Abstract
Objective
Both short and long interpregnancy intervals (IPI) are believed to present possible adverse conditions for fetal development. Short IPI has recently been associated with increased risk of autism, but whether long IPI increases risk for autism spectrum disorders (ASD) has not been thoroughly investigated. We investigated the association between short and long IPI in a Finnish population-based study.
Method
This study was conducted in the Finnish Prenatal Study of Autism, which is based in a national birth cohort. Children born in Finland in 1987–2005 and diagnosed with ASD by 2007 were identified through the Finnish Hospital Discharge Register. 2,208 non-firstborn patients with ASD and 5,163 matched controls identified from the Finnish Medical Birth Register were included in the primary analysis. The association between IPI and ASD was determined using conditional logistic regression and adjusted for potential confounders.
Results
Relative to births with an IPI of 24–59 months, those with the shortest IPI (<12 months) had an increased risk of ASD (OR [95% CI], 1.50 [1.28, 1.74]) in confounder-adjusted models, while the ORs (95% CI) for longer IPI births (60–119 months and ≥120 months) were 1.28 (1.08, 1.52) and 1.44 (1.12, 1.85), respectively.
Conclusion
This study provides evidence that risk of ASD is increased at long as well as short IPI.
Keywords: autism spectrum disorders, autism, interpregnancy interval, interbirth interval, birth spacing
Introduction
Autism spectrum disorders (ASD) are developmental conditions involving impairments in social communication and patterns of restricted interests or repetitive behaviors. The etiology of ASD is believed to stem from a complex combination of genetic and environmental factors.1 It is generally believed that the prenatal time period is most relevant for the potential impact of non-genetic factors.
Interpregnancy interval (IPI) is a potentially modifiable factor influencing the prenatal environment. Both short and long IPI are believed to present possible adverse conditions for fetal development. Short IPI may result in the depletion of maternal nutrient reserves, in particular folate,2 or in conditions of unresolved inflammation from the preceding pregnancy.3 Longer IPI is associated with increased risk of pre-eclampsia4 and has been hypothesized to result in a “physiologic regression” caused by a loss of maternal vascular adaptation from the prior pregnancy.5 Pregnancies with short or long IPI may also be more likely to be unintended.6 Unintended pregnancies bear a higher risk of exposure to maternal risk behaviors including prenatal smoking and alcohol consumption; and inadequate use of prenatal care and folic acid supplementation.7,8
An epidemiologic study from California reported an inverse association between IPI less than 36 months and the risk of autism among second-born children.9 The finding was replicated in a study from Norway, where a 2-fold increased risk of autistic disorder was found in pregnancies with IPI less than 9 months.10 Likewise, a multivariate analysis of obstetric and perinatal factors in children from Nova Scotia, Canada reported a relative risk for ASD of 1.51 for IPI less than versus greater than 18 months.11 However, it has not yet been determined whether and to what extent this association holds consistently across a variety of different populations. Also, an association between long IPI and increased risk of autistic disorder was suggested graphically by the study from Norway,10 but this has not yet been tested rigorously.
In order to test the hypotheses that both short and long IPI are associated with increased risk of ASD, we used data from the Finnish Prenatal Study of Autism (FIPS-A), a population-based patient-control study. In this population, a diagnosis of ASD is facilitated by universal access to health care and regular childhood developmental screenings, and data on participants as well as their parents and siblings are available through linkage of several national registers. A key strength of the present study was the availability of a sufficient number of participants with long IPI (60 or more months).
Method
Two sets of analyses were conducted. The primary analysis used non-firstborn patients with ASD and unrelated matched controls and is described in detail below. A complementary patient-sibling analysis addressing the potential for confounding by family-level factors used pairs of first- and second-born siblings where one sibling was diagnosed with ASD. Given the data available, interbirth interval, rather than IPI, served as the exposure variable for the patient-sibling analysis. The selection of participants, definition of exposure, and statistical methods for the patient-sibling analysis are described in detail in Supplement 1, available online.
