Abstract
We report a long-term follow-up (median 11.8 years) of the First North American Intergroup Study. 379 patients were randomized to induction with ATRA or to chemotherapy. All complete responders (CR) received consolidation chemotherapy, then randomized to 1 year ATRA or observation. 245 patients received ATRA sometime during the study: 195 (80%) achieved a CR. Nine (4.6%) relapsed late (>3 years from CR), the last occurred after 4.6 years; 7 of them were still alive after 5.5–15 years. In APL patients, late relapses are uncommon, and those who sustain CR >5 years can be considered cured.
Keywords: Acute promyelocytic leukemia, All-trans retinoic acid, Late relapse
1. Introduction
All-trans retinoic acid (ATRA) was shown in the late 1980s in China [1] to induce a complete remission (CR) as a single agent in most patients with acute promyelocytic leukemia (APL). These results shortly thereafter were confirmed in France and the USA [2,3]. ATRA acts by targeting the APL-specific gene product PML/RARα, derived from the t(15:17) chromosomal translocation, which is primarily manifested by APL cell differentiation [4,5] As a single agent, ATRA is not active in any other acute leukemia subtype. Although remarkably effective in inducing CR in APL, the duration of CR with ATRA alone is brief and chemotherapy is necessary to prevent relapses. Two randomized clinical trials, the European APL91 [6] and the North American Intergroup Study I0129 [7], were the first to report that when ATRA is combined with chemotherapy, the outcome of APL patients markedly improved compared with treatment with chemotherapy alone. Additional large randomized trials [8,9] and single arm studies [10–14] from around the world confirmed this impact of ATRA, while optimizing its schedule of administration. In addition to preventing death during induction, the advantage of adding ATRA was generally due to fewer relapses [15] and consequently ATRA plus chemotherapy became the standard of care in APL [16].
Although updated reports of these two early trials [6,7] have been published [17,18], very long follow-up to determine the durability of remission achieved with the combination of ATRA and chemotherapy in newly diagnosed APL patients is limited [14,19,20]. Although ATRA plus chemotherapy remains the standard of care, strategies minimizing or eliminating chemotherapy by combining ATRA with arsenic trioxide are being explored [21,22], and data on the long-term durability of treatment approaches has become even more relevant.
The North American Intergroup Study I0129 is the larger of the two earliest randomized APL clinical trials, the first from North America, and the last to include patients with no ATRA treatment. Now, 18 years after the last patient was enrolled and with a median follow-up of 11.8 years, we present results of the longest follow-up in the ATRA era and report the rate and characteristics of patients with late relapses in comparison to those patients with early relapses.
2. Patients and methods
2.1. Patients
Between April 1992 and February 1995, 397 patients with newly diagnosed, previously untreated APL were registered from 6 cooperative oncology groups. Eligibility included a diagnosis of APL established by morphology with central review, no prior chemotherapy (except hydroxyurea), normal liver and kidney function, ECOG performance status of 0–3, and mandatory cytogenetic testing for t (15;17), although the results did not affect eligibility.
Of the 397 registered patients, 18 patients were excluded; Of the 18 patients excluded, 14 did not receive any study medication and 4 were ineligible. A total of 379 patients were included in this analysis.
2.2. Protocol design
The details of the protocol design, including the pediatric dosing, were previously reported [7,18] (Fig. 1). Briefly, patients were randomized to receive induction treatment with ATRA alone, 45 mg/m2/d in two divided doses orally until CR or a maximum of 90 days; or to chemotherapy induction with daunorubicin 45 mg/m2 intravenously on days 1–3 and cytarabine 100mg/m2 by continuous intravenous infusion on days 1–7. No chemotherapy except hydroxyurea, was allowed with ATRA even for a rise in the white blood cell (WBC) count. All patients who achieved CR received two cycles of anthracycline-containing consolidation chemotherapy. The first cycle was identical to the induction chemotherapy, and the second cycle included high dose cytarabine 2 g/m2 as a 1 h intravenous infusion every 12 h for four consecutive days plus daunorubicin 45mg/m2 intravenously on days 1–2. Patients in CR, irrespective of their induction arm, were then randomized to receive 1 year of maintenance with ATRA in two divided doses orally or to observation. The treatment at relapse was left to the discretion of the treating physician.
