Abstract
The present study investigates if genetic variation in the serotonergic system interacts with early adversity to predict changes in the Behavioral Approach System (BAS), a system that taps into reward processing. In a sample of community adults (N= 236) the influence of single serotonergic candidate polymorphisms on BAS was analyzed, we also examined the aggregate contribution of these genetic variants by creating a Cumulative Genetic Score (CGS). A CGS quantifies an individual’s cumulative risk by aggregating the number of risk alleles across the candidate polymorphisms. After individual gene analysis, three candidate genes rs7305115 (TPH2), rs6311 (HTR2A), and rs6295 (HTR1A) were combined into the CGS. There were no significant interactions between individual candidate polymorphisms and childhood adversity, but the CGS interacted with childhood adversity to explain a significant amount of variance (11.6%) in the BAS. Findings suggest that genetic variations in the serotonergic system in combination with childhood adversity contribute to individual differences in reward sensitivity.
Keywords: Behavioral Approach System, Reward sensitivity, Serotonin function, Environmental adversity, GxE
Introduction
The Behavioral Approach System (BAS; Carver & White, 1994) is a bio-behavioral system related to reward processes and positive feelings. The BAS may be a fundamental dimension of personality and has associations with dimensions such as Cloninger’s novelty seeking (Cloninger, 1986) and Eysenck’s extraversion (Eysenck, 1947). Individuals with greater BAS sensitivity should experience more positive emotions and goal directed activity when they are exposed to a potentially rewarding stimuli.
The BAS is implicated in diverse psychopathologies (Johnson, Turner & Iwata, 2003). For example, high BAS levels may signal vulnerability to mania (Alloy & Abramson, 2010), whereas low BAS levels are common among depressed individuals (Kasch, Rottenberg, Arrow & Gotlib, 2002). Identifying factors that contribute to individual differences in BAS would advance our understanding of the etiologies of these psychopathologies, which is also in line with efforts to understand dimensional constructs that cut-across psychopathology and traditional diagnostic categories (Insel et al. 2010).
Early adverse environments can alter reward processes central to the BAS. For instance, in rodents repeated neonatal maternal separation can cause decreased responsiveness to rewards in adulthood (Matthews & Robbins, 2003). In humans, children with a history of trauma were less sensitive to reward values compared to controls (Guyer et al., 2006). Similar differences emerged in studies examining brain activation in regions associated with reward processes. Compared to controls, individuals with exposure to early life adversity showed less activity in the left basal ganglia (Dillon et al., 2009) and failed to recruit the ventral striatum (Mehta et al. 2010) during the anticipation of reward. Thus, there is substantial evidence that environmental adversity has a long-term, negative impact on reward processing.
Of course, not all individuals exposed to negative environments experience decreases in reward sensitivity. Individual differences in neurobiology may also contribute to the etiology of aberrant reward processing. Most research on the neurobiological mechanisms underlying reward processes focuses on the dopamine and opioid systems (Spanagel & Weiss, 1999); however, there is a growing awareness of the important role serotonin plays in these processes (Carver & Miller, 2006).
Serotonin function is implicated in psychopathologies associated with maladaptive reward processes, notably bipolar disorder (Nugent et al. 2013) and depression (Owens & Nemeroff, 1994). Reducing serotonin function by acute trypthopan depletion makes it more challenging to assess the magnitude of anticipated rewards (Rogers et al., 2003). Rodent models also indicate that enhancing serotonergic function impacts activity in the nucleus accumbens, a central brain reward system, which in turn alters reward behavior. More specifically, selective serotonin reuptake inhibitors normalized impairment in the nucleus accumbens and restored motivation and goal directed activity (Zangen, Nakash, Overstreet, & Yadid, 2001). Thus, serotonergic function appears to have an important role in reward sensitivity.
Numerous genetic variants related to the reuptake, synthesis and binding of serotonin have been identified. Within the serotonin transporter (5-HTT) gene two polymorphisms: the 5-HTT gene linked promoter region (5-HTTLPR) and STin2 have been the main focus. There are two variants of 5-HTTLPR, a short (S) and a long (L) allele. In a primed gambling task rhesus macaques carrying the S-allele were more risk averse when presented with a rewarding stimuli than monkeys homozygous for the L allele, who were more risk seeking (Watson, Ghodasra, & Platt, 2009). In humans, following acute trypthopan depletion S allele carriers showed attenuated motivational responses to reinforcing cues on a reaction time task when compared to L allele homozygotes (Roiser et al., 2006). For STin2, carriers of the 12-repeat were more risk-averse in a gambling task (Zhong et al. 2009).
