Abstract
Immunization with a combination melanoma helper peptide (6MHP) vaccine has been shown to induce CD4+ T cell responses, which are associated with patient survival. In the present study, we define the relative immunogenicity and HLA allele promiscuity of individual helper peptides and identify helper peptide-mediated augmentation of specific CD8+ T cell responses. Thirty-seven participants with stage IIIB-IV melanoma were vaccinated with 6MHP in incomplete Freund’s adjuvant. The 6MHP vaccine is comprised of 6 peptides representing melanocytic differentiation proteins gp100, tyrosinase, Melan-A/MART-1, and cancer testis antigens from the MAGE family. CD4+ and CD8+ T cell responses were assessed in peripheral blood and in sentinel immunized nodes (SIN) by thymidine uptake after exposure to helper peptides and by direct interferon-γ ELIspot assay against 14 MHC class I-restricted peptides. Vaccine-induced CD4+ T cell responses to individual epitopes were detected in the SIN of 63 % (22/35) and in the peripheral blood of 38 % (14/37) of participants for an overall response rate of 65 % (24/37). The most frequently immunogenic peptides were MAGE-A3281–295 (49 %) and tyrosinase386–406 (32 %). Responses were not limited to HLA restrictions originally described. Vaccine-associated CD8+ T cell responses against class I-restricted peptides were observed in 45 % (5/11) of evaluable participants. The 6MHP vaccine induces both CD4+ and CD8+ T cell responses against melanoma antigens. CD4+ T cell responses were detected beyond reported HLA-DR restrictions. Induction of CD8+ T cell responses suggests epitope spreading and systemic activity mediated at the tumor site.
Keywords: Melanoma, Peptide vaccines, CD4 T cells, Immunogenicity
Introduction
The role of CD4+ T lymphocytes in tumor immunotherapy is incompletely explored and may include augmentation of cytotoxic T lymphocyte (CTL) responses, licensing of antigen-presenting cells, or direct cytotoxic function [1]. In murine models, tumor control can be achieved by immunotherapy independent of CD8 T cells [2], and in humans, adoptive transfer of antigen-specific CD4+ T cells can mediate durable complete regression of advanced melanoma [3]. Thus, there is rationale for developing effective immune therapies targeting CD4+ T cells.
Thus far, most melanoma peptide vaccines have focused on CTL epitopes, as early studies utilizing individual HLA-DR restricted peptides showed disappointing immunogenicity [4–6]. However, in a phase I/II clinical trial, we demonstrated the safety, immunogenicity, and clinical activity of a mixture of 6 HLA-DR restricted melanoma helper peptides (6MHP) administered in combination with GM-CSF in an emulsion with Montanide ISA-51 adjuvant [7]. In a follow-up, randomized phase II study in which 6MHP was administered with CTL epitopes among patients with stage IV melanoma (ECOG 1602), a striking finding was a strong association between immune responses to 6MHP and patient survival [8]. While two of the peptides in the 6MHP combination were weakly immunogenic in prior studies, 4 additional peptides had not previously been tested in humans. These recent results show that there is promising immunogenicity and evidence of clinical activity for the 6MHP combination peptide vaccine.
We hypothesized that within this combination vaccine, there exists an immunological hierarchy, in terms of the ability of each peptide to elicit CD4+ T cell responses in vivo. Because the 6MHP component peptides had pre-existing reports of restriction by specific HLA-DR molecules, we had limited enrollment to patients expressing at least one of the reported restricting alleles (HLA-DR-1, −4, −11, −13, or −15). However, peptides that stimulate helper T cells are frequently promiscuous in their binding to HLA-DR molecules [9]; so, we hypothesized that each peptide would be immunogenic in the context of a broad array of HLA-DR molecules.
The intent of the present study is therefore threefold. First, we aim to provide a more comprehensive evaluation of the immunogenicity of each of these 6 melanoma-associated helper peptides derived from cancer testis antigens and melanocytic differentiation proteins. Second, the promiscuity of these peptides for specific HLA-DR molecules will be assessed. Last, we will determine whether helper peptide vaccination can induce CD8 T cell responses to class I-restricted melanoma peptides.
Materials and methods
Patients and vaccination
Thirty-seven evaluable participants with American Joint Committee on Cancer stage IIIB, IIIC or IV melanoma with (n = 17) or without (n = 20) measurable disease were vaccinated with an experimental melanoma vaccine comprised of 6 peptides 14–23 amino acids in length (Table 1). The patients were required to express HLA-DR1, DR4, DR11, DR13, or DR15. The peptides were administered with 110 mcg GM-CSF (Berlex, Seattle, WA) in a stable emulsion with 1 ml Montanide ISA-51 adjuvant (IFA, Seppic, Inc., Paris, France/Fairfield, NJ) at weeks 0, 1, 2, 4, 5, and 6. Following the third vaccination, a sentinel immunized node (SIN) draining the replicate vaccine site was removed surgically. Additional enrollment and study design details have been reported previously [7]. Patients’ blood and tissue samples were studied following informed consent, and with Institutional Review Board (HIC#10464) and FDA approval (BB-IND #1825).
