Abstract
Passive immunotherapy with monoclonal antibodies represents a cornerstone of human anticancer therapies, but has not been established in veterinary medicine yet. As the tumor-associated antigen EGFR (ErbB-1) is highly conserved between humans and dogs, and considering the effectiveness of the anti-EGFR antibody cetuximab in human clinical oncology, we present here a “caninized” version of this antibody, can225IgG, for comparative oncology studies. Variable region genes of 225, the murine precursor of cetuximab, were fused with canine constant heavy gamma and kappa chain genes, respectively, and transfected into Chinese hamster ovary (CHO) DUKX-B11 cells. Of note, 480 clones were screened and the best clones were selected according to productivity and highest specificity in EGFR-coated ELISA. Upon purification with Protein G, the recombinant cetuximab-like canine IgG was tested for integrity, correct assembly, and functionality. Specific binding to the surface of EGFR-overexpressing cells was assessed by flow cytometry and immunofluorescence; moreover, binding to canine mammary tissue was demonstrated by immunohistochemistry. In cell viability and proliferation assays, incubation with can225IgG led to significant tumor cell growth inhibition. Moreover, this antibody mediated significant tumor cell killing via phagocytosis in vitro. We thus present here, for the first time, the generation of a canine IgG antibody and its hypothetical structure. On the basis of its cetuximab-like binding site, on the one hand, and the expression of a 91% homologous EGFR molecule in canine cancer, on the other hand, this antibody may be a promising research compound to establish passive immunotherapy in dog patients with cancer.
Introduction
Malignant diseases are major health problems in humans, as well as in the area of veterinary medicine. Although pets and owners share several environmental (1) as well as genetic risk factors (2) to develop cancer, comparative oncology approaches treating human and pet patients with similar drugs to gain more insight into biologic activity, pharmacokinetics, or therapeutic indices have only recently been initiated (3, 4).
Standard therapy regimens for veterinary patients with cancer comprise surgery, radiotherapy, and chemotherapy with drugs that have been long established in human medicine, for example, cyclophosphamide, 5-fluorouracil, and doxorubicin for treatment of canine mammary carcinoma (5). Modern targeted approaches, such as receptor tyrosine kinase inhibitors (6, 7) or cancer vaccines (8), have just been introduced into veterinarian medicine in recent years, but these have shown promising results so far.
Passive immunotherapy with monoclonal antibodies is a key element in therapy guidelines on human oncology. However, immunoglobulins, such as cetuximab (Erbitux; Merck) for the treatment of colorectal carcinoma overexpressing EGFR (epidermal growth factor receptor, ErbB-1; ref. 9) or trastuzumab (Herceptin; Genentech) for metastatic breast cancer overexpressing HER2 (human epidermal growth factor receptor-2, ErbB-2; ref. 10), have not yet been introduced in veterinary medicine at all.
Our group could previously demonstrate that both of these targets, EGFR and HER2, have close sequential and structural homologs in dogs. More specifically, even the relevant epitopes for targeting with cetuximab and trastuzumab are conserved and lead upon targeting to similar biologic events in vitro (11). The growth-inhibitory effect of EGFR and HER2 targeting is due to the silencing of important signaling pathways [PI3K, Ras–Raf (MAPK), JNK, and PLCγ] of growth factors [EGF; ref. 12; and transforming growth factor-α (TGFα); refs. 13–15]. Signaling via EGFR mediates characteristic features of malignancy, such as higher proliferation, but is also associated with higher genomic instability and hormone therapy resistance resulting in poorer overall prognosis in clinics (16).
Both cetuximab and trastuzumab attract immune effector cells to the site of the tumor and elicit tumor cell death via antibody-dependent cell-mediated phagocytosis (ADCP) or antibody-dependent cell-mediated cytotoxicity (ADCC; refs. 17–19). Growth signal inhibition, as well as immune cell-mediated tumor cell death, contribute to high efficacy of cetuximab and trastuzumab in clinical use and lead to clear benefits for patients with advanced colorectal carcinoma with wild-type KRAS status in case of cetuximab treatment (20, 21), as well as longer progression-free and overall survival in patients with metastatic breast cancer with trastuzumab treatment, respectively (22).
As most clinically applied monoclonal antibodies were originally generated in mice, their murine constant regions had to be replaced by human ones (“chimerization”) to avoid immunogenicity and rendering them fully functional. One step further, when only the murine complementarity-determining region (CDR) is grafted into the framework of a consensus human IgG, a “humanized” antibody, such as trastuzumab, results, which is even less immunogenic (23). In the case of cetuximab, chimerization of its mouse precursor antibody “225” (24) led to a 5-fold higher relative affinity toward EGFR as a positive “side effect” and higher biologic efficacy in a human tumor xenograft model (25).
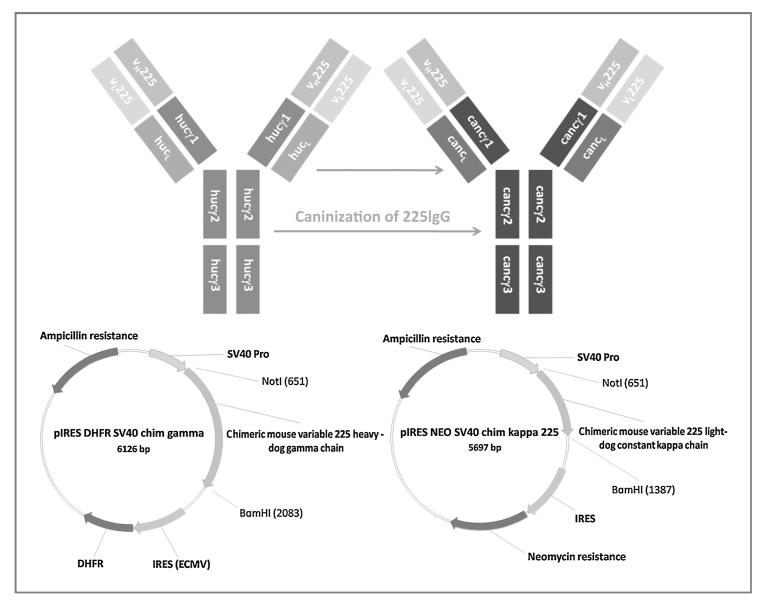
Consequently, the “caninization” of monoclonal antibodies must take place when approaching canine patients with cancer (see the schematic in Fig. 1). Antibodies against oncogenic proteins can act as either tumor promoting (e.g., via cross-linking growth factor receptors and thereby activating the receptors) or tumor inhibiting (e.g., via interference of binding of growth factors), depending on their epitope specificity (26, 27). For 225, it could be demonstrated that upon binding of the antibody to EGFR, EGF-mediated growth signals are inhibited, and the chimerized cetuximab proved to be highly efficacious in clinical trials and clinical use (28). Thus, it was of eminent importance for this study to use the specificity of this successfully applied antibody.
Figure 1.
Schematic overview of antibody generation. For generation of can225IgG, variable heavy chain gene regions of 225 were fused to canine gamma-immunoglobulin C constant regions genes and introduced into pIRES DHFR_SV40 using the restriction sites NotI and BamHI. Similarly, variable light chain gene regions of 225 were combined with dog kappa light chain constant regions and cloned into the vector pIRES_NEO SV40, using again NotI and BamHI as restriction sites. Subsequently, CHO DUKX-B11 cells were cotransfected with both plasmids for production of recombinant antibodies.
In addition to the growth-inhibitory action due to growth signal deprivation (11), a caninized cetuximab-like “225 IgG” antibody would lead to the attraction of immune effector cells toward the site of the malignancy. Several studies reported cytotoxic activity of macrophages against canine osteosarcoma (29), melanoma (30), or lymphoma (31), as well as cytotoxic activity of canine natural killer (NK) cells against leukemia blasts (32), two immune effector cell populations, which express Fcγ receptors. Moreover, monocytes, which are known to have tumoricidal properties in humans, could be recruited into tumors (33–35); similarly, neutrophilic granulocytes could also exert ADCC against malignant cells (36). Furthermore, it could be demonstrated that cross-presentation of tumor-associated antigens via Fc receptors on dendritic cells could lead to activation of tumor-reactive T cells and subsequent tumor regression (17).
In summary, we propose the generated canine anti-EGFR IgG to be a potentially highly effective immunotherapeutical tool for clinical veterinary oncology. This antibody could serve as a candidate-research molecule to establish passive immunotherapy for canine patients with cancer as well as a useful tool for proof-of-concept studies in comparative oncology settings. Pharmacokinetic, pharmacodynamic, and pharmacovigilance data of clinical trials in dog patients with cancer will render in return important information for a large number of researchers working on the ErbB-1/EGFR for human anticancer therapy.
Materials and Methods
Cells, monoclonal antibodies, and recombinant proteins
The Chinese hamster (Cricetulus griseus) ovary cell line, Chinese hamster ovary (CHO) DUKX-B11, was purchased from the American Type Culture Collection (ATCC; Cat. No. CRL-9096) and cultivated in Pro CHO 5 Medium (Lonza Group AG) supplemented with Phenol Red (15 mg/L), 0.5 mg/mL Geneticin (G418), 4 mmol/L l-glutamine, and methotrexate (0.038 μmol/L). CHO DUKX-B11 cells were cultivated under serum-free conditions to ensure that they grow in suspension and that an easier purification of IgG upon large-scale production is allowed.
