Abstract
Background
Lymph node counts are a measure of quality assurance and are associated with prognosis for numerous malignancies. To date, investigations of lymph node counts in testis cancer are lacking.
Methods
Using the Memorial Sloan-Kettering Testis Cancer Database, we identified 255 patients treated with primary retroperitoneal lymph node dissection (RPLND) for nonseminomatous germ cell tumors (NSGCT) between 1999 and 2008. Features associated with node counts, positive nodes, number of positive nodes, and risk of positive contralateral nodes were evaluated with regression models.
Results
Median (IQR) total node count was 38 (27–53) and was 48 (34 – 61) during the most recent 5 years. Features associated with higher node count on multivariate analysis included high volume surgeon (p=0.034), clinical stage (p=0.036), and more recent year of surgery (p<0.001) while pathologist was not significantly associated with node count (p=0.3). Clinical stage (p<0.001) and total node count (p=0.045) were significantly associated with finding positive nodes on multivariate analysis. The probability of finding positive nodes were 23%, 23%, 31%, and 48% if the total node count was <21, 21 – 40, 41 – 60, and >60, respectively. With a median follow-up of 3.0 years all patients were still alive and 16 patients relapsed while no patient relapsed in the paracaval, interaortocaval, paraaortic, or iliac regions.
Conclusion
Our results suggest that >40 lymph nodes removed at RPLND improves the diagnostic efficacy of the operation. These results will be useful for future trials comparing RPLND, especially when assessing the adequacy of lymph node dissection.
Keywords: Testicular neoplasms, Lymph nodes, Lymph node excision, Neoplasm staging
Introduction
Cancer-specific death from clinical stage I or IIA nonseminomatous germ cell tumors (NSGCT) should be the rare exception with proper observation, retroperitoneal lymph node dissection (RPLND), and/or chemotherapy. For patients with clinical stage I or IIA NSGCT, the most likely site of metastatic spread is the retroperitoneum, with up to 90% of patients having disease limited to the retroperitoneum. Thus, a properly performed RPLND has both a diagnostic and therapeutic role and is the preferred treatment modality for high risk clinical stage I and all clinical stage IIA NSGCT patients at Memorial Sloan-Kettering Cancer Center (MSKCC) in the setting of normal serum tumor markers. However, the potential risks of an uncontrolled retroperitoneum are late relapse,1 need for reoperation,2 salvage chemotherapy, and malignant transformation of unresected teratoma.
Historically, RPLND was associated with ejaculatory morbidity due to interruption of the sympathetic trunks, hypogastric plexus, and/or postganglionic efferent nerve fibers. In effort to thwart these side effects, multiple modified templates were proposed which limited the contralateral dissection to preserve antegrade ejaculation. However, the oncologic efficacy of template-limited dissection has recently been reassessed due to concerns regarding extra-template disease;3, 4 in fact, recent observations suggest that 3 – 23% of patients (depending on template utilized) with clinical stage I or IIA NSGCT actually harbor extra-template disease.4 Moreover, with improved surgical technique utilizing a nerve sparing approach, there is near uniform preservation of antegrade ejaculation without the need to modify a standard bilateral template.5
More recently, laparoscopic RPLND has been reported as technically feasible, associated with improved pain control, and shorter convalescence.6–9 However, the number of lymph nodes removed with laparoscopic RPLND (mean 16 total nodes in a recent meta-analysis)6 seems much lower than our anecdotal experience. Additionally, there is a substantial volume of literature demonstrating that lymph node counts are have important staging and clinical implications in numerous malignancies;10–17 however, a thorough investigation of lymph node counts is lacking for testis cancer. Therefore, this study was undertaken to evaluate contemporary node counts with open RPLND to provide a benchmark for comparison with novel surgical approaches. We also investigated features associated with node counts and analyzed which factors predict for identifying patients with positive nodes.
