Abstract
P2Y receptors (P2YRs), a family of purinergic G-protein-coupled receptors (GPCRs), are activated by extracellular nucleotides. There are a total of eight distinct functional P2YRs expressed in human, which are subdivided into P2Y1-like receptors and P2Y12-like receptors1. Their ligands are generally charged molecules with relatively low bioavailability and stability in vivo2, which limits our under-standing of this receptor family. P2Y12R regulates platelet activation and thrombus formation3,4, and several antithrombotic drugs targeting P2Y12R—including the prodrugs clopidogrel (Plavix) and prasugrel (Effient) that are metabolized and bind covalently, and the nucleoside analogue ticagrelor (Brilinta) that acts directly on the receptor—have been approved for the prevention of stroke and myocardial infarction. However, limitations of these drugs (for example, a very long half-life of clopidogrel action and a characteristic adverse effect profile of ticagrelor)5,6 suggest that there is an unfulfilled medical need for developing a new generation of P2Y12R inhibitors7,8. Here we report the 2.6 Å resolution crystal structure of human P2Y12R in complex with a non-nucleotide reversible antagonist, AZD1283. The structure reveals a distinct straight conformation of helix V, which sets P2Y12R apart from all other known class A GPCR structures. With AZD1283 bound, the highly conserved disulphide bridge in GPCRs between helix III and extracellular loop 2 is not observed and appears to be dynamic. Along with the details of the AZD1283-binding site, analysis of the extracellular interface reveals an adjacent ligand-binding region and suggests that both pockets could be required for dinucleotide binding. The structure provides essential insights for the development of improved P2Y12R ligands and allosteric modulators as drug candidates.
P2Y12R, a member of the P2Y purinergic GPCR family stimulated by adenosine diphosphate (ADP), is a major player in platelet aggregation and granule secretion and supports the formation of a thrombus9. Ethyl 6-(4-((benzylsulphonyl)carbamoyl)piperidin-1-yl)-5-cyano-2-methylnicotinate (AZD1283) (Extended Data Fig. 1) was recently revealed by AstraZeneca as a novel P2Y12R antagonist for the treatment of arterial thrombosis. AZD1283 efficiently inhibits platelet activation in vivo with only a limited increase in the bleeding time10.
To understand how antithrombotic drugs recognize their purinoceptor target, we solved the structure of an engineered human P2Y12R by inserting a thermostabilized apocytochrome, b562 RIL (BRIL), in the third intracellular loop (ICL3)11 in complex with AZD1283 at 2.6 Å (Extended Data Table 1). A point mutation—D2947.49N (superscript indicates residue numbering using Ballesteros–Weinstein nomenclature12)—in the N[D]P7.50xxY motif of helix VII was introduced to improve the purified protein yield twofold (Extended Data Fig. 2). Saturation and competition binding assays indicate that neither the BRIL fusion nor the point mutation significantly influenced ligand binding of P2Y12R (Extended Data Table 2).
The overall fold of the P2Y12R structure consists of a canonical seven transmembrane (7TM) bundle of α-helices and a carboxy-terminal helix VIII that is parallel to the membrane bilayer (Fig. 1a). Several loops, especially ECL2, appear to be flexible and result in a total of 24 unmodelled loop residues (88–91 in ECL1, 133–135 intracellular loop 2 (ICL2), 163–178 ECL2 and 230 in ICL3). Only one disulphide bond is clearly observed in the structure, connecting the amino terminus (C17) with helix VII (C2707.25). Two receptor molecules from adjacent unit cells form receptor–receptor interactions in a parallel orientation mediated by helix V. Two cholesterol molecules are observed bound to each receptor: one is at the interface of helices III and V, stabilizing the receptor–receptor interaction, and the other is at the interface of helices I and VII and does not participate in crystal contacts (Extended Data Fig. 3).
Figure 1. Overview of the P2Y12R–AZD1283 complex structure.
a, Cartoon representation of P2Y12R. P2Y12R is coloured green. AZD1283 is shown as magenta spheres. Cholesterol and lipids have yellow carbons. The disulphide bridge is shown as lime sticks. Missing loops and membrane boundaries are indicated as black and blue dashed lines, respectively. b, c, Side (b) and top (c) views of P2Y12R (green cylinders) compared with β2AR (PDB accession 2RH1, brown) and PAR1 (PDB accession 3VW7, blue). The ligands AZD1283, carazolol and vorapaxar are shown as sticks with magenta, cyan and yellow carbons, respectively. Other elements are coloured as follows: oxygen, red; nitrogen, dark blue; sulphur, yellow. d, Comparison of P2Y12R with β2AR, PAR1, A2A adenosine receptor (A2AAR; PDB accession 3EML), neurotensin receptor (NTSR1; PDB accession 4GRV) and κ opioid receptor (κ-OR; PDB accession 4DJH). P2Y12R is shown in green and the other GPCRs are in grey.
The P2Y12R structure has important features that set it apart from another representative of the δ group of class A GPCRs13, protease activated receptor 1 (PAR1, ~24% sequence identity; transmembrane Cα root mean squared deviation (r.m.s.d.) ~2.2 Å), and other known class A structures, such as the β2-adrenergic receptor (β2AR, transmembrane Cα r.m.s.d. 2.6–3.5 Å) (Fig. 1b–d). Whereas helix V in most of the class A GPCR structures that have been determined so far is bulged and bent at a highly conserved P5.50, P2Y12R has N2015.50 (and V5.50 for the other P2Y12R-like receptors, P2Y13R and P2Y14R), and, consequently, its structure lacks the corresponding helical bend. Moreover, P2Y12R has no other proline or glycine residues in helix V that could destabilize its straight α-helical conformation. Besides P2Y12R, the only other receptor structure solved so far that also lacks the conserved proline and corresponding bend in helix V is the sphingosine 1-phosphate receptor 1 (S1P1)14. However, compared with S1P1, helix V of P2Y12R extends ~2 additional helical turns above the extracellular side of the membrane, and thus is also ~1–2 helical turns longer than in other known GPCR structures. The unique straightening and elongation of helix V observed in the P2Y12R structure results in a shift of its extracellular end towards helix IV by more than 6 Å in comparison with other known class A structures, leading to rearrangements in other helices and extracellular loops.