Two commonly examined genes, 5-HTR1A and 5-HTR2A (serotonin receptor 1A and 2A), code for serotonin receptors. Within these genes two single nucleotide polymorphisms (SNPs), rs6295 and rs6311, code for receptor 5-HT1A and receptor 5-HT2A, respectively. Administration of a 5-HT1A agonist, 8-OH-DPAT, increased reward in rodents (Ahn et al., 2005), and reinforced the rewarding properties of cocaine in monkeys (Czoty, McCabe & Nader, 2005). The 5-HT2A seems integral in mediating the effects of some abused drugs such as 3,4-methylenedioxy-N-methylamphetamine (MDMA) (Liechti, Saur, Gamma, Hell & Vollenweider, 2000). The 5-HT2A rs6311 C-allele has been associated with decreased novelty seeking (Andre et al. 2010), while the 5-HT1A rs6295 C-allele has been linked to depression (Kishi et al. 2009).
Tryptophan hydroxlase (TPH) is the rate-limiting enzyme of the biosynthesis of serotonin, and thus plays an important role in serotoninergic functioning. In rodent studies the activity and expression of TPH2, a brain specific TPH isoform, is high in the ventral tegmental area, a brain region implicated in reward processes and motivation (Carkaci-Salli et al. 2011). Three SNPs found within TPH2 are rs4570625, rs7305115 and rs1386494. Although the relationship between these SNP’s and reward processes is unclear, we include these SNP’s here because of their putative association with serotonin functioning (Zhang, Beaulieu, Sotnikova, Gainetdinov & Caron, 2004). Furthermore, there is an association between the G allele of rs7305115 (Zhang et al. 2011) and the G-allele of rs1386494 (Zill et al. 2004) and depression, which in turn is implicated in reward processes (e.g., Juckel et al. 2005).
Thus, there is reasonable evidence that variation within the identified serotonergic genes, perhaps in concert with adverse environments, may contribute to individual differences in reward sensitivity. Nevertheless, in addition to single polymorphism analyses, we will also examine the aggregate contribution of these genetic variants to reward sensitivity. A Cumulative Genetic Score (CGS) includes two or more SNPs, and allows for the quantification of an individual’s cumulative risk by aggregating the number of risk alleles across the candidate polymorphisms. In the present study, we will aggregate alleles that putatively impair reward function. This CGS technique has been successfully employed in other studies (Disner, McGeary, Wells, Ellis & Beevers, 2014).
Thus, the aim of this study is to investigate environmental and genetic factors that contribute to individual differences in the BAS. First, interactions between each single polymorphism and adverse environment on the BAS will be examined. We hypothesize that carriers of vulnerability alleles will report lower BAS, especially when a history of environmental adversity is also reported. Second, the most promising polymorphisms will be included in a Cumulative Genetic Score (CGS). We hypothesize that those individuals with greater genetic risk (and thus a higher CGS) will report lower BAS, especially when a history of environmental adversity is also reported.
Methods
Participants
Participants were 263 community members, who did not meet criteria for serious current psychopathology, as determined by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I; First et al. 2002). Of the 263 participants, 27 had incomplete genetic or self-report data leaving 236 participants (mean age= 28.3, SD= 8.42, 53% Caucasian, 61% female) for inclusion in this study. We selected healthy individuals with no past or current psychopathology in an effort to isolate the genetic and environmental contributions to the BAS while removing third variable explanations, such as the presence of psychopathology, which may be related to both environmental adversity and variation within the selected genes.
Measures
Genotyping
Genotyping procedures and assaying procedures for 5-HTTLPR, HTR1A and HTR2A are described elsewhere (Disner et al., 2014) The Stin polymorphism was assayed using methods described by Bah and colleagues (2008). The primer sequences were: forward, 5′-GTCAGTATCACAGGCTGCGAG-3′, and reverse, 5′-TGTTCCTAGTCTTACGCCAGTG-3′. The 17 bp repetitive element of the STin2 polymorphism was measured as 7 to 12 copies of the repeat, 214bp (7), 248bp (9), 265bp (10) or 299bp (12) respectively. The genotypes were coded as to the number of copies of the 10-repeat allele.
