Abstract
Dengue virus infection induces a dramatic expansion of B cell plasmablasts. In this issue, Kwissa et al. (2014) begin with transcriptomic analysis and then integrate studies in human clinical samples, non-human primates, and co-culture to identify a role for CD14+CD16+ monocytes in generating plasmablast responses during dengue virus infection.
Dengue virus (DENV) continues to expand as a major global health problem, with up to 100 million dengue cases per year. The four DENV serotypes (DENV1-4) are transmitted by Aedes mosquitoes and can result in a self-limiting but debilitating acute febrile illness (dengue fever) or the more severe and potentially life-threatening plasma leakage syndrome, dengue hemorrhagic fever/dengue shock syndrome. The primary risk factor for developing severe disease is a secondary infection with a different DENV serotype than that causing the previous infection. During acute DENV infection, a dramatic expansion of plasmablasts occurs (Wrammert et al., 2012; Zompi et al., 2012). However, what factors play a role in this expansion are not known. During secondary DENV infection, these plasmablasts demonstrate a greater cross-reactive response to DENV serotype(s) from a previous infection than to the current infecting serotype (Zompi et al., 2012). It is thought that poorly neutralizing serotype-cross-reactive antibodies can enhance viral uptake through Fcγ receptors found on DENV target cells such as macrophages and monocytes through the mechanism of antibody-dependent enhancement, which, along with cross-reactive T cells and host genetic factors, contribute to dengue disease severity. However, the antibody and T cell response can also play a protective role. What tips the balance of the adaptive immune response towards protection versus pathogenesis is an area of active investigation. In addition, which innate immune factors contribute to control of viral load and disease severity are not well understood.
In this issue of Cell Host & Microbe, Kwissa et al. (Kwissa et al., 2014) demonstrate how a particular subset of monocytes modulates humoral responses during DENV infection and thus unveil an important link between the innate and adaptive immune response. The authors utilize an integrated systems biology approach, in which they combine results from studies in human populations, non-human primates, and in vitro analyses. First, they performed transcriptomic analysis of samples from Thai patients with either dengue fever or dengue hemorrhagic fever. Transcriptional signatures from these patients during DENV infection correlated with viral load and duration of illness, but not disease severity --likely because the study population did not include patients with the most severe disease, dengue shock syndrome. Previous reports have associated changes in transcriptional profile in dengue with the occurrence of shock (Loke et al., 2010; Simmons et al., 2007). Dengue patients experience high viral load early after symptom onset; DENV viremia decreases over the course of illness so that samples taken at later time-points (≥6 days) have low viral load. Kwissa et al. conducted parallel analyses to evaluate gene expression signatures in patients with high versus low viral load and in samples collected early versus late after symptom onset, and overall they observed similar patterns in samples with high viral load early in disease versus low viral load late in disease progression. These findings are consistent with previous studies, in which gene expression patterns exhibited temporal changes over the course of illness (Popper et al., 2012; Sun et al., 2013). Interestingly, when Kwissa et al. focused only on early time-points post-symptom onset and compared transcriptomic analysis in patients with high or low viral load, they found that viral load can influence gene expression patterns independently of time post-onset of illness – an observation worthy of future study.
Samples with high viremia and/or collected early after symptom onset positively correlated with innate immune responses associated with type I interferon genes, where as genes involved in cell cycle, proliferation and metabolism correlated with lower viremia and later stages of illness. Gene set enrichment analysis detected genes associated with monocytes, dendritic cells (DCs) and macrophages in patients with high viremia early in illness, whereas genes related to NK cells, B cells, and CD4+ and CD8+ T cells were associated with low viremia laterin the course of disease. Thus, samples with high viremia from early after symptom onset expressed genes associated with innate immune sensing of viruses and inflammatory responses to viral infection.
Kwissa et al. next examined alterations in blood cells during acute DENV infection and found an increased frequency of monocytes and a reduction in the proportion and absolute number of a classical dendritic cell subset, BDCA-1+ mDC-1 cells, especially in patients with high viral load at early stages of illness. This suggests that changes within the peripheral blood may be related to changes within the monocyte compartment. Monocytes are the predominate target for DENV infection in peripheral blood mononuclear cells, and monocyte activation has been implicated in DENV pathogenesis (Durbin et al., 2008). Expression of the surface markers CD14 and CD16 divides monocytes into three subsets that display distinct ligand sensing and cytokine/chemokine secretion patterns: “classical” CD14+CD16−, “intermediate” CD14+CD16+, and “non-classical” CD14dimCD16++ monocytes (Wong et al., 2011). Kwissa et al. found genes associated with the CD14+CD16+ monocyte subset to be enriched in patients with high viral load/early disease stage when monocyte-specific datasets were used to analyze the whole blood transcriptome. These data were confirmed when monocyte populations were evaluated via flow cytometry in both humans and a non-human primate model of DENV infection. Non-human primates infected with DENV demonstrated an increase in CD14+CD16+ monocytes in peripheral blood between days 1 and 3 post-infection and were found in the lymph nodes by day 3. In addition, DENV infection of CD14+ monocytes from healthy human donors increased the frequency of CD14+CD16+ monocytes. These DENV-infected monocytes secreted MCP-1 (CCL-2), IP-10 (CXCL-10), IL-6, IL-8 and IL-10, similar to the pattern of cytokine and chemokine secretion observed in the plasma of patients with high viral load early in disease. These factors were previously shown to be associated with CD14+CD16+ monocytes (Wong et al., 2011). Taken together, these data provide strong evidence that DENV infection results in increased frequency of CD14+CD16+ monocytes and suggest that they may play a role in shaping the immune response to DENV.