Fig. 1.
CONSORT diagram showing late and early relapses according to randomization.
2.3. Response and late relapse criteria
In this study patients in durable clinical CR, but lacking bone marrow confirmation were nonetheless considered non-responders. Only hematological and clinical relapses were considered. Late relapse was defined as occurring 3 or more years from achievement of CR based on the ECOG published definition for long term survival [23]. Late relapses were identified in the EGOG follow up database and individual patient information derived by tracing the medical records at the site where they relapsed.
2.4. Statistical methods
The distribution of baseline characteristics was summarized for early versus late relapses using descriptive statistics. Univariate associations between dichotomous variables were evaluated by Fisher's exact test [24]. Associations involving continuous variables were evaluated by the Wilcoxon Rank Sum test. Overall survival was defined as the time from study registration to death from any cause or date last known alive. Duration of remission was defined as the time from CR to relapse. The methods of Kaplan and Meier [25] were used to estimate survival curves and the significance was tested by logrank tests. P-values were reported for two-sided tests. The median follow-up was 11.8 years (range, 0.4–18.4 years).
3. Results
3.1. Patient characteristics
Patient characteristics and karyotype information were described in detail in the original report of this study and were similar in both induction arms [7]. Briefly, among the 379 patients, the median age was 39 years (range 1–81 years), 49 (13%) patients were children younger than age 15, 48% were women, the median WBC count at diagnosis was 2,200 mm3 (range, 0–550,000), and 84 patients (22%) had a WBC count ≥10,000 mm3.
3.2. Treatment outcome based on induction randomization
Of the 397 patients, 206 were last known alive; the 12 year overall survival (OS) rate was 51% (95% CI: 46%, 56%). Among all patients, 271 (72%) achieved a CR. Of the 191 patients who were randomized to induction chemotherapy, 129 (68%) achieved a CR which was not statistically different from 142 (76%) of the 188 patients randomized to ATRA induction p = 0.52). Of those who received induction chemotherapy, 85 (45%) were known to be alive with a median OS of 3.6 years, while of those induced with ATRA, 116 (62%) were known to be alive, with median a OS that has not yet been reached (p = 0.0007). The 12-year OS rate for DA induction patients was 42% (95% CI: 35%, 49%). The 12 year OS rate for ATRA induction patients was 60% (95% CI: 53%, 67%) (Fig. 2).
Fig. 2.
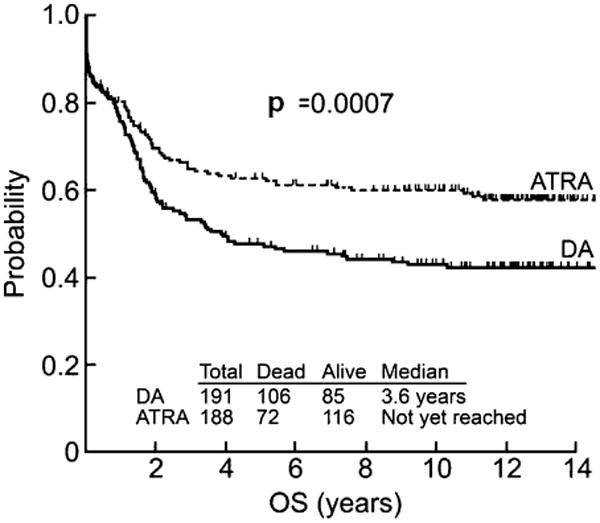
Kaplan-Meier overall survival (OS) curves for the total 379 patients by induction arm.
