Abstract
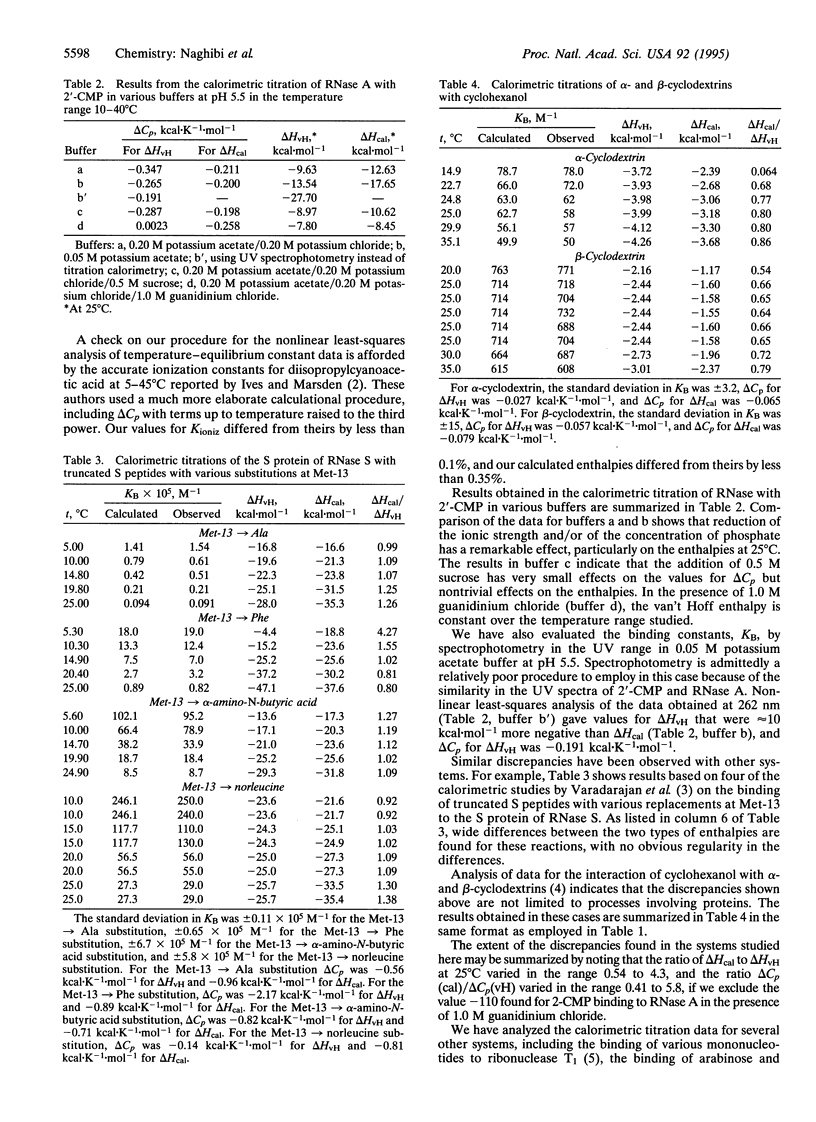
In this paper we show that the usual assumption in studies of the temperature variation of equilibrium constants for equilibria of the form A+B <-->AB that a plot of ln K vs. 1/T (K = equilibrium constant, T = temperature in degrees kelvin) is a straight line with slope equal to -delta HvH/R (delta HvH = van't Hoff or apparent enthalpy, R = gas constant) is not valid in many cases. In all the cases considered here, delta HvH is temperature dependent and is significantly different from the true or calorimetrically measured enthalpy, and the respective values for delta Cp are also significantly different.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fukada H., Sturtevant J. M., Quiocho F. A. Thermodynamics of the binding of L-arabinose and of D-galactose to the L-arabinose-binding protein of Escherichia coli. J Biol Chem. 1983 Nov 10;258(21):13193–13198. [PubMed] [Google Scholar]
- Ladbury J. E., Sturtevant J. M., Leontis N. B. The thermodynamics of formation of a three-strand, DNA three-way junction complex. Biochemistry. 1994 Jun 7;33(22):6828–6833. doi: 10.1021/bi00188a011. [DOI] [PubMed] [Google Scholar]
- Varadarajan R., Connelly P. R., Sturtevant J. M., Richards F. M. Heat capacity changes for protein-peptide interactions in the ribonuclease S system. Biochemistry. 1992 Feb 11;31(5):1421–1426. doi: 10.1021/bi00120a019. [DOI] [PubMed] [Google Scholar]