Data sources
Patients with ASD and controls were identified through the FIPS-A, a nested patient-control study based on a national birth cohort, which has been described in detail previously.12 Data in the FIPS-A were linked between registries using unique personal identity codes, which are given at birth to every Finnish resident and remain the same throughout life. The registries used in this study collect and maintain information about Finnish residents and their use of health services. Data used for the current study came from three national registries. The Finnish Hospital Discharge Register (FHDR) is maintained by the National Institute of Health and Welfare (THL) and includes all public and private inpatient diagnoses since January 1, 1967 and outpatient diagnoses in specialized public hospital units since January 1, 1998. The Finnish Medical Birth Register (FMBR; also maintained by THL, established in 1987) includes comprehensive data, collected using a standardized form, on the pre-, peri-, and neonatal periods up to age 7 days for all births in Finland. The Finnish Central Population Register (CPR) is a computerized national register that contains basic information about Finnish citizens and foreign citizens residing permanently in Finland, including name, personal identity code, address, municipality of residence, citizenship, family relations, and dates of birth and death. The study received approval from the Ministry of Social Affairs and Health of Finland and from the Institutional Review Board of the New York State Psychiatric Institute.
Identification of participants
Under the recently released DSM-5, ASD constitutes a single diagnosis, but it has historically encompassed the related diagnoses of childhood autism or autistic disorder (depending on whether the DSM or International Classification of Diseases [ICD] is used), Asperger syndrome, and pervasive developmental disorder not otherwise specified (PDD-NOS). In the FIPS-A, children born in 1987–2005 and diagnosed with ASD by the end of 2007 were identified through the FHDR. Diagnoses in the FHDR are based on the ICD. ICD-9 was used from 1987–1995 and ICD-10 was used beginning in 1996. The most recently registered diagnosis was used. The diagnostic categories included in this study and their ICD-10 codes were: childhood autism (F84.0), Asperger syndrome (F84.5), and other pervasive developmental disorder (PDD) and PDD-NOS (F84.8 and F84.9). Diagnoses based on ICD-9 codes (299.0, 299.8 and 299.9) were used to identify a small number (n=19) of patients, and their diagnoses were updated to the latest ICD-10 classifications. A validation study has shown high specificity for the childhood autism diagnosis in the FHDR.13 Four controls were selected from the FMBR for each patient, matched by date of birth (+/− 30 days), place of birth (birth hospital or regional hospital district), sex, and residence in Finland. The exclusion criteria for population controls were ASD or severe/profound intellectual disability (ID) according to the FHDR.
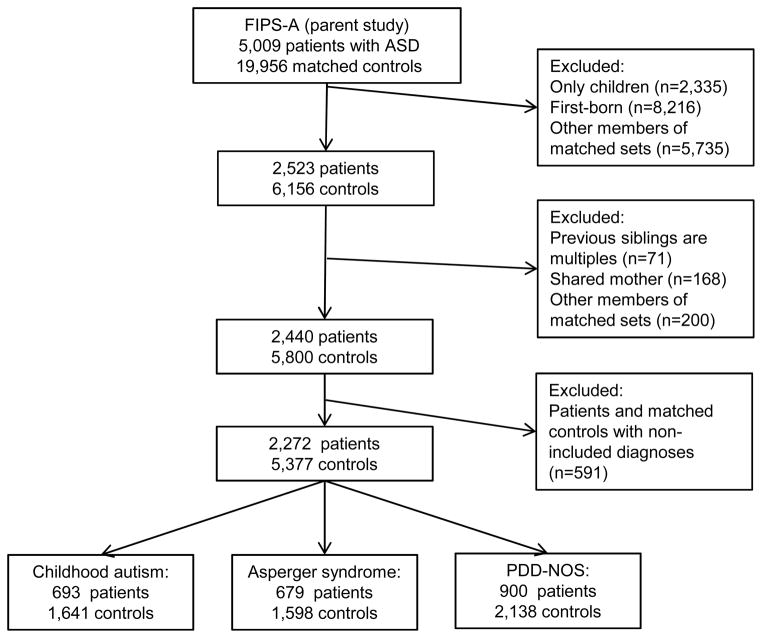
Selection of patients and controls is illustrated in Figure 1. 5,009 matched sets, comprising 5,009 patients with ASD and 19,956 matched controls were initially identified. For the current investigation, we excluded participants without any siblings (n=2,335) and all participants who were first-born based on maternal parity (n=8,216) because the exposure of interest, IPI, is not defined for these births. We also excluded participants born following a multiple birth (n=71), those sharing a mother with another participant (n=168), and those where the patient had a diagnosis other than those included in our outcome definition (see above; n=591). Remaining members of matched sets were excluded if the patient or all matched controls had previously been excluded (total n=5,935). This resulted in a total of 2,272 matched sets (2,272 patients with ASD and 5,377 controls) for the current investigation.