Materials and Methods
Patient Selection
After obtaining Institutional Review Board approval, we queried the prospective MSKCC Testis Cancer Registry and identified 273 patients treated with primary RPLND for NSGCT between the years 1999 and 2008. Eighteen patients who did not have a discernable lymph node count (i.e. “multiple benign lymph nodes”) were excluded, leaving 255 patients available for analysis. The MSKCC Testis Cancer Registry is prospectively maintained with >100 variables collected and recorded for all patients who undergo RPLND at MSKCC. For this project, charts were reviewed for all patients to ensure that the location and number of lymph nodes recorded in the registry was accurate.
Operative Boundaries and Pathologic Sectioning
For nearly all patients, the upper limits of dissection included the skeletonized renal vessels and crus of the diaphragm while lower limits included the external iliac vessels on the ipsilateral side and the bifurcation of the great vessel on the contralateral side. Packets consisting of the paracaval, interaortocaval, paraortic, and ipsilateral iliac lymph nodes were routinely submitted for pathologic examination. On occasion, interiliac nodes or contralateral iliac were removed and submitted separately. The nodal packets are received in pathology from the operating room in a fresh state. Using visual inspection, palpation and blunt dissection with a scalpel blade, the adipose tissue is dissected free and all firm, rubbery areas suspicious for a lymph node are then separated and submitted for microscopic examination. If no nodes are palpated, the tissue is submitted for microscopic examination in its entirety.
Clinical and Pathologic Features
The clinical and pathologic features studied included lymph node counts from the paracaval, interaortocaval, and paraaortic regions, total lymph node count, pathologist, surgeon, clinical stage, primary tumor histology, year of surgery, pathologic stage, number of positive nodes, location of positive nodes, and timing and location of relapse. For simplicity and when performed, right iliac lymph nodes were added to the paracaval node region, interiliac lymph nodes were added to the interaortocaval region, and left iliac nodes were added to the paraaortic region. A high volume surgeon was defined as performing >10 primary RPLND’s and a high volume pathologist was defined as evaluating >10 primary RPLND’s over the study time period.
Statistical Analysis
The clinical and pathologic features were summarized with median and interquartile rage (IQR) or frequency and percentage as appropriate. Features thought to be associated with higher lymph node counts were evaluated using linear regression models both in a univariate and a multivariate model adjusting for year of surgery, surgeon (dichotomized as high volume vs low volume), clinical stage (stage I vs II), and pathologist (high volume vs low volume). To evaluate features associated with the finding of positive nodes, we performed logistic regression models with total node count adjusting for features associated with total node count. In these models, total node count was treated as both a continuous variable and as a binary variable split at 40 which seemed appropriate given that the mean total node count was 38 and the median total node count was 42. We then evaluated if total node count is associated with number of positive nodes using linear regression models univariately and after adjusting for features significantly associated with total node count in a multivariate analysis. Next, we used logistic regression models to evaluate if positive ipsilateral/In-template nodes is associated with positive outside-of-template nodes (defined as positive paraaortic nodes with a right sided primary tumor or positive interaortocaval or paracaval nodes with a left sided primary NSGCT) both univariately and after adjusting for clinical stage, total number of nodes, side of primary tumor, and year of surgery. Statistical analyses were performed with Stata v8.2, all tests were two-sided, and p-values <0.05 were considered statistically significant.
Results
A summary of baseline features for the 255 patients studied is detailed in Table I. Median (IQR) age at RPLND was 30 (23 – 37). The most common primary tumor histology was mixed NSGCT with embryonal predominance, noted in 112 (44%) patients. Only 26 (10%) patients were clinical stage IA while the remaining were IB (n=147, 58%) or IIA (n=82, 32%). Among the 26 patients with clinical stage IA NSGCT, 21 had either embryonal and/or teratoma features in the primary tumor. Overall, positive lymph nodes were found in 75 patients, including 56 (75%) with viable tumor only, 10 (13%) with viable tumor and teratoma, and 9 (12%) with teratoma only. Positive nodes were found in 2 (8%), 34 (23%), and 39 (48%) of patients with clinical stage IA, IB, and IIA NSGCT.