Table 1.
6MHP helper peptide pool, derived from melanocytic differentiation antigens and cancer testis antigens. Immunogenicity presented as proportion of all test subjects, unrestrained by HLA-DR expression
| Sequence | Proposed HLA-DR Allele | Epitope | Immunogenic (PBMC or SIN) (n = 37) | PBMC (%) | SIN (%) |
|---|---|---|---|---|---|
| AQNILLSNAPLGPQFP [13] | HLA-DR4 | Tyrosinasea56–70 | 5 % (2) | 3 | 3 |
| FLLHHAFVDSIFEQWLQRHRP [14] | HLA-DR15 | Tyrosinase386–406 | 32 % (12) | 19 | 29 |
| RNGYRALMDKSLHVGTQCALTRR [12] | HLA-DR4 | Melan-A/MART-151–73 | 24 % (9) | 16 | 20 |
| TSYVKVLHHMVKISG [15] | HLA-DR11 | MAGE-A3281–295 | 49 % (18) | 27 | 46 |
| LLKYRAREPVTKAE [26] | HLA-DR13 | MAGE-A1,2,3,6121–134 | 22 % (8) | 5 | 23 |
| WNRQLYPEWTEAQRLD [17, 18] | HLA-DR1 & 4 | gp10044–59 | 5 % (2) | 3 | 6 |
aAn alanine residue was added to the N-terminus to prevent cyclization
Peptides for laboratory use
The 6MHP melanoma helper vaccine was comprised of six peptides, and our prior report focused on the overall response to the mixture of all 6 peptides. In the present report, we present data on CD4+ T cell responses to each of the 6 peptides individually: gp10044–59 (first 3 amino acids: WNR), Tyrosinase56–70 (AQN), Tyrosinase386–406 (FLL), Melan-A/MART151–73 (RNG), MAGE-A3281–295 (TSY), and MAGE-A1, 2,3,6121–134 (LLK) [7]. Additionally, a tetanus helper peptide AQYIKANSKFIGITEL [10] and the irrelevant peptide from HIV gag protein, SLYNTVATL [11] were included in the laboratory analyses. MHC class I-restricted peptides used to evaluate CD8+ T cell responses are listed in Table 2.
Table 2.
Class I MHC-restricted peptides used to detect de novo CD8+ T cell response by IFN-γ direct ELIspot assay
| Allele | Sequence | Epitope |
|---|---|---|
| HLA-A1 | DAEKSDICTDEY [27] | Tyrosinasea240–251S |
| SSDYVIPIGTY [28] | Tyrosinase146–156 | |
| EADPTGHSY [29] | MAGE-A1161–169 | |
| EVDPIGHLY [30, 31] | MAGE-A3168–176 | |
| HLA-A2 | YMDGTMSQV [32] | Tyrosinaseb369–377D |
| IMDQVPFSV [33] | gp100c209–217-2M | |
| YLEPGPVTA [34] | gp100280–288 | |
| GLYDGMEHL [35] | MAGE-A10254–262 | |
| AAGIGILTV [36] | MART-1/MelanA27–35 | |
| SLLMWQITA [37] | NY-ESO-1d157–165 | |
| HLA-A3 | ALLAVGATK [38] | gp10017–25 |
| LIYRRRLMK [28] | gp100614–622 | |
| SLFRAVITK [26] | MAGE-A196–104 | |
| ASGPGGGAPR [39, 40] | NY-ESO-153–62 |
aSubstitution of S for C at residue 244
bPost-translational change of N to D at residue 370
c 209-2M, substitution of M for T at position 210
dModified
Proliferation assays
While only a subset of patients expressed the HLA-DR allele reported to restrict each peptide, to test peptide promiscuity to HLA-DR restriction, we evaluated the immunogenicity of each peptide in patients with both matching and unmatched HLA-DR types. Detailed assay methodology has been reported previously [7]. In brief, proliferation in response to helper peptides was assessed in vitro after exposure to each of the following 11 conditions: media only; bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO); tetanus peptide; each of the six melanoma helper peptides; six melanoma helper pool (6MHP); and phytohaemagglutinin (PHA, Sigma-Aldrich, St. Louis, MO; at 5 mcg/ml). Each peptide was assayed at 10 mcg/ml. Two normal donors (Virginia Blood Services, Charlottesville, VA) were included as controls. Stimulation index (SI) was determined based on the following definitions: N vax = stimulation in vaccine peptide; N neg = stimulation in negative control; and stimulation index = R vax = N vax/N neg.
