of University Hospitals Cleveland Case Medical Center approved both the follow-up study and the chart review.[CWRU6]
Data Analysis
Statistical comparisons were made using t tests for continuous variables and χ2 analyses for categorical variables. Degree of discordance in birth weight was calculated as the difference in birth weight between co-twins divided by the birth weight of the larger co-twin and expressed as a percentage.21 Pairs with a discordance value greater than 15% were considered birth weight discordant.2 Neonatal risk factors considered included severe cerebral ultrasound abnormality, BPD, and sepsis and/or NEC. These two latter risk factors were considered together due to the fact that most cases of NEC are blood culture positive. Neurodevelopmental outcomes considered included cerebral palsy, subnormal MDI (MDI< 70), and neurodevelopmental impairment. We considered twin pairs to be discordant for each of the neonatal risk factors or neurodevelopmental outcomes when one co-twin had the risk factor or adverse outcome while the other did not.
Logistic regression analyses using generalized estimating equations (GEE) were conducted to examine the association of gender, birth order (first versus second twin), and neonatal risk factors with neurodevelopmental outcomes within the pairs discordant for each of the neurodevelopmental outcomes. Twin pairing was taken into account to adjust for the non-independence of outcomes within twin pairs. Risk factors were analyzed individually due to the small sample size of pairs discordant for each outcome. GEE is a regression method that extends generalized linear models for examining associations between variables to allow for correlated observations (e.g. twin pairings) and is especially useful in examining predictors of binary outcomes (e.g., the presence versus the absence of a given developmental outcome). Because co-twins have the same gestational age, race, and maternal socioeconomic status, these were not adjusted for in this analysis. Gender was not significant in univariate analysis and thus was not adjusted for in analysis of other risk factors among discordant pairs.
For the pairs with known chorionicity (n= 83), GEE were used to examine the association of chorionicity (monochorionic versus dichorionic) with neonatal risk factors and neurodevelopmental outcomes. We controlled for gestational age, gender, and maternal education in this analysis. Monochorionic-monoamniotic and monochorionic-diamniotic pairs were considered together due to the small number of monoamniotic pairs. P values <0.05 were considered significant in all analyses.
RESULTS
Sociodemographic, Perinatal, and Birth Data
Table 1 presents a description of the sociodemographic, perinatal, and birth data. Mean birth weight and gestational age for the sample were 1018g and 27.6 weeks respectively with 18% SGA. The majority of twin pairs had dichorionic-diamniotic placentas. For pairs born by Cesarean section, equal numbers of first- and second-born co-twins had low five-minute Apgars scores [4/50 (8%)] with data missing for 1 pair. For those born vaginally 5/37 (14%) of first-born and 9/37 (24%) of second-born twins had low Apgar scores. Significantly fewer second-born twins born via Cesarean section had low Apgar scores than those born vaginally [4/50 (8%) vs. 9/37 (24%), p=0.04].
TABLE 1.
POPULATION DEMOGRAPHICS, PERINATAL, AND BIRTH DATA
| Twin pairs (n=88) | |
|---|---|
| Maternal Sociodemographic Factors | |
| Age, mean +/− SD, yr | 29.1 ± 6.0 |
| Black race, n (%) | 40 (45%) |
| Education, n (%) | |
| Less than high school | 11 (13%) |
| Completed high school | 29 (33%) |
| Greater than high school | 48 (55%) |
| Infertility treatment, n (%) | 19 (22%) |
| Perinatal Factors | |
| Antenatal steroid therapy, n (%) | 59 (67%) |
| Outborn in community hospital, n (%) | 10 (11%) |
| Cesarean delivery, n (%) | 51 (58%) |
| Chorionicity, n (%) | |
| Dichorionic-diamniotic | 61 (69%) |
| Monochorionic-diamniotic | 20 (23%) |
| Monochorionic-monoamniotic | 2 (2%) |
| Unknown | 5(6%) |
| Infant Birth Data | |
| Birth weight, mean +/− SD, g | 1018 ± 225a |
| Gestational age, mean +/− SD, wk | 27.6 ± 2.0 |
| Small for gestational age, n (%) | 31 (18%)a |
| Male gender, n (%) | 92 (53%)a |
Data for total population, n=176 infants
Compared to the 88 twin pairs included in the study, the 61 surviving pairs of whom both had a birth weight less than 1.5 kg and were free of major congenital malformations but were lost to follow up had a significantly higher mean birth weight (1202 ±194g vs. 1018±225g; p<0.001) and gestational age (29.2±1.9 weeks vs. 27.6±2.0 weeks; p<0.001) as well as lower rates of BPD (16% vs. 30%; p<0.01) and sepsis/NEC (27% vs. 44%; p<0.01).