Figure 1. Selection of patients and controls.
Note: ASD = autism spectrum disorder; FIPS-A = Finnish Prenatal Study of Autism; PDD-NOS = pervasive developmental disorder, not otherwise specified.
Exposure
The primary exposure, IPI, was calculated using data from the FMBR and CPR. Sibships were identified for patients and matched controls by linkage to the CPR and were identified based on a shared biological mother. IPI was first calculated in days as the difference between each participant’s birth date and the birth date of their preceding sibling, minus the gestational age of the participant at birth. IPI was converted to months according to the following, where M=months and D=days: M= 12*(D/365.25). IPI in months was categorized into 5 levels (<12; 12-<24; 24-<60; 60-<120, >=120) in order to capture intervals typically considered both short and long. Where relevant, 24-<60 months served as the reference category.
Covariates
Data on covariates were obtained from the FMBR, the CPR, and the FHDR. Maternal and paternal ages at birth, maternal parity, previous miscarriages/abortions, maternal socioeconomic status (SES), and infant preterm birth and low birth weight were obtained from the FMBR. Maternal or paternal psychiatric diagnoses and any ASD diagnosis in a prior sibling were determined through linkage to the FHDR based on the following ICD-10/9 codes: F10-F99 (excluding F70-F79)/291-316 (excluding 293-294). Variables were categorized as shown in Table 1. Diagnoses of co-morbid ID in patients were determined using the FHDR, based on the following ICD-10/9 codes: F70/317, F71/318.0, F72/318.1, F73/318.2, F78 (no ICD-9 code), and F79/319.
Table 1.
Frequencies of Characteristics of Singleton, Non-Firstborn Patients With Autism Spectrum Disorders (ASD) and Matched Controls From Finnish Births, 1987–2005.
| %, Controls (n=5,377) | %, ASD (n=2,272) | χ2 p-value | n missing | |
|---|---|---|---|---|
| Maternal age | <.0001 | 0 | ||
| 15–24 | 9.5 | 9.5 | ||
| 25–29 | 31.5 | 28.2 | ||
| 30–34 | 36.4 | 33.9 | ||
| 35–39 | 18.2 | 22 | ||
| 40+ | 4.4 | 6.4 | ||
| Paternal age | ||||
| 15–24 | 4.2 | 4.4 | .00 | 80 |
| 25–29 | 22.3 | 19.6 | ||
| 30–34 | 36.1 | 34.6 | ||
| 35–39 | 24.5 | 24.7 | ||
| 40–49 | 12 | 15.3 | ||
| 50+ | 0.9 | 1.5 | ||
| Parental psychiatric disorder | 12.6 | 19.8 | <.0001 | 80 |
| Maternal parity, 2+ (reference=1) | 41 | 37.4 | .00 | |
| History of miscarriage/abortion | 29 | 30.5 | .19 | 66 |
| ASD diagnosis in any prior sibling | 0.5 | 4.5 | <.0001 | 0 |
| Maternal SES | ||||
| Upper white collar | 15.9 | 13.4 | .01 | 1785 |
| Lower white collar | 44.7 | 43.5 | ||
| Blue collar | 20.8 | 23.9 | ||
| Other | 18.6 | 19.3 | ||
| Preterm birth | 3.9 | 5.7 | .00 | 47 |
| Low birth weight | 1.9 | 4 | <.0001 | 31 |
Note: SES = socioeconomic status.
Statistical Methods
Population characteristics were tabulated and compared between patients and controls using chi-squared tests. To determine whether covariates were associated with IPI in the general population, they were tabulated by IPI level among controls, and chi-squared tests were conducted.