Other distinct conformational features of the P2Y12R structure are observed in helices VII and VI. The intracellular tip of helix VII is closer to the axis of the 7TM bundle than in most class A GPCR structures, in an ‘inward’ position similar to PAR1 (ref. 15). The helix VI intracellular tip is shifted slightly outward, and the whole helix VI is translated along its axis towards the intracellular surface by a half α-helical turn as compared with other known GPCR structures (Fig. 1d and Extended Data Fig. 4d). Owing to this shift, the conserved R1223.50 in the D(E)R3.50Y motif, which forms an ionic or a hydrogen-bonding lock with E6.30 or T6.34 in rhodopsin and several other GPCRs, is actually located at the same level as a hydrophobic side-chain V2386.37 in the P2Y12R structure (Extended Data Fig. 4a–c). This residue arrangement excludes formation of an ionic lock or polar interaction between helix VI and the DRY motif. The lack of an ionic lock or a polar interaction between helix VI and the DRY motif, as well as active-like conformations of the intracellular side of helices VI and VII, suggest that P2Y12R might be more prone to activation, in agreement with previous reports that this receptor exhibits high levels of basal activity16,17. Another key structural difference in helix VI occurs in the centre of the 7TM helical bundle at position 6.48, which in many class A GPCRs contains a tryptophan residue, involved in ligand binding and implicated in signal transduction18. In all P2YRs and PARs, however, W6.48 is replaced with F(Y)6.48, which displays the same orientation in both structures that might be a common feature within the δ group (Extended Data Fig. 4e).
AZD1283 binds to P2Y12R in a pocket that is very distinct in location and shape from that seen in previously solved GPCRs (Fig. 2). The elongated ligand stretches over more than 17 Å between helices IV and VII, making a number of polar and hydrophobic interactions with side chains from helices III–VII (Fig. 2a, b). The orientation of this ligand is orthogonal to a general ligand position in solved receptor complexes from α, β and γ groups in class A GPCRs, although it shares some similarity with the orientation of vorapaxar in PAR1, which belongs to the δ group (Extended Data Fig. 4). The piperidinyl–nicotinate group of the antagonist inserts into a sub-pocket formed by helices III, IV and V, whereas the benzylsulphonyl group mainly interacts with helices VI and VII. The structure reveals at least seven polar and ionic interactions between P2Y12R and AZD1283 (Fig. 2b). Additionally, the benzene ring of Y1053.33 forms a π–π interaction with the nicotinate and a hydrophobic interaction with the piperidine group, and the phenyl group of the ligand inserts into a hydrophobic pocket formed by the side chains of F2526.51, R2566.55, Y2596.58, L2767.31 and K2807.35.
Figure 2. P2Y12R ligand-binding pocket for AZD1283.
a, Key residues in P2Y12R for AZD1283 binding. AZD1283 (magenta carbons) and receptor residues (green carbons) involved in ligand binding are shown in stick representation. b, Schematic representation of interactions between P2Y12R and AZD1283. Polar interactions are shown as red dashed lines. c, d, Side view (c) and top view (d) comparison of ligand-binding sites in P2Y12R (green) and PAR1 (blue). The antagonists AZD1283 and vorapaxar have magenta and yellow carbons, respectively. e, Side view of the P2Y12R ligand-binding pocket. The receptor is shown in both green surface and grey ribbons, and AZD1283 is shown in sticks. Pocket 1 and pocket 2 are indicated by black lines.
Although the AZD1283-binding pocket of P2Y12R and its equivalent in PAR1 are formed by the same helices, there are significant differences between them. Whereas in PAR1, 24 residues of ECL2 cover the entire binding pocket and participate in extensive ligand interactions, the unresolved 16 residues of ECL2 in P2Y12R are likely to be more flexible and to have a lesser role in interactions with AZD1283. Moreover, the extracellular ends of helices IV, VI and VII in P2Y12R are shifted outwards compared with PAR1, making the ligand-binding pocket of P2Y12R more open and allowing AZD1283 to bind deeper in the 7TM domain (Fig. 2c, d). Within the P2Y12R extracellular cavity formed by all 7TM helices, two residues, Y1053.33 and K2807.35, form a barrier separating the cavity into two pockets. Pocket 1 is composed of helices III–VII, forming the binding site of AZD1283, whereas pocket 2 consists of helices I–III and VII and is not occupied in the structure (Fig. 2e).