Table I.
Summary of baseline features for 255 patients treated with primary RPLND for NSGCT.
| Feature | No. | % |
|---|---|---|
|
| ||
| Primary Tumor | ||
| Right | 133 | 52 |
| Left | 119 | 47 |
| Bilateral | 3 | 1 |
|
| ||
| Primary Histology | ||
| Mixed | 210 | 82.4 |
| Pure Embryonal | 41 | 16.0 |
| Pure Teratoma | 3 | 1.2 |
| Pure Malignant Transformation | 1 | 0.4 |
|
| ||
| Clinical Stage | ||
| IA | 26 | 10 |
| IB | 147 | 58 |
| IIA | 82 | 32 |
|
| ||
| Year of RPLND | ||
| 1999 | 26 | 10 |
| 2000 | 19 | 7 |
| 2001 | 31 | 12 |
| 2002 | 23 | 9 |
| 2003 | 27 | 11 |
| 2004 | 27 | 11 |
| 2005 | 16 | 6 |
| 2006 | 28 | 11 |
| 2007 | 22 | 9 |
| 2008 | 36 | 14 |
|
| ||
| pN Stage | ||
| N0 | 180 | 70 |
| N1 | 50 | 20 |
| N2 | 25 | 10 |
|
| ||
| RPLND Histology | ||
| Benign | 180 | 70 |
| Viable GCT (only) | 56 | 22 |
| Viable GCT and Teratoma | 10 | 4 |
| Teratoma (only) | 9 | 4 |
A summary of node counts by region is detailed in Table II. Median (IQR) total lymph node count was 38 (27–53). In the subset of 129 patients treated over the last 5 years (2004 – 2008), the median (IQR) total lymph node count increased to 48 (34 – 61). Features predictive of higher total lymph node count on univariate and multivariate analysis included year of surgery, surgeon, and clinical stage (Table III). On average, 2.5 additional nodes were counted with each increasing year during the study time frame, 8.3 additional nodes were counted if a high volume surgeon performed the RPLND, and 5.3 additional nodes were counted if clinical stage was IIA vs I. While high volume pathologists were significantly more likely to have a higher node counts on univariate analysis, after adjusting for year of surgery, surgeon, and clinical stage, pathologist no longer remained significantly associated with higher lymph node counts (p=0.3).
Table II.
Node count by region, surgeon, and pathologist for 255 patients treated with primary RPLND
| Feature | Median | IQR |
|---|---|---|
|
| ||
| Paracaval Lymph Node Count | 5 | 2–9 |
|
| ||
| Interaortocaval Lymph Node Count | 13 | 9–20 |
|
| ||
| Paraaortic Lymph Node Count | 20 | 11–28 |
|
| ||
| Total Lymph Node Count | 38 | 27–53 |
|
| ||
| Surgeon | ||
| High Volume | 40 | 28–55 |
| Low Volume | 28 | 16–36 |
|
| ||
| Pathologist | ||
| High Volume | 43 | 30–58 |
| Low Volume | 34 | 25–49 |
IQR = Interquartile range
Table III.