Neonatal Risk Factors and Neurodevelopmental Outcomes
Of the total population of 176 infants, 19 (11%) had a severely abnormal cerebral ultrasound, 52 (30%) BPD, and 77 (44%) sepsis/NEC. On follow up at 20 months corrected age, 16 (9%) had cerebral palsy, 47 (27%) MDI<70, 7 (4%) deafness, 1 (1%) blindness, and 53 (30%) neurodevelopmental impairment. The mean MDI for the population was 81 (SD: 17).
Discordance within Twin Pairs
The mean birth weight difference between co-twins was 121g (SD: 106; range: 4–672) with a birth weight discordance of greater than 15% in 17 (19%) pairs. The mean difference in MDI between co-twins was 10 points (SD: 10; range 0–46) with a difference greater than 1SD in 27% of pairs.
To examine rates of discordance between co-twins in the 88 pairs, each pair was classified as both co-twins having the specific neonatal risk factor or neurodevelopmental outcome, as discordant with only one co-twin affected and the other not affected, or as neither having the risk factor or outcome (Table 2). Rates of discordance between co-twins for neonatal risk factors ranged from 17% for abnormal cerebral ultrasound to 42% for sepsis/NEC. For neurodevelopmental outcomes, rates of discordance were 18% for cerebral palsy, 28% for MDI <70, and 31% for neurodevelopmental impairment.
TABLE 2.
RATES OF DISCORDANCE IN NEONATAL RISK FACTORS AND NEURODEVELOPMENTAL OUTCOMES WITHIN TWIN PAIRS
| Co-twins affected n=88 pairs | |||
|---|---|---|---|
| Both | One (discordant) | Neither | |
| Neonatal Risk Factors | |||
| SGAa | 5 (6%) | 21 (24%) | 62(70%) |
| Abnormal cerebral ultrasoundb | 2 (2%) | 15 (17%) | 71 (81%) |
| BPDc | 18 (21%) | 16 (18%) | 54 (61%) |
| Sepsis/NEC | 20 (23%) | 37 (42%) | 31 (35%) |
| Neurodevelopmental Outcomes | |||
| Cerebral Palsyd | 0 (0%) | 16 (18%) | 72 (82%) |
| MDI <70 | 11 (13%) | 25 (28%) | 52 (59%) |
| Deafnesse | 1 (1%) | 5 (6%) | 81 (93%) |
| Blindnessf | 0 (0%) | 1 (1%) | 87 (99%) |
| Neurodevelopmental impairmentg | 13 (15%) | 27 (31%) | 48 (55%) |
Abbreviations: BPD: Bronchopulmonary Dysplasia; MDI: Bayley Mental Development Index; NEC: Necrotizing Enterocolitis; SGA: Small for Gestational Age
< 10%tile21
grade III–IV intraventricular hemorrhage, periventricular leukomalcia, or ventricular dilation at discharge
oxygen requirement at 36 weeks corrected age
hemiplegia, diplegia, or quadriplegia
requiring amplification
unilateral or bilateral
one or more of the following: MDI<70, cerebral palsy, deafness requiring amplification, and/or unilateral or bilateral blindness
Factors Associated with Discordant Neurodevelopmental Outcomes
Table 3 shows the comparison of the rates of each neonatal risk factor for the co-twins with and without the adverse outcomes in the discordant pairs and the results of the regression analysis. GEE regression analysis revealed that the only neonatal risk factor that was significantly associated with discordance in 20 month outcomes was a severely abnormal cerebral ultrasound. This was associated with both cerebral palsy (OR 13.00, 95% CI: 2.22–76.03; p<0.01) and neurodevelopmental impairment (OR 4.00, 95% CI: 1.13–14.18; p<0.05) in pairs discordant for these outcomes. Among the 16 pairs discordant for cerebral palsy, 75% of the children with cerebral palsy had an abnormal ultrasound compared to 19% of those without cerebral palsy. Similarly, in the 27 pairs discordant for neurodevelopmental impairment, significantly more co-twins with impairment had an abnormal ultrasound (33%) compared to those without impairment (11%). There was a trend (p=0.06) for SGA to be associated with an MDI <70 in discordant pairs; however, the number of infants who were SGA in pairs discordant for MDI<70 was low[CWRU7] (n=6).