A patient is considered to have a proliferative response to vaccination by meeting all of the following criteria: R vax ≥ 4; (N vax − 1 SD) ≥ (N neg + 1 SD); and R vax post-vaccination ≥4 × R vax pre-vaccination. To compare proliferative responses to individual epitopes versus responses to the 6MHP mixture, we first calculated the ratio between the sum of the stimulation indices of individual epitopes (SIE) and the stimulation index for the 6MHP mixture (SI6MHP). We then found the average SIE:SI6MHP ratio across all samples for each responsive patient and finally, the average ratio across all responsive patients.
Flow cytometry and ELIspot assays
To determine the percent of CD8 T cells in patients’ peripheral blood specimens used in the ELIspot assay, PBMC were labeled with CD3-PE, CD4-FITC, CD8-PECy7, and CD56-APC antibodies (BD Biosciences, San Jose, CA) [7]. ELIspot assays of T cell function were performed directly ex vivo after thawing cryopreserved cells. Lymphocytes were plated (200,000 cells/well) and pulsed with peptide (10 mcg/ml) in quadruplicate at each of two dilutions. A patient is considered to have a T cell response if all of the following criteria have been met: N vax − Nneg > 20 cells/100,000 CD8+ cells (CD8 percentage based on flow cytometry), R vax ≥ 2, (Nvax − 1 SD) ≥ (Nneg + 1 SD), and R vax post-vaccination ≥2 × R vax pre-vaccination. As was the case in the proliferation assays, pre-vaccine fold-increases <1 were converted to one.
Results
Immunogenicity of individual peptides
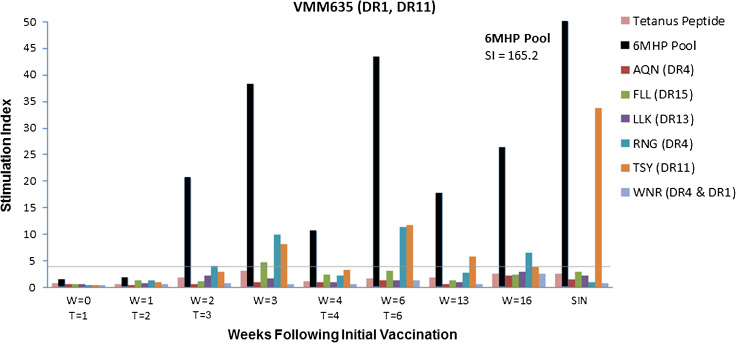
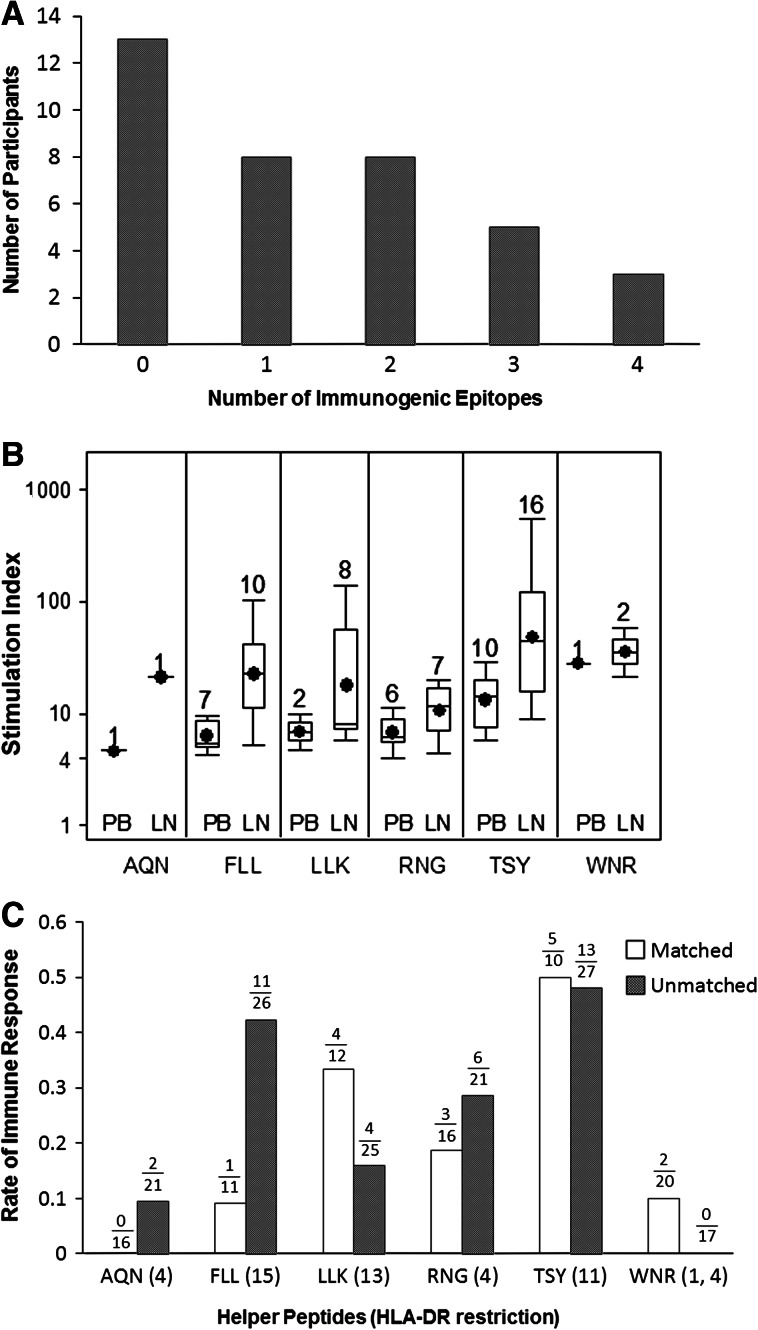
Immune responses were detected to at least one individual epitope in the SIN of 63 % of participants [22/35, 95 % CI (45, 79 %)], in the PBMC of 38 % of participants [14/37, 95 % CI (22, 55 %)], and in either the PBMC or the SIN of 65 % of participants [24/37, 95 % CI (47, 89 %)]. Rate of immune response did not differ between patients with and without measurable disease for the 6MHP mixture (82.4 %, 14/17 vs. 80 %, 16/20, p = 1.0) or for any of the individual epitopes (data not shown). Each of the six peptides was immunogenic in one or more participants. An example of a vaccine-induced immune response to multiple antigens is shown in Fig. 1. In this example, reactivity was first evident 2 weeks after the first vaccine, persisted through week 6 and was still evident at weeks 13 and 16 (Fig. 1). Durability of response to the 6MHP pool for the study group as a whole has been presented previously [7]. Among responders to at least one individual peptide, the median number of peptides responded to was 2 (range 1–4, Fig. 2a).
Fig. 1.