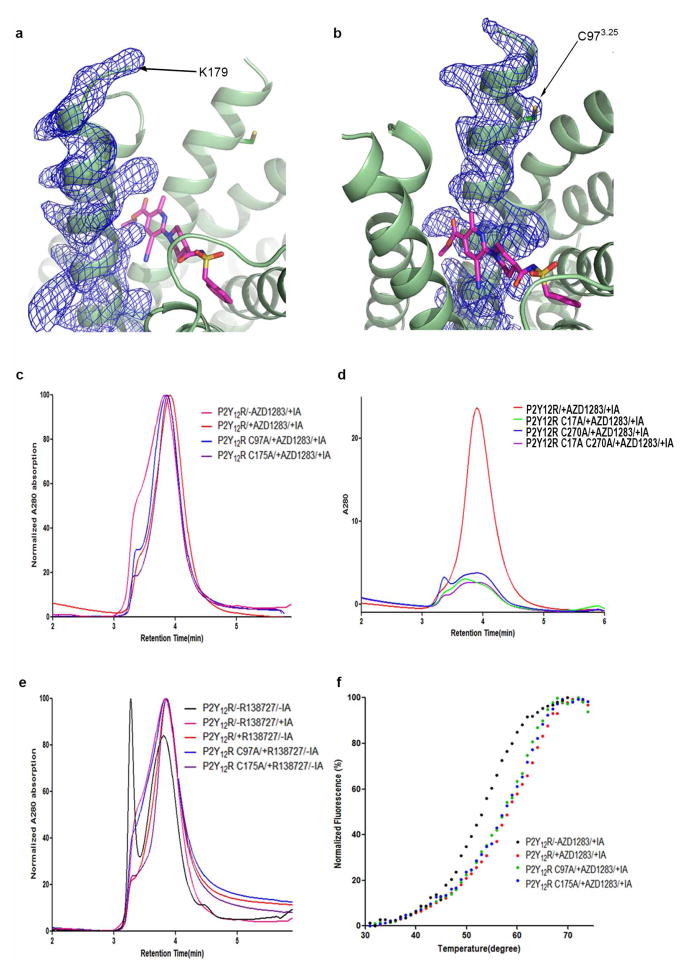
Two cysteine residues in helix III and ECL2 (C973.25 and C175 in P2Y12R) are highly conserved throughout the GPCR superfamily19, and, when present, they are observed to form a disulphide bond in all the GPCR structures solved so far20,21. To our knowledge, the only receptor structure that lacks the conserved Cys3.25 and the corresponding disulphide bridge is S1P1 (ref. 14), and it also lacks any secondary structure in ECL2. In the crystal structure of P2Y12R, no electron density is observed for most of ECL2 or for a potential disulphide bond at C97, suggesting the possibility of a labile or dynamic disulphide bond in P2Y12R, a feature that would be different from other known GPCRs and may be important functionally. Consistent with the labile nature of a disulphide in the structure, C973.25A and C175A mutations retain similar protein yield and stability, with no obvious aggregation, according to analytical size-exclusion chromatography (aSEC) (Extended Data Fig. 5c). In addition, the melting temperatures of both mutants are substantially higher in complex with AZD1283 than in the apo form and are equivalent to that of the native constructs (Extended Data Fig. 5f), suggesting that neither the receptor’s conformation nor its ability to bind AZD1283 is affected by the cysteine mutations. On the contrary, alanine mutations of C17 or C2707.25, which disrupt the disulphide bond that is clearly observed in the structure, lead to a marked receptor aggregation (Extended Data Fig. 5d). These data suggest that the disulphide bridge between ECL2 and helix III may be labile in native P2Y12R, thus leaving C973.25 and C175 available for interactions with the thiol moieties of drug metabolites.
Residue C973.25 has been previously implicated by functional assays as a covalent binding site for the active metabolites of P2Y12R drugs8,22. Our aSEC results are consistent with C973.25 being the primary attachment site for the active metabolite of prasugrel, R-138727 (Extended Data Fig. 5e). The suggestion of a dynamic disulphide bridge in our structure correlates with the previous observation that thiol-reactive reagents such as p-chloromercuribenzene sulphonate (pCMBS) specifically inhibit activation of P2Y12R but not other receptors such as P2Y1R23. In addition, these observations are consistent with the absolute selectivity of the active metabolites of prasugrel and clopidogrel for the P2Y12R; so far, there have been no reports that they exhibit detectable binding to any other receptor. The structure of P2Y12R opens up more possibilities to explain these previous observations, but additional evidence is needed to fully understand their mechanisms.
As residue C973.25 belongs to pocket 2 in the AZD1283-bound P2Y12R, the active metabolites of drugs that covalently link to this residue may occupy pocket 2 as well. This is consistent with the docking analysis, from which pocket 2 seems to be an energetically favourable binding site for these active metabolites (Fig. 3a, b). However, docking of nucleotide agonists into the crystal structure of antagonist-bound P2Y12 is less definitive, which probably reflects high conformational plasticity of the ligand-binding region in this receptor. Although limited mutagenesis data are consistent with binding of the nucleotides in pocket 2, docking allows for 2-methylthio-adenosine 5′-diphosphate (2MeSADP) binding in either pocket (Extended Data Fig. 6). Mutations of a few residues that belong to pocket 2, K802.60A or E2817.36A, or a residue at the interface of the two pockets, K2807.35A, decrease the binding affinity of the nucleotide radioligand [3H]2MeSADP (Extended Data Table 3 and Extended Data Fig. 7). In both docking models, R2566.55, which was previously reported to be important for the activation of P2Y12R24,25, potentially makes contact with the phosphate groups of 2MeSADP. This is consistent with pharmacological and biochemical data showing that mutations of this residue affect both the number of binding sites and their affinities for radio-labelled 2MeSADP in patient platelets and in transfected CHO cells26–28. Another residue, R265ECL3, which was previously reported to affect P2Y12R activation, is located on ECL3 and should not directly interact with 2MeSADP; the effect of mutations in R265ECL3 impairing receptor activation is probably due to their effects on the conformational states of thereceptor, rather than on agonist binding. Dinucleotide recognition at P2YRs is dependent on an optimal polyphosphate chain length and on precise substituents of both nucleoside moieties, suggesting that both pockets of the extended binding site participate in recognition. This is also consistent with dinucleotide docking to the AZD1283-bound P2Y12R structure, in which one nucleotide may bind in pocket 1 while the second half of the dinucleotide molecule is predicted to wrap around helix III to reach pocket 2, and the polyphosphate moiety occupies a highly cationic region of the binding site (Fig. 3c, d). The possibility of multiple binding pockets in the P2Y12R structure extends our knowledge of purinoceptor family ligand recognition mechanisms, and will serve as a template for designing improved orthosteric and allosteric drug candidates. The high conformational flexibility of the extracellular region suggests the potential for large conformational changes on nucleotide binding and activation, the understanding of which requires further structural studies of P2Y12R agonist complexes.
Figure 3. Hypothetical binding modes of other ligands to P2Y12R obtained by docking simulations.
a, b, Electrostatic surface representation (a) and cartoon representation (b) of the docking model of R-138727, with (R)-configuration of the reactive thiol-group and (S)-configuration at the benzylic position29, binding to P2Y12R (before covalent bond formation). The surface is coloured according to its electrostatic potential from red (negative) to blue (positive). The receptor is shown in green and R-138727 is represented as sticks with blue carbons. Side chains of residues involved in the binding of R-138727 are shown as sticks (yellow carbons). AZD1283 is shown as sticks with magenta carbons. c, d, Electrostatic surface representation (c) and cartoon representation (d) of the docking model of diadenosine tetraphosphate (Ap4A) binding to P2Y12R. Ap4A is represented as sticks with blue carbons, and the receptor and residues involved in binding are coloured as in b). Other elements are coloured as follows: red, oxygen; dark blue, nitrogen; yellow, sulphur; orange, phosphorus.