Univariate and multivariate features predictive of higher lymph node counts in 255 patients treated with primary RPLND
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Feature | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value |
| Year of Surgery | 3.0 (2.2 – 2.8) | <0.001 | 2.5 (1.6 – 3.5) | <0.001 |
| Surgeon (High vs Low Volume) | 15.0 (7.0 – 23.0) | <0.001 | 8.3 (0.6 – 16.0) | 0.034 |
| Clinical stage (I vs II) | 5.8 (0.3 – 11.4) | 0.027 | 5.4 (0.4 – 10.4) | 0.036 |
| Pathologist (High vs Low Volume) | 9.7 (4.6 – 14.9) | <0.001 | 2.8 (−2.5 – 8.1) | 0.3 |
We then evaluated features associated with positive nodes (vs pN0) at time of RPLND. As demonstrated in Table IV, clinical stage and total node count were significantly associated with positive nodes while year of surgery and surgeon were not. The odds of finding positive nodes was >3 for patients with clinical stage II compared with clinical stage I NSGCT (p<0.001). Each additional lymph node removed was associated with a nearly 2% increase odds of finding a positive node (p=0.029). In a pre-determined analysis of dichotomizing node count, patients with a total node count of >40 had nearly 2 times increased odds of having positive nodes compared with a total node count of 40 or less (odds ratio 1.9; 95% CI 1.1 – 3.3; p=0.019). In a multivariate analysis, total node count >40 remained significantly associated with positive nodes even after adjusting for year of surgery, clinical stage, and surgeon (odds ratio 2.0; 95% CI 1.1 – 3.7; p=0.026). Figure I demonstrates the frequency of positive nodes by total node count. The chances of finding positive nodes were 23%, 23%, 31%, and 48% with a total node count of <21, 21 – 40, 41 – 60, and >60, respectively.
Table IV.
Univariate and multivariate features predictive of positive lymph nodes in 255 patients treated with primary RPLND
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Feature | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value |
| Year of Surgery | 1.0 (0.9 – 1.1) | 0.7 | 0.9 (0.8 – 1.0) | 0.145 |
| Surgeon (High vs Low Volume) | 1.4 (0.6 – 3.3) | 0.5 | 1.3 (0.5 – 3.5) | 0.6 |
| Clinical stage (I vs II) | 3.5 (2.0 – 6.1) | <0.001 | 3.3 (1.8 – 5.9) | <0.001 |
| Total lymph node count | 1.01 (1.00 – 1.03) | 0.029 | 1.02 (1.00 – 1.03) | 0.045 |
Figure I.
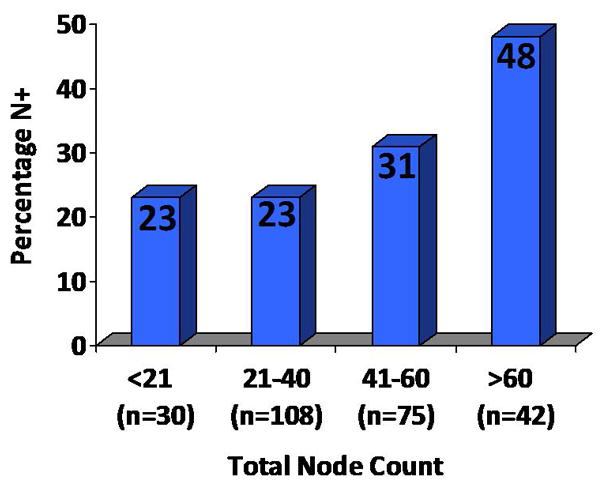
Percentage of patients with positive nodes by total node count
Next, we evaluated if total node count is associated with the absolute number of positive nodes. A total of 75 patients had positive nodes; among them, 27, 20, 11, and 17 had 1, 2, 3, and >3 positive nodes. One notable patient with clinical stage IB NSGCT had 37 positive nodes among 112 counted. In a linear regression model, higher total node count was significantly associated with higher total positive nodes (coefficient 0.03 for each additional node counted; 95% CI 0.01 – 0.05; p=0.005; Figure II). Higher total node count remained significantly associated with higher number of positive nodes even after adjusting for year of surgery, surgeon, and clinical stage in a multivariate analysis (coefficient 0.03 for each additional node counted; 95% CI 0.01 – 0.05; p=0.004).
Figure II.