TABLE 3.
ASSOCIATIONS BETWEEN NEONATAL RISK FACTORS AND NEURODEVELOPMENTAL OUTCOMES IN DISCORDANT PAIRS
| Discordant Cerebral Palsy (N=16 pairs, 32 infants) | Discordant MDI (N=25 pairs, 50 infants) | Discordant Neurodevelopmental Impairment (N=27 pairs, 54 infants) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk factors | Cerebral Palsyd n=16 infants |
No Cerebral Palsy n= 16 infants |
Odds Ratio (95% Confidence Interval) | MDI <70 n=25 infants |
MDI >70 n= 25 infants |
Odds Ratio (95% Confidence Interval) | Neurodev Impairmente n=27 infants |
No Neurodev Impairment n= 27 infants |
Odds Ratio (95% Confidence Interval) |
| Male Gender | 10 (63%) | 12 (75%) | 0.56 (0.14–2.23) | 20 (80%) | 16 (64%) | 2.25 (0.87–5.79) | 21 (78%) | 18 (67%) | 1.75 (0.67–4.55) |
| Second born | 7 (44%) | 9 (56%) | 0.60 (0.08–4.36) | 15 (60%) | 10 (40%) | 2.25 (0.45–11.15) | 13 (48%) | 14 (52%) | 0.86 (0.19–3.90) |
| SGAa | 3 (19%) | 2 (13%) | 1.62 (0.32–8.22) | 5 (20%) | 1 (4%) | 6.00 (0.96–37.53) | 5 (19%) | 2 (7%) | 2.84 (0.61–13.27) |
| Abnormal cerebral ultrasoundb | 12 (75%) | 3 (19%) | 13.00 (2.22–76.03)** | 7 (28%) | 3 (12%) | 2.85 (0.82–9.89) | 9 (33%) | 3 (11%) | 4.00 (1.13–14.18)* |
| BPDc | 7 (44%) | 7 (44%) | 1.00 f | 12 (48%) | 12 (48%) | 1.00 f | 11 (41%) | 12 (44%) | 1.16 (0.60–2.26) |
| Sepsis/NEC | 8 (50%) | 8 (50%) | 1.00 f | 9 (36%) | 12 (48%) | 1.64 (0.57–4.77) | 12 (44%) | 13 (48%) | 1.16 (0.44–3.06) |
Abbreviations: BPD: Bronchopulmonary Dysplasia; MDI: Bayley Mental Development Index; NEC: Necrotizing Enterocolitis; Neurodev: Neurodevelopmental; SGA: Small for Gestational Age
p < 0.05;
p<0.01
< 10%tile (Yudkin)
grade III–IV intraventricular hemorrhage, periventricular leukomalcia, or ventricular dilation at discharge
oxygen requirement at 36 weeks corrected age
hemiplegia, diplegia, or quadriplegia
one or more of the following: MDI<70, cerebral palsy, deafness requiring amplification, and/or unilateral or bilateral blindness
Equal numbers of infants with and without CP had BPD and sepsis and/or NEC. Equal numbers with and without MDI<70 had BPD.
Rates of discordant outcomes in the 17 pairs with birth weight discordance greater than 15% were not significantly different from discordance rates in the 71 pairs with concordant birth weights for cerebral palsy [3/17 (18%) vs. 13/71 (18%)], MDI<70 [3/17 (18%) vs. 22/71 (31%)], or neurodevelopmental impairment [3/17 (18%) vs. 24/71 (34%)].
Chorionicity
For the twins with known chorionicity (n=83 pairs; 166 infants), there were no differences in rates of neonatal risk factors or neurodevelopmental outcomes between the 44 monochorionic (22 pairs) and the 122 dichorionic (61 pairs) twin infants with the exception that significantly more infants from monochorionic pairs were born SGA [14/44 (32%) vs. 13/122 (11%)] (Supplemental Table). Rates of discordance for neurodevelopmental outcomes between co-twins were also not significantly different for the 22 monochorionic versus the 61 dichorionic pairs [4/22 (18%) vs. 11/61 (18%) for discordance in cerebral palsy; 5/22 (23%) vs. 18/61 (30%) for discordance in MDI<70; and 5/22 (23%) vs. 19/61 (31%) for discordance in neurodevelopmental impairment].