Canine (Canis lupus familiaris) mammary carcinoma cell lines P114 and Sh1b were a kind gift of Dr. Gerard Rutteman (Department of Clinic Science and Companion Animals, University of Utrecht, Utrecht, the Netherlands). Both cell lines were maintained in DMEM/F12 supplemented with 10% fetal calf serum (FCS), 2 mmol/L l-glutamine, and 10 μg/mL gentamicin sulfate.
Canine (Canis lupus familiaris) cell lines CF33 and CF41, derived from carcinoma of the mammary gland, were obtained from ATCC (Cat. No. CRL-6227 for CF33 and CRL-6232 for CF41) and cultivated in DMEM supplemented with 10% FCS, 2 mmol/L l-glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL).
The canine cell line TLM1 was used as an EGFR-negative model cell. This oral malignant melanoma cell line (37) was kindly provided by Jaime F. Modiano (Masonic Cancer Center, University of Minnesota, Minneapolis, MN) and kept in DMEM, 10% FCS, 2 mmol/L l-glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL).
The human (Homo sapiens) EGFR-overexpressing cell line A431, derived from an epidermoid carcinoma, and HT29, colorectal adenocarcinoma cells, were purchased from ATCC (Cat. No. CRL-1555 for A431 and HTB-38 for HT29). A431 cells were allowed to grow in DMEM supplemented with 10% FCS, penicillin (100 U/mL), and streptomycin (100 μg/mL). HT29 cells were maintained in RPMI-1640 medium, augmented with 10% FCS, 2 mmol/L l-glutamine, and 10 μg/mL gentamicin sulfate. The human mammary carcinoma cell line BT474 was a kind gift of Prof. Dr. Thomas Grunt (Institute for Cancer Research, Medical University of Vienna, Vienna, Austria) and was kept in α-MEM (Minimum Essential Medium) supplemented with 10% FCS, 2 mmol/L l-glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL). All cells were kept at 37°C in a humidified atmosphere of 5% CO2.
The human cell lines BT474 and A431 were authenticated by single-nucleotide polymorphism (SNP) profiling (Multiplexion GmbH) and HT29 by short tandem repeat (STR) profiling (DDC Medical); non-human cell lines were not authenticated.
All cell lines were grown initially, and multiple aliquots were cryopreserved for long-term storage in liquid nitrogen. After resuscitation, cells were passaged no more than 10 times before a new aliquot was thawed for use. For flow cytometric and cell proliferation/viability assays, cells were used between passages 5 and 10.
Blood samples from dogs, in the course of diagnostic work-ups or routine check-ups at the oncology unit at the Small Animal Hospital, University of Veterinary Medicine Vienna (Vienna, Austria), were used for peripheral blood mononuclear cell (PBMC) purification. The study was discussed and approved by the Institutional Ethics Committee in accordance with Good Scientific Practice (GSP) guidelines and national legislation.
Cetuximab (Erbitux), a chimeric IgG1 anti-EGFR monoclonal antibody, was acquired from Merck KGaA, and trastuzumab (Herceptin), a humanized IgG1 monoclonal anti-HER2 antibody, was obtained from Roche. Rituximab (MabThera), a chimeric IgG1 anti-CD20 monoclonal antibody, served as an isotype control (Roche). Dog IgG (mixed breed, affinity purified from serum, produced by Innovative Research, and distributed by Dunn Labortechnik GmbH) was used as a canine antibody isotype control (canine IgG standard).
Recombinant human extracellular domain (ECD)-EGFR was purchased from ACROBiosystems. Recombinant human ECD-HER2 was purified via its His-tag from supernatants of transfected Lec-1 cells, which were a kind gift of Prof. Daniel Leahy (Johns Hopkins University School of Medicine, Baltimore, MD). Bovine serum albumin (BSA) served as an additional negative control protein and was obtained from Sigma-Aldrich.
Cloning of canine Fc regions from PBMCs, cetuximab-variable regions from 225, and vector design
Using real-time PCR, four canine IgG heavy chain genes (IgG A, B, C, and D) were obtained from normal canine PBMC cDNA. Of these subclasses, IgGC was chosen for all consecutive cloning approaches.
Oligonucleotide primers for obtaining the IgGC constant region sequence were gatcctcgagcgcctccaccacggccc (forward sequence containing an XhoI site) and gatcggcccagccggcctcaatggtggtgatggtgtttacccggagaatgggag (reverse sequence providing an SfiI site). In addition, primers gatcggcgcgccagccgtctatttgttccaaccatct (forward sequence with an SgsI site included) and gatctctagagcctgttagtccactctctgacact (reverse sequence with an XbaI site) were used for canine Ig kappa.
The cetuximab heavy chain variable region was amplified from cDNA of its murine precursor hybridoma cell line 225 (ATCC no. HB-8508), using the primers gatcatttaaatgtgtccagtgtcaggtgcagctgaagcagtcag and gatcctcgagccgacagtgaccagagtcccttg and incorporating an SwaI and an XhoI site. The variable light chain region was amplified using the primers gatccctgcagggtgccagatgtgacatcttgctgactcagtctc and gatcggcgcgcctttcagctccagcttggtccc, providing an SbfI and an SgsI site.
Final sequences were optimized for expression in Cricetulus griseus and synthesized by GeneArt (Gene Art AG). Completely fused gamma heavy chain product (1.4 kbp) was introduced into the vector pIRES_dhfr_SV40, applying NotI and BamHI restriction sites (Fig. 1, bottom right). Similarly, fused kappa light chain gene product (0.7 kbp) was cloned into the vector pIRES_NEO_SV40, again using NotI and BamHI as restriction sites (Fig. 1, bottom left).
The newly generated constructs were transformed into Escherichia coli DH5α cells, and DNA sequences of the inserts were verified by Sanger sequencing (Microsynth, The Swiss DNA Company). Subsequently, large-scale vector DNA was produced and purified using the PureLink HiPure Plasmid Midiprep Kit (Invitrogen, Life Technologies) according to the manufacturer’s instructions.
Model generation
The model of can225IgG antibody was based on the crystal structure of an intact mouse IgG1 monoclonal antibody (38) with the PDB ID: 1IGY (resolution: 3.2 A). Modeling was carried out with MODELLER (version 9v8; ref. 39) using the automodel protocol. Fifty models were generated. Model quality was assessed using the DOPE score (40) and ProCheck (41). The model with the best DOPE score was selected for visualization and analysis.
Conservation mapping
Sequences of human anti-EGFR IgG [assembled of the cetuximab variable regions from PDB ID: 1YY8 (RCSB PDB-database, The Research Collaboratory for Structural Bioinformatics), the constant heavy chain region P01857 (Uniprot database), and the constant kappa light chain region P01834 (Uniprot database)] were aligned to those of can225IgG antibody using Muscle 3.7 (42) and analyzed using Clustal X (43). The sequence conservation scores, as defined by Clustal, were then mapped onto the model of the can225IgG antibody.
Production of recombinant antibodies
Purified plasmids were transfected into CHO DUKX-B11 cells using polyethylenimine (PEI; 25-kD linear, Poly-sciences Inc.) as transfection agent and seeded onto 96-well cell culture plates to generate 480 different clones in total. Clones of interest were selected by G418 and increasing concentrations of methotrexate (methotrexate hydrate; Sigma-Aldrich) and screened by ELISA for antibody production yields as well as for specificity against EGFR.
ELISA
For productivity screening of the clones, ELISA plates (Immuno MaxiSorp; Nunc) were coated overnight at 4°C with 1 μg/mL rabbit anti-dog IgG (Fc; Acris Antibodies GmbH) or 0.5 μg/mL goat anti-dog light chain antibodies (Bethyl Laboratories, Inc.) in carbonate buffer, respectively. After a blocking step, cell culture supernatants of clones of interest were diluted at 1:5 in phosphate-buffered saline (PBS), 0.05% Tween20 (PBST) + 1% BSA (Sigma-Aldrich). Following application, the plates were incubated for 1 hour at room temperature. Rabbit anti-dog IgG horseradish peroxidase (HRP; Jackson ImmunoResearch Europe Ltd.) was applied at a 1:5,000 dilution in PBST + 1% BSA, again for 1 hour at room temperature. Between all incubation steps, plates were washed three times with 200 μL PBST. For detection, 3,3′,5,5′-tetramethylbenzidine (TMB; BD Biosciences) was added, and after stopping the reaction with 1.8 mol/L H2SO4, the optical density (OD) absorbencies of the wells were measured at 450 nm (with a reference at 620 nm) with a multiwell plate reader (Infinite M200 PRO; Tecan Group AG).