METHODS
Protein engineering for structural studies
Human wild-type P2Y12R DNA (HUGO Gene Nomenclature Committee (HGNC) accession 18124) was codon optimized and synthesized by Genewiz for insect cell expression, and cloned into a modified pFastBac1 vector (Invitrogen) containing an expression cassette with a haemagglutinin (HA) signal sequence followed by a Flag tag at the N terminus and a PreScission protease site followed by a 10×His tag at the C terminus. To facilitate crystallogenesis, thermostabilized BRIL (PDB accession 1M6T) was fused into ICL3 of P2Y12R (T223–R224) with intact N and C termini excluding the start codon. The P2Y12R-BRIL gene was further modified by introducing the D2947.49N mutation based on the sequence alignment of conserved motifs.
Expression and purification of Sf9-expressed P2Y12R constructs for crystallization
High-titre recombinant baculovirus (>108 viral particles per ml) was obtained using the Bac-to-Bac Baculovirus Expression System (Invitrogen). Sf9 cells at a cell density of 2–3 ×106 cells ml−1 were infected with virus at a multiplicity of infection (m.o.i.) of 5. Cells were harvested by centrifugation at 48 h post-infection and stored at −80 °C until use. Insect cell membranes were disrupted by thawing frozen cell pellets in a hypotonic buffer containing 10 mM HEPES, pH 7.5, 10 mM MgCl2, 20 mM KCl and protease inhibitor cocktail (Roche) with the ratio of 1 tablet per 100 ml lysis buffer. Extensive washing of the raw membranes was performed by repeated centrifugation in the same buffer and then in a high salt buffer containing 50 mM HEPES, pH 7.5, 10 mM MgCl2, 20 mM KCl and 1 M NaCl (three times each).
Purified membranes were thawed on ice in the presence of 200 μM AZD1283, 2 mg ml−1 iodoacetamide, and EDTA-free protease inhibitor cocktail (Roche), and incubated at 4 °C for 30 min before solubilization. P2Y12R-BRIL was extracted from the membrane by adding n-dodecyl-β-D-maltopyranoside (DDM; Affymetrix) and cholesteryl hemisuccinate (CHS; Sigma) to the membrane solution to a final concentration of 0.5% (w/v) and 0.1% (w/v), respectively, and stirring was continued at 4 °C for 2.5 h. The supernatant was isolated by centrifugation at 160,000g for 30 min and incubated with TALON IMAC resin (Clontech) overnight at 4 °C. The resin was then washed with twenty column volumes of 50 mM HEPES, pH 7.5, 1 M NaCl, 10% (v/v) glycerol, 0.05% (w/v) DDM, 0.01% (w/v) CHS and 20 mM imidazole. The protein was then eluted with 5 column volumes of 50 mM HEPES, pH 7.5, 1 M NaCl, 10% (v/v) glycerol, 0.05% (w/v) DDM, 0.01% (w/v) CHS, 300 mM imidazole and 200 μM AZD1283. A PD MiniTrap G-25 column (GE Healthcare) was used to remove imidazole. The protein was then treated overnight with His-tagged PreScission protease (20 μg per 500 ml of expressed material) and His-tagged PNGase F (20 μg per 500 ml of expressed material) to remove the C-terminal His tag and deglycosylate the receptor. PreScission protease, PNGase F and the cleaved 10×His tag were removed from the sample by passing the sample over Ni-NTA superflow resin (Qiagen). The receptor was then concentrated to 20–30 mg ml−1 with a 100 kDa molecular weight cut-off concentrator (Millipore). Protein purity and monodispersity was tested by SDS–PAGE and aSEC. Typically, the protein purity exceeded 95% and the aSEC profile showed a single peak, indicative of receptor monodispersity.
For aSEC analysis of P2Y12R in complex with R-138727 (Alsachim), the receptor was first treated with 100 μM R-138727 on insect cell membrane at 4 °C for 1 h and then purified under a similar protocol without further supplement with ligand thereafter.
Lipidic cubic phase crystallization of P2Y12R
The P2Y12R–BRIL construct was crystallized using the lipidic cubic phase (LCP) method by mixing 40% of ~40 mg ml−1 protein with 60% lipid (monoolein and cholesterol 10:1 by mass) using a syringe lipid mixer as described previously30. After a clear LCP formed, the mixture was dispensed onto glass sandwich plates (Shanghai FAstal BioTech) into 40 nl drops and overlaid with 800 nl precipitant solution using a Mosquito LCP robot (TTP LabTech). Crystals appeared after 3 days and reached their full size within 2 weeks in 0.05–0.15 M ammonium formate, 0.1 M sodium cacodylate, pH 6.0–6.5, 25–35% PEG400 and 200 μM AZD1283. Crystals were harvested directly from LCP using 100–150 μm micro-loops (M2-L19-100/150, MiTeGen) and flash frozen in liquid nitrogen.
Data collection and structure solution
X-ray data were collected on the 23ID-B/D beamline (GM/CA CAT) at the Advanced Photon Source using a 10 μm mini-beam (at a wavelength of 1.0330 Å) and a MarMosaic 300 CCD detector. Among the crystal samples screened, most crystals diffracted to 3.0–2.6 Å resolution when exposed to 1 s of unattenuated beam using 1° oscillation. Data from the 15 best-diffracting crystals were integrated and scaled to an overall 2.6 Å resolution using HKL200031. Initial phase information was obtained by molecular replacement using the receptor portion of PAR1 (PDB accession 3VW7) and BRIL (PDB accession 1M6T) independently with the program Phaser32. All refinements were performed with Refmac533 and Buster34 followed by manual examination and rebuilding of the refined coordinates in the program Coot35 using both 2mFo − DFc and mFo − DFc maps.