Immunogenicity profile of a study participant (VM635) to individual peptides and to the 6MHP pool. Immune responses are evident to MAGE-A3281–295 (TSY) weeks 3, 6, and 13 in peripheral blood mononuclear cells (PBMC) and in sentinel immunized nodes (SIN), and to MART-151–73 (RNG) weeks 3, 6, and 16 in PBMC. VMM635 expresses HLA-DR1 and DR11; response to TSY matches previously reported HLA-DR11 restriction; response to RNG reflects promiscuity on DR alleles not originally reported (unmatched DR). W denotes weeks following initial vaccination. T denotes administration of 6MHP vaccine. SIN sampling was performed at W = 3. Stimulation index (SI) >4 is criterion for positive response (gray line)
Fig. 2.
Hierarchy of 6MHP epitope immunogenicity and promiscuity. a Response rate to individual 6MHP epitopes was 65 % (24/37). Of responsive participants, most showed proliferative responses to multiple epitopes. b Stimulation index data are plotted for responders to the respective peptides, using SIN (LN) and maximum PBMC (PB) data for each peptide, corrected for pre-vaccine reactivity. Boxes represent IQR25–75, and lines represent the range. Mean values are shown with a solid black circle, and median values with a horizontal line. The number of immune responders is recorded above each box plot. c Comparison of matched and unmatched proliferative responses demonstrates evidence of helper peptide promiscuity. Response rates shown as proportions of available participants in each group. Best response of PBL or SIN was used
The two most frequently immunogenic peptides were TSYVKVLHHMVKISG (MAGE-3281–295) and FLLHHAFVDSIFEQWLQRHRP (Tyrosinase386–406). Overall, for responses in PBMC and/or SIN, vaccine-induced immune responses were detected in 49 % of subjects to TSY, 32 % to FLL, 24 % to RNG, 22 % to LLK, 5 % to WNR, and 5 % to AQN (Table 1), with TSY producing the highest mean stimulation index (Fig. 2b). Within PBMC of patients with a positive immunogenic response to 6MHP, the sum of stimulation indices of individual epitopes tended to be less than the index of the 6MHP mixture, with a mean SIE: SI6MHP ratio of 0.71 [95 % CI (0.61, 0.80)]. Within SIN samples, a more consistent synergistic pattern was apparent, with a mean SIE: SI6MHP ratio of 0.45 [95 % CI (0.29, 0.61)].
Promiscuity of helper peptides for HLA-DR molecules
For each peptide, immunogenicity was evaluated for patients who expressed the reported restricting class II MHC molecule as well as for those who did not. Findings suggested that HLA-DR restrictions were not limited to the MHC molecules originally described as the restriction element for each peptide. For instance, patient VMM635 (HLA-DR1+ and HLA-DR11+) developed persistent immune responses to peptides TSY and RNG (Fig. 1). Prior evidence exists for TSY restriction by HLA-DR11. However, RNG was reported to be presented by HLA-DR4 [12], which was not expressed by this patient; thus, immunogenicity of RNG represents additional restriction by HLA-DR1 or DR11. Overall, immune response rates were not noticeably different between patients whose HLA-DR expressions matched the originally reported restrictions (matched patients) and unmatched patients (Fig. 2c); however, subgroup analysis for each epitope was not performed due to inadequate enrollment.