Behavioral Approach System Scale
Behavioral approach sensitivity was measured by the BAS scale of Carver and White’s BIS/BAS scale (1994). The BAS consists of 13-items and is made up of 3 scales; BAS fun seeking (four items), BAS reward responsiveness (five items) and BAS drive (four items). Both total BAS and subscale scores were considered in this study. The fun seeking subscale measures a desire for new rewards and the willingness to impulsively approach potentially rewarding activities (e.g., “I will often do things for no other reason than that they might be fun”). The reward responsiveness subscale measures positive affect in response to the experience or anticipation of reward (e.g., “When I get something I want, I feel excited and energized.”). The drive subscale measures activity towards desired outcomes (e.g., “I go out of my way to get things I want.”). Items are scored on a four-point Likert scale ranging from “very true for me” to “very false for me”. Psychometric properties of the BAS are satisfactory, and the scale has demonstrated convergent and discriminant validity (Carver & White, 1994).
Childhood Trauma Questionnaire
Environmental adversity was measured with the brief version of the Childhood Trauma Questionnaire (CTQ; Bernstein et al. 2003). The CTQ consists of three validity items and five subscales (emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect) made up of five items each. For this study subscales scores were aggregated into a total CTQ score. Items are scored on a five-point Likert scale ranging from “never true” to “very often true”. The CTQ demonstrated good reliability and validity (Bernstein et al., 2003).
Procedure
All study procedures were completed at the University of Texas’ Mood Disorders Laboratory. Participants provided informed consent and study eligibility was confirmed by structured clinical interview. The self-report measures and a saliva sample for genetic analysis were collected during the baseline assessment. This study was approved by the Institutional Review Board at the University of Texas Austin.
Statistical Analysis
All analyses were performed in Stata 12 (StataCorp, 2011). The assumptions underlying regression were tested and confirmed for all analyses.
Results
Linear regression models examined the relationship between genetic variants, childhood adversity, and total score for the BAS. In single polymorphism analyses, genotype groups were modeled independently as group variables. Childhood adversity was modeled as a continuous variable. Each model contained genetic variant and childhood adversity main effects as well as their interaction. For the CGS analysis, the CGS variable also modeled as a group variable based on number of risk alleles. This allowed the slopes to vary across groups rather than forcing the groups to fit a pre-specified pattern (e.g., linear effect of CGS). Gene-environment correlations could threaten the validity of inference made about the relationship between genes and the BAS. Therefore, correlations between single genetic variants and the CTQ and the CGS and the CTQ were also examined. None of these correlations reached significance at the corrected (p < 0.007) or non-corrected (p < 0.05) alpha threshold. Genotype frequencies were consistent with HWE (p > 0.05) for all genes.
Single genetic variants
To minimize the likelihood of a Type I error, we adjusted our alpha based on the number of genetic polymorphisms we planned to examine. Given that we are investigating seven polymorphisms, we used a conservative alpha of .007 (α = .05/7) to determine statistical significance.
The results for each model testing the genotype x childhood adversity interaction for the prediction of BAS total scores is shown in Table 1. None of the models passed the adjusted threshold (p < .007) for statistical significance. However, three candidates, rs7305115 (TPH2), rs6311 (HTR2A), and rs6295 (HTR1A) approached statistical significance.
Table 1.