Further evaluation of DENV-infected non-human primates indicated that CD14+CD16+ monocytes express CD163 and CD169, markers of tissue subcapsular sinus macrophages. Subcapsular sinus macrophages localize to the subcapsular region of the lymph node, phagocytose viruses, and stimulate virus-specific B cell responses (Junt et al., 2007). To evaluate the ability of the CD14+CD16+ monocyte population to stimulate DENV-specific B cell responses, Kwissa et al. co-cultured DENV-infected CD14+CD16+ monocytes with autologous B cells. DENV-infected CD14+CD16+ monocytes stimulated resting B cells to differentiate into CD27++CD38++ plasmablasts that secreted IgM and IgG. Further analysis of the in vitro co-culture system identified the B-cell-modulating cytokines BAFF, APRIL and IL-10, but not IL-6, as potential mechanisms by which this monocyte population stimulates plasmablast formation. Furthermore, expression of genes encoding BAFF and APRIL was increased in dengue patients with high viral load early in disease progression. These data suggest that early during DENV infection, monocytes differentiate into CD14+CD16+ monocytes and migrate to draining lymphnodes where they may encounter and activate antigen-specific memory B cells.
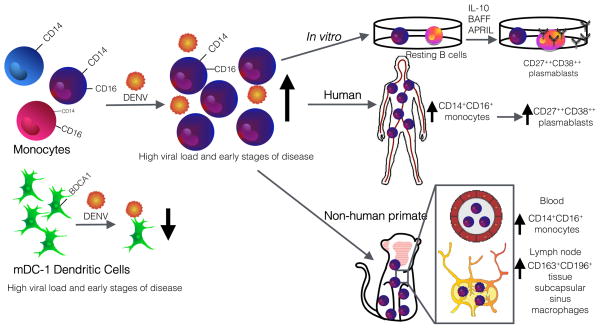
In summary, Kwissa et al. utilized an elegant approach integrating immunoprofiling of DENV-infected human clinical samples with studies in non-human primates and in vitro co-culture systems to identify a monocyte subset that modulates humoral responses (Figure). These novel data show that following DENV infection, CD14+CD16+ monocytes increase in the peripheral blood of both humans and non-human primates and then localize to the lymph nodes. Whether the increase in CD14+CD16+ monocytes is due to proliferation, mobilization from the bone marrow or spleen, or differentiation remains to be determined. The CD14+CD16+ monocytes express CD138 and CD169, surface markers of tissue subcapsular sinus macrophages, which in the lymph node capture viruses from tissues to present to B cells. The data suggest that this CD14+CD16+ monocyte population is capable of stimulating the expansion of CD38++CD27++ plasmablasts following DENV infection through the secretion of the cytokines BAFF, APRIL and IL-10. These data provide an exciting new perspective into how the innate immune response plays a role in shaping the magnitude and quality of the adaptive immune response; future studies addressing the relation of these findings with disease severity are now warranted.
Figure. Identification of CD14+CD16+ monocytes involved in plamablast differentiation.
CD14+CD16+ monocytes increase in humans and non-human primates following DENV infection. This monocyte population migrates to the lymph node and expresses markers of subcapsular sinus macrophages, which are capable of activating antigen-specific B cells. CD14+CD16+ monocytes stimulate plasmablast formation in vitro, driven by BAFF, APRIL, and IL-10. Illustrated by Sara Watson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, type setting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Durbin A, Vargas MJ, Thumar B, Hammond SN, Gordon G, Rocha C, Balmaseda A, Harris E. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology. 2008;376:429–435. doi: 10.1016/j.virol.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kwissa M, Nakaya HI, Onlamoon N, Wrammert J, Villinger F, Perng GC, Yoksan S, Pattanapanyasat K, Chokephaibulkit K, Ahmed R, et al. Dengue virus infection induces expansion of a CD14+CD16+ monocyte population that stimulates plasmablast differentiation. Cell Host Microbe. 2014 doi: 10.1016/j.chom.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke P, Hammond SN, Leung JM, Kim CC, Batra S, Rocha C, Balmaseda A, Harris E. Gene expression patterns of dengue virus-infected children from Nicaragua reveal a distinct signature of increased metabolism. PLoS Negl Trop Dis. 2010;4:e710. doi: 10.1371/journal.pntd.0000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper SJ, Gordon A, Liu M, Balmaseda A, Harris E, Relman DA. Temporal dynamics of the transcriptional response to dengue virus infection in Nicaraguan children. PLoS Negl Trop Dis. 2012;6:e1966. doi: 10.1371/journal.pntd.0001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CP, Popper S, Dolocek C, Chau TN, Griffiths M, Dung NT, Long TH, Hoang DM, Chau NV, Thao le TT, et al. Patterns of host genome-wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J Infect Dis. 2007;195:1097–1107. doi: 10.1086/512162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Garcia J, Comach G, Vahey MT, Wang Z, Forshey BM, Morrison AC, Sierra G, Bazan I, Rocha C, et al. Sequential waves of gene expression in patients with clinically defined dengue illnesses reveal subtle disease phases and predict disease severity. PLoS Negl Trop Dis. 2013;7:e2298. doi: 10.1371/journal.pntd.0002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, Kwissa M, Pulendran B, Wilson P, Wittawatmongkol O, et al. Rapid and massive virus specific plasmablast responses during acute dengue virus infection in humans. J Virol. 2012;86:2911–2918. doi: 10.1128/JVI.06075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zompi S, Montoya M, Pohl MO, Balmaseda A, Harris E. Dominant cross-reactive B cell response during secondary acute dengue virus infection in humans. PLoS Negl Trop Dis. 2012;6:1–14. doi: 10.1371/journal.pntd.0001568. [DOI] [PMC free article] [PubMed] [Google Scholar]