To assess the association between IPI and ASD, conditional logistic regression models were fit. The first model was unadjusted. A second model adjusted for the following potential confounders selected based on prior association with ASD in this and other populations14–17 and potential association with IPI: maternal age, paternal age, parental psychiatric disorders, parity, previous miscarriage/abortions, and any ASD diagnosis in a prior sibling.
A series of supplementary models were fit to test the sensitivity of the results to changes in the analysis. These models are as follows: 1) To test for potential confounding by maternal SES, indicator variables for each maternal SES category were added to a model including the subset of observations for which SES data were available. 2) To test whether the association between IPI and ASD was attributable to low birth weight or pre-term birth, which have each previously been associated with IPI5, dichotomous terms for these variables were added to the model. 3) To eliminate possible effects of ASD in a prior child on IPI, a model was fit restricted to observations with no ASD diagnosis in a prior sibling. 4) Because the IPI is measured between two live-birth pregnancies, women experiencing a miscarriage or abortion during this interval will have spent some of the time pregnant and may have different exposures or physiologic parameters than women who are non-pregnant during the interval. To address the possibility that this influenced our results, we fit a model restricted to the observations with no prior reported miscarriage or abortion. 5) To address the potential for residual confounding by parental ages, we fit a model with continuous (linear), rather than categorical, terms for maternal and paternal age. 6) To test for differences in the associations between IPI and ASD across subtypes and by the presence or absence of an ID diagnosis in the patients, stratified models were estimated and p-values for heterogeneity18 were computed. All analyses were conducted using SAS statistical software (SAS Version 9.3; SAS Institute Inc., Cary, NC).
Results
Patients in the sample were 78.6% male, with a mean birth year of 1994 and age of diagnosis of 7.4. Characteristics of the patients and controls are shown in Table 1. Participants with ASD were more likely to have older parents, parents with a history of psychiatric disorder, an older sibling with an ASD diagnosis, preterm birth, and low birth weight. Participants with ASD were less likely to be third or later versus second-born and to have mothers who were white-collar workers (Table 1). Maternal history of miscarriage or abortion was similar for patients and controls in the overall sample; however, these covariates were significantly more frequent among mothers of patients with Asperger syndrome than matched control mothers (32.6% vs. 27.9%; p=.02).
Among the controls, IPI was positively correlated with the ages of both parents. IPI of less than 24 months occurred more frequently among controls who were second- (versus third- or later-) born, and who had maternal SES categorized as “other” (entrepreneurs and people outside the labor force, i.e. students or unemployed persons). Parental psychiatric disorder, maternal history of prior miscarriage/abortion, preterm birth, low birth weight, and maternal SES categorized as “blue collar workers” were most common in pregnancies following longer IPI of 60 months or more (Table S1, available online).
Table 2 shows ORs for the association between IPI and ASD. Relative to births with an IPI of 24–59 months, those with the shortest IPI (<12 months) had an increased risk of ASD (OR [95% CI], 1.50 [1.28, 1.74]) in confounder-adjusted models, while the ORs (95% CI) for longer IPI births (60–119 months and ≥120 months) were 1.28 (1.08, 1.52) and 1.44 (1.12, 1.85), respectively. Results from models applying additional adjustments or restrictions (see Methods) were consistent with those presented in Table 2 (also see Table S2, available online).
Table 2.
Odds Ratios and 95% Confidence Intervals for the Association Between Interpregnancy Interval (IPI) and Autism Spectrum Disorders (ASD) in Finnish Births, 1987–2005.
| Controls (%) n=5,163 |
Patients (%) n=2,208 |
cOR | 95% CI | aOR | 95% CI | |
|---|---|---|---|---|---|---|
| IPI (months) | ||||||
| <12 | 19.2 | 23.1 | 1.47 | (1.27, 1.70) | 1.50 | (1.28, 1.74) |
| 12–23 | 28.4 | 26.4 | 1.13 | (0.99, 1.29) | 1.13 | (0.98, 1.30) |
| 24–59 | 34.3 | 28.6 | 1.00 | Ref | 1.00 | Ref |
| 60–119 | 13.6 | 15.2 | 1.38 | (1.17, 1.62) | 1.28 | (1.08, 1.52) |
| >=120 | 4.4 | 6.7 | 1.82 | (1.44, 2.28) | 1.44 | (1.12, 1.85) |
Note: adjusted odds ratio (aOR) adjusted for maternal age, paternal age, parental psychiatric disorders, parity, previous miscarriage/abortions, any ASD diagnosis in a prior sibling. cOR = crude (unadjusted) odds ratio; Ref = reference category.