CD8 T cell responses to MHC class I-associated melanoma peptides
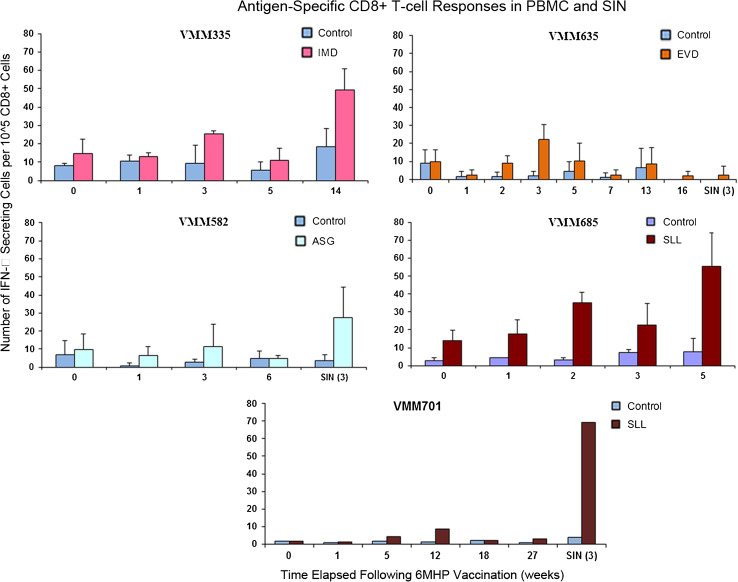
To test whether vaccination with a panel of melanoma helper peptides may induce CD8+ T cell responses to melanoma antigens, PBMC and SIN samples from eleven patients who expressed HLA-A1, A2, or A3 were selected and tested for reactivity using a direct IFNγ ELIspot assay against a panel of 14 melanoma-associated peptides restricted by the those HLA alleles. PBMC samples were available from all eleven patients, and SIN samples were available from seven. Novel reactivity following 6MHP vaccination was observed in 5 of 11 patients [46, 95 % CI (17, 77 %), 3 in PBMC and 2 in SIN]. Responses were to the HLA-A1 restricted EVDPIGHLY (EVD, MAGE-A3168–176), the HLA-A2 restricted IMDQVPFSV (IMD, gp100 209-2 M), and SLLMWQITA (SLL, NY-ESO-1157–165 modified), and the HLA-A3/A31 restricted ASGPGGGAPR (ASG, NY-ESO-153–62) (Fig. 3). None of these CD8-directed peptides overlapped with the amino acid sequences of the helper peptides used for vaccination.
Fig. 3.
T cell responses to class I MHC-restricted epitopes were identified by direct IFN-γ ELIspot assay in 5/11 participants (46 %). VMM335, VMM635, and VMM685 developed responses in peripheral blood; VMM582 and VMM701 developed a response in SIN
Discussion
Few studies have evaluated the immunogenicity of peptides for inducing CD4+ T cell responses to melanoma antigens. Here, we report the immunogenicity of each of 6 intermediate-length peptides first described as HLA-DR restricted epitopes for CD4+ T cells [12–18]. These peptides represent naturally occurring sequences of melanocytic differentiation proteins (gp100, MelanA/MART-1, and tyrosinase) and cancer testis antigens (MAGE proteins). Among these 6 peptides, two had previously been evaluated for immunogenicity in human clinical trials: gp10044–59 and MART-1/MelanA51–73. Two studies had failed to demonstrate immunogenicity of the gp10044–59 peptide [5, 6]; one study showed MART-1/MelanA51–73 to be immunogenic [12]. The other 4 peptides, to our knowledge, had not previously been evaluated in human trials. We have found that, when administered together, each of the 6 peptides was able to induce CD4 T cell proliferation, but with a range of immunogenicity. Fluctuations in stimulation index do occur over the course of vaccination (Fig. 1). These may be temporally related to the vaccination schedule, to patient-specific fluctuations in immune function, or to minor variations in assay technique. The most immunogenic were FLLHHAFVDSIFEQWLQRHRP (Tyrosinase386–406) and TSYVKVLHHMVKISG (MAGE-3281–295). In our hands, gp10044–59 was immunogenic, but in the SIN of only two patients and in the PBMC of only one of these two (Table 1). Interestingly, one of the patients’ responses in PBMC was of high magnitude (max SI 28.0). Our experience with the MART-1 peptide corroborated the prior study, with immune responses detected in 24 % of patients. The two most immunogenic peptides were 15- and 21-mers, the two with intermediate immunogenicity were 14- and 23-mers, and the two least immunogenic were 15- and 16-mers; thus, there was no clear association between peptide length and immunogenicity. Given that the two most dominant epitopes were a melanocytic differentiation antigen (tyrosinase) and a cancer testis antigen (MAGE-A3), the source protein also did not appear to influence immunogenicity.