DISCUSSION
Twins have an increased risk of preterm birth and VLBW which are associated with risks of both neonatal and neurodevelopmental morbidity. Given the high percentage of twins born preterm, specific information on their outcomes is needed to provide anticipatory guidance and support for families. We thus sought to describe the rates of neonatal risk factors and 20 month neurodevelopmental outcomes in a cohort of VLBW twin pairs in which both co-twins were followed through 20 months corrected age and to examine discordance in outcomes between co-twins. Our results revealed that despite having shared genetic, demographic, and environmental risk factors, between 17 and 42% of VLBW twin pairs were discordant in neonatal risk factors and between 18 and 31% had discordant neurodevelopmental outcomes. Discordance for cerebral palsy and neurodevelopmental impairment was associated with discordance in abnormal neonatal cerebral ultrasound findings.
The only study of discordance in neonatal risk factors and neurodevelopmental outcomes has been that of Resch et al.15 who followed 18 preterm twin pairs discordant for cystic periventricular leukomalacia and found significantly higher rates of cerebral palsy and intellectual disability in the affected compared to unaffected co-twins. Previous older studies which included both term and preterm report discordance rates for cerebral palsy in the range of 45 to 75%.22 Information on neonatal risk factors was not reported in these studies.
We hypothesized that discordance in neurodevelopmental outcomes within pairs would be associated with discordance in neonatal risk factors. We confirmed this for an abnormal cerebral ultrasound but not for BPD or sepsis/NEC. Rates of neonatal cerebral injury on ultrasound were significantly higher for affected compared to unaffected co-twins in pairs discordant for cerebral palsy or for neurodevelopmental impairment. Our results are consistent with those of Resch et al.15 as well as other investigations showing a strong association between cerebral ultrasound lesions and adverse neurodevelopmental outcomes.3, 23
We also found a trend for an association between being born SGA and having an MDI<70 in our twin pairs discordant for MDI<70. Intrauterine growth restriction is a risk factor for poorer cognitive outcome.24 In this regard, Monset-Couchard et al.25 reported more speech and behavioral problems in SGA twins and triplets compared to their appropriately-grown co-twin/triplets.
Birth weight discordance in twin pairs may indicate growth restriction in the smaller co-twin2, 21. Previous studies have shown a correlation between birth weight differences in term and preterm twins and differences in results of cognitive or developmental testing with smaller co-twins having lower scores.14, 26 In our study, discordance in birth weight greater than15% was not associated with discordance for 20 month outcomes. Because infants with birth weights above 1.5 kg are not included in the follow-up program at our institution, we were not able to examine discordant outcomes in pairs in which only one co-twin was VLBW. This limited our ability to detect effects of birth weight discordance. As growth of twins diverges more as gestation advances,27 birth weight discordance may be a more important contributor to discordant developmental outcomes in twins born at later gestational ages than our population. Adegbite et al.9 report an increased risk of adverse neurodevelopmental outcomes for both the growth-restricted and appropriately-grown co-twins from birth weight discordant pairs, but differences within pairs were not examined.
Birth order potentially influences outcomes for twins with an increased risk of adverse perinatal outcomes for second born twins.28–29 Similar to our findings, Wadhawan et al.30 did not find significant differences in rates of adverse outcomes in first versus second born extremely low birth weight twins. As suggested by the lower rates of low Apgar scores in our second-born twins born via Cesarean section than in those born vaginally, the lack of an effect of birth order on outcome discordance is likely related to the fact that during recent years a large percentage of preterm twins are delivered via cesarean section.