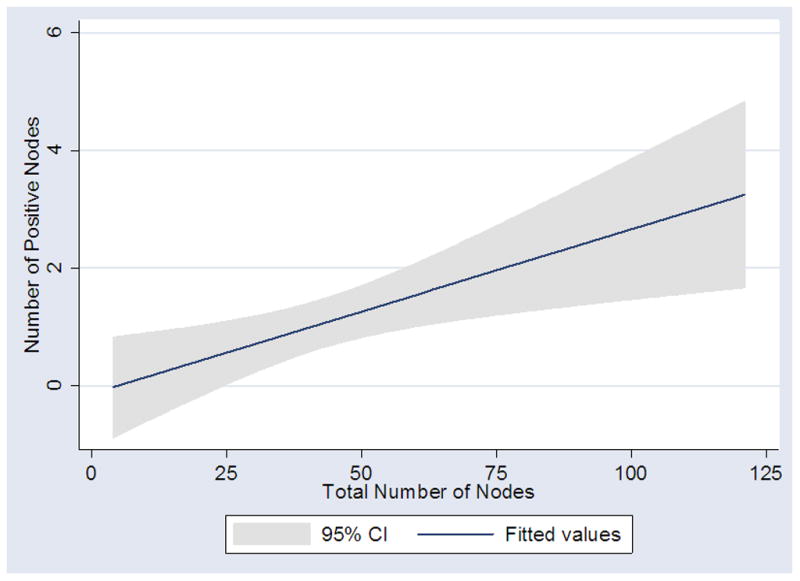
Relationship between total number of nodes removed and number of positive nodes at primary RPLND (p=0.005).
We then evaluated features predictive of positive nodes outside the surgical template (i.e. positive paraaortic nodes with a right sided primary and positive paracaval or interaortocaval nodes with a left sided primary). Among the 75 patients with positive nodes, 39 had right side primary and 36 had left side primary tumors. The number of patients with right and left sided primary tumors who had positive nodes outside the template was 7 (18%) and 8 (22%), respectively. Among all patients, those with positive in-field nodes were more than 6 times more likely to have positive contralateral nodes compared with patients who had benign in-field nodes (odds ratio 6.28; 95% CI 2.06 – 19.11; p=0.001; Figure II). This association remained significant even after adjusting for clinical stage, total number of nodes, side of primary tumor, and year of surgery (odds ratio 4.7; 95% CI 1.5 – 15.0; p=0.009) in a multivariate analysis. Among the 15 patients with contralateral positive nodes, the median total positive node count was 4 (IQR 3–11) including 7 patients who had 3 or less total positive nodes. Also of note, 5 (2%) patients had positive nodes outside the template without having positive nodes inside the template.
In general, patients with viable germ cell tumor who were pN1 were observed following RPLND while those with pN2 disease received good-risk chemotherapy in the form of 4 cycles of etoposide and cisplatin.18 At last follow-up, all patients were still alive and 16 patients relapsed at a median of 3.2 months (range 1 – 20 months). Median follow-up for patients who did not relapse was 3.0 years. Most relapses were in the lung (n=11, 69%) and no patient had a relapse in the paracaval, interaortocaval, paraaortic, or iliac regions. Due to the limited number of relapses and 100% survival at last follow-up, outcome analyses were not performed.
Discussion
There is a substantial volume of literature demonstrating that the number of lymph nodes removed is paramount for many malignancies including bladder,10 lung,11 esophageal,12 pancreatic,13 breast,14 gastric,15 and colon cancers.16, 17 While the mechanisms underlying the association between survival and lymph node count remain unknown for many of these malignancies, it seems intuitive for testicular cancer- a cancer that primarily spreads via the lymphatic channels. Thus, when the decision to perform a primary RPLND is made, it is important that both the diagnostic and therapeutic benefits of the operation be maximized. This will not only identify patients who would benefit from adjuvant chemotherapy but it will also serve to prevent overtreatment for those who do not need chemotherapy. Young men with early stage testis cancer should expect near 100% cure and a life expectancy of 50+ years and a suboptimal RPLND risks late relapse,1 need for reoperation,2 salvage chemotherapy, and malignant transformation of unresected teratoma. However, a systematic investigation of normal lymph node counts during primary RPLND is lacking. Herein, we present the first evaluation of normal lymph node counts during primary RPLND at a center that routinely performs a full bilateral dissection.