The immune response rates to individual peptides were lower than the immunogenicity rate of the 6MHP pool. We reported previously that immune responses to the 6MHP pool were induced in the peripheral blood in 57 % of patients and in the SIN in 78 % of patients, with an overall immunogenicity rate (PBMC and/or SIN) of 81 % [7]. By comparison, only 65 % of patients had immune responses detected to one or more individual peptides. The criteria for immune responses toward the 6MHP pooled peptide mixture were the same as for individual peptides in the present study. Thus, expecting at minimum an additive effect of individual peptides in the immune response to the 6MHP, it is not surprising that, in some cases, the contributions of individual peptides to the total 6MHP response may each fall below our immunogenicity threshold even if the cumulative effect of all 6 succeeded in satisfying those criteria. Our results suggest that the effect of multiple melanoma epitopes may actually result in a synergistic effect on T cell proliferation within both PBMC and SIN environments.
Each of the helper peptides was originally described as an epitope for melanoma-reactive CD4+ T cells from a specific patient, for whom the restricting HLA-DR molecule was known [12–18]. We had hypothesized that the peptides would be promiscuous, presenting antigen across a range of HLA-DR molecules. Thus, for each peptide, we compared the immunogenicity rate among patients expressing the originally reported HLA-DR allele (e.g., HLA-DR11 for MAGE-A3281–295, TSY) to that among patients lacking that HLA-DR allele. We found that immunogenicity rates were similar between patients whose HLA-DR expression matched the original restricting molecules and those whose HLA-DR expression did not. Therefore, our results support the presence of promiscuity of these helper peptides, in concordance with existing evidence of a hierarchy of HLA-DR promiscuity among a set of tetanus peptides, with some epitopes appearing to be universally immunogenic across all HLA-DR alleles [9]. To our knowledge, TSY was the only component peptide within the 6MHP vaccine with prior in vitro evidence of HLA-DR binding promiscuity (to alleles DR1, 4, 11) [19]. Our results suggest that potential clinical application of helper peptides may not need to be limited to those patients with MHC class II alleles matching previously described restrictions.
A limitation of peptide vaccines is that they can only include a small subset of the potentially relevant tumor antigens for a given patient. However, if immune responses to the vaccine peptides contribute to expansion of immune responses to other epitopes from the same proteins or to other antigens (epitope spreading), there is greater potential for clinical benefit. CD4+ T cells offer the potential to support expansion of CD8+ T cell responses by providing “help”; thus, we hypothesized that immune responses to 6MHP would be associated with induction of CD8+ T cell responses to class I MHC-restricted peptides. To obtain preliminary evidence toward that hypothesis, we tested whether immune responses were induced to any of a panel of 14 class I MHC-restricted melanoma-derived peptides, depending on the patients’ expression of HLA-A1, A2, or A3 (Table 2). We observed de novo reactivity to class I MHC-restricted epitopes in 5 of 11 patients evaluated (46 %). These were responses against peptide sequences not contained in the 6MHP, and reactivity to a protein not represented in the vaccine (NY-ESO-1) was observed. This suggests that induction of a CD4 response may provide help toward antitumor activity via CTLs reactive to different proteins than those represented by the helper epitopes. With the rapid emergence of alternative modalities for CTL activation through innovative approaches to dendritic cell antigen presentation [20, 21], our results advocate for the design of multi-modality trials.
It should be noted that the vaccines in this study were administered with GM-CSF in addition to IFA. In previous studies, GM-CSF administered locally with a multipeptide melanoma-associated vaccine resulted in a reduction in both CD8 and CD4 T cell responses compared with the use of IFA alone [22]. Therefore, the ability of helper peptides to induce CD4 responses and de novo CD8 T cell anti-tumor responses in the absence of GM-CSF may exceed those observed in the present study. Recent evidence also suggests that T cells primed with peptides in IFA may not migrate to tumor locations, but may instead be retained at the vaccine site, where they undergo dysfunction and subsequent apoptosis [23, 24]. Although this has been demonstrated with short (9-mer) peptides in IFA, such an effect may be circumvented by employing longer peptide epitopes due to requisite presentation by dendritic cells in local lymph nodes, away from the effects of IFA at the vaccine site [25]. In our study, the presence of CD8+ T cell responses in peripheral blood and the high frequency of CD4+ T cell responses in the vaccine-draining node suggest that antigen presentation may occur outside of the vaccine site alone.