Ligand-binding assays
Wild-type and mutant P2Y12R plasmids with single amino acid substitutions (Extended Data Table 3) were cloned into PCDNA3.0 and transfected into COS7 cells using Lipofectamine 2000 (Life Technologies). Sf9 cells were harvested 48 h after transfection. After harvesting, cells were homogenized for 15 s and then centrifuged for 10 min at 1,000g. The suspension was re-centrifuged at 20,000g for 60 min. The resulting pellet was re-suspended, homogenized, split into aliquots and maintained at −80 °C in a freezer until use. Protein concentrations were measured using Bio-Rad protein assay reagents. Membranes for binding with the constructs containing BRIL and the point mutation (Extended Data Table 2) were prepared following the same procedure using Sf9 cells.
For saturation experiments, 50 μl [3H]2MeSADP (3.5 Ci mmol−1, from 0.4 to 46 nM; Moravek) was incubated with 100 μl wild-type and mutant P2Y12R membrane preparations (5 μg per tube) in a total assay volume of 200 μl Tris-HCl buffer containing 10 mM MgCl2. AZD1283 (10 μM) was used to determine non-specific binding. For displacement experiments, increasing concentrations of AZD1283 were incubated with wild-type or mutant membrane preparations (5–10 μg) and [3H]2MeSADP (10 nM) at 25 °C for 30 min. ADP, 2MeSADP and 2MeSATP were obtained from Sigma PSB-0739 was obtained from Tocris. The reaction was terminated by harvesting with a 24-channnel Brandel cell harvester and followed by washing twice with 5 ml cold Tris-HCl buffer containing 10 mM MgCl2. Radioactivity was measured using a scintillation counter (Tri-Carb 2810TR). Data were analysed using Prism 6 (GraphPad).
Docking and molecular modelling
The P2Y12R structure was prepared using the Protein Preparation Wizard36 tool implemented in the Schrödinger suite, adding all the hydrogen atoms and the missing side chains of residues whose backbone coordinates were observed in the structure. The orientation of polar hydrogens was optimized, the protein protonation states were adjusted and the overall structure was minimized with harmonic restraints on the heavy atoms, to remove strain. Then, all the hetero groups and water molecules were deleted.
The SiteMap tool of the Schrödinger suite was used to identify potential binding sites in the structure. A bifurcated cavity was identified on the extracellular side of the receptor and was selected as the docking site. Molecular docking of selected compounds (ADP, 2MeSADP, AZD1283, Ap4A and the active metabolites of clopidogrel and prasugrel) at the P2Y12R structure was performed by means of the Glide package from the Schrödinger suite. In particular, a Glide Grid was centred on the centroid of residues located within 6 Å from the previously identified cavity (considering both pocket 1 and pocket 2). The Glide Grid was built using an inner box (ligand diameter midpoint box) of 14 Å × 14 Å × 14 Å (so that both pockets could be explored) and an outer box that extended 10 Å in each direction from the inner one (so that ligands up to 20 Å could be docked). Docking of ligands was performed in the rigid binding site using the standard precision procedure. The top scoring docking conformations for each ligand were subjected to visual inspection and analysis of protein–ligand interactions to select the final binding conformations in agreement with the experimental data.
DNA sequence of the crystallization construct
ATGAAGACGATCATCGCCCTGAGCTACATCTTCTGCCTGGTGTTCGCCGACTACAAGGACGATGATGACGGCGCGCCGCAAGCCGTGGACAACCTCACATCAGCCCCTGGCAACACCTCCCTCTGTACCCGCGACTACAAGATCACACAAGTTCTCTTCCCCCTCCTCTACACAGTGTTGTTCTTCGTCGGCCTCATCACCAACGGATTGGCTATGCGTATCTTCTTCCAGATCCGCTCCAAGTCTAACTTCATCATCTTCCTGAAGAACACTGTGATCTCGGACCTGCTCATGATCCTCACATTCCCATTCAAGATCCTGTCAGATGCCAAGCTCGGTACTGGCCCGTTGCGTACATTCGTCTGCCAGGTTACCTCTGTGATCTTCTACTTCACTATGTACATCAGCATCTCATTCCTGGGTCTCATCACCATCGACAGGTACCAAAAGACCACTAGACCCTTCAAGACTAGCAACCCTAAGAACTTGCTGGGCGCTAAGATCCTGAGCGTGGTCATCTGGGCCTTCATGTTCCTCTTGTCACTGCCCAACATGATCCTCACCAACAGGCAGCCTAGAGATAAGAACGTGAAGAAGTGTTCATTCCTCAAGTCGGAGTTCGGATTGGTTTGGCACGAAATCGTGAACTACATCTGCCAAGTCATCTTCTGGATCAACTTCCTGATCGTTATCGTGTGTTACACATTGATCACCAAGGAGCTCTACAGGTCCTACGTCCGTACTGCTGATCTGGAAGACAATTGGGAAACTCTGAACGACAATCTCAAGGTGATCGAGAAGGCTGACAATGCTGCACAAGTCAAAGACGCTCTGACCAAGATGAGGGCAGCAGCCCTGGACGCTCAGAAGGCCACTCCACCTAAGCTCGAGGACAAGAGCCCAGATAGCCCTGAAATGAAAGACTTTCGGCATGGATTCGACATTCTGGTGGGACAGATTGATGATGCACTCAAGCTGGCCAATGAAGGGAAAGTCAAGGAAGCACAAGCAGCCGCTGAGCAGCTGAAGACCACCCGGAATGCATACATTCAGAAGTACCTGCGCGGAGTCGGCAAGGTTCCTAGGAAGAAGGTCAACGTTAAGGTGTTCATCATCATCGCTGTCTTCTTCATCTGCTTCGTTCCATTCCACTTCGCCCGTATCCCGTACACTTTGTCCCAAACACGCGACGTGTTCGATTGTACCGCTGAGAACACTCTGTTCTACGTCAAGGAATCCACATTGTGGCTGACCTCTCTGAACGCCTGCCTCAACCCATTCATCTACTTCTTCCTCTGTAAGTCTTTCCGCAACTCGTTGATCTCCATGCTGAAGTGCCCTAACTCTGCTACCAGCCTGTCCCAAGATAACAGAAAGAAGGAGCAGGACGGAGGCGACCCGAACGAGGAAACCCCGATGGGCCGGCCTCTGGAAGTTCTGTTCCAGGGGCCCCATCATCATCATCATCATCATCATCATCATTAG.