3.3. Relapse rate
Of 271 patients in CR, 112 patients are known to have relapsed. Only 9 (3.3%) patients relapsed after 3 years from achieving CR, while 103 (38%) relapsed earlier. After excluding patients who never received ATRA, the rate of late and early relapses remained unchanged. Of the 245 patients who received ATRA at any time (induction, maintenance or both), 195 (80%) achieved CR and 69 are known to have relapsed: 9 (4.6%) patients relapsed late all of whom had received ATRA at some time during treatment. One of the late relapses received ATRA in induction but was not randomized to maintenance therapy. Sixty (31%) patients relapsed early (two of them were not randomized to maintenance therapy).
3.4. Characteristics of late and early relapse
A comparison of the clinical characteristics between the patients with late and early relapses is presented in Table 1. With a cutoff at 3 years [23], the median time to early relapse was 0.9 (range 0.3–2.9) years compared to 3.9 in late relapses (range 3.1–4.6). Six relapses occurred between 3 and 4 years, 3 relapses after 4 years and the last relapse was after 4.6 years from achieving CR.
Table 1.
Characteristics of patients known to have relapsed.
| Late N = 9 | Early all patients N = 103 | Early ATRA anytimea N = 60 | p Valueb | |
|---|---|---|---|---|
| CR1 duration, years | ||||
| Median | 3.9 | 0.9 | 1.1 | |
| Range | 3.1–4.6 | 0.3–2.9 | 0.5–2.9 | |
| Gender, no (%) | ||||
| Females | 4 (44%) | 35 (34%) | 19 (32%) | 1.00 |
| Age, years | ||||
| Median | 45 | 36 | 32 | 0.56 |
| Range | 14–72 | 2–75 | 2–75 | |
| WBC × 1,000/mm3 | ||||
| Median | 1.9 | 4.3 | 3.2 | 0.51 |
| Range | 1.1–30 | 0–106 | 0–93 | |
| >10,000, no (%) | 3 (33%) | 30 (29%) | 19 (32%) | 1.00 |
Early ATRA anytime - patients who received ATRA at any time (induction, maintenance or both).
p value between late relapses and all early relapses (N = 103).
There were no statistically significant differences in age or WBC count at time of diagnosis between early and late relapse patients. About 30% of both groups had high risk APL with a WBC at diagnosis ≥10,000/mm3 .Results were the same after we excluded patients who never received ATRA (Table 1).
Of the 9 late relapses, 7 occurred in bone marrow, one occurred only in the central nervous system and one site of relapse is unknown. All relapses had the typical APL morphology and 5 of the known 7 bone marrow relapses were confirmed by cytogenetics for t15:17) or by PCR for PML/RAR transcripts. Seven were salvaged with chemotherapy, (4 together with ATRA), and one with ATRA only. Five patients underwent hematopoietic cell transplantation in second remission (4 allogeneic, 1 autologous) (Table 2). No patients received arsenic trioxide probably because they relapsed prior to the standard use of this treatment for APL in the USA.
Table 2.
Late relapses: patient characterization and treatment at first relapse.
| Patient | Age* years | CR1 duration years | Survival years | Treatment at 1st relapse | HSCT at CR2 | |
|---|---|---|---|---|---|---|
|
| ||||||
| After relapse | Overall | |||||
| 1 | 62 | 3.1 | 8 | 11.1 | Unknown | Unknown |
| 2 | 45 | 4.1 | 14.3 | 18.4 | ATRA + chemotherapy | Autologous |
| 3 | 46 | 4.1 | 0.1b | 4.2 | ATRA + chemotherapy | No |
| 4 | 38 | 4.0 | 13.4 | 17.4 | ATRA + chemotherapy | No |
| 5 | 62 | 3.3 | 0.1b | 3.4 | Chemotherapy | No |
| 6 | 58 | 4.6 | 5.4 | 10 | ATRA + chemotherapy | Allogeneic |
| 7 | 15 | 3.8 | 12.8 | 16.6 | Chemotherapy | Allogeneic |
| 8 | 14 | 3.7 | 14.8 | 18.5 | ATRA | Allogeneic |
| 9c | 16 | 3.9 | 13.1 | 17 | IT chemotherapy ATRA + chemotherapy | Allogeneic |
Age at diagnosis.
Patients who died after relapse.