Previous studies have reported shorter gestations and lower birth weights7–10 in monochorionic versus dichorionic twins as well as a higher incidence of intrauterine death8, 10 and neonatal7–8 and neurodevelopmental morbidity9–10 in monochorionic pairs. We found similar rates of neonatal risk factors and adverse neurodevelopmental outcomes in the infants from monochorionic and dichorionic pairs with the exception of a significantly higher rate of SGA births in infants from monochorionic pairs. Others also report higher rates of SGA in monochorionic twins7, 31 related to factors such as placental sharing and abnormal umbilical cord insertion.21, 32 The lack of an effect of chorionicity on other neonatal morbidities and later outcomes in our study may be due to our focus on a population of VLBW infants while many previous investigations have included twins born at a wider range of gestational age. Adegbite et al.9 found higher rates of neurodevelopmental morbidity for monochorionic than dichorionic twins with birth weights greater than 1 kg, but rates did not differ by chorionicity for twins with birth weights less than 1 kg. Another factor that may have contributed to the lack of differences in our study is that we excluded pairs in which one co-twin was stillborn. Intrauterine death of a co-twin is a known risk factor for cerebral palsy,33–34 especially in monochorionic twins.9
Some of the increased morbidity for monochorionic twins in previous studies is attributable to complications specific to monochorionic pregnancy such as twin-twin transfusion syndrome.9–10 While some of our monochorionic pairs (7/22) were known to have such complications, we did not account for this in our analysis as we could not reliably identify these complications in the other monochorionic pairs.
This study was unique in that it focused on twins born of VLBW and examined rates of discordance within pairs of both neonatal risk factors and neurodevelopmental outcomes. Complete neonatal and 20 month outcome data was available for each of the included pairs and was prospectively collected. We were also able to examine effects of chorionicity as data was available regarding placental type for 94% of the pairs.
Limitations include the fact that our outcome rates for VLBW twins are only for pairs in which both co-twins survived and that the children were only followed through 20 months. Cognitive scores may improve in later childhood,35 and some neurodevelopmental sequelae of prematurity such as learning disabilities may not be apparent until school age.6
This study represents a predominantly urban population and may not be generalizable to more suburban or rural populations. The twin pairs who were lost to follow up had a higher mean birth weight and gestational age than the pairs included in the study and thus likely had lower rates of adverse neurodevelopmental outcomes. Another limitation of this study is that the number of pairs with discordance for specific outcomes provided limited power to detect risk factor differences.
Previous studies have shown elevated rates of parental stress and depression associated with twin birth,36–37 VLBW,38 and neurodevelopmental impairment.39 Discordance in neonatal morbidity and neurodevelopmental outcomes may increase stress on families who not only have a child with a disability but also have twins with vastly different developmental abilities. Differences in health and ability may also influence the parents’ relationships with the twins.40 Improved understanding of outcomes for VLBW twins can lead to more informed anticipatory guidance, counseling, and support for their families. Additionally, knowing which children are most at risk can help focus intervention efforts.
Future research should include examination of the effect of discordant twin outcomes on the family, assessment of discordance in neurodevelopment outcomes in older children, and investigation of other factors that may contribute to discordant outcomes such as twin-twin transfusion syndrome.
Supplementary Material
Acknowledgments
Sources of Support: HRSA/MCHB (T77MC00004B0); NICHD
This project was supported by the Developmental Behavioral Pediatrics Fellowship Training Grant from the Health Resources and Services Administration/Maternal and Child Health Bureau (T77MC00004B0). This project was also partially supported by the NICHD Neonatal Research Network.