In this report, we detail our experience with lymph node counts during primary RPLND for patients treated at MSKCC over the last decade. Median total node count was 38 over the last 10 years and increased to 48 (mean 50) during the most recent 5 years. In a recent meta-analysis of 34 contemporary laparoscopic RPLND articles, the mean lymph node count was 16 (among the 3 articles that reported node count).6 It should be noted, however, that modified templates were utilized in all of the laparoscopic RPLND reports6 although more recent laparoscopic RPLND data suggest that a nerve-sparing bilateral template RPLND is technically feasible in experienced hands.19 However, 16 lymph nodes falls far below the value of 40 nodes which we found to be significant in terms of finding positive nodes and properly staging patients. Every effort should be made to not only remove the lymph nodes but to evaluate them morphologically as this has important ramifications when deciding upon adjuvant chemotherapy following primary RPLND.
In this report, we observed that surgeon was significantly associated with lymph node counts. In fact, median total node count was 40 for high volume surgeons compared with 28 for low volume surgeons. While this may be explained, in part, by the fact that low volume surgeons were more likely to perform RPLND earlier in the study time period (a feature strongly associated with diminished total node counts), surgeon remained significantly associated with higher node counts on multivariate analysis (including adjustment for year of surgery). We believe that this observation, if validated by others, will serve to set benchmarks for not only surgeons at our institution but for all surgeons who perform primary RPLND. We also evaluated pathologist, including 39 different pathologists who reviewed primary RPLND slides over the last decade. Importantly, we did not observe a significant difference in total node counts among high and low volume pathologists in a multivariate analysis. We believe that this information demonstrates a relative uniformity among pathologists at a specialized cancer center and we postulate that this uniformity would equally apply to many academic centers of specialized care.
Our data also suggest that higher total node count is significantly associated with the finding of more positive lymph nodes. This finding has important clinical implications since observation is generally recommended for pathologic IIA disease while chemotherapy is given for IIB (which includes >5 positive nodes) or greater NSGCT.18 Additionally, positive nodes on the ipsilateral side of the primary tumor were significantly associated with finding positive contralateral nodes and 2% of patients harbored positive nodes restricted to the contralateral side. Thus, a full bilateral RPLND with nerve sparing maximizes both diagnostic and therapeutic goals surgery with the benefit of minimizing ejaculatory morbidity and reducing the need for chemotherapy and its associated long-term sequelae.19, 20
In a recent randomized controlled trial comparing primary RPLND to chemotherapy in the community setting, Albers et al reported that disease recurrence following RPLND was significantly higher compared with one cycle of bleomycin, etoposide, and cisplatin.21 Among 191 patients treated with primary RPLND, 13 recurrences were noted including 9 who recurred in the retroperitoneum or scrotal region.21 Notably, all recurrences following RPLND occurred within 17 months and modified templates were utilized. In contrast, we did not observe a single recurrence in the paracaval, interaortocaval, or paraaortic regions and only one of 255 patients recurred in the inguinal/scrotal region following RPLND with a bilateral nerve-sparing dissection. As stated by Albers et al, the results of their trial demonstrate the importance of experienced surgeons if RPLND is used as a treatment option.21 It should also be noted that chemotherapy or observation for stage I NSGCT requires surveillance of the retroperitoneum. Recent evidence suggests that radiation exposure from repeated computerized tomography may increase the risk of secondary malignancies.22–24 In fact, Tarin et al suggest that the relative risk of a secondary malignancy in a surveillance protocol for clinical stage I NSGCT patients is approximately 15 compared with primary RPLND.23 In our practice, pN0 patients only receive one scheduled CT scan following primary RPLND assuming chest x-ray and tumor markers remain normal.