Isolated CNS relapse; all other relapses were in the bone marrow.
3.5. Relapse based on randomization arm
The rate of early and late relapses did not differ significantly according to the induction or maintenance randomization arm. Among 142 CR patients after ATRA induction, 5 (3.5%) have relapsed (one of the 5 was not randomized to maintenance). It appeared, however, that patients who relapsed early were more likely to be on the observational maintenance arm (59%) than on the ATRA arm (33%) (p = 0.12) (Table 3).
Table 3.
Relapse based on randomization arm.
| Study arm | Late relapse | Early relapse | p Value |
|---|---|---|---|
| Induction arm (N = 271)a | |||
| DA (N = 129) | 4 (3.1%) | 63 (48%) | 0.48 |
| ATRA (N = 142) | 5 (3.5%) | 40 (28%) | |
| Maintenance arm (N = 218)b | |||
| Observation (N = 117) | 3 (2.6%) | 69 (59%) | 0.12 |
| ATRA N = (1 01) | 5 (4.9%) | 32 (31%) | |
Of 271 patients who achieved a CR, 53 were not randomized to the maintenance because of: relapse – 14 patients; intolerant to induction ATRA – 4 patients; discontinued protocol treatment after cycle 1 of consolidation – 9 patients. Unknown reason – 26 patients.
Three patients were not randomized to maintenance.
3.6. Survival of relapsed patients
The median follow-up of patients last known alive is more than 11 years for both relapse groups (Table 4). The median OS was 2.1 years for all early relapse patients and 2.5 years for the 60 patients who had received any ATRA (34% vs. 37%, respectively, last known alive). In contrast, of the 9 late relapses, 2 died from disease progression, and 7 were last known alive. Within the small number of late relapses, OS for late relapse patients was not dependent on the duration of first remission. The 12-year OS rate of the 159 patients who are not known to have relapsed is 81% (95% CI 74%, 87%) compared to 76% (95% CI: 33%, 94%) in patients with the late relapses and 31% (95% CI: 22%, 41%) in patients with early relapses (Fig. 3). Three second malignancies (Breast-2; Skin-1) were identified and all occurred in patients who have not relapsed.
Table 4.
Survival of patients known to have relapsed.
| Late N = 9 | Early All patients N = 103 | ATRA anytimea N = 60 | |
|---|---|---|---|
| Alive after relapse, N (%) | 7 (78%) | 35 (34%) | 22 (37%) |
| Follow-up of known to be alive, yrs. | |||
| Median | |||
| Range | 17.7 | 11.5 | 11.5 |
| 7.7–18.4 | 0.7–14 | 8.3–14 | |
| Overall survival after CR1, yrs. | |||
| Median | NR | 2.1 | 2.5 |
| 95% Confidence intervals | 3.3, ∞ | 1.7, 3.4 | 1.6, 5.3 |
Early ATRA anytime– patients who received ATRA at any time (induction, maintenance or both).
Fig. 3.
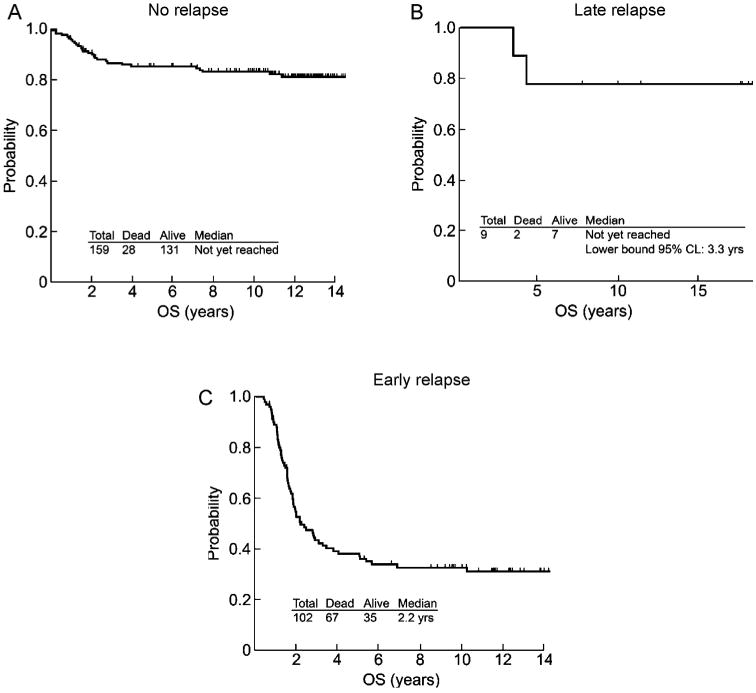
Overall survival (OS) of patients who achieved first complete remission (A) patients who are not known to have relapsed (n = 159) (B) patients who are known to have relapsed late, after 3 years or more (n = 9) (C) patients who are known to have relapsed early (n = 102). Note: One patient was missing a registration date and excluded from plot c.