To test the secreted antibodies for their selectivity toward EGFR, cell culture supernatants of clones displaying the highest yields of total IgG were examined in EFGR-specific ELISA as well. Immunoassay plates (Immuno MaxiSorp; Nunc) were coated overnight at 4°C with 0.5 μg/mL recombinant human EGFR (Acro Biosystems) in carbonate buffer. To prevent unspecific binding, blocking was carried out for 2 hours at room temperature with Tris-buffered saline (TBS), 0.05% Tween20 (TBST) + 1% dried milk powder (DMP). Cell culture supernatants were diluted at 1:5 in TBST + 0.1% DMP. Control antibodies cetuximab (positive) and trastuzumab (serving as negative control) were applied in four serial dilution steps (1:2), starting with 0.5 μg/mL, again in TBST + 0.1% DMP. Plates were incubated for 2 hours at room temperature. Rabbit anti-dog IgG HRP (1:5,000) or anti-human IgG HRP, at a 1:8,000 dilution, were applied to the respective wells in TBST + 0.1% DMP, following an incubation period of 1 hour at room temperature. Between each step, plates were washed three times with 200 μL TBST. HRP signal was again detected with TMB substrate.
PAGE and immunoblotting
For protein gel electrophoresis, self-cast 10% SDS–acrylamide gels were used.
Semi-dry blotting was performed according to the standard blotting protocols, using nitrocellulose membranes. Blocking was carried out by incubation in TBS, 0.1% Tween20 (TBST; 50 mmol/L, 150 mmol/L NaCl; pH 7.5) + 5% DMP for 2 hours at room temperature. For detection of the canine heavy gamma Fc chain, HRP-labeled rabbit anti-dog IgG (Fc) antibodies (Jackson ImmunoResearch Europe Ltd.) were used at a 1:5,000 dilution. After washing three times with TBST for 10 minutes, binding of the HRP-conjugated antibodies was detected by incubation with ECL BD OptEIA substrate (BD Biosciences), using a VersaDoc Imaging System (Bio-Rad Laboratories, Inc.).
For detection of the canine kappa light chain, goat anti-dog light chain (Bethyl Laboratories, Inc.) and rabbit anti-goat IgG alkaline phosphatase (AP; Sigma-Aldrich) antibodies were consecutively used at a 1:2,000 dilution. Binding of AP-labeled antibodies was detected using nitro blue tetrazolium chloride/5-bromo-4chloro-3-indosyl phosphate, toluidine salt solution (NBT/BCIP; Roche Diagnostics GmbH).
Purification of recombinant proteins
The can225IgG cell culture supernatant of the chosen clone 3A3 (subclone 3A3/14E1, respectively) was dialyzed before purification in 20 mmol/L sodium phosphate buffer (pH 7.0) overnight at 4°C under gentle stirring. Dialysis buffer was exchanged at least twice. For purification of the dialyzed supernatant, the Fast Protein Liquid Chromatography system (FPLC; ÄKTA; GE Healthcare Europe GmbH) was applied, using either a 1 mL HiTrap Protein A HP column (GE Healthcare), a 1 mL recombinant Protein A column (UNOsphere SUPrA; Bio-Rad Laboratories, Inc.), or a 5 mL HiTrap Protein A HP column (GE Healthcare). Furthermore, several smallscale purification attempts using Protein G Sepharose 4 Fast Flow beads (GE Healthcare) were performed before FPLC purification with HiTrap Protein G HP 5 mL column (GE Healthcare) was established, with a flow rate of 5 mL/min. To elute bound can225IgG, 0.1 mol/L Glycine–HCl buffer (pH 2.7) was applied. Upon elution, samples were immediately neutralized with 1 mol/L Tris–HCl (pH 9.0).
Circular dichroism spectroscopy
Circular dichroism (CD) spectroscopic analyses were performed as described previously (44, 45). In short, dog antibody samples (c = 100 μg/mL ddH2O) were assessed at a J-715 spectropolarimeter (Jasco), using a 1-mm path-length quartz cuvette (Hellma) equilibrated at 20°C. Scan speed for spectra measurement was 50 nm/min at a 0.2-nm resolution. The average of five scans was taken as result after subtraction of the baseline spectrum and is shown as mean residue ellipticity (h) at the respective wavelengths.
Flow cytometry
To assess specific binding of the generated can225IgG to natural canine EGFR expressed on the surface of canine mammary carcinoma cell lines, flow cytometric analyses were performed. Cells (300,000/tube) were washed three times with FACS buffer (PBS + 2% normal goat serum). Afterward, cells were incubated for 30 minutes at 4°C in the dark with 200 μL of 10 μg/mL can225IgG as primary antibody and 200 μL of 10 μg/mL canine IgG standard as isotype control, in FACS staining buffer (PBS +2% normal goat serum +1% normal rabbit serum). Upon washing with FACS buffer, cells were incubated with 200 μL of 10 μg/mL rabbit anti-dog IgG FITC antibodies (Jackson ImmunoResearch Europe Ltd.) for 30 minutes at 4°C. After another washing step, cells were analyzed using the dual-laser FACSCalibur (BD Biosciences).
To evaluate binding properties of can225IgG to dog monocytes, PBMCs were isolated from canine patients with carcinoma using Ficoll-Paque separation (GE Healthcare Europe GmbH). PBMCs were incubated with 200 μL of 10 μg/mL can225IgG as primary antibody and 200 μL of 10 μg/mL canine IgG standard as positive control, respectively. Subsequently, cells were incubated with 200 μL of 10 μg/mL anti-dog IgG FITC and mouse anti-human CD14 phycoerythrin (PE; clone TÜK4; Life Technologies), which is cross-reactive to dog CD14 to identify monocytic cells. Analysis was again carried out using a dual-laser FACSCalibur (BD Biosciences). Histograms of FACS data were plotted with the FlowJo 10.0.6 software (TreeStar, Inc.).
Viability assays
To assess the tumor-inhibitory properties of the recombinant can225IgG, tetrazolium-based cell viability assays were performed according to the manufacturer’s instructions (EZ4U The 4th Generation Non Radioactive Cell Proliferation & Cytotoxicity Assay Kit; Biomedica). A431 cells were seeded at a density of 3 × 104 per well in a 96-well plate (round-bottomed). After overnight incubation for proper adhesion to the plate, cells were treated with either 5 μg/mL can225IgG, 5 μg/mL cetuximab, 5 μg/mL dog IgG standard, or 5 μg/mL rituximab for 48 hours. Subsequently, tetrazolium substrate was added, and after another 3 hours of incubation, OD was measured at 450 nm with 620 nm as the reference wavelength.
Proliferation assays
To directly measure DNA replication, BrdU Proliferation assays (Roche) were performed. BrdU (bromodeoxyuridine) is a nonradioactive compound, which is incorporated into the genomic DNA of proliferating cells during DNA synthesis. EGFR-expressing canine cell lines P114 and Sh1b, as well as the negative control cell line TLM1, were seeded onto 96-well cell culture plates at a density of 3 × 103 cells per well. After 18 hours of growing in full medium, cells were incubated with 5 μg/mL concentrations of can225IgG or cetuximab for 24 hours before labeling with BrdU. After a labeling period of 4 hours, incorporated BrdU was detected with an HRP-conjugated anti-BrdU antibody. Bound antibodies were detected with TMB, and color reaction was measured at 450 nm in an ELISA reader, with 630 nm as a reference value (Infinite M200 PRO; Tecan Group AG).
ADCC/ADCP assay
To determine the levels of immune cell-mediated tumor cell killing via ADCC and ADCP, a three-color flow cytometric assay (46) was adapted to the canine system. EGFR-expressing canine P114 cells were stained with CFDA [Spiro(isobenzofuran-1(3H),9′-(9H)xanthene)-5-carboxylic acid, 3′,6′-bis(acetyloxy)-oxo-, (acetyloxy) methyl ester; Invitrogen, Life Technologies] and served as target cells. PBMCs, purified from canine patients with cancer, served as effector cells. Effector (E) and target (T) cells were coincubated at a ratio of 3:1 (300,000 E:100,000 T; total volume = 400 μL) in the presence or absence of can225IgG (c = 2.5 μg/mL). After 2.5 hours of incubation, the killing assay was stopped by adding ice-cold FACS buffer. Effector cells were labeled with anti-CD14 PE (clone TÜK4; Life Technologies). After another 30 minutes of incubation at 4°C and subsequent washing, dead cells were labeled with 7-amino-actinomycin D (7-AAD; eBioscience) for 15 minutes at 4°C. After a final washing step, cells were resuspended in 200 μL FACS buffer and analyzed on a dual-laser FACSCalibur (BD Biosciences).
Data handling and statistical analysis
Flow cytometric experiments of receptor binding and competitive binding were repeated at least three times, and histograms display one representative example. In cell viability assays, each treatment group consisted of eight samples, statistical analyses of assays were performed by means of a two-sided t test, and significance was accepted at *, P < 0.05; **, P < 0.01; and ***, P < 0.001. In proliferation assays, each treatment group consisted of six samples, and statistical analyses were performed analogously. In ADCC/ADCP assays, 3 canine patients with cancer were investigated, and three samples were measured from each dog. Statistical analyses were performed again by using the two-sided t test. All statistic calculations were performed using the GraphPad Prism 4 software (GraphPad).