In summary, vaccination with 6MHP in IFA plus GM-CSF induced immune responses against each of the 6 peptides, but there was a hierarchy of immunodominance. The most immunogenic peptides were from tyrosinase and MAGE-A3. There was no evidence that peptide length or the type of source tumor antigen predicted immunodominance. Immune responses to class I MHC-restricted peptides were observed in 46 % of patients evaluated, suggesting that CD8 T cell reactivity was induced by epitope spread. This mixture of 6 helper peptides has been shown to be immunogenic and safe [7], and immune responses to these peptides correlate strongly with survival of patients with advanced melanoma [8]. By defining the immunogenic hierarchy and promiscuity of the peptides comprising the 6MHP mixture, we hope to promote future clinical research with these epitopes through personalized immunotherapy.
Conflicts of interest
The authors declare that they have no conflict of interest.
Abbreviations
- 6MHP
Combination melanoma helper peptide
- CTL
Cytotoxic T lymphocyte
- PBMC
Peripheral blood mononuclear cell
- SI
Stimulation index
- SIN
Sentinel immunized node
References
- 1.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, et al. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PD, Kern DE, Cheever MA. Therapy of disseminated murine leukemia with cyclophosphamide and immune lyt-1+, 2-T cells. Tumor eradication does not require participation of cytotoxic T cells. J Exp Med. 1985;161:1122–1134. doi: 10.1084/jem.161.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khong HT, Yang JC, Topalian SL, Sherry RM, Mavroukakis SA, White DE, et al. Immunization of HLA-A*0201 and/or HLA-DPbeta1*04 patients with metastatic melanoma using epitopes from the NY-ESO-1 antigen. J Immunother. 2004;27:472–477. doi: 10.1097/00002371-200411000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phan GQ, Touloukian CE, Yang JC, Restifo NP, Sherry RM, Hwu P, et al. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother. 2003;26:349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong R, Lau R, Chang J, Kuus-Reichel T, Brichard V, Bruck C, et al. Immune responses to a class II helper peptide epitope in patients with stage III/IV resected melanoma. Clin Cancer Res. 2004;10:5004–5013. doi: 10.1158/1078-0432.CCR-04-0241. [DOI] [PubMed] [Google Scholar]
- 7.Slingluff CL, Jr, Petroni GR, Olson W, Czarkowski A, Grosh WW, Smolkin M, et al. Helper T-cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. J Clin Oncol. 2008;26:4973–4980. doi: 10.1200/JCO.2008.17.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slingluff CL, Jr, Lee S, Zhao F, Chianese-Bullock KA, Olson WC, Butterfield LH, et al. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602) Clin Cancer Res. 2013;19:4228–4238. doi: 10.1158/1078-0432.CCR-13-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 10.Slingluff CL, Jr, Yamshchikov G, Neese P, Galavotti H, Eastham S, Engelhard VH, et al. Phase I trial of a melanoma vaccine with gp100(280-288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–3024. [PubMed] [Google Scholar]
- 11.Johnson RP, Trocha A, Yang L, Mazzara GP, Panicali DL, Buchanan TM, et al. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- 12.Zarour HM, Kirkwood JM, Kierstead LS, Herr W, Brusic V, Slingluff CL, Jr, et al. Melan-A/MART-1(51-73) represents an immunogenic HLA-DR4-restricted epitope recognized by melanoma-reactive CD4(+) T cells. Proc Natl Acad Sci USA. 2000;97:400–405. doi: 10.1073/pnas.97.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Gonzales MI, Parkhurst M, Li YF, Southwood S, Sette A, et al. Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes. J Exp Med. 1996;183:1965–1971. doi: 10.1084/jem.183.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi H, Kokubo T, Sato K, Kimura S, Asano K, Takahashi H, et al. CD4+ T cells from peripheral blood of a melanoma patient recognize peptides derived from nonmutated tyrosinase. Cancer Res. 1998;58:296–301. [PubMed] [Google Scholar]
- 15.Manici S, Sturniolo T, Imro MA, Hammer J, Sinigaglia F, Noppen C, et al. Melanoma cells present a MAGE-3 epitope to CD4(+) cytotoxic T cells in association with histocompatibility leukocyte antigen DR11. J Exp Med. 1999;189:871–876. doi: 10.1084/jem.189.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaux P, Vantomme V, Stroobant V, Thielemans K, Corthals J, Luiten R, et al. Identification of MAGE-3 epitopes presented by HLA-DR molecules to CD4(+) T lymphocytes. J Exp Med. 1999;189:767–778. doi: 10.1084/jem.189.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halder T, Pawelec G, Kirkin AF, Zeuthen J, Meyer HE, Kun L, et al. Isolation of novel HLA-DR restricted potential tumor-associated antigens from the melanoma cell line FM3. Cancer Res. 1997;57:3238–3244. [PubMed] [Google Scholar]