Table 3 shows estimates for the association between IPI and ASD stratified by: a) ASD subtype and b) the presence or absence of intellectual disability in the patient. The patients with ASD were comprised of 674 (30.5%) individuals diagnosed with childhood autism, 665 (30.1%) with Asperger syndrome, and 869 (39.4%) with PDD-NOS. Three hundred forty-one patients (15.4%) had a comorbid diagnosis of ID. Tests of heterogeneity between categories indicated significant (p<.05) differences between childhood autism and Asperger syndrome for shorter IPI (<12 months and 12–23 months) whereby the increased risk of ASD appeared to be accounted for by the childhood autism diagnosis. The estimates for PDD-NOS varied significantly from those for childhood autism for IPI of 12–23 months only. When stratified by ASD subtype, the associations between IPI>60 months and increased risk of ASD were statistically significant for Asperger syndrome only. However, the differences between estimates for longer IPIs were not significant, nor was there evidence for significant heterogeneity of association for patients with versus without ID.
Table 3.
Odds Ratios and 95% Confidence Intervals, Stratified by Intellectual Disability and Autism Spectrum Disorder (ASD) Subtype, and P-Values From Test of Heterogeneity
| IPI (months) | Childhood autism | Asperger syndrome | pheta | PDD-NOS | phetb | Patient has ID | Patient does not have ID | phetc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| <12 | 1.89 | (1.42, 2.50) | 1.13 | (0.84, 1.50) | .01 | 1.55 | (1.22, 1.97) | .30 | 1.91 | (1.29, 2.84) | 1.47 | (1.25, 1.73) | .23 |
| 12–23 | 1.51 | (1.18, 1.94) | 0.96 | (0.73, 1.24) | .01 | 0.99 | (0.79, 1.23) | .01 | 1.40 | (0.98, 1.99) | 1.13 | (0.97, 1.31) | .27 |
| 24–59 | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | |||
| 60–119 | 1.11 | (0.79, 1.54) | 1.55 | (1.15, 2.08) | .14 | 1.14 | (0.87, 1.49) | .89 | 1.20 | (0.77, 1.88) | 1.28 | (1.07, 1.53) | .80 |
| >=120 | 1.08 | (0.65, 1.80) | 1.71 | (1.13, 2.60) | .17 | 1.38 | (0.90, 2.09) | .48 | 1.50 | (0.79, 2.87) | 1.39 | (1.06, 1.82) | .83 |
Note: ID = intellectual disability; IPI = interpregnancy interval; PDD-NOS = pervasive developmental disorder, not otherwise specified; Ref = reference category.
P-value for heterogeneity between estimates for Asperger syndrome and childhood autism.
P-value for heterogeneity between estimates for PDD-NOS and childhood autism.
P-value for heterogeneity between estimates for patients with versus without intellectual disability.
Results from the patient-sibling control analysis of interbirth intervals are presented in Table S3 (available online). The second-born sibling was more likely to be affected than the first when separated by long birth intervals of 69–128 months and ≥129 months. These differences were significant (p<.05) and persisted after adjustment for covariates. For short interbirth intervals of less than 21 months, the odds of ASD in the second-born sibling were increased (OR=1.23), though the difference was not statistically significant.
Discussion
This population-based study, nested within a cohort of all births in Finland, provides evidence that IPI of <24 or >60 months is associated with an increased risk of ASD among second- and later-born children. The highest risk of ASD was found among children with IPI <12 months or >=120 months. Here, we confirm prior findings that short IPI is associated with increased risk of ASD9–11 and additionally report that long IPI is associated with increased ASD risk. We observed limited evidence for heterogeneity of association between ASD subtypes, whereby the association of shorter IPI (<24 months) with ASD appears to be accounted for more strongly by childhood autism and PDD-NOS than by Asperger syndrome.