4. Discussion
This report focuses on the long-term benefit of adding ATRA to intensive chemotherapy in patients with untreated APL. After a median follow-up of almost 12 years, to our knowledge the longest follow up published, we conclude that the significantly higher OS rate after induction with ATRA followed by chemotherapy consolidation is sustained; the 12-year OS rate was 60% versus 42% for the ATRA versus the chemotherapy induction arms, respectively. This extends the observation made in a previous update of this trial after a median follow-up of 6.2 years [18] and is in agreement with updated results of the 101 patient randomized APL 91 study [17], which reported a 4-year OS rate of 76% among patients induced with ATRA alone compared to 49% of those induced with chemotherapy.
Our current analysis together with its earlier update now clearly establishes that the relapse rate continues to decrease with time, such that after 4 years relapses are very rare. The relapse rate in the previous update of this trial [18] was 9% after 2 years, and in this study it decreased to 4.6% and to 1.6%, after 3 years, and 4.1 years, respectively. ATRA is obligatory in all current standard induction regimens [16]. The late relapse rate was low (3.5%) when only patients on the ATRA induction arm were analyzed. In addition, because of the double randomization design of our study, patients in one of the four arms (DA induction and observation maintenance) never received ATRA throughout the trial. After excluding the patients on this arm, the low relapse rate was confirmed in the patients who had received ATRA at sometime (during induction and/or maintenance), which interestingly included all 9 relapses. Overall, the very low relapse rate after 3–4 years confirms the results of the smaller randomized APL 91 trial with a shorter follow up [17], and the later large studies in which all patients had received ATRA after median follow-ups of 5.5–10 years [14,19,20,26,27]. Based on our data we can conclude that almost all APL patients treated with ATRA and chemotherapy who had not relapsed by 5 years after achieving CR can be considered cured.
While we recognize that only 9 patients on I0129 study experienced a late relapse, we found no particular characteristics that predicted for late relapse. There was no differences between patients with early and late relapses with respect to gender, age, or WBC count at diagnosis; in both relapse groups, 30% had high-risk APL, presenting with WBC count ≥10,000/mm3. These findings remained unchanged after excluding patients who had never received ATRA.
Our study, with its long term perspective, also contributes to the ongoing debate on the role of maintenance treatment in APL.When reported initially and at its first follow-up analysis, the one year ATRA maintenance was beneficial compared to observation [7,18]. Now, after much longer follow-up, patients who relapsed early appeared more likely to have been on the observation arm than on ATRA maintenance (59% versus 31%, respectively) though not significantly different, possibly because of the small number of patients analyzed. However late relapses did not depend on maintenance randomization. Interestingly, the 4-arm randomized maintenance GIEMEMA AIDA 0493 protocol initially reported more relapses in the two arms not containing ATRA but with longer follow-up, no difference was seen between the four arms [14]. In contrast, the APL 93 study with the same 4 randomization maintenance arms reported a persistent benefit of ATRA containing maintenance after a very long follow-up [19], but yet did not affect late relapses. Both studies demonstrate that maintenance reduced the incidence of early relapses, and the rate of late relapses. Taken together, we can now suggest that ATRA containing maintenance therapy prevents early relapses rather than delaying them. Recently, the maintenance randomization part of the second North American Intergroup study C9710, resulted in no difference in overall outcome between ATRA maintenance alone and ATRA with chemotherapy. The better overall outcome was dependent on earlier randomization to consolidation treatment that included arsenic trioxide [28].