Effect of individual polymorphisms and childhood trauma on BAS total scores
| Genotype | rs | R2 | p |
|---|---|---|---|
| 5-HTTLPR | 0.0089 | 0.7618 | |
| TPH2 | rs4570625 | 0.0130 | 0.2955 |
| TPH2 | rs7305115 | 0.0578 | 0.0961 |
| TPH2 | rs1386494 | 0.0239 | 0.9799 |
| HTR2A | rs6311 | 0.0569 | 0.0102 |
| HTR1A | rs6295 | 0.0551 | 0.0080 |
| STin2 | rs57098334 | 0.0129 | 0.4199 |
Cumulative Genetic Risk score (CGS)
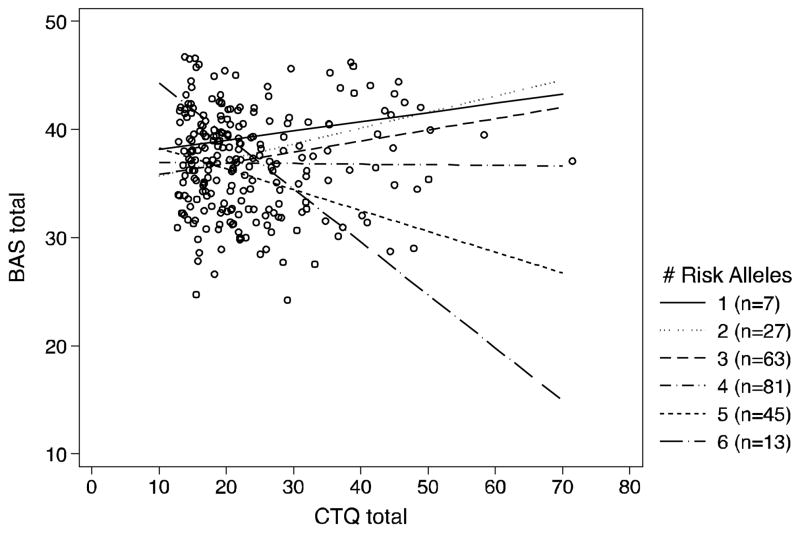
The interaction between early adversity and three polymorphisms—rs7305115, rs6311, and rs6295—from TPH2, HTR1A and HTR2A genes, respectively, approached statistical significance for the prediction of BAS total scores. Therefore, the aggregate effect of these three polymorphisms and childhood adversity on BAS total and subscale scores was examined. The CGS was computed by simply summing the number of risk variants (0, 1, or 2) across the three identified polymorphisms. See Table 2 for how each allele group across genetic variants contributed to the CGS. Participants ranged from 1 to 6 risk alleles (see Figure 1 legend for distribution).
Table 2.
Composition of CGS
| Genotype | Score=2 | Score=1 | Score=0 |
|---|---|---|---|
| rs7305115 | G/G | G/A | A/A |
| rs6311 | C/C | C/G | G/G |
| rs6295 | C/C | C/G | G/G |
Figure 1.
Relationship between BAS total scores and CTQ presented as function of CGS groups.
Regression analyses indicated a significant interaction between CGS and childhood adversity for predicting BAS total score (F(5, 224) = 4.08, p=0.001; see Figure 1). The entire model (main effects and interactions) accounted for 11.63% of the variance and the interaction in isolation accounted for 8.04% of the variance in BAS total scores. To understand the form of the interaction, we first examined whether the slopes for each CGS group differed from 0. The negative slopes for groups with 5 (b = −.19, SE = .07, p = .004) and 6 (b = −.49, SE = .20, p = .015) risk alleles were both significantly different from 0. Further, the positive slope for CGS group 3 was also significantly different from 0 (b = .10, SE = .05, p = .047). Slopes for the other CGS groups were not significant (ps ranged from .15 – .93). We next examined whether the slopes were significantly different from each other. As can be seen in Table 3, the slopes for CGS groups 5 and 6 differed from almost all other groups but not each other.
Table 3.
p-values for pairwise comparisons of slopes for CTQ total predicting BAS total across CGS groups.
| CGS1 | CGS2 | CGS3 | CGS4 | CGS5 | CGS6 | |
|---|---|---|---|---|---|---|
| CGS1 | .75 | .92 | .61 | .12 | .028 | |
| CGS2 | .70 | .19 | .005 | .005 | ||
| CGS3 | .17 | .001 | .004 | |||
| CGS4 | .04 | .02 | ||||
| CGS5 | .16 | |||||
| CGS6 |
Taken together, these analyses suggest that increased childhood adversity significantly predicted lower BAS for individuals with 5 or 6 risk alleles. For individuals with fewer risk alleles, childhood adversity was not strongly associated with BAS total, although there was a trend for increasing BAS as childhood adversity increased among people with 3 risk alleles.