While the mechanisms explaining these associations may differ for short and long IPI, one potentially unifying factor is unintended (unwanted or mistimed) pregnancy. Unintended pregnancy may be more common among both short6 and long IPI pregnancies, although there is less information available about long IPI pregnancies in general. Women with an unintended pregnancy are more likely to smoke, to use alcohol, medications, or illicit drugs, to delay prenatal care, and are less likely to take folic acid or prenatal vitamin supplements,7,8,19,20 both before and after the recognition of pregnancy.19 Periconceptional folic acid supplementation has been associated with decreased ASD risk,21,22 and prenatal exposure to certain medications including valproate23 and antidepressants,24,25 with increased risk. Moreover, ASD has been associated with a range of prenatal and obstetric complications,26–28 which may be exacerbated by late or inadequate prenatal care.
Another possible factor linking short and long IPI is inflammation. Short IPI may result in unresolved inflammation from the prior pregnancy,3 while longer IPI may lead to increased inflammation through its impact on BMI. Parenthood has been associated with an increased risk of obesity 29 and in a Swedish study of interpregnancy weight change, longer IPI was associated with a greater increase in BMI between pregnancies.30 Obesity and increased BMI are consistently associated with higher levels of C-reactive protein, a marker of low-grade systemic inflammation.31 Both maternal obesity32 and higher levels of maternal C-reactive protein during pregnancy33 have previously been associated with increased risk of ASD or autism.
Upon stratifying by ASD subtypes, the association of shorter IPI with ASD appeared to be accounted for by childhood autism and PDD-NOS, but not Asperger syndrome. Because ID is much more prevalent among patients with childhood autism and PDD-NOS (29.4% and 14.9% in our sample) relative to Asperger syndrome (1.6%), one explanation could be that the association of ASD with short IPI occurs specifically among the subset of patients with ID. This does not appear to be the case, however, given that the magnitude of association between IPI and ASD did not differ between those with and without ID. In Norway, the association between short IPI and increased risk of autistic disorder (analogous to our category of “childhood autism”) was not observed for PDD-NOS or Asperger syndrome,10 in partial concordance with our findings. Patients with childhood autism comprised a similar percentage of all patients with ASD in our study (30.5%) as in the Norway study (31.3%), suggesting reasonable similarity between the populations in terms of the distribution of ASD subtypes. Although it is now widely believed that ASD is better conceptualized as a single spectrum than as distinct disorders,34 considerable heterogeneity remains within the diagnosis. The association of shorter IPI with lower-functioning categories of ASD suggests the possibility of an impact on functional domains that may overlap between disorders rather than being specific to ASD. Notably, short IPI has also been associated with increased risk of schizophrenia,35 and some studies suggest limited diagnostic overlap between the disorders.36,37
The use of national registry data in this study provided a large sample size, essentially encompassing an entire country for over a decade of births. A limitation of this method is that it does not allow for individual diagnostic confirmation. The specificity of the registry-based childhood autism diagnosis used in this study was previously found to be 96% 13. However, sensitivity of each of the ASD diagnoses may be imperfect, so differential ascertainment of ASD by IPI cannot be ruled out, for example if the spacing of children affects parental help-seeking for more mildly affected individuals. We suggest that as evidence for similar IPI-ASD associations accrues across different diagnostic contexts (i.e. universally-available, minimal-cost health care versus private insurance systems with wide inequality of access), this explanation would appear less likely; however, we cannot directly test it in our study.
A second strength of this study was the use of registry data linked by personal identification numbers to accurately match siblings. This should have resulted in low misclassification with respect to IPI. While misclassification of IPI due to mis-estimation of gestational age may still have particularly impacted shorter IPI pregnancies, this is expected to be non-differential with respect to outcome and may have biased estimates of association for shorter IPI pregnancies toward the null. Furthermore, the accurate identification of siblings, and the availability of data on psychiatric diagnoses for participants’ family members allowed us to examine the association between IPI and ASD excluding any participants where a prior sibling had an ASD diagnosis. The similarity of these results to those obtained using the full sample suggests that the association we observed was not due to altered reproductive behavior (changes in the numbers or spacing of pregnancies) as a result of having a previously born child with an ASD.