Results
Construction and modeling of can225IgG
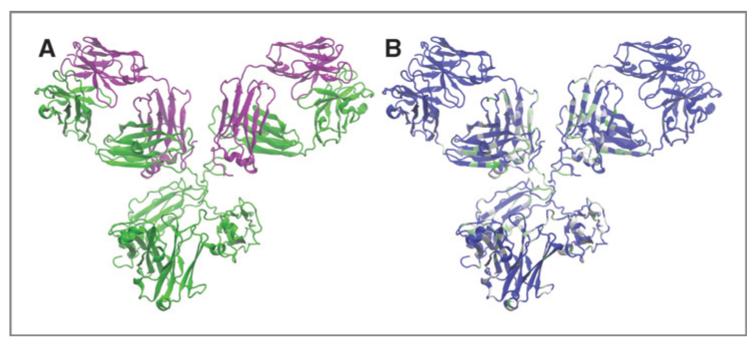
Figure 2 shows the topography of the constructed can225IgG antibody in comparison with human cetuximab IgG. Thus, the variable region and EGFR-binding site was grafted on the canine IgG backbone (Supplementary Table S1). Sequence comparisons suggested an approximately 60% homology across heavy and light chains. On the basis of this information, we approached in silico the hypothetical structure of the canine cetuximab counterpart. The information was collected by assembling cetuximab variable regions from PDB (ID: 1YY8) with amino acid translations of the constant canine heavy chain region C gene AAL35303.1/AF354266.1, and the canine constant kappa light chain region gene, XP_532962.3 (Fig. 2A). Upon closer investigation of conserved structures between human and canine immunoglobulin sequences, “conservation mapping” could be performed on the modeled antibody. As can be seenin Fig. 2B, regions with identical amino acids are depicted in blue, whereas amino acid changes are indicated in various shades of green, depending on the grade of discrepancy of amino acids. As the variable regions of both heavy and light chains were taken from the original cetuximab sequence, no differences can be observed in this part of the molecule. Regions with highest amino acid variability include the hinge region and parts of the constant regions that do not bind to Fcγ receptors.
Figure 2.
Molecular modeling of newly generated can225IgG antibody. A, model of canine antibody, based on human crystallographic structures; heavy chain shown in green, light chain in pink. B, conservation map of can225IgG antibodies; identical amino acids of human and canine molecules are shown in blue, and varying amino acids are depicted in shades from light gray to green, based on the degree of variation (gray, similar; dark green, highly different).
Productivity and specificity screening of transfected clones in ELISA
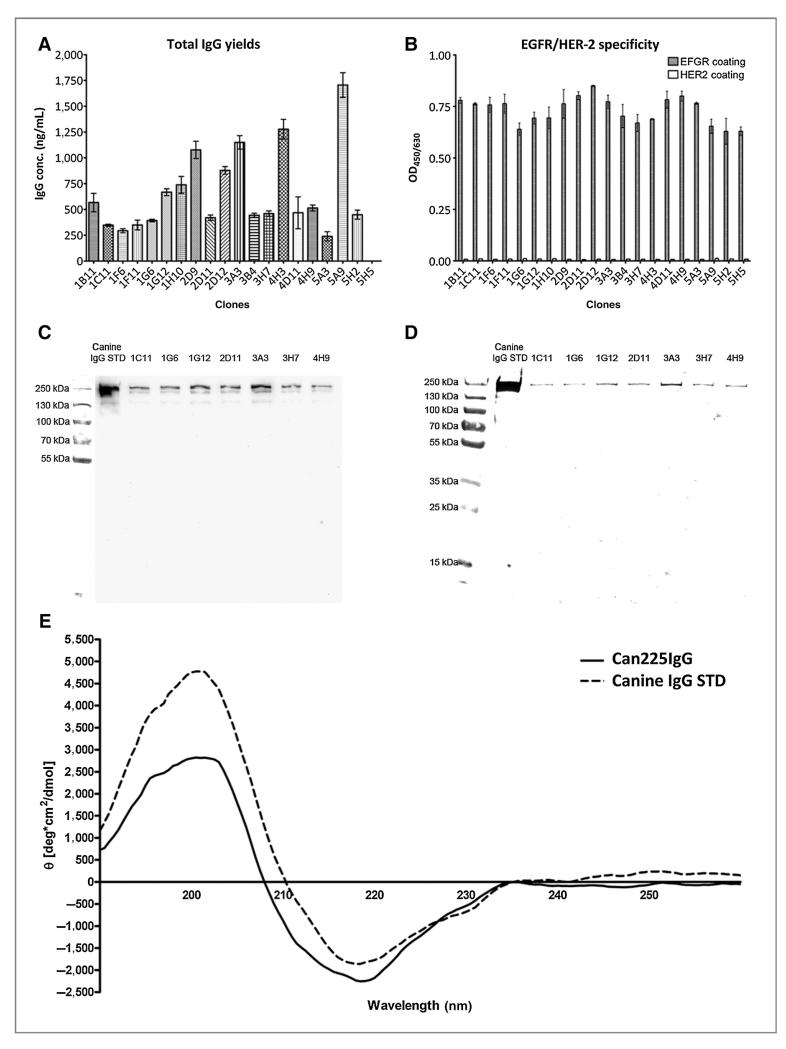
Plasmids for heavy and light chains of can225IgG were constructed on the basis of the above information and transfected into CHO DUKX-B11 cells. Clones with highest production yields (Fig. 3A) were screened for specificity in ELISA coated with the ECD of human recombinant EGFR, and recombinant human HER2 for control purposes. Original cetuximab antibody was used as a positive control. All tested clones produced IgG highly specific to EGFR, whereas none of it bound to HER2, a closely related and highly homologous molecule that does not display the 225 epitope (Fig. 3B).
Figure 3.
Integrity testing of can225IgG. A, productivity testing of selected clones in ELISA. Antibody yields of 20 selected supernatants from transfected CHO-cell clones. Productivity ranged from 200 to almost 1,900 ng/mL. Four clones showed remarkably higher levels by producing more than 1,000 ng/mL of can225IgG. Finally, clone 3A3 was chosen for large-scale production. Displayed are mean values ± standard error of the mean (SEM; n = 4). B, specificity testing of selected clones toward EGFR in ELISA. Clones displaying high levels of IgG production were screened for EGFR specificity. All selected clones displayed high specificity selectively toward EGFR and only background signal toward the control protein HER2. Displayed are mean OD values ± SEM (n = 2). C, Western blot analysis of selected cell culture supernatants testing for presence of dog gamma heavy chain. Clones that underwent positive productivity and specificity screening in ELISA were also tested for integrity in Western blot analysis. All selected clones displayed a sharp band at approximately 250 kD, the same height as canine IgG standard, the purified control IgG from dog serum. D, Western blot analysis of selected cell culture supernatants testing for presence of dog kappa light chain. Clones that displayed canine gamma heavy chain in cell culture supernatants were tested for kappa light chain presence. All tested clones displayed a sharp band at approximately 250 kD, the same height as canine IgG standard. E, CD spectrometry of can225IgG in comparison with purified canine IgG standard protein. Spectra are represented as the mean residue ellipticity (θ; y-axis) at respective wave lengths (x-axis). Analysis of the far-UV CD spectrum of can225IgG showed a strong maximum at approximately 200 nm and a minimum at approximately 220 nm, comparable with purified canine IgG standard.
Integrity testing of secreted antibodies in cell culture supernatants
Seven highly productive and specific clones were selected for further analysis. The integrity of the generated antibodies was proven by Western blot analysis, detecting the canine heavy gamma and kappa light chain. The signals were observed at the correct molecular size in comparison with canine IgG standard (canine IgG STD), and no unassembled single heavy chains were detectable (Fig. 3C). In parallel, the detection of light chain displayed sharp bands at the same molecular size as that of purified standard canine IgG, and again no unassembled single light chains were traceable (Fig. 3D). Therefore, each selected clone produced intact, correctly assembled antibodies.
Purification of recombinant proteins
Having displayed high productivity, specific binding to recombinant EGFR, as well as intact and correct assembly of cetuximab-like can225IgG, clone 3A3 was subjected to amplification and supernatants to purification via Protein A affinity chromatography. As can be seen from Supplementary Fig. S1A, unexpectedly and despite published work (47–49), can225IgG did not bind to Protein A.
In contrast, substantial binding to Protein G Sepharose beads could be observed (Supplementary Fig. S1B), resulting in proper purification of the recombinant can225IgG antibodies from cell culture supernatant (Supplementary Fig. S2). Thus, FPLC in combination with Protein G columns was chosen as the method for large-scale purification.
Integrity testing of purified antibodies
As an additional step of quality control, we assessed the correct folding of the purified antibodies in CD spectroscopy. The secondary structures of can225IgG cannot be expected to be identical to the purified canine IgG STD, due to its different specificity and the presence of kappa and lambda light chains. Nonetheless, Fig. 3E still displays comparable traces of molecular ellipticity between the two molecules.