The European APL91 and North American Intergroup I0129 trials are the only randomized trials that studied the role of ATRA in APL, in comparison to chemotherapy alone throughout the trial. Both studies provide a unique opportunity to compare late relapses with or without ATRA. In the APL91 trial after 4 years, 2 patients relapsed on the ATRA plus chemotherapy arm, but none on the chemotherapy alone arm [17]. In our study with its larger patient population, the rate of early relapses in those who never received ATRA was very high (56%) (data not shown), but none of them relapsed beyond 3 years; all late relapses occurred in patients who received ATRA. Therefore, we can now conclude, that late relapses are rare even without ATRA consistent with rare relapses beyond 4 years observed in the era before ATRA [17,20,29,30]. This suggests that the effect of ATRA in APL is probably relatively early in remission. Since in both the APL91 and our trial all late relapses occurred only in patients who had received ATRA treatment, we raise the possibility that ATRA may select or induce a molecular aberration – in some instances a mutation of PML–RARα fusion gene – in the ultimate relapse clone that was not eliminated by chemotherapy [31]. In addition as relapses beyond 4 years are rare regardless of ATRA treatment, we hypothesize that the APL clone is probably eradicated by then in the vast majority of cases. Yet, later relapses of APL have been observed even after 4 [19,20,25] or more years of continuous CR. The outcome of patients with late compared to those with early relapses was significantly better. Salvage chemotherapy, often with transplantation, had a durable effect and after a long follow-up (median 11 years), 7 of the 9 patients with late relapses were in continuous remission, even before arsenic trioxide was approved in the USA. The same favorable outcome of patients with late relapses after a very long follow up has been reported in the APL 93 study in which all patients received frontline ATRA [19,20]. This is analogous to the reported correlation between a longer duration of first remission and better outcome after relapse in AML in general.
The strengths of this study are its very long follow-up, the very large patient sample size, and the ability to compare patients with and without ATRA treatment in the same study. The study has several limitations. Although we contacted the individual sites, the clinical and laboratory characterization of late relapses was not more complete due to limitations of gathering retrospective documentation from patients treated more than a decade ago. In addition although all our late relapses had APL, late myelodysplastic syndromes or therapy-related non-APL AML may not have been well documented. However, this does not distract from the conclusions of the study that in APL late relapses are uncommon.
Because of the remarkable success of curing most APL patients since ATRA entered clinical practice, future well conducted systematic studies of long term survivorship, quality of life, treatment-related complications (e.g. cardiac function), and therapy-related AML are now needed. Most importantly, if the rate of early death can be reduced [32,33], even more patients may be cured with ATRA based therapies as demonstrated by the results of this study.
Acknowledgments
Funding: This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA21115, CA23318, CA66636, CA17145, CA14958, CA77658, CA32102, CA 37027, CA12213 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. Research support for the study: CA021115 and CA114737 were awarded to EP and CA56771 to REG.
Footnotes
Conflict of interest statement: All authors have no conflict of interest to report.
Contributions. D.D performed research, analyzed and interpreted data, wrote the manuscript; L.N.Z. analyzed and interpreted data, performed statistical analysis. C.A.S helped conceive the study design, performed research; F.R.A. helped conceive the original study design; J.H.F study design, performed research; LS performed research; C.W. SWOG coordinator of correlative studies, analyzed data, reviewed manuscript. C.D.B study design, collected data, approved final manuscript; E.P. collected data, approved final manuscript; REG helped interpret data and finalize manuscript; J.H.P. helped writing the paper; J.M.R analyzed and interpreted data; P.H.W study design, performed research; M.S.T national principal investigator, performed research, analyzed and interpreted data.