As with any observational study, we cannot rule out the possibility of bias due to uncontrolled confounding, if for example, parents with higher levels of autistic traits are also more likely to space their children outside of conventional population norms. We were able to partially address confounding by family-level factors, such as the overall genetic background of parents, through a patient-sibling control analysis. This analysis was limited by the use of interbirth, rather than interpregnancy, intervals and by the potential bias inherent in sibling comparison designs.38 Nonetheless, the consistency of a U-shaped pattern in the risk of ASD associated with IPI observed across analytic methods supports our primary finding.
In conclusion, this study adds to the growing evidence that risk for ASD is associated with short IPI and provides the first statistically confirmed indication that long IPI also may confer increased risk. Three large population-based studies have now reported that the risk of autism is increased among pregnancies following short IPI. While obstetric factors as a category have reliably been associated with ASD, the specific types of such factors vary between studies.26–28 Given this, the consistency of the IPI findings reported thus far suggests that IPI has the potential to provide unique insight into non-genetic risk factors for ASD. Additional research to elucidate the mechanisms responsible for the association will be key to this endeavor and may have the potential to identify means to reduce risk of ASD among some pregnancies.
Supplementary Material
Clinical Guidance.
Births following both short and long interpregnancy intervals have been associated with increased risk of autism spectrum disorders.
The mechanism(s) behind these observed associations are not yet known, but clinicians should be aware of the potential increased risk of ASD in patients born following short or long intervals.
Elucidation of the mechanisms responsible for the association may eventually yield targets for intervention.
Acknowledgments
This study was funded by the National Institutes of Health (NIEHS R01ES019004 [A.S.B.], NIMH K02 MH065422 [A.S.B.], and NIMH T32-13043 [K.C.P.]), the Turku University Foundation (E.J.), and the Finnish Epilepsy Society (E.J.).
Footnotes
Clinical guidance is available at the end of this article.
Supplemental material cited in this article is available online.
Dr. McKeague served as the statistical expert for this research.
Disclosure: Drs. Cheslack-Postava, Lehti, McKeague, Sourander, Brown, and Mss. Suominen and Jokiranta report no biomedical financial interests or potential conflicts of interest.
This article is discussed in an editorial by Dr. Armin Raznahan on page xx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Eijsden M, Smits LJ, van der Wal MF, Bonsel GJ. Association between short interpregnancy intervals and term birth weight: the role of folate depletion. Am J Clin Nutr. 2008;88:147–153. doi: 10.1093/ajcn/88.1.147. [DOI] [PubMed] [Google Scholar]
- 3.Shachar BZ, Lyell DJ. Interpregnancy interval and obstetrical complications. Obstet Gynecol Surv. 2012;67:584–596. doi: 10.1097/OGX.0b013e31826b2c3e. [DOI] [PubMed] [Google Scholar]
- 4.Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002;346:33–38. doi: 10.1056/NEJMoa011379. [DOI] [PubMed] [Google Scholar]
- 5.Zhu BP, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med. 1999;340:589–594. doi: 10.1056/NEJM199902253400801. [DOI] [PubMed] [Google Scholar]
- 6.Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstet Gynecol. 2013;122:64–71. doi: 10.1097/AOG.0b013e3182955e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng D, Schwarz EB, Douglas E, Horon I. Unintended pregnancy and associated maternal preconception, prenatal and postpartum behaviors. Contraception. 2009;79:194–198. doi: 10.1016/j.contraception.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 8.McCrory C, McNally S. The effect of pregnancy intention on maternal prenatal behaviours and parent and child health: results of an Irish cohort study. Paediatr Perinat Epidemiol. 2013;27:208–215. doi: 10.1111/ppe.12027. [DOI] [PubMed] [Google Scholar]
- 9.Cheslack-Postava K, Liu K, Bearman PS. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics. 2011;127:246–253. doi: 10.1542/peds.2010-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunnes N, Suren P, Bresnahan M, et al. Interpregnancy interval and risk of autistic disorder. Epidemiology. 2013;24:906–12. doi: 10.1097/01.ede.0000434435.52506.f5. [DOI] [PubMed] [Google Scholar]