Flow cytometric analysis of can225IgG binding to EGFR-expressing cells
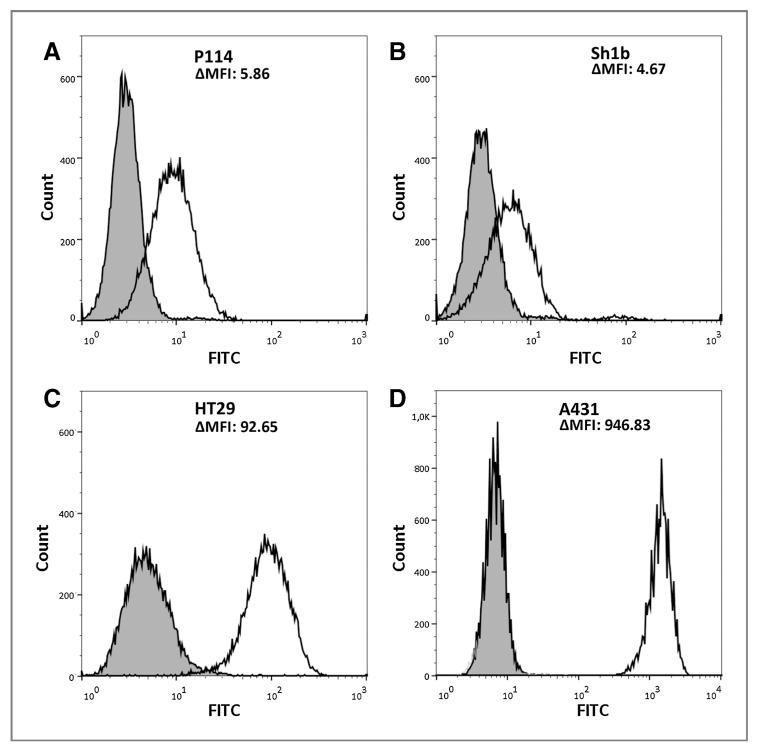
For specificity testing of the generated can225IgG toward natural EGFR on the surface of cells, flow cytometric analyses were performed. EGFR-overexpressing canine mammary carcinoma cells P114 and Sh1b, as well as the established human EGFR-overexpressing model cell lines A431 and HT29, were used (Fig. 4). The newly generated can225IgG specifically stained all four cell lines, depending on the amount of expressed proteins. P114 cells were stained with a shift in median fluorescence intensity (ΔMFI) of 5.86 (4.44 for isotype control to 10.30 for can225IgG). Sh1b cells showed a ΔMFI of 4.67, HT29 of 92.65, and A431 had a ΔMFI of 946.83. In the case of the CF33 and CF41 cells, which express low levels of EGFR (11), specific staining could again be observed (Supplementary Fig. S3A and S3B). The specificity of can225IgG was further affirmed by staining EGFR-negative canine melanoma cells TLM1, rendering only background signal (Supplementary Fig. S3C).
Figure 4.
Flow cytometric assessment of can225IgG binding to EGFR on cells. A, canine P114 cells could be specifically stained with can225IgG (black line), compared with isotype control staining (gray histogram); difference in mean fluorescence intensity (ΔMFI) = 5.86. B, canine Sh1b cells, ΔMFI = 4.67. C, human colorectal cancer cells HT29, ΔMFI = 92.65. D, human A431 epidermoid carcinoma cells, ΔMFI = 946.83.
Possible affinity differences of can225IgG binding toward canine and human EGFR were addressed by competitive flow cytometry on canine P114 and human BT474 cells (a human mammary carcinoma cell line that shows similar EGFR expression as the investigated canine mammary carcinoma cells; see Supplementary Fig. S3D). Supplementary Fig. S4A–S4C displays that A431 cells would not have been applicable for this assay, as their EGFR density is more than 100-fold.
The known affinity of cetuximab binding to human EGFR has a Kd value of 0.39 nmol/L (50). As can be seen from Supplementary Fig. S4D, can225IgG binding to canine cancer cells can be removed by molar excess of soluble human EGFR, whereas this is not possible when can225IgG binds to human cancer cells (Supplementary Fig. S4E). It can, thus, be concluded that due to the four amino acid changes in the 225 epitope (11), there is a difference in the affinity of the newly generated can225IgG toward human and canine EGFR; however, the affinity is still high enough to bind and exert function.
Microscopy
Specific binding of purified can225IgG antibodies was also confirmed by microscopy. Whereas neither canine (Supplementary Fig. S5A, top left) nor human isotype controls (Supplementary Fig. S5A, bottom left) showed any signal, both can225IgG (Supplementary Fig. S5A, top right) and the original antibody cetuximab (Supplementary Fig. S5A, bottom right) displayed strong, membrane-specific staining of A431 cells.
To further investigate the binding properties of can225IgG on malignant tissue, canine mammary carcinoma samples were tested for EGFR expression with the FDA-approved in vitro diagnostic EGFR pharmDx kit. Positive specimens, which show a strong and complete membrane-specific staining (Supplementary Fig. S5B, left), were also used for staining with purified dog IgG standard and can225IgG (Supplementary Methods). The control dog IgG standard did not stain any structures specifically (Supplementary Fig. S5B, middle), whereas incubation with can225IgG resulted in EGFR staining, comparable with the diagnostic kit, though less intense (Supplementary Fig. S5B, right).
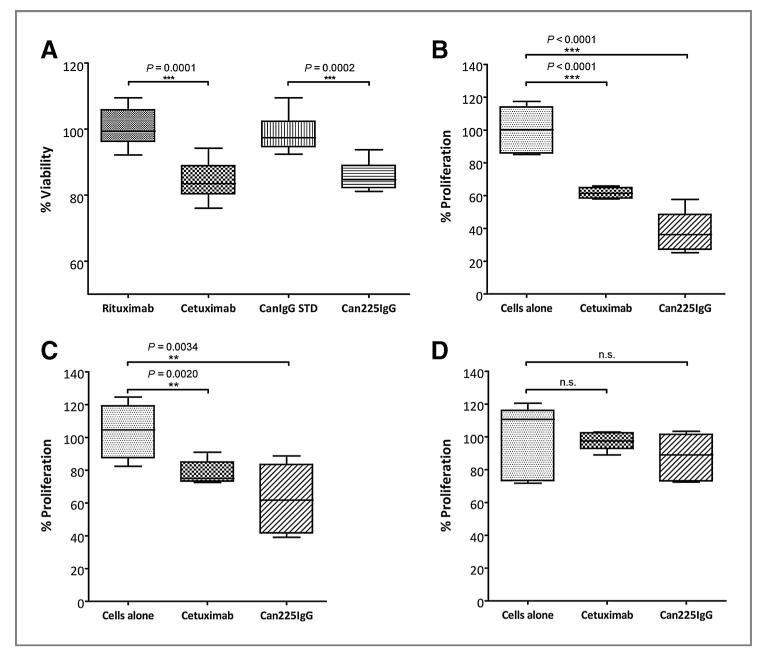
Cell viability and proliferation assays
For assessment of the tumor-inhibitory properties of the newly generated can225IgG antibody, cell viability assays with highly EGFR-overexpressing A431 cells were performed (Fig. 5A). After 48 hours of treatment with can225IgG, only 85.80% of viable cells could be detected (compared with untreated A431 cells), whereas incubation with purified dog IgG standard led to normal cell growth (98.79%). Thus, significant growth inhibition could be observed (P = 0.0002).
Figure 5.
Effects of can225IgG treatment on viability and proliferation of cancer cells. A, cell viability assay of human EGFR-overexpressing A431 cells. Isotype controls rituximab and canine IgG STD showed no growth inhibition on EGFR-overexpressing A431 cells in comparison with untreated cells (100%). In contrast, incubation with can225IgG led to significant growth inhibition (85.80%; P = 0.0002) similar to cetuximab treatment (84.44%; P = 0.0001). Box and whiskers plot, whiskers displaying minimum and maximum values (n = 8). B, cell proliferation assay of canine EGFR-overexpressing P114 cells. Newly generated can225IgG and cetuximab, which served as positive control, led to strong and significant growth inhibition after 24 hours of treatment. Box and whiskers plot, whiskers displaying minimum and maximum values (n = 6). C, cell proliferation assay of canine EGFR-overexpressing Sh1b cells. Newly generated can225IgG (P = 0.0034) and cetuximab (P = 0.002), which served as positive control, led to strong and significant growth inhibition after 24 hours of incubation, compared with untreated cells. Box and whiskers plot, whiskers displaying minimum and maximum values (n = 6). D, cell proliferation assay of canine EGFR-negative TLM1 cells. Neither treatment with can225IgG nor cetuximab led to significant changes in growth of canine EGFR-negative TLM1 melanoma cells. Box and whiskers plot, whiskers displaying minimum and maximum values (n = 6). n.s., not significant.
For control purposes, the same experiment was conducted with cetuximab and rituximab, respectively (in same concentrations, 5 μg/mL). Again rituximab, the unspecific isotype control, showed no growth-inhibitory effect (100.53%), whereas treatment with cetuximab led to the expected significant growth inhibition (84.44%; P = 0.0001).