When the BAS subscales were examined separately, a significant interaction between CGS and childhood adversity was observed for the BAS drive subscale (F(5,224) = 5.19, p= 0.000, R2(model)= 0.1192) and BAS reward subscale (F(5, 224)= 2.79, p= 0.02, R2(model) =0.081). The pattern of these interactions was consistent with the interaction depicted in Figure 1 for total BAS scale. The interaction effect of childhood adversity and CGS on BAS fun subscale scores was not significant (p=0.253).
Discussion
We examined the contribution of variation in genes that putatively influence serotonergic function and exposure to early adversity to individual differences in the BAS. When polymorphisms were examined in isolation, there was no evidence of a significant interaction between genetic variation and early adversity. However, a CGS comprising of three polymorphisms—rs7305115, rs6311, and rs6295 from TPH2, HTR1A and HTR2A genes, respectively—did indeed interact with childhood adversity to explain a significant amount of variance in the BAS. Furthermore, this model explained 11.6% of the variance in BAS, which is significantly more variance explained than typically observed in candidate gene studies.
The results support the hypothesis that serotonergic systems and environment interact to influence reward processing. In the context of an adverse childhood, a high CGS is associated with reduced reward sensitivity. However, it should be noted that in the absence of maltreatment, higher CGS score was associated with greater reward sensitivity. This finding is consistent with the recent conceptualization that low serotonergic function may be associated with greater responsiveness to the environment (Carver, Johnson & Joormann, 2008). These individuals may be particularly impulsive and have poor cognitive control in response to affective stimuli, leading them to pursue incentives/rewards with relatively low restraint.
Results from this study clearly suggest that the aggregate effect of multiple serotonergic SNPs was a more robust predictor than any one single SNP alone. However, this raises the question of why the CGS was more strongly associated with BAS than any single SNP. One possibility is that the CGS is capturing synergistic effects across multiple genes on reward sensitivity. Exploring specific gene x gene x environment interactions would be ideal but would also require a much larger sample size than was obtained for the current study. Another possibility is that CGS accounts for aspects of genetic background, whereas a single polymorphism cannot. The associations of a single candidate polymorphism may be obscured when there are multiple other variants contributing to a phenotype, but modeling them concurrently might allow a stronger and more reliable estimate of genetic influence (Gibb, Beevers & McGeary, 2012).
Future work in this area may also examine dopaminergic polymorphisms. Genes influencing the expression of dopamine are linked to reward processes (Hahn et al. 2011). Furthermore, there is evidence suggesting that the interaction of dopamine and serotonin is especially relevant for the investigation of reward processing (Zangen et al. 2001). Research exploring how dopaminergic and serotonergic genes interact (with environment) to influence reward processes might provide further insights into the biological pathways that contribute to impairment in these processes.
Although in this study impairment in reward processes is mostly conceptualized as a hyporesponsiveness to reward (low BAS), it is important to note that hyper-responsiveness to reward (high BAS) is also associated with adverse outcomes (e.g., Alloy & Abramson, 2010). Nevertheless, low CGS (even in combination with an adverse childhood) was not associated with dramatically elevated BAS (i.e., slopes did not differ from 0; see figure 1). This may not be surprising, as the genes selected for the current study were linked with hyporesponsiveness to reward and psychiatric disorders (such as depression) marked by deficiencies in reward responsivity. Other gene sets and environmental contexts may need to be examined to identify etiological factors that contribute to hyperresponsiveness to reward.
Several limitations of this study should be noted. First, this study has a relatively small sample size. As with many psychiatric genetics studies, there is a strong need to replicate these results in a larger sample. In this study we also relied on participants’ self-reported early environment and BAS, which can be prone to bias. Including behavioral measures of reward processing and gathering collateral on reports of environment would strengthen the methodology. Finally, we only examined a relatively limited number of polymorphisms in the current study. Future work may consider investigating a larger number of polymorphisms and using more sophisticated statistical modeling of the genetic effects (e.g., Holmes et al., 2012).