Thank you to Harriet Friedman, MA, for performing the developmental assessments, to Mark Schluchter, PhD, for statistical consultation, and to Angelia Williams and Bonnie Siner, RN, for their help in conducting this research.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Kochanek KD, Kirmeyer SE, Martin JA, Strobino D, Buyer B. Annual summary of vital statistics 2009. Pediatrics. 2012;129:338–348. doi: 10.1542/peds.2011-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. Multiple gestation: complicated twin, triplet, and high-order multifetal pregnancy. ACOG Practice Bulletin Number. 2004;56 doi: 10.1097/00006250-200410000-00046. [DOI] [PubMed] [Google Scholar]
- 3.Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998. Pediatrics. 2005;116:635–643. doi: 10.1542/peds.2004-2247. [DOI] [PubMed] [Google Scholar]
- 4.Heron M, Sutton P, Xu J, Ventura S, Strobino S, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 5.Wilson-Costello D, Friedman J, Minich N, Siner B, Taylor G, Schluchter M, et al. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000–2002. Pediatrics. 2007;119:37–45. doi: 10.1542/peds.2006-1416. [DOI] [PubMed] [Google Scholar]
- 6.Vohr B, Wright LL, Hack M, Aylward G, Hirtz D. Follow-up care of high-risk infants. Pediatrics. 2004;114:1377–1397. [Google Scholar]
- 7.Leduc L, Takser L, Rinfret D. Persistence of adverse obstetric and neonatal outcomes in monochorionic twins after exclusion of disorders unique to monochorionic placentation. Am J Obstet Gynecol. 2005;193:1670–1675. doi: 10.1016/j.ajog.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Hack KEA, Derks JB, Elias SG, Franx A, Roos EJ, Voerman SK, et al. Increased perinatal mortality and morbidity in monochorionic versus dichorionic twin pregnancies: clinical implications of a large Dutch cohort study. BJOG. 2008;115:58–67. doi: 10.1111/j.1471-0528.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 9.Adegbite A, Castille S, Ward S, Bajoria R. Neuromorbidity in preterm twins in relation to chorionicity and discordant birth weight. Am J Obstet Gynecol. 2004;190:156–16. doi: 10.1016/j.ajog.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Minakami H, Honma Y, Matsubara S, Uchida A, Shiraishi H, Sata I. Effects of placental chorionicity on outcome in twin pregnancies. J Reprod Med. 1999;44:595–600. [PubMed] [Google Scholar]
- 11.Amaru RC, Bush MC, Berkowitz RL, Lapinski RH, Gaddipati S. Is discordant growth in twins as independent risk factor for adverse neonatal outcome? Obstet Gynecol. 2004;103:71–76. doi: 10.1097/01.AOG.0000104060.37475.29. [DOI] [PubMed] [Google Scholar]
- 12.Hollier LM, McIntire DD, Leveno KJ. Outcome of twin pregnancies according to intrapair birth weight differences. Obstet Gynecol. 1999;94:1006–1010. doi: 10.1016/s0029-7844(99)00500-1. [DOI] [PubMed] [Google Scholar]
- 13.Nawab US, Greenspan JS, Kirkby S, Culhane JF, Kornhauser M. Differences in short-term neonatal outcomes between discordant twins. Adv Neonatal Care. 2008;8:334–340. doi: 10.1097/01.ANC.0000342765.71864.61. [DOI] [PubMed] [Google Scholar]
- 14.Goyen T, Veddovi M, Lui K. Developmental outcome of discordant premature twins at 3 years. Early Hum Dev. 2003;73:27–37. doi: 10.1016/s0378-3782(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 15.Resch B, Jammernegg A, Vollaard E, Maurer U, Meuller WD, Pertl B. Preterm twin gestation and cystic periventricular leucomalacia. Arch Dis Child Fetal Neonatal Ed. 2004;89:F315–320. doi: 10.1136/adc.2003.037309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yudkin PL, Aboualfa M, Eyre JA, Redman CWG, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24–42 weeks. Early Hum Dev. 1987;15:45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 17.Amiel-Tison C. Neurological evaluation of the maturity of newborn infants. Arch Dis Child. 1968;43:89–93. doi: 10.1136/adc.43.227.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bax M. Terminology and classification of cerebral palsy. Dev Med Child Neurol. 1964;6:295–297. doi: 10.1111/j.1469-8749.1964.tb10791.x. [DOI] [PubMed] [Google Scholar]
- 19.Bayley N. Bayley Scales of Infant Development. Psychological Corp; New York, NY: 1969. [Google Scholar]
- 20.Bayley N. Bayley Scales of Infant Development. 2. Psychological Corp; San Antonio, TX: 1993. [Google Scholar]