This report is not without limitations. While the data was collected in a prospective fashion, it was analyzed retrospectively and is subject to the many inherent biases associated with this approach. For example, surgical decisions such as performing a more extensive dissection if suspicious nodes are found intra-operatively cannot be accounted for in this analysis. Additionally, pathologic evaluation of more nodes once positive nodes are found could impact the results presented. Furthermore, there are limitations of using node count as surrogates for oncologic efficacy and further follow-up with additional events is needed to determine if node count is associated with improved outcomes. Nevertheless, as part of a quality assurance, the pathology reports for all 273 patients initially identified were reviewed to ensure the accuracy regarding the number and location of lymph node counts. Furthermore, while a majority of RPLND’s at our institution were performed by a single surgeon who utilized similar boundaries of dissection over the entire study time period, node counts clearly increased over the last decade. We believe this observation likely reflects increased emphasis on nodal counts by all pathologists for many malignancies that occurred during the last 10 years.10–17
One additional point deserves mention. While chemotherapy has revolutionized the field of testis cancer, providing cure for many of the most advanced stages, it should be emphasized that chemotherapy is not without long term adverse consequences. Increased risk of endothelial dysfunction, infertility, pulmonary fibrosis, cardiovascular disease, and secondary malignancies are recently reported long-term side effects of chemotherapy for NSGCT.25, 26 A properly performed RPLND can obviate the need for chemotherapy for patients with pathologic stage IIA disease, reduce the need for intense radiographic surveillance, and reduce the risk of malignant transformation from unresected teratoma. For these reasons, we advocate a thorough dissection, including the paracaval, interaortocaval and paraaortic regions, which should yield on average 40 – 50 lymph nodes in contemporary times for patients undergoing primary RPLND.
Acknowledgments
Support: Sidney Kimmel Foundation for Urologic Cancer and the NIH T32 CA082088
Footnotes
There are no financial disclosures associated with this project
References
- 1.Baniel J, Foster RS, Gonin R, et al. Late relapse of testicular cancer. J Clin Oncol. 1995;13:1170–1176. doi: 10.1200/JCO.1995.13.5.1170. [DOI] [PubMed] [Google Scholar]
- 2.McKiernan JM, Motzer RJ, Bajorin DF, et al. Reoperative retroperitoneal surgery for nonseminomatous germ cell tumor: clinical presentation, patterns of recurrence, and outcome. Urology. 2003;62:732–736. doi: 10.1016/s0090-4295(03)00579-x. [DOI] [PubMed] [Google Scholar]
- 3.Carver BS, Shayegan B, Eggener S, et al. Incidence of metastatic nonseminomatous germ cell tumor outside the boundaries of a modified postchemotherapy retroperitoneal lymph node dissection. J Clin Oncol. 2007;25:4365–4369. doi: 10.1200/JCO.2007.11.2078. [DOI] [PubMed] [Google Scholar]
- 4.Eggener SE, Carver BS, Sharp DS, et al. Incidence of disease outside modified retroperitoneal lymph node dissection templates in clinical stage I or IIA nonseminomatous germ cell testicular cancer. J Urol. 2007;177:937–942. doi: 10.1016/j.juro.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 5.Donohue JP, Foster RS, Rowland RG, et al. Nerve-sparing retroperitoneal lymphadenectomy with preservation of ejaculation. J Urol. 1990;144:287–291. doi: 10.1016/s0022-5347(17)39434-x. [DOI] [PubMed] [Google Scholar]