Because one cannot assign individuals to genotypes, genetic association studies are quasi-experimental in design. This bespeaks a vulnerability to unmeasured third variables that would otherwise have been randomly assigned to groups. Accordingly, these results may reflect relationships that driven by other factors. These other factors may be genetic (e.g., linkage disequilibrium of measured variants with unmeasured, causal variants, population stratification, etc.) or environmental. Future studies that use pharmacological or dietary probes to manipulate the serotonergic system would provide supporting evidence of the findings reported here. Additionally, the CGS strategy reported here makes assumptions of additive and equivalent (i.e., unweighted) genetic effects within and across genotypes and does not allow for epistatic effects. Moreover, the use of a CGS strategy in a gene by environment design makes additional assumptions that the constituent polymorphisms all interact similarly with the environmental exposure.
Nevertheless, to our knowledge this is the first study investigating the combined effect of serotonergic polymorphisms and adverse environment on reward sensitivity. These results suggest that genetic variation within the serotonergic system and adverse childhoods combine to produce individual differences in reward sensitivity. Deficiencies within this reward system, in turn, may increase a person’s risk for psychopathology.
Highlights.
We examined whether serotonergic polymorphisms and early adversity predict BAS.
A cumulative genetic score aggregated the contribution of three polymorphisms.
More serotonergic risk alleles interacted with early adversity to predict lower BAS.
Serotonergic genetic variation and early adversity contribute to reward sensitivity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn KC, Pazderka-Robinson H, Clements R, Ashcroft R, Ali T, Morse C, Greenshaw AJ. Differential effects of intra-midbrain raphe and systemic 8-OH-DPAT on VTA self-stimulation thresholds in rats. Psychopharmacology. 2005;178:381–388. doi: 10.1007/s00213-004-2031-3. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. The role of the behavioral approach system (BAS) in bipolar spectrum disorders. Current Directions in Psychological Science. 2010;19:189–194. doi: 10.1177/0963721410370292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Andre K, Kampman O, Setälä-Soikkeli E, Vikkii M, Poutanen O, Nuolivirta T, Illi A. Temperament profiles, 5-HT2A genotype, and response to treatment with SSRIs in major depression. Journal of Neural Transmission. 2010;117:1431–1434. doi: 10.1007/s00702-010-0512-6. [DOI] [PubMed] [Google Scholar]
- Antilla S, Viikki M, Huuhka K, Huuhka M, Huhtala H, Rontu R, Lehtimaki T, Leinone E. TPH2 polymorphisms may modify clinical picture in treatment resistant depression. Neuroscience Letters. 2009;464:43–46. doi: 10.1016/j.neulet.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Bah J, Lindström M, Westberg L, Mannerås L, Ryding E, Henningsson S, Eriksson E. Serotonin transporter gene polymorphisms: Effect on serotonin transporter availability in the brain of suicide attempters. Psychiatry Research Neuroimaging. 2008;162:221–229. doi: 10.1016/j.pscychresns.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Canli T, Congdon E, Gutknecht L, Constable RT, Lesch KP. Amygdala responsiveness is modulated by tryptophan hydroxylase-2 gene variation. Journal of Neural Transmissions. 2005;112:1479–1485. doi: 10.1007/s00702-005-0391-4. [DOI] [PubMed] [Google Scholar]
- Carver CS. Negative Affects Deriving From the Behavioral Approach System. Emotion. 2004;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Miller CJ. Relations of serotonin function to personality: current views and a key methodological issue. Psychiatry Research. 2006;144:1–15. doi: 10.1016/j.psychres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Carkaci-Salli N, Salli U, Kuntz-Melcavage KL, Pennock MM, Ozgen H, Tekin I, Vrana KE. TPH2 in the ventral tegmental area of the male rat brain. Brain Research Bulletin. 2011;84:376–380. doi: 10.1016/j.brainresbull.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatric Developments. 1986;4:167–226. [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Effects of the 5-HT1A agonist (±)-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on cocaine choice in cynomolgus monkeys. Behavioral Pharmacology. 2005;16:197–91. doi: 10.1097/00008877-200505000-00008. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, McGeary JE, Wells TT, Ellis AJ, Beevers CG. 5-HTTLPR, HTR1A, HTR2A cumulative genetic score interacts with mood reactivity to predict mood congruent gaze bias. Cognitive Affective Behavioral Neuroscience. 2014 doi: 10.3758/s13415-014-0267-x. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, Hudson JL, Creswell C, Tropeano M, Lester KJ, Cooper P, et al. Therapygenetics: the 5HTTLPR and response to psychological therapy. Molecular Psychiatry. 