- 18.Li K, Adibzadeh M, Halder T, Kalbacher H, Heinzel S, Muller C, et al. Tumour-specific MHC-class-II-restricted responses after in vitro sensitization to synthetic peptides corresponding to gp100 and annexin II eluted from melanoma cells. Cancer Immunol Immunother. 1998;47:32–38. doi: 10.1007/s002620050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Consogno G, Manici S, Facchinetti V, Bachi A, Hammer J, Conti-Fine BM, et al. Identification of immunodominant regions among promiscuous HLA-DR-restricted CD4+ T-cell epitopes on the tumor antigen MAGE-3. Blood. 2003;101:1038–1044. doi: 10.1182/blood-2002-03-0933. [DOI] [PubMed] [Google Scholar]
- 20.Benteyn D, Van Nuffel AM, Wilgenhof S, Corthals J, Heirman C, Neyns B, et al. Characterization of CD8+ T-cell responses in the peripheral blood and skin injection sites of melanoma patients treated with mRNA electroporated autologous dendritic cells (TriMixDC-MEL) Biomed Res Int. 2013;2013:976383. doi: 10.1155/2013/976383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilgenhof S, Van Nuffel AM, Benteyn D, Corthals J, Aerts C, Heirman C, et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol. 2013;24:2686–2693. doi: 10.1093/annonc/mdt245. [DOI] [PubMed] [Google Scholar]
- 22.Slingluff CL, Jr, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salerno EP, Shea SM, Olson WC, Petroni GR, Smolkin ME, McSkimming C, et al. Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete freund’s adjuvant, with or without peptide. Cancer Immunol Immunother. 2013;62:1149–1159. doi: 10.1007/s00262-013-1435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179:5033–5040. doi: 10.4049/jimmunol.179.8.5033. [DOI] [PubMed] [Google Scholar]
- 26.Chaux P, Luiten R, Demotte N, Vantomme V, Stroobant V, Traversari C, et al. Identification of five MAGE-A1 epitopes recognized by cytolytic T lymphocytes obtained by in vitro stimulation with dendritic cells transduced with MAGE-A1. J Immunol. 1999;163:2928–2936. [PubMed] [Google Scholar]
- 27.Kittlesen DJ, Thompson LW, Gulden PH, Skipper JC, Colella TA, Shabanowitz J, et al. Human melanoma patients recognize an HLA-A1-restricted CTL epitope from tyrosinase containing two cysteine residues: implications for tumor vaccine development. J Immunol. 1998;160:2099–2106. [PubMed] [Google Scholar]
- 28.Kawakami Y, Robbins PF, Wang X, Tupesis JP, Parkhurst MR, Kang X, et al. Identification of new melanoma epitopes on melanosomal proteins recognized by tumor infiltrating T lymphocytes restricted by HLA-A1, -A2, and -A3 alleles. J Immunol. 1998;161:6985–6992. [PubMed] [Google Scholar]
- 29.Traversari C, van der Bruggen P, Luescher IF, Lurquin C, Chomez P, Van Pel A, et al. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992;176:1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaugler B, Van den Eynde B, van der Bruggen P, Romero P, Gaforio JJ, De Plaen E, et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celis E, Tsai V, Crimi C, DeMars R, Wentworth PA, Chesnut RW, et al. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc Natl Acad Sci USA. 1994;91:2105–2109. doi: 10.1073/pnas.91.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skipper JC, Hendrickson RC, Gulden PH, Brichard V, Van Pel A, Chen Y, et al. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- 34.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, et al. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 35.Huang LQ, Brasseur F, Serrano A, De Plaen E, van der Bruggen P, Boon T, et al. Cytolytic T lymphocytes recognize an antigen encoded by MAGE-A10 on a human melanoma. J Immunol. 1999;162:6849–6854. [PubMed] [Google Scholar]
- 36.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero P, Dutoit V, Rubio-Godoy V, Lienard D, Speiser D, Guillaume P, et al. CD8+ T-cell response to NY-ESO-1: relative antigenicity and in vitro immunogenicity of natural and analogue sequences. Clin Cancer Res. 2001;7:766s–772s. [PubMed] [Google Scholar]
- 38.Skipper JC, Kittlesen DJ, Hendrickson RC, Deacon DD, Harthun NL, Wagner SN, et al. Shared epitopes for HLA-A3-restricted melanoma-reactive human CTL include a naturally processed epitope from pmel-17/gp100. J Immunol. 1996;157:5027–5033. [PubMed] [Google Scholar]
- 39.Hogan KT, Sutton JN, Chu KU, Busby JA, Shabanowitz J, Hunt DF, et al. Use of selected reaction monitoring mass spectrometry for the detection of specific MHC class I peptide antigens on A3 supertype family members. Cancer Immunol Immunother. 2005;54:359–371. doi: 10.1007/s00262-004-0592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang RF, Johnston SL, Zeng G, Topalian SL, Schwartzentruber DJ, Rosenberg SA. A breast and melanoma-shared tumor antigen: T cell responses to antigenic peptides translated from different open reading frames. J Immunol. 1998;161:3598–3606. [PubMed] [Google Scholar]