- 21.Miller J, Chauhan SP, Abuhamad AZ. Discordant twins: diagnosis, evaluation and management. Am J Obstet Gynecol. 2012;206:10–20. doi: 10.1016/j.ajog.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 22.Laplaza FJ, Root L, Tassanawipas A, Cervera P. Cerebral palsy in twins. Dev Med Child Neurol. 1992;34:1053–1063. doi: 10.1111/j.1469-8749.1992.tb11417.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuban KCK, Allred EN, O’Shea TM, Paneth N, Pagano M, Dammann O, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24:63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz JM, Whitaker AH, Feldman JF, Yudkin PL, Shen S, Blong A, et al. Indices of body and brain size at birth and age the age of 2 years: relations to cognitive outcome at the age of 16 years in low birth weight infants. J Dev Behav Pediatr. 2009;30:535–543. doi: 10.1097/DBP.0b013e3181c35ee4. [DOI] [PubMed] [Google Scholar]
- 25.Monset-Couchard M, de Bethmann O, Relier JP. Long term outcome of small versus appropriate size for gestational age co-twins/triplets. Arch Dis Child Fetal Neonatal Ed. 2004;89:F310–F314. doi: 10.1136/adc.2002.021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newcombe R, Milne B, Caspi A, Poulton R, Moffitt T. Birthweight predicts IQ: fact or artefact? Twin Res Hum Genet. 2007;10:581–586. doi: 10.1375/twin.10.4.581. [DOI] [PubMed] [Google Scholar]
- 27.Garite TJ, Clark RH, Elliott JP, Thorp JA Pediatrix/Obstetrix Perinatal Research Group. Twins and triplets: the effect of plurality and growth on neonatal outcome compared with singleton infants. Am J Obstet Gynecol. 2004;191:700–707. doi: 10.1016/j.ajog.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 28.Armson BA, O’Connell C, Persad V, Joseph KS, Young DC, Baskett TF. Determinants of perinatal mortality and serious neonatal morbidity in the second twin. Obstet Gynecol. 2006;108:556–564. doi: 10.1097/01.AOG.0000227747.37184.0a. [DOI] [PubMed] [Google Scholar]
- 29.Shinwell ES, Blickstein I, Lusky A, Reichman B. Effect of birth order on neonatal morbidity and mortality among very low birthweight twins: a population based study. Arch Dis Child Feteal Neonatal Ed. 2004;89:F145–F148. doi: 10.1136/adc.2002.021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadhawan R, Oh W, Perritt R, McDonald S, Das A, Poole W, et al. Twin gestation and neurodevelopmental outcome in extremely low birth weight infants. Pediatrics. 2009;123:e220–227. doi: 10.1542/peds.2008-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blickstein I, Mincha S, Goldman RD, Machin GA, Keith LG. The Northwestern twin chorionicity study: testing the ‘placental crowding’ hypothesis. J Perinat Med. 2006;34:158–161. doi: 10.1515/JPM.2006.028. [DOI] [PubMed] [Google Scholar]
- 32.Redline RW, Shah D, Sakar H, Schluchter M, Salvator A. Placental lesions associated with abnormal growth in twins. Pediatr Dev Path. 2001;4:473–481. doi: 10.1007/s10024001-0044-z. [DOI] [PubMed] [Google Scholar]
- 33.Scher A, Petterson B, Blair E, Ellenberg J, Grether J, Haan E, et al. The risk of mortality or cerebral palsy in twins: a collaborative population-based study. Pediatr Res. 2002;52:671–681. doi: 10.1203/00006450-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Bonelli S, Chalmers J. Comparison of risk factors for cerebral palsy in twins and singletons. Dev Med Child Neurol. 2005;47:587–591. [PubMed] [Google Scholar]
- 35.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor Predictive Validity of the Bayley Scales of Infant Development for Cognitive Function of Extremely Low Birth Weight Children at School Age. Pediatrics. 2005;116:334–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 36.Vilska S, Unkila-Kallio L, Punamaki RL, Poikkeus P, Repokari L, Sinkkonen J, et al. Mental health of mothers and fathers of twins conceived via assisted reproduction treatment: a 1-year prospective study. Hum Reprod. 2009;24:367–377. doi: 10.1093/humrep/den427. [DOI] [PubMed] [Google Scholar]
- 37.Choi Y, Bishai D, Minkovit CS. Multiple births are a risk factor for postpartum maternal depressive symptoms. Pediatric. 2009;123:1147–1154. doi: 10.1542/peds.2008-1619. [DOI] [PubMed] [Google Scholar]
- 38.Moore M, Taylor HG, Klein N, Minich N, Hack M. Longitudinal changes in family outcomes of very low birth weight. J Pediatr Psychol. 2006;31:1024–1035. doi: 10.1093/jpepsy/jsj075. [DOI] [PubMed] [Google Scholar]
- 39.Drotar D, Hack M, Taylor G, Schluchter M, Andreias L, Klein N. The impact of extremely low birth weight on the families of school-aged children. Pediatrics. 2006;117:2006–2013. doi: 10.1542/peds.2005-2118. [DOI] [PubMed] [Google Scholar]
- 40.Feldman R, Eidelman A. Does a Triplet Birth Pose a Special Risk for Infant Development? Assessing Cognitive Development in Relation to Intrauterine Growth and Mother-Infant Interaction Across the First 2 Years. Pediatrics. 2005;115:443–453. doi: 10.1542/peds.2004-1137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.