2012;17:236–237. doi: 10.1038/mp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ. Dimensions of personality. 1. London: Paul, Trench, Truber & Co; 1947. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Gibb BE, Beevers CG, McGeary JE. Toward an integration of cognitive and genetic models of risk for depression. Cognition and Emotion. 2012;27:193–216. doi: 10.1080/02699931.2012.712950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, Ernst M. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. Journal of the American Acadamy of Child and Adolescent Psychiatry. 2006;45:1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- Hettema JM, An SS, van den Oord EJCG, Neale MC, Kendler KS, Chen X. Asosciation study between the Serotonin 1A receptor (HTR1A) gene and neuroticism, major depression, and anxiety disorders. American Journal of Medical Genetics Part B: Neuropsychiatry. 2010;147B:661–666. doi: 10.1002/ajmg.b.30656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, Buckner RL. Individual differences in amygdala-medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. Journal of Neuroscience. 2012;32:18087–18100. doi: 10.1523/JNEUROSCI.2531-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Heinzel S, Dresler T, Plitcha MM, Tenner TJ, Markulin F, Fallgater J. Association between reward-related activation in the ventral stiatum and trait reward sensitivity is moderated by dopamine transporter genotype. Human Brain Mapping. 2011;32:1557–1565. doi: 10.1002/hbm.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research Domain Criteria (RDoC): Towards a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Turner RJ, Iwata N. BIS/BAS levels and psychiatric disorder: An epidemiological study. Journal of Psychopathology and Behavioral Assessment. 2003;25:25–36. [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Heinz A. Dusfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2005;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arrow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsunaka T, Ikeda M, Kawashima K, Kinoshita Y, Okumura T, Iwata N. Serotonin 1A receptor gene and major depressive disorder: an association study and meta-analysis. Journal of Human Genetics. 2009;54:629–633. doi: 10.1038/jhg.2009.84. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neuroscience & Biobehavioral Reviews. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colbert E, Williams SCR, Sonuga-Barke E. Journal of Cognitive Neuroscience. 2010;22:2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Munafo MR. Reliability and replicability of genetic association studies. Addiction. 2009;104:1439–1440. doi: 10.1111/j.1360-0443.2009.02662.x. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Brain EE, Carlson PJ, Neurmeister A, Bonne O, Carson RE, Drevets WC. Reduced post-synaptic serotonin type 1A reception binding in bipolar depression. European Neuropsychopharmacology. 2013;23:822–829. doi: 10.1016/j.euroneuro.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clinical Chemistry. 1994;40:288–295. [PubMed] [Google Scholar]
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28:153–162. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Blackwell AD, Cools R, Clark L, Rubinsztein DC, Robbins TW, Sahakian BJ. Serotonin transporter polymorphism mediates vulnerability to loss of incentive motivation following acute tryptophan depletion. Neuropsychopharmacology. 2006;31:2264–2272. doi: 10.1038/sj.npp.1301055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends in Neuroscience. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. College Stataion, TX: StataCorp LP; 2013. [Google Scholar]
- Sullivan PF. Spurious genetic associatins. Biological Psychiatry. 2007;15:1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Watson KK, Ghodasra JH, Platt ML. Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS One. 2009;4:e4156. doi: 10.1371/journal.pone.0004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Nakash R, Overstreet D, Yadid G. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology. 2001;155:434–439. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Tupprecht R, Ackenheil M. SNP and haplotype analysis of a noval trytophan hydroxylase isoform TPH2_ gnee provide evidence for association with major depression. Molecular Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu J, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan Hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- Zhang U, Zhang C, Yuan G, Yao J, Zaohuo, Cheng Z, Li L. Effect of trytophan hydroxylase-2 rs 7305115 SNP on suicide attempt risk in major depression. Behavioral and Brain Functions. 2011;6:49. doi: 10.1186/1744-9081-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Israel S, Xue H, Sham PC, Ebstein RP, Chew S. A neurochemical approach to valuation sensitivity over gains and losses. Preceedings of the Royal Society of Biological Sciences. 2009;276:4181–4188. doi: 10.1098/rspb.2009.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]