In addition to the tetrazolium-based cell viability assays, BrdU incorporation experiments were carried out to measure the direct impact of the newly generated can225 IgG on the proliferation of canine cancer cells. Thus, P114 and Sh1b cells were incubated for 24 hours with can225IgG as well as cetuximab, which served as positive control. As can be seen in Fig. 5B and C, both canine cell lines could be significantly inhibited in their growth by cetuximab and can225IgG (P114: P < 0.0001 for both antibody-treated groups compared with untreated cells; Sh1b: P = 0.002 for cetuximab-treated and P = 0.0034 for can225IgG-treated cells compared with untreated ones). In contrast, treatment of EGFR-negative canine TLM1 cells did not lead to any significant change in proliferation (Fig. 5D).
Flow cytometric analysis of can225IgG binding to canine monocytes
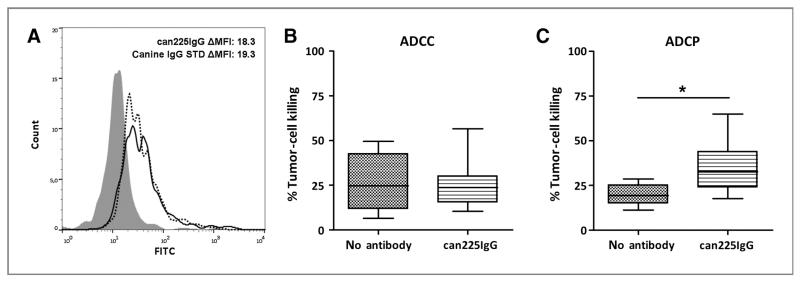
Functionality of the newly generated antibody with respect to binding to Fcγ receptors on dog immune cells was tested by flow cytometry. Thus, PBMCs of canine patients with cancer (n = 3) were purified and stained with can225IgG or dog IgG standard as positive control. Monocytes, important effector cells in tumor immunotherapy, were identified by staining with anti-CD14 PE and as shown in Fig. 6A, can225IgG binds to the same extent on monocytes as the purified canine IgG standard.
Figure 6.
Binding of can225IgG to canine monocytes and assessment of immune cell–mediated cancer cell death. A, evaluation of can225IgG binding to Fcγ receptors on monocytic cells purified from dog patients with cancer. Monocytes (gated as CD14 PE–positive) were stained with anti-dog IgG FITC (gray histogram). Can225IgG (dotted line) as well as canine IgG STD (black line, serving as positive control) are able to bind specifically; ΔMFI for can225IgG = 18.3; ΔMFI for canine IgG STD = 19.3 (n = 3, one representative example depicted). B, measurement of ADCC of P114 cells mediated by can225IgG. PBMCs were purified from dog patients with cancer (n = 3) and incubated with P114 cells for 2.5 hours in the presence or absence of can225IgG, which led to no significant change of cytotoxicity levels. C, measurement of ADCP of P114 cells mediated by can225IgG. PBMCs were purified from dog patients with cancer (n = 3) and incubated with P114 cells for 2.5 hours in the presence or absence of can225IgG, which led to a significant increase in phagocytosis of cancer cells (P = 0.0153).
ADCC/ADCP assays
As one of the major mechanisms of cetuximab is to confer immune-mediated tumor cell death, the newly generated can225IgG was also assessed in this regard. Therefore, a three-color flow cytometric method was applied, which is able to measure ADCC and ADCP simultaneously. Again, EGFR-overexpressing canine mammary carcinoma cells P114 were investigated. A total of 1 × 105 cells were coincubated with three times the amount of PBMCs, isolated from dog patients with cancer. After 2.5 hours of incubation in the presence of can225IgG, a significant difference in the level of ADCP could be observed (P = 0.0153; Fig. 6C). For ADCC (Fig. 6B), no significant difference could be displayed.
Discussion
As human and veterinary oncology face similar challenges, such as comparable incidence rates in certain tumor types (51), with studies even reporting higher rates in dogs for mammary cancer in the same geographic area (52), comparative approaches could be highly valuable for both human and veterinary oncology. Recent studies recognized the high similarities between human and canine genes as a result to the unraveling of the canine genome (53). Similar genetic risk factors were identified for humans and dogs contributing to breast cancer development, including BRCA-1 and BRCA-2 (2). In addition, clinical features of the disease, such as metastatic behavior (54) or dissemination into the bone marrow and circulation, have been closely investigated (55). Several tumor-associated antigens, particularly important for targeted therapies in human oncology, could also be identified in canine malignancies, such as CD20 (56), EGFR (57–59), HER2 (60), or VEGF (61). Our group revealed in a previous study that close homologs of EGFR (ErbB-1) and HER2 (ErbB-2) are overexpressed in canine mammary carcinoma lesions; and more importantly, that the relevant epitopes for the clinically applied monoclonal antibodies cetuximab and trastuzumab are highly conserved between the two species (11). Both antibodies are effective in human clinical use (20, 22), but they have as well a tumor-inhibitory potential on canine mammary carcinoma cells in vitro (11).
Thus, targeting of EGFR in veterinary clinical oncology could contribute to new insights into cancer biology, development of resistance mechanisms, or safety and efficacy of targeted therapies of the next generation. By testing new anti-EGFR agents head-to-head in human and canine patients, state-of-the-art therapies could be provided simultaneously for veterinary medicine. This goal is strongly fostered by the comparative oncology trials consortium, founded by the National Cancer Institute (NCI, Bethesda, Maryland) in 2003 (3).
Cetuximab (Erbitux), a monoclonal mouse–human chimeric IgG1 antibody directed against human EGFR (ErbB-1), could serve as a promising lead compound for comparative studies, because its activity is not limited to growth signal inhibition mediated via its Fab region, but it is also able to elicit immune cell–mediated tumor cell death via its Fc regions. 225, the murine precursor of cetuximab, was chimerized leading to higher relative affinity toward EGFR and higher biologic efficacy in in vivo studies with human tumor xenografts (25). Thus, we aimed to similarly generate a “caninized” 225 IgG antibody, termed can225IgG, by applying the same variable regions as 225, but fuse them with canine Fcγ regions (Fig. 1) to exploit the canine cellular effector mechanisms. Therefore, its variable regions were amplified from the cDNA of the hybridoma cell line 225 and reassessed with the published amino acid sequence of the cetuximab-Fab cocrystallized with human EGFR (62). As dogs display four different isotypes of γ-immunoglobulins (IgG A, B, C, and D; ref. 63), specific primers against each subclass were designed and gene sequences were obtained from canine PBMC cDNA. Gamma heavy chain protein of IgGC displayed 67.7% identity to the amino acid sequence of the human IgG1 heavy chain (RCSB pdb-database, No. 3RY6), an isotype long known for mediating cytotoxicity in humans (64) and representing the isotype of almost all FDA-approved monoclonal antibodies (65). Thus, IgGC was chosen for all subsequent cloning steps and for production of the chimeric can225IgG. To match the obtained gamma heavy chain C sequence with published ones, alignment against the published protein sequence AAL35303.1/AF354266.1 (63) was performed. This alignment displayed four amino acid mutations between the two sequences, which could be explained by genetic divergence due to breeding. As a complete analysis of the influence of race and breeding would have exceeded the focus of this study, we decided to use the published sequence from NCBI’s Protein database (National Center for Biotechnology Information, Bethesda, MD). Similarly, the amino acid sequence of the extracted kappa constant region was aligned to XP_532962.3, which resulted in complete accordance. Final sequences were optimized for production in Cricetulus griseus and transfected into CHO DUKX-B11 cells. Having established stable-transfected cell lines, clones were screened for productivity (Fig. 3A) and specificity (Fig. 3B).
Supernatants of clones that have undergone positive ELISA screening were again tested by Western blot analysis to determine the biochemical properties of secreted proteins. Figure 3C shows that all selected clones displayed a sharp band at the same molecular mass as control IgG standard, thus representing fully assembled canine immunoglobulins (Fig. 3D). As the positive control IgG comprises kappa and lambda light chains, and the detection antibody is directed against both, the signal intensity of the can225IgG band, comprising only kappa, cannot be compared par for par.
According to the literature, we expected medium-to-strong binding affinity of Protein A to canine IgG (47–49). Thus, purification of the recombinant can225IgG antibodies via Protein A affinity chromatography was our first method of choice, but can225IgG did not bind to recombinant Protein A (Supplementary Fig. S1A). This prompted us to purify via Protein G (66). Supplementary Fig. S1B demonstrates that can225IgG bound to Protein G and could be eluted via a pH shift with 0.1 mol/L glycine (pH 2.5) buffer. Again, SDS–PAGE affirmed the stability and purity of eluted proteins. Purified can225IgG showed the same sharp band as can225IgG in cell culture supernatants before purification, indicating proper refolding after neutralization in Tris–HCl buffer (pH 9.0; Supplementary Fig. S2). Furthermore, still no unassembled heavy or light chains could be detected in the gel.