- 11.Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. 2011;41:891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- 12.Lampi KM, Banerjee PN, Gissler M, et al. Finnish Prenatal Study of Autism and Autism Spectrum Disorders (FIPS-A): overview and design. J Autism Dev Disord. 2011;41:1090–1096. doi: 10.1007/s10803-010-1132-6. [DOI] [PubMed] [Google Scholar]
- 13.Lampi KM, Sourander A, Gissler M, et al. Brief report: validity of Finnish registry-based diagnoses of autism with the ADI-R. Acta Paediatr. 2010;99:1425–1428. doi: 10.1111/j.1651-2227.2010.01835.x. [DOI] [PubMed] [Google Scholar]
- 14.Jokiranta E, Brown AS, Heinimaa M, Cheslack-Postava K, Suominen A, Sourander A. Parental psychiatric disorders and autism spectrum disorders. Psychiatry Res. 2013;207:203–211. doi: 10.1016/j.psychres.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampi KM, Hinkka-Yli-Salomaki S, Lehti V, et al. Parental age and risk of autism spectrum disorders in a Finnish national birth cohort. J Autism Dev Disord. 2013;43:2526–35. doi: 10.1007/s10803-013-1801-3. [DOI] [PubMed] [Google Scholar]
- 16.Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheslack-Postava K, Jokiranta E, Suominen A, Lehti V, Sourander A, Brown AS. Variation by diagnostic subtype in risk for autism spectrum disorders associated with maternal parity among Finnish births. Paediatr Perinat Epidemiol. 2014;28:58–66. doi: 10.1111/ppe.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dott M, Rasmussen SA, Hogue CJ, Reefhuis J. Association between pregnancy intention and reproductive-health related behaviors before and after pregnancy recognition, National Birth Defects Prevention Study, 1997–2002. Matern Child Health J. 2010;14:373–381. doi: 10.1007/s10995-009-0458-1. [DOI] [PubMed] [Google Scholar]
- 20.Han JY, Nava-Ocampo AA, Koren G. Unintended pregnancies and exposure to potential human teratogens. Birth Defects Res A Clin Mol Teratol. 2005;73:245–248. doi: 10.1002/bdra.20132. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80–89. doi: 10.3945/ajcn.110.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suren P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570–577. doi: 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen J, Gronborg TK, Sorensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 25.Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ. 2013;346:f2059. doi: 10.1136/bmj.f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195:7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128:344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guinchat V, Thorsen P, Laurent C, Cans C, Bodeau N, Cohen D. Pre-, peri- and neonatal risk factors for autism. Acta Obstet Gynecol Scand. 2012;91:287–300. doi: 10.1111/j.1600-0412.2011.01325.x. [DOI] [PubMed] [Google Scholar]
- 29.Weng HH, Bastian LA, Taylor DH, Jr, Moser BK, Ostbye T. Number of children associated with obesity in middle-aged women and men: results from the health and retirement study. J Womens Health (Larchmt) 2004;13:85–91. doi: 10.1089/154099904322836492. [DOI] [PubMed] [Google Scholar]
- 30.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368:1164–1170. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- 31.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14:232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 32.Krakowiak P, Walker CK, Bremer AA, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–e1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown AS, Sourander A, Hinkka-Yli-Salomaki S, McKeague IW, Sundvall J, Surcel HM. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry. 2014;19:259–64. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witwer AN, Lecavalier L. Examining the validity of autism spectrum disorder subtypes. J Autism Dev Disord. 2008;38:1611–1624. doi: 10.1007/s10803-008-0541-2. [DOI] [PubMed] [Google Scholar]
- 35.Gunawardana L, Smith GD, Zammit S, et al. Pre-conception inter-pregnancy interval and risk of schizophrenia. Br J Psychiatry. 2011;199:338–339. doi: 10.1192/bjp.bp.111.092916. [DOI] [PubMed] [Google Scholar]
- 36.Konstantareas MM, Hewitt T. Autistic disorder and schizophrenia: diagnostic overlaps. J Autism Dev Disord. 2001;31:19–28. doi: 10.1023/a:1005605528309. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan S, Rai D, Golding J, Zammit S, Steer C. The association between autism spectrum disorder and psychotic experiences in the Avon longitudinal study of parents and children (ALSPAC) birth cohort. J Am Acad Child Adolesc Psychiatry. 2013;52:806–814. doi: 10.1016/j.jaac.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Frisell T, Oberg S, Kuja-Halkola R, Sjolander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.