References
- 1.Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L. Use of all trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–72. [PubMed] [Google Scholar]
- 2.Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, et al. All trans retinoic acid as a differentiating therapy for acute promyelocytic leukemias I. Clinical results. Blood. 1990;76:1704–9. [PubMed] [Google Scholar]
- 3.Wanell RP, Frankel SR, Miller W, Scheinberg DA, Itri LM, Hittelman WH, et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all trans retinoic acid) N Engl J Med. 1991;324:1385–93. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- 4.Melnick A, Licht JD. Deconstructing a disease: RARα, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–215. [PubMed] [Google Scholar]
- 5.Nasr R, Lallemand-Breitenbach L, Zhu J, Marie-Claude Guillemin MC, de Thé H. Therapy induced PML/RARA proteolysis and acute promyelocytic leukemia cure. Clin Cancer Res. 2009;15:6321–6. doi: 10.1158/1078-0432.CCR-09-0209. [DOI] [PubMed] [Google Scholar]
- 6.Fenaux P, Le Deley MC, Castaigne S, Archimbaud E, Chomienne C, Link H, et al. Effect of all-trans retinoic acid in newly diagnosed acute promyelocytic leukemia, results of a multicenter randomized trial. Blood. 1993;82:3241–9. [PubMed] [Google Scholar]
- 7.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. All-trans retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–8. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 8.Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, et al. A randomized comparison of ATRA followed by chemotherapy and ATRA plus chemotherapy, and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. Blood. 1999;94:1192–200. [PubMed] [Google Scholar]
- 9.Burnett AK, Grimwade D, Solomon E, Wheatley K, Goldstone AH. On behalf of the MRC adult leukaemia working party, presenting white blood cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: result of the randomized MRC trial. Blood. 1999;93:4131–43. [PubMed] [Google Scholar]
- 10.Kanamaru A, Takemoto Y, Tanimoto M, Murakami H, Asou N, Kobayashiet T, et al. All-trans retinoic acid for the treatment of newly diagnosed acute promyelocytic leukemia, Japan adult leukemia study group. Blood. 1995;85:1202–6. [PubMed] [Google Scholar]
- 11.Sanz MA, Lo-Coco F, Martin G, Avvisati G, Rayón C, Barbui T, et al. Definition of relapse risk and role of non-anthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96:1247–52. [PubMed] [Google Scholar]
- 12.Lengfelder E, Reichert A, Schoch C, Haase D, Haferlach T, Löffler H, et al. Double induction strategy including high dose cytarabine in combination with all-trans retinoic acid: effects in patients with newly diagnosed acute promyelocytic leukemia. Leukemia. 2000;14:1362–70. doi: 10.1038/sj.leu.2401843. [DOI] [PubMed] [Google Scholar]
- 13.Lo-Coco F, Avvisati G, Vignetti M, Breccia M, Gallo E, Rambaldi A, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults patients younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116:3171–9. doi: 10.1182/blood-2010-03-276196. [DOI] [PubMed] [Google Scholar]
- 14.Avvisati G, Lo-Coco F, Paoloni FP, Petti MC, Diverio D, Vignetti M, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117:4716–25. doi: 10.1182/blood-2010-08-302950. [DOI] [PubMed] [Google Scholar]
- 15.Fenaux P, Castaigne S, Dombret H, Archimbaud E, Duarte M, Morel P, et al. All-trans retinoic acid followed by intensive chemotherapy gives a high complete remission rate and may prolong remissions in newly diagnosed acute romyelocytic leukemia: a pilot study on 26 cases. Blood. 1992;80:2176–218. [PubMed] [Google Scholar]
- 16.Sanz MA, Grimwade, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European leukemianet. Blood. 2009;113:1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 17.Fenaux P, Chevret S, Guerci A, Fegueux N, Dombret H, Thomas X, et al. Long-term follow-up confirms the benefit of all-trans retinoic acid in acute promyelocytic leukemia European APL group. Leukemia. 2000;14:1371–7. doi: 10.1038/sj.leu.2401859. [DOI] [PubMed] [Google Scholar]