To confirm the proper folding of can225IgG after the pH drop during purification, CD spectroscopy was performed (Fig. 3E), displaying again comparable results for molecular ellipticity between can225IgG and canine IgG standard.
For closer examination of tertiary structures of the newly generated can225IgG, molecular modeling was applied on the basis of crystallographic structures of human antibodies. Figure 2A displays predicted structures of can225IgG and the assembly of heavy and light chains based on these assumptions. To localize differences, “conservation mapping” was performed, displaying high variability between human and canine molecules, especially in the hinge region of the antibodies (Fig. 2B).
Next, the binding capacity of can225IgG to EGFR was tested by flow cytometric analyses with EGFR-overexpressing human as well as canine carcinoma cell lines. As illustrated in Fig. 4 and Supplementary Fig. S3, can225IgG is capable of detecting EGFR on the surface of cell lines (such as A431, HT29, P114, Sh1b, CF33, and CF41 cells) that have been previously reported to express this receptor; yet, no binding was seen on TLM1 canine melanoma cells (Supplementary Fig. S3C). Also, in immunofluorescence, can225IgG displayed the same membrane-specific staining pattern on A431 cells as that of cetuximab (Supplementary Fig. S5A).
Moreover, binding of can225IgG to EGFR expressed on malignant canine mammary cancer lesions could be demonstrated by immunohistochemistry, again leading to a comparable staining pattern like the FDA-approved diagnostic EGFR pharmDx test (Supplementary Fig. S5B). Different staining intensities of can225IgG and EGFR pharmDx can, at least in part, be explained by a different epitope specificity of 2-18C9, the monoclonal antibody used in the diagnostic kit (2-18C9 binds to the extracellular cysteine-rich region of the molecule spanning domain II proximal to the transmembrane region, whereas 225 has its epitope in domain III of EGFR), as it was also demonstrated previously that distinct antibodies against EGFR lead to different staining intensities in immunohistochemistry (67, 68).
The most important aim of this study was to demonstrate the tumor-inhibitory potential of can225IgG. Indeed, both can225IgG and cetuximab rendered comparable levels of growth inhibition in vitro via growth signal depletion after 24 or 48 hours of incubation, respectively (Fig. 5).
All cellular assays clearly illustrate that can225IgG shows the same or comparable biochemical and functional properties as original cetuximab.
Moreover, we also addressed the immune-mediated tumoricidal effects of can225IgG. Therefore, we proved its capability to bind to Fcγ receptors on canine monocytic cells (Fig. 6A), which are known as important effector cells in tumor immunotherapy (35). Indeed, coincubated with canine PBMCs, can225IgG was able to mediate significant levels of phagocytosis (ADCP) in canine mammary carcinoma cells (Fig. 6C). Yet, no significant ADCC could be recorded (Fig. 6B), possibly due to partially high background cytotoxicity and large variation in the samples caused by a high diversity of the canine patients with respect to age, sex, and breed. However, the observation of an IgG antibody mediating significant amounts of ADCP, but not ADCC, in this three-color flow cytometric method was also previously described for trastuzumab and HER2-overexpressing cancer cells in the human setting (18, 69).
In summary, this newly generated “caninized” anti-EGFR antibody seems to be highly specific as well as effective in targeting EGFR-overexpressing canine tumor cells. Its caninization prevents adverse reactions, such as anaphylaxis or serum sickness in treated dogs, making this antibody a safe research lead compound for the first passive immunotherapy approaches in canine patients with cancer.
Supplementary Material
Acknowledgments
The authors thank all members of the Jensen-Jarolim laboratory for inspiring discussions and support. They also thank Prof. Wrba for help in detection of EGFR in canine cancer samples, Judith Frei for assisting in ADCC/ADCP assays, and Michael Schranz for excellent advice in amplifying immunohistochemical signals with tyramide.
Grant Support This work was supported by grant P23398-B11 (to E. Jensen-Jarolim) of the Austrian Science Fund (FWF) and J. Singer and J. Fazekas were supported by the CCHD PhD program, FWF project W1205-B09 (to E. Jensen-Jarolim).
Footnotes
Disclosure of Potential Conflicts of Interest E. Spillner has ownership interest in a patent. No potential conflicts of interest were disclosed by the other authors.
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
References
- 1.Takashima-Uebelhoer BB, Barber LG, Zagarins SE, Procter-Gray E, Gollenberg AL, Moore AS, et al. Household chemical exposures and the risk of canine malignant lymphoma, a model for human non-Hodgkin’s lymphoma. Environ Res. 2012;112:171–6. doi: 10.1016/j.envres.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera P, von Euler H. Molecular biological aspects on canine and human mammary tumors. Vet Pathol. 2011;48:132–46. doi: 10.1177/0300985810387939. [DOI] [PubMed] [Google Scholar]
- 3.Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009;6:e1000161. doi: 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–56. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 5.Sorenmo K. Canine mammary gland tumors. Vet Clin North Am Small Anim Pract. 2003;33:573–96. doi: 10.1016/s0195-5616(03)00020-2. [DOI] [PubMed] [Google Scholar]
- 6.London CA, Malpas PB, Wood-Follis SL, Boucher JF, Rusk AW, Rosenberg MP, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15:3856–65. doi: 10.1158/1078-0432.CCR-08-1860. [DOI] [PubMed] [Google Scholar]
- 7.Lachowicz JL, Post GS, Brodsky E. A phase I clinical trial evaluating imatinib mesylate (Gleevec) in tumor-bearing cats. J Vet Intern Med. 2005;19:860–4. doi: 10.1892/0891-6640(2005)19[860:apicte]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Bergman PJ, Camps-Palau MA, McKnight JA, Leibman NF, Craft DM, Leung C, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine. 2006;24:4582–5. doi: 10.1016/j.vaccine.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso F, Fallowfield L, Costa A, Castiglione M, Senkus E. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi25–30. doi: 10.1093/annonc/mdr372. [DOI] [PubMed] [Google Scholar]
- 11.Singer J, Weichselbaumer M, Stockner T, Mechtcheriakova D, Sobanov Y, Bajna E, et al. Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol Immunol. 2012;50:200–9. doi: 10.1016/j.molimm.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983;1:511–29. [PubMed] [Google Scholar]
- 13.Eccles SA. The epidermal growth factor receptor/Erb-B/HER family in normal and malignant breast biology. Int J Dev Biol. 2011;55:685–96. doi: 10.1387/ijdb.113396se. [DOI] [PubMed] [Google Scholar]
- 14.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 15.Yarden Y, Shilo BZ. SnapShot: EGFR signaling pathway. Cell. 2007;131:1018. doi: 10.1016/j.cell.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Rimawi MF, Shetty PB, Weiss HL, Schiff R, Osborne CK, Chamness GC, et al. Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer. 2010;116:1234–42. doi: 10.1002/cncr.24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Zhang X, Mortenson ED, Radkevich-Brown O, Wang Y, Fu YX. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol Ther. 2013;21:91–100. doi: 10.1038/mt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karagiannis P, Singer J, Hunt J, Gan SK, Rudman SM, Mechtcheriakova D, et al. Characterisation of an engineered trastuzumab IgE antibody and effector cell mechanisms targeting HER2/neu-positive tumour cells. Cancer Immunol Immunother. 2009;58:915–30. doi: 10.1007/s00262-008-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spillner E, Plum M, Blank S, Miehe M, Singer J, Braren I. Recombinant IgE antibody engineering to target EGFR. Cancer Immunol Immunother. 2012;61:1565–73. doi: 10.1007/s00262-012-1287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vale CL, Tierney JF, Fisher D, Adams RA, Kaplan R, Maughan TS, et al. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev. 2012;38:618–25. doi: 10.1016/j.ctrv.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Tol J, Punt CJ. Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clin Ther. 2010;32:437–53. doi: 10.1016/j.clinthera.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Harris CA, Ward RL, Dobbins TA, Drew AK, Pearson S. The efficacy of HER2-targeted agents in metastatic breast cancer: a meta-analysis. Ann Oncol. 2011;22:1308–17. doi: 10.1093/annonc/mdq593. [DOI] [PubMed] [Google Scholar]
- 23.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov. 2003;2:52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 24.Mendelsohn J. Epidermal growth factor receptor inhibition by a monoclonal antibody as anticancer therapy. Clin Cancer Res. 1997;3:2703–7. [PubMed] [Google Scholar]
- 25.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–8. [PubMed] [Google Scholar]