Extended Data
Extended Data Figure 1. Chemical structures of different P2Y12R ligands.
a, AZD1283. b, ADP. c, 2MeSADP. d, R-138727. e, Diadenosine tetraphosphate (Ap4A).
Extended Data Figure 2. Size-exclusion chromatography traces, crystals and overall structure of P2Y12R–AZD1283 complex.
a, aSEC traces of P2Y12R–BRIL (black) and P2Y12R(D294N)–BRIL (red) purified in complex with AZD1283. The samples are expressed and purified in parallel from roughly the same amount of cells. b, Crystals of P2Y12R–BRIL and AZD1283 complex. The size of crystals is roughly 150 × 5 × 5 μm. c, Crystals of P2Y12R(D294N)–BRIL and AZD1283 complex. The size of crystals is roughly 150 × 15 × 15 μm. d, Cartoon representation of P2Y12R(D294N)–BRIL. The P2Y12R is shown in pale green ribbons, BRIL is in wheat ribbons, AZD1283 is magenta carbons, and cholesterols and lipids are yellow carbons. e, The 2mFo − DFc electron density map of the ligand-binding pocket contoured at 1.2σ. f, The 2mFo − DFc electron density map of the DRY motif region contoured at 1.2σ.
Extended Data Figure 3. Dimeric receptor association and interactions with cholesterols in the crystals of P2Y12R.
a, Two P2Y12R molecules make contact with each other, as mediated by helix V and two molecules of cholesterol related by a two-fold axis. a, b, The detailed interactions of the cholesterol molecules on helices III and V are shown in surface (b) and in cartoon (c) representations. The cholesterol molecule is coloured in yellow carbons, and P2Y12R is shown in pale green (surface) or grey (cartoon). Residues within 4 Å of cholesterols are represented as green sticks. Hydrogen-bond interactions are indicated by dashed lines. As the interactions of the two cholesterols at the interface are identical, only one is revealed in detail. d, e, The binding site of cholesterol between helices I and VII is shown in surface (d) and in cartoon (e) representation. AZD1283 is shown in magenta carbons.
Extended Data Figure 4. Comparison of relative residue positions between helix III and helix VI of P2Y12R, PAR1 and β2AR.
a, P2Y12R. b, PAR1. c, β2AR. The receptors are shown in grey ribbon representation. The DR3.50Y(F) motif and corresponding 6.37 (or 6.34) positions are shown as sticks of green, slate and blue carbons, respectively. d, Superimposition of P2Y12R with other GPCR structures. P2Y12R (pale green), β2AR (PBD accession 2RH1; blue), A2AAR (PDB accession 3EML; orange), κ-OR (PDB accession 4DJH; pink), NTSR1 (PDB accession 4GRV; yellow) and PAR1 (PDB accession 3VW7; slate) are superimposed and shown as ribbons. Transmembrane helices I, II and VII overlay relatively well, whereas the position of helix VI is substantially different in P2Y12R. e, Comparison of W(F)6.48 positions in P2Y12R (pale green), β2AR (blue), A2AAR (orange), κ-OR (pink) and PAR1 (light blue). For receptors other than P2Y12R, only helix VI is shown, and the residues at position 6.48 are shown as sticks. f, The comparison of ligand-binding sites of GPCRs from α (β2AR and A2AAR), β (NTSR1), γ (CXCR4; PDB accession 3OE0) and δ (P2Y12R and PAR1) subgroups. The structure of P2Y12R is shown in grey cartoon representation and AZD1283 is shown as magenta sticks. The ligands from other receptors (carazolol, cyan; ZM241385, green; neurotensin, red cartoon; IT1t, purple; vorapaxar, yellow) are placed at their corresponding positions in the 7TM bundle.
Extended Data Figure 5. The conserved helix III–ECL2 disulphide bond might be dynamic in AZD1283-bound P2Y12R.
a, b, The electron density of helix V (a) and helix III (b). Electron density is represented by a 2mFo − DFc map countered at 1.2σ. The K179 and C973.25 side chains are indicated with black arrows. c–f, Effects of cysteine mutations on P2Y12R–BRIL. As the stability of wild-type P2Y12R is very poor, all the constructs here contain fusion protein and D294N mutation as described in the crystallization of the receptor. c, Comparison of aSEC traces of P2Y12R apo and P2Y12R, C97A, C175A in complex with AZD1283. The samples were treated with iodoacetamide (IA) before extraction. d, Comparison of aSEC traces of P2Y12R, C17A, C270A and C17A/C270A in complex with AZD1283. e, aSEC curves of purified P2Y12R (red), P2Y12R C97A (blue) and P2Y12R C175A (purple) with R-138727, the active metabolite of prasugrel, which binds irreversibly to P2Y12R by interacting with its cysteine residue(s). aSEC curve of apo P2Y12R is shown in black. It is obvious that treatment of R-138727 greatly improves the homogeneity of P2Y12R, which is not affected by the C175A mutation. However, the C97A mutation almost completely abolishes the effect of R-138727, indicating that C973.25 is the binding site of this compound. f, The melting curves of P2Y12R, C97A, C175A binding with AZD1283 and P2Y12R in the apo form.
Extended Data Figure 6. The hypothetical binding modes of 2MeSADP to antagonist-bound state P2Y12R.
a, b, An electrostatics surface representation (a) and a cartoon representation (b) of the hypothetical docking model of P2Y12R with bound 2MeSADP in pocket 2. The agonist 2MeSADP is shown as sticks (deep blue carbons). The side chains of residues that are involved in the binding of 2MeSADP are also labelled and shown as sticks (yellow carbons). c, d, An electrostatics surface representation (c) and a cartoon representation (d) of the hypothetical docking model of P2Y12R with bound 2MeSADP in pocket 1.
Extended Data Figure 7. Representative saturation curves of [3H]2MeSADP-specific binding to wild-type P2Y12R and various mutant receptors at a concentration range of 0.4–46 nM.
The calculated Kd values from 3–6 independent experiments are listed in Extended Data Table 3. Non-specific binding was determined using 10 μM AZD1283.
Extended Data Table 1.