- 18.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Woodset WG, et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American intergroup protocol. Blood. 2002;100:4298–302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- 19.Adès L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL group experience. Blood. 2010;115:1690–6. doi: 10.1182/blood-2009-07-233387. [DOI] [PubMed] [Google Scholar]
- 20.Kelaidi C, Ades L, Chevret S, Sanz M, Guerci A, Thomas X, et al. Late first relapses in APL treated with all-trans-retinoic acid- and anthracycline-based chemotherapy: the European APL group experience (APL 91 and APL 93 trials) Leukemia. 2006;20:905–7. doi: 10.1038/sj.leu.2404158. [DOI] [PubMed] [Google Scholar]
- 21.Ravandi F, Estey E, Jones D, Faderl S, O'Brien S, Fiorentino J, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504–10. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American leukemia intergroup study C9710. Blood. 2010;116:3751–7. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett JM, Young ML, Andersen JW, Cassileth PA, Tallman MS, Paietta E, et al. Cancer Long-term survival in acute myeloid leukemia: the Eastern cooperative oncology group experience. Cancer. 1997;80(Suppl 11):2205–9. [PubMed] [Google Scholar]
- 24.Agresti A. Categorical data analysis. New York: John Wiley & Sons; 1990. [Google Scholar]
- 25.Kaplan E, Meier P. Non parametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457. [Google Scholar]
- 26.Sanz MA, Montesinos P, Vellenga E, Rayón C, de la Serna J, Parody R, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA99 multicenter study by the PETHEMA group. Blood. 2008;112:3130–4. doi: 10.1182/blood-2008-05-159632. [DOI] [PubMed] [Google Scholar]
- 27.Lengfelder E, Haferlach C, Saussele S, Haferlach T, Schultheis B, Schnittger S, et al. High dose ARA-C in the treatment of newly diagnosed acute promyelocytic leukemia: long-term results ofthe German AMLCG. Leukemia. 2009;23:2248–58. doi: 10.1038/leu.2009.183. [DOI] [PubMed] [Google Scholar]
- 28.Powell BL, Moser BK, Stock W, et al. Adding mercaptopurine and methotrexate to alternate week ATRA maintenance therapy does not improve the outcome for adults with acute promyelocytic leukemia (APL) in first remission: results from North American leukemia intergroup trial c9710. Blood. 2011;118(118) abstr 258. [Google Scholar]
- 29.Marty M, Ganem G, Fischer J, Flandrin G, Berger R, Schaison G, et al. Acute promyelocytic leukemia: retrospective study of 119 patients treated with daunorubicin. Nouv Rev Fr Hematol. 1984;26:371–8. [PubMed] [Google Scholar]
- 30.Avvisati G, Petti MC, Lo-Coco F, Vegna ML, Amadori S, Baccarani M, et al. GIMEMA (Gruppo Italiano Malattie Ematologische dell'Adulto) Italian cooperative group, induction therapy with idarubicin alone significantly influences event-free survival duration in patients with newly diagnosed hypergranular acute promyelocytic leukemia: final results of the GIMEMA randomized study LAP 0389 with 7 years of minimal follow-up. Blood. 2002;100:3141–7. doi: 10.1182/blood-2002-02-0352. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher RE, Schachter-Toarz EL, Zhou DC, Ding W, Kim SH, Sankoorikal BJ, et al. Relapse of acute promyelocytic leukemia with PML-RARa mutant subclones independent of proximate all-trans retinoic acid selection pressure. Leukemia. 2006;20:556–62. doi: 10.1038/sj.leu.2404118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118:1248–54. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altman JK, Rademaker A, Cull E, Weitner B, Ofran Y, Rosenblat T, et al. Administration of all-trans retinoic acid (ATRA) to newly diagnosed patients (pts) with acute promyelocytic leukemia (APL) is delayed even at experienced centers and associated with an increased early death rate (EDR): a retrospective analysis of 205 pts. Blood. 2011;118(429) abstr 942. [Google Scholar]