- 6.Rassweiler JJ, Scheitlin W, Heidenreich A, et al. Laparoscopic retroperitoneal lymph node dissection: does it still have a role in the management of clinical stage I nonseminomatous testis cancer? A European perspective. Eur Urol. 2008;54:1004–1015. doi: 10.1016/j.eururo.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen ME, Lima G, Schaeffer EM, et al. Oncologic efficacy of laparoscopic RPLND in treatment of clinical stage I nonseminomatous germ cell testicular cancer. Urology. 2007;70:1168–1172. doi: 10.1016/j.urology.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Cresswell J, Scheitlin W, Gozen A, et al. Laparoscopic retroperitoneal lymph node dissection combined with adjuvant chemotherapy for pathological stage II disease in nonseminomatous germ cell tumours: a 15-year experience. BJU Int. 2008;102:844–848. doi: 10.1111/j.1464-410X.2008.07754.x. [DOI] [PubMed] [Google Scholar]
- 9.Castillo OA, Alvarez JM, Vitagliano G, et al. Retroperitoneal laparoscopic lymphadenectomy for stage I non seminomatous testicular cancer. Arch Esp Urol. 2007;60:59–66. doi: 10.4321/s0004-06142007000100010. [DOI] [PubMed] [Google Scholar]
- 10.Koppie TM, Vickers AJ, Vora K, et al. Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer. 2006;107:2368–2374. doi: 10.1002/cncr.22250. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128:1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 12.Bollschweiler E, Baldus SE, Schroder W, et al. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 2006;94:355–363. doi: 10.1002/jso.20569. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 14.Woodward WA, Vinh-Hung V, Ueno NT, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24:2910–2916. doi: 10.1200/JCO.2005.03.1526. [DOI] [PubMed] [Google Scholar]
- 15.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 16.Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–441. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 17.Ricciardi R, Baxter NN. Association versus causation versus quality improvement: setting benchmarks for lymph node evaluation in colon cancer. J Natl Cancer Inst. 2007;99:414–415. doi: 10.1093/jnci/djk106. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson AJ, Bosl GJ, Bajorin DF, et al. Retroperitoneal lymph node dissection in patients with low stage testicular cancer with embryonal carcinoma predominance and/or lymphovascular invasion. J Urol. 2005;174:557–560. doi: 10.1097/01.ju.0000165163.03805.37. [DOI] [PubMed] [Google Scholar]
- 19.Steiner H, Zangerl F, Stohr B, et al. Results of bilateral nerve sparing laparoscopic retroperitoneal lymph node dissection for testicular cancer. J Urol. 2008;180:1348–1352. doi: 10.1016/j.juro.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 20.Stephenson AJ, Bosl GJ, Motzer RJ, et al. Retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer: impact of patient selection factors on outcome. J Clin Oncol. 2005;23:2781–2788. doi: 10.1200/JCO.2005.07.132. [DOI] [PubMed] [Google Scholar]
- 21.Albers P, Siener R, Krege S, et al. Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I Nonseminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group. J Clin Oncol. 2008;26:2966–2972. doi: 10.1200/JCO.2007.12.0899. [DOI] [PubMed] [Google Scholar]
- 22.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 23.Tarin TV, Sonn G, Shinghal R. Estimating the risk of cancer associated with imaging related radiation during surveillance for stage I testicular cancer using computerized tomography. J Urol. 2009;181:627–632. doi: 10.1016/j.juro.2008.10.005. discussion 632–623. [DOI] [PubMed] [Google Scholar]
- 24.Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251:175–184. doi: 10.1148/radiol.2511081296. [DOI] [PubMed] [Google Scholar]
- 25.Haugnes HS, Aass N, Fossa SD, et al. Pulmonary function in long-term survivors of testicular cancer. J Clin Oncol. 2009;27:2779–2786. doi: 10.1200/JCO.2008.18.5181. [DOI] [PubMed] [Google Scholar]
- 26.Feldman DR, Bosl GJ, Sheinfeld J, et al. Medical treatment of advanced testicular cancer. Jama. 2008;299:672–684. doi: 10.1001/jama.299.6.672. [DOI] [PubMed] [Google Scholar]