Data collection and refinement statistics
| Data Collection* | |
| Space group | C2 |
| Cell dimensions | |
| a, b, c (Å) | 98.6, 156.4, 47.8 |
| α, β, γ (°) | 90.0, 111.1, 90.0 |
| Number of reflections processed | 73,502 |
| Number of unique reflections | 19,116 |
| Resolution (Å) | 50.0-2.6 (2.7-2.6)† |
| Rmerge (%) | 10.3 (95.8) |
| CC1/2 | 0.99 (0.64) |
| Mean I/σ(I) | 14.4 (1.2) |
| Completeness (%) | 94.1 (79.5) |
| Redundancy | 3.8 (3.1) |
| Refinement | |
| Resolution (Å) | 50.0-2.6 |
| Number of reflections (test set) | 19,094 (990) |
| Rwork/Rfree (%) | 22.0/24.6 |
| Number of atoms | |
| Protein | 2,886 |
| Ligand | 33 |
| Cholesterol | 56 |
| Lipids, PEG and waters | 50 |
| Overall B values (Å2) | |
| P2Y12R | 107.4 |
| BRIL | 99.7 |
| Ligand | 95.6 |
| Cholesterol | 133.2 |
| Lipids and waters | 119.1 |
| RMSD | |
| Bond lengths (Å) | 0.010 |
| Bond angles (°) | 1.05 |
| Ramachandran plot statistics (%) ‡ | |
| Favored regions | 96.4 |
| Allowed regions | 3.6 |
| Disallowed regions | 0.0 |
Fifteen crystals were used for data processing.
Values in parentheses are for highest-resolution shell.
As defined in MolProbity.
Extended Data Table 2.
Binding affinities for different P2Y12R constructs
| Inhibition of binding | Ki (nM), WT | Ki (nM), WT+BRIL | Ki (nM), WT+D294N mutation | Ki (nM), WT+BRIL +D294N mutation |
|---|---|---|---|---|
| ADP | 332±35 | 405±122 | 169±81 | 280±85 |
| 2MeSADP | 120±7.3 | 91.3±30.7 | 80.8±16.8 | 128±48 |
| 2MeSATP | 119±19.8 | 68.0±20.8 | 82.4±28.5 | 93.2±19.9 |
| AZD1283 | 31.5±11.9 | 39.3±9.9 | 42.2±27.7 | 43.9±10.0 |
| PSB-0739 | 275±58 | 155±73 | 72.8±16.7 † | 157±58 |
| Ticagrelor | 454±110 | 299±138 | 424±193 | 182±70 |
| Saturation binding | WT | WT+BRIL | WT+D294N mutation | WT+BRIL+D294N mutation |
|---|---|---|---|---|
| Kd (nM) | 35.9±6.3 | 36.7±3.6 | 33.6±10.2 | 51.5±12.0 |
| Bmax (pmol/mg protein) | 6.34±0.27 | 6.87±0.78 | 11.4+1.1 | 11.9±0.63 |
Data represent mean ± standard error of the mean (s.e.m.) from 3–8 separate experiments performed in duplicate.
One-way analysis of variance with post-hoc test yielded significant difference (P<0.05) compared with wild type (WT) but not with WT+BRIL and WT+BRIL+D294N groups (P>0.05). The affinities of other ligands at wild type are not significantly different from their affinities at mutants (P > 0.05).
Extended Data Table 3.
[3H]2MeSADP binding affinities for different P2Y12R mutants
| Constructs | [3H]2MeSADP (Kd, nM) |
|---|---|
| WT | 4.25 ± 0.87 |
| K80A | N.S.* |
| S156A | 5.66 ± 0.72 |
| N159A | 6.39 ± 0.94 |
| K280A | N.S. |
| E281A | N.S. |
N.S., not saturable or negligible specific binding within the radioligand concentrations used (0.4–46 nM). Results are expressed as mean ± s.e.m. from 3–6 independent experiments performed in duplicate.
Acknowledgments
This work was supported by National Basic Research Program of China grants 2012CB910400 and 2012CB518000 (B.W., Q.Z.), National Institutes of Health (NIH) grants R01 AI100604 (B.W., Q.Z.) and U54 GM094618 (V.C., V.K., R.C.S.; Target GPCR-87), National Science Foundation of China grants 31370729 and 31170683 (B.W., Q.Z.), the National Institute of General Medical Sciences Postdoctoral Research Associate program (E.K.) and the NIH National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program (K.A.J.). The authors thank AstraZeneca for their gift of AZD1283, and thank S. Nylander, F. Giordanetto and H. van Giezen for careful review and scientific feedback on the manuscript, A. Walker for assistance with manuscript preparation, and C. Wang and D. Wacker for help on collection of X-ray diffraction data.
Footnotes
Online Content Any additional Methods, Extended Data display items and Source Data are available in the online version of the paper; references unique to these sections appear only in the online paper.
Author Contributions K.Z. optimized the construct, expressed and purified human P2Y12R–BRIL for crystallization, developed the purification procedure, performed crystallization trials and optimized crystallization conditions. J.Z. helped in construct and crystal optimization, and collected diffraction data. Z.-G.G. designed, performed and analysed ligand-binding and competition assays of wild-type and mutant P2Y12R. D.Z. helped in expression and purification. L.Z. designed and made constructs for baculoviral expression. G.W.H. solved and refined the structure. S.M.M. performed and analysed ligand-binding assays. S.P. performed and analysed docking assays. E.K. helped in ligand synthesis of P2Y12R. W.L. helped in crystal optimization. G.F. helped in crystallographic data collection. W.Z. developed the initial expression and purification protocol for P2Y12R. C.E.M. provided compounds and discussed results. H.Y. helped to design and analysed docking assays. H.J. oversaw design and validation of P2Y12R models. V.C. helped to design and optimize LCP crystallization trials, collected and processed crystallographic data and wrote the manuscript. V.K. performed and analysed molecular modelling simulations, and wrote the manuscript. K.A.J. oversaw, designed and analysed ligand-binding assays and docking, and assisted with manuscript preparation. R.C.S. oversaw expression, purification and crystallization, and structure analysis/interpretation of P2Y12R. B.W. and Q.Z. initiated the project, planned and analysed experiments, supervised the research and wrote the manuscript.