- 26.Chang C, Takayanagi A, Yoshida T, Shimizu N. Recombinant human IgG antibodies recognizing distinct extracellular domains of EGF receptor exhibit different degrees of growth inhibitory effects on human A431 cancer cells. Exp Cell Res. 2013;319:1146–55. doi: 10.1016/j.yexcr.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Knittelfelder R, Riemer AB, Jensen-Jarolim E. Mimotope vaccination—from allergy to cancer. Expert Opin Biol Ther. 2009;9:493–506. doi: 10.1517/14712590902870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You B, Chen EX. Anti-EGFR monoclonal antibodies for treatment of colorectal cancers: development of cetuximab and panitumumab. J Clin Pharmacol. 2011 Mar 11; doi: 10.1177/0091270010395940. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Kurzman ID, Shi F, Vail DM, MacEwen EG. In vitro and in vivo enhancement of canine pulmonary alveolar macrophage cytotoxic activity against canine osteosarcoma cells. Cancer Biother Radiopharm. 1999;14:121–8. doi: 10.1089/cbr.1999.14.121. [DOI] [PubMed] [Google Scholar]
- 30.Soergel SA, MacEwen EG, Vail DM, Potter DM, Sondel PM, Helfand SC. The immunotherapeutic potential of activated canine alveolar macrophages and antitumor monoclonal antibodies in metastatic canine melanoma. J Immunother. 1999;22:443–53. doi: 10.1097/00002371-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Steplewski Z, Rosales C, Jeglum KA, McDonald-Smith J. In vivo destruction of canine lymphoma mediated by murine monoclonal antibodies. In Vivo. 1990;4:231–4. [PubMed] [Google Scholar]
- 32.Nariai N, Kitagawa K, Nariai K, Kosaka T, Kuwabara M, Kiuchi Y. Active-oxygen involvement in canine NK-mediated cytotoxicity. J Vet Med Sci. 2000;62:457–60. doi: 10.1292/jvms.62.457. [DOI] [PubMed] [Google Scholar]
- 33.Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–54. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Washburn B, Weigand MA, Grosse-Wilde A, Janke M, Stahl H, Rieser E, et al. TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle disease virus. J Immunol. 2003;170:1814–21. doi: 10.4049/jimmunol.170.4.1814. [DOI] [PubMed] [Google Scholar]
- 35.Dalle S, Thieblemont C, Thomas L, Dumontet C. Monoclonal antibodies in clinical oncology. Anticancer Agents Med Chem. 2008;8:523–32. doi: 10.2174/187152008784533071. [DOI] [PubMed] [Google Scholar]
- 36.Challacombe JM, Suhrbier A, Parsons PG, Jones B, Hampson P, Kavanagh D, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123–32. doi: 10.4049/jimmunol.177.11.8123. [DOI] [PubMed] [Google Scholar]
- 37.Ritt MG, Wojcieszyn J, Modiano JF. Functional loss of p21/Waf-1 in a case of benign canine multicentric melanoma. Vet Pathol. 1998;35:94–101. doi: 10.1177/030098589803500202. [DOI] [PubMed] [Google Scholar]
- 38.Harris LJ, Skaletsky E, McPherson A. Crystallographic structure of an intact IgG1 monoclonal antibody. J Mol Biol. 1998;275:861–72. doi: 10.1006/jmbi.1997.1508. [DOI] [PubMed] [Google Scholar]
- 39.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 40.Shen MY, Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006;15:2507–24. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–86. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 42.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 44.Swoboda I, Bugajska-Schretter A, Verdino P, Keller W, Sperr WR, Valent P, et al. Recombinant carp parvalbumin, the major cross-reactive fish allergen: a tool for diagnosis and therapy of fish allergy. J Immunol. 2002;168:4576–84. doi: 10.4049/jimmunol.168.9.4576. [DOI] [PubMed] [Google Scholar]
- 45.Starkl P, Felix F, Krishnamurthy D, Stremnitzer C, Roth-Walter F, Prickett SR, et al. An unfolded variant of the major peanut allergen Ara h 2 with decreased anaphylactic potential. Clin Exp Allergy. 2012;42:1801–12. doi: 10.1111/cea.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bracher M, Gould HJ, Sutton BJ, Dombrowicz D, Karagiannis SN. Three-colour flow cytometric method to measure antibody-dependent tumour cell killing by cytotoxicity and phagocytosis. J Immunol Methods. 2007;323:160–71. doi: 10.1016/j.jim.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Warr GW, Hart IR. Binding of canine IgM and IgG to protein A of Staphylococcus aureus: a simple method for the isolation of canine immunoglobulins from serum and the lymphocyte surface. Am J Vet Res. 1979;40:922–6. [PubMed] [Google Scholar]
- 48.Yamamoto S, Omura M, Hirata H. Isolation of porcine, canine and feline IgG by affinity chromatography using protein A. Vet Immunol Immunopathol. 1985;9:195–200. doi: 10.1016/0165-2427(85)90019-4. [DOI] [PubMed] [Google Scholar]
- 49.Scott MA, Davis JM, Schwartz KA. Staphylococcal protein A binding to canine IgG and IgM. Vet Immunol Immunopathol. 1997;59:205–12. doi: 10.1016/s0165-2427(97)00073-1. [DOI] [PubMed] [Google Scholar]
- 50.Kim GP, Grothey A. Targeting colorectal cancer with human anti-EGFR monoclonocal antibodies: focus on panitumumab. Biologics. 2008;2:223–8. doi: 10.2147/btt.s1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marconato L, Gelain ME, Comazzi S. The dog as a possible animal model for human non-Hodgkin lymphoma: a review. Hematol Oncol. 2013;31:1–9. doi: 10.1002/hon.2017. [DOI] [PubMed] [Google Scholar]
- 52.Owen LN. A comparative study of canine and human breast cancer. Invest Cell Pathol. 1979;2:257–75. [PubMed] [Google Scholar]
- 53.Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–19. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 54.Cooley DM, Waters DJ. Skeletal metastasis as the initial clinical manifestation of metastatic carcinoma in 19 dogs. J Vet Intern Med. 1998;12:288–93. doi: 10.1111/j.1939-1676.1998.tb02124.x. [DOI] [PubMed] [Google Scholar]
- 55.Jaillardon L, Barthelemy A, Goy-Thollot I, Pouzot-Nevoret C, Fournel-Fleury C. Mammary gland carcinoma in a dog with peripheral blood and bone marrow involvement associated with disseminated intravascular coagulation. Vet Clin Pathol. 2012;41:261–5. doi: 10.1111/j.1939-165X.2012.00433.x. [DOI] [PubMed] [Google Scholar]
- 56.Jubala CM, Wojcieszyn JW, Valli VE, Getzy DM, Fosmire SP, Coffey D, et al. CD20 expression in normal canine B cells and in canine non-Hodgkin lymphoma. Vet Pathol. 2005;42:468–76. doi: 10.1354/vp.42-4-468. [DOI] [PubMed] [Google Scholar]
- 57.Gama A, Gartner F, Alves A, Schmitt F. Immunohistochemical expression of epidermal growth factor receptor (EGFR) in canine mammary tissues. Res Vet Sci. 2009;87:432–7. doi: 10.1016/j.rvsc.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 58.Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak A, Ren SG, Bruyette D, et al. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest. 2011;121:4712–21. doi: 10.1172/JCI60417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sabattini S, Mancini FR, Marconato L, Bacci B, Rossi F, Vignoli M, et al. EGFR overexpression in canine primary lung cancer: pathogenetic implications and impact on survival. Vet Comp Oncol. 2012 Sep 20; doi: 10.1111/vco.12002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Ferreira E, Gobbi H, Saraiva BS, Cassali GD. Columnar cell lesions of the canine mammary gland: pathological features and immunophenotypic analysis. BMC Cancer. 2010;10:61. doi: 10.1186/1471-2407-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Millanta F, Caneschi V, Ressel L, Citi S, Poli A. Expression of vascular endothelial growth factor in canine inflammatory and non-inflammatory mammary carcinoma. J Comp Pathol. 2010;142:36–42. doi: 10.1016/j.jcpa.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–11. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Tang L, Sampson C, Dreitz MJ, McCall C. Cloning and characterization of cDNAs encoding four different canine immunoglobulin gamma chains. Vet Immunol Immunopathol. 2001;80:259–70. doi: 10.1016/s0165-2427(01)00318-x. [DOI] [PubMed] [Google Scholar]
- 64.Bruggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351–61. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reichert JM, Wenger JB. Development trends for new cancer therapeutics and vaccines. Drug Discov Today. 2008;13:30–7. doi: 10.1016/j.drudis.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 66.Peng ZK, Simons FE, Becker AB. Differential binding properties of protein A and protein G for dog immunoglobulins. J Immunol Methods. 1991;145:255–8. doi: 10.1016/0022-1759(91)90335-d. [DOI] [PubMed] [Google Scholar]
- 67.Buffet W, Geboes KP, Dehertogh G, Geboes K. EGFR-immunohistochemistry in colorectal cancer and non-small cell lung cancer: comparison of 3 commercially available EGFR-antibodies. Acta Gastroenterol Belg. 2008;71:213–8. [PubMed] [Google Scholar]
- 68.Lee HJ, Xu X, Choe G, Chung DH, Seo JW, Lee JH, et al. Protein overexpression and gene amplification of epidermal growth factor receptor in nonsmall cell lung carcinomas: comparison of four commercially available antibodies by immunohistochemistry and fluorescence in situ hybridization study. Lung Cancer. 2010;68:375–82. doi: 10.1016/j.lungcan.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 69.Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, et al. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med. 2013;11:307. doi: 10.1186/1479-5876-11-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.