Atomic coordinates and structure factors have been deposited in the PDB under accession number 4NTJ.
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of the paper.
References
- 1.Abbracchio MP, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010;15:570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach P, et al. Synthesis, structure–property relationships and pharmacokinetic evaluation of ethyl 6-aminonicotinate sulfonylureas as antagonists of the P2Y12 receptor. Eur J Med Chem. 2013;65:360–375. doi: 10.1016/j.ejmech.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Jagroop IA, Burnstock G, Mikhailidis DP. Both the ADP receptors P2Y1 and P2Y12, play a role in controlling shape change in human platelets. Platelets. 2003;14:15–20. doi: 10.1080/0953710021000062914. [DOI] [PubMed] [Google Scholar]
- 5.Wallentin L, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 6.Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27:259–274. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savi P, et al. The active metabolite of clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci USA. 2006;103:11069–11074. doi: 10.1073/pnas.0510446103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Algaier I, Jakubowski JA, Asai F, von Kügelgen I. Interaction of the active metabolite of prasugrel, R-138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. J Thromb Haemost. 2008;6:1908–1914. doi: 10.1111/j.1538-7836.2008.03136.x. [DOI] [PubMed] [Google Scholar]
- 9.Nylander S, Mattsson C, Ramstrom S, Lindahl TL. Synergistic action between inhibition of P2Y12/P2Y1 and P2Y12/thrombin in ADP- and thrombin-induced human platelet activation. Br J Pharmacol. 2004;142:1325–1331. doi: 10.1038/sj.bjp.0705885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach P, et al. Lead optimization of ethyl 6-aminonicotinate acyl sulfonamides as antagonists of the P2Y12 receptor. Separation of the antithrombotic effect and bleeding for candidate drug AZD1283. J Med Chem. 2013;56:7015–7024. doi: 10.1021/jm400820m. [DOI] [PubMed] [Google Scholar]
- 11.Chun E, et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure. 2012;20:967–976. doi: 10.1016/j.str.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballesteros J, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 13.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 14.Hanson MA, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, et al. High-resolution crystal structure of human protease-activated receptor 1. Nature. 2012;492:387–392. doi: 10.1038/nature11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chee MJ, et al. The third intracellular loop stabilizes the inactive state of the neuropeptide Y1 receptor. J Biol Chem. 2008;283:33337–33346. doi: 10.1074/jbc.M804671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz A, Schöneberg T. The structural evolution of a P2Y-like G-protein-coupled receptor. J Biol Chem. 2003;278:35531–35541. doi: 10.1074/jbc.M303346200. [DOI] [PubMed] [Google Scholar]
- 18.Audet M, Bouvier M. Restructuring G-protein- coupled receptor activation. Cell. 2012;151:14–23. doi: 10.1016/j.cell.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Rader AJ, et al. Identification of core amino acids stabilizing rhodopsin. Proc Natl Acad Sci USA. 2004;101:7246–7251. doi: 10.1073/pnas.0401429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan Q, et al. Structure of the CCR5 chemokine receptor–HIV entry inhibitor maraviroc complex. Science. 2013;341:1387–1390. doi: 10.1126/science.1241475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 2013;53:531–556. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Z, Bynagari YS, Mada SR, Jakubowski JA, Kunapuli SP. Studies on the role of the extracellular cysteines and oligomeric structures of the P2Y12 receptor when interacting with antagonists. J Thromb Haemost. 2009;7:232–234. doi: 10.1111/j.1538-7836.2008.03202.x. [DOI] [PubMed] [Google Scholar]
- 23.Ohlmann P, et al. The platelet P2Y12 receptor under normal and pathological conditions. Assessment with the radiolabeled selective antagonist [3H]PSB-0413. Purinergic Signal. 2013;9:59–66. doi: 10.1007/s11302-012-9329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cattaneo M. The P2 receptors and congenital platelet function defects. Semin Thromb Hemost. 2005;31:168–173. doi: 10.1055/s-2005-869522. [DOI] [PubMed] [Google Scholar]
- 25.Ignatovica V, Megnis K, Lapins M, Schiöth HB, Klovins J. Identification and analysis of functionally important amino acids in human purinergic 12 receptor using a Saccharomyces cerevisiae expression system. FEBS J. 2012;279:180–191. doi: 10.1111/j.1742-4658.2011.08410.x. [DOI] [PubMed] [Google Scholar]
- 26.Cattaneo M. The platelet P2Y12 receptor for adenosine diphosphate: congenital and drug-induced defects. Blood. 2011;117:2102–2112. doi: 10.1182/blood-2010-08-263111. [DOI] [PubMed] [Google Scholar]
- 27.Cattaneo M, et al. Molecular bases of defective signal transduction in the platelet P2Y12 receptor of a patient with congenital bleeding. Proc Natl Acad Sci USA. 2003;100:1978–1983. doi: 10.1073/pnas.0437879100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao Y, Zhang L, Jin J, Ashby B, Kunapuli SP. Mutational analysis of residues important for ligand interaction with the human P2Y12 receptor. Eur J Pharmacol. 2010;644:10–16. doi: 10.1016/j.ejphar.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa M, et al. Stereoselective inhibition of human platelet aggregation by R-138727, the active metabolite of CS-747 (prasugrel, LY640315), a novel P2Y12 receptor inhibitor. Thromb Haemost. 2005;94:593–598. doi: 10.1160/TH05-03-0208. [DOI] [PubMed] [Google Scholar]
- 30.Caffrey M, Cherezov V. Crystallizing membrane proteins using lipidic mesophases. Nature Protocols. 2009;4:706–731. doi: 10.1038/nprot.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 32.McCoy AJ, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vagin AA, et al. REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr D. 2004;60:2184–2195. doi: 10.1107/S0907444904023510. [DOI] [PubMed] [Google Scholar]
- 34.Smart OS, et al. Exploiting structure similarity in refinement: automated NCS and target–structure restraints in BUSTER. Acta Crystallogr D. 2012;68:368–380. doi: 10.1107/S0907444911056058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madhavi Sastry G, Adzhigirey M, Day T, Annabhimoju R, Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]