Abstract
Background
The Phambili study, conducted in South Africa amongst a predominantly heterosexual population, evaluated the efficacy of the MRK Ad5 gag/pol/nef subtype B HIV-1 preventive vaccine. Enrollment and vaccinations were stopped, participants unblinded, and follow-up extended when the Step study evaluating the same vaccine in the Americas, Caribbean and Australia was unblinded for non-efficacy with more HIV infections amongst vaccinee than placebo recipients [ZM1]. Extensive analyses over the complete follow-up period, most of which was unblinded, are reported.
Methods
Phambili participants were HIV-1 uninfected, sexually active men and women aged 18–35 years, followed for 3.5 years. HIV testing and risk reduction counseling occurred at weeks 0, 12, 30 and were switched to a 3 monthly schedule after unblinding. Cox proportional hazards models were used to estimate HIV-1 infection hazard ratios (HR) comparing vaccine to placebo recipients, overall and within subgroups. Long-term vaccine efficacy was evaluated in participants who were unblinded early in follow-up.
Results
Of the 801 participants enrolled (400 vaccine, 401 placebo), 112 (28%) received 1 vaccination, 259 (65%) 2 vaccinations and 29(7%) 3 vaccinations. More infections occurred in vaccinees (n=63) as compared to placebo (n=37) (adjusted HR (vaccine:placebo) 1.70, 95% CI 1.13–2.55, p = 0.01). We found no increase in infections with the number of vaccinations received and that the HRs did not differ by gender, circumcision, or Ad5 serostatus. Differences in risk behavior at baseline or during the study, or differential drop-out (p=0.40) are unlikely explanations for the increased rate of HIV-1 infections seen in vaccinees.
Conclusion
The increased HR of HIV-1 acquisition, irrespective of number of doses received, warrants further investigation to understand the biological mechanism. Further use of the Ad5 vector for HIV vaccines is not warranted
Keywords: HIV, rAd5 HIV vaccines, HIV-1 vaccine efficacy studies, South Africa
Introduction
Recombinant adenovirus type 5 (rAd5) vectors have been described as ideal platforms for use in HIV-1 vaccine research because they are highly immunogenic, able to express large amounts of antigen/s, and are easily manufactured1. Despite well-documented pre-clinical studies2,3,4,5,6,7,8,9, clinical safety and immunogenicity in multiple phase1/2 studies10,11,12,13,14,15,16,17,18, two phase 2b proof-of-concept trials of a rAd5-vectored HIV-1vaccine failed to show vaccine efficacy19,20,21. The first of these trials, the Step study19, raised concerns about vaccine enhanced HIV-1 acquisition. Step tested the MRK rAd5 polyvalent HIV-1 gag/pol/nef subtype B vaccine in a cohort consisting primarily of men who had sex with men (MSM) and at risk women in the Americas, Caribbean and Australia, where the circulating HIV-1 subtype is B. In September 2007, the first interim efficacy analysis concluded that the trial met the pre-specified criteria for futility, thus vaccinations were halted, follow-up continued, and participants were informed of their treatment assignments. Unplanned subgroup analyses of Step showed that the increased susceptibility to HIV-1 acquisition appeared restricted to vaccinated Ad5 seropositive and/or uncircumcised men (based on interaction tests).19 A subsequent analysis of the study follow-up showed a higher risk of HIV-1 infection among male vaccinees as compared to male placebo recipients over all the follow-up time (hazard ratio [HR]1.40; 95% CI 1.03–1.92; p=0.03).22 The vaccine-induced enhanced risk of HIV-1 acquisition seen in the uncircumcised and/or Ad5 seropositive men was more evident in the first 18 months and appeared to wane over time, whereas for circumcised, Ad5 seronegative men, the HR increased over time from 0.38 (95% CI 0.16–0.90) in the first 18 months to 2.18 (95% CI (0.97–4.92) after 18 months of follow-up.
The second trial of the MRK rAd5 vaccine, HVTN 503 or Phambili,20 was undertaken in South Africa, where clade C is the predominant HIV-1 subtype, in a principally heterosexual population. Enrollment and vaccinations were halted in September 2007 when Step met the non-efficacy criteria. Because of the increased susceptibility to HIV-1 acquisition seen in certain sub-groups of Step, participants were rapidly unblinded in Phambili and follow-up was extended. The initial analysis of Phambili based on an average of 22.5 months of follow-up found that although there were more infections amongst vaccinees as compared to placebos, this was not statistically significant [HR 1.25 adjusted for gender (95% CI 0.76–2.05)]. Because of the concern for increased acquisition and the lack of sufficient HIV-1 infections in the Step trial to assess the effect of vaccination in women, long-term follow-up of the Phambili participants occurred. This report describes HIV-1 acquisition over the 3.5 years of participant follow-up and recent recall of the Phambili cohort.
Methods
Study Design and Population
This two-arm, double-blind, placebo controlled randomized clinical trial, initiated in January 2007 was designed to enroll 3000 healthy HIV-1 uninfected, heterosexual adults aged 18–35 at 5 sites within SA (Soweto, Cape Town, Klerksdorp-Orkney-Stilfontein-Hartbeesfontein [KOSH], eThekwini, and MEDUNSA).20 On 19 September 2007, enrollment and vaccinations were halted and in October 2007, unblinding of participants began, with concomitant HIV-1 testing, risk evaluation and counseling.
The study was registered with the Food and Drug Administration in the USA; and approved by the SA Medicines Control Council; the Genetically Modified Organism Review Committee of the SA Department of Agriculture; and the ethical review committees and institutional biosafety committees of the University of the Witwatersrand, University of Cape Town, University of Limpopo and the University of KwazuluNatal. Participants provided written informed consent in English or their local language.
Products and Procedures
MRKAd5 HIV-1 gag/pol/nef vaccine (Merck and Co., Inc) was given as a dose of 1.5×1010 Ad viral genomes/1mL. Placebo was a 1ml solution of the vaccine diluent with no Ad5 vector. Study products were administered by intramuscular injection on a 0, 1, 6 month schedule.
Initially, HIV-1 evaluation was done on blood drawn on the day of first vaccination, weeks 12, 30, 52, and every six months. Risk was assessed six months prior to screening and at HIV-1 evaluation. After unblinding and receipt of appropriate approvals, follow-up visits were changed to every three monthly for HIV-1 evaluation, risk assessment, and risk reduction counseling. In June 2013, a follow-up study of the 695 Phambili participants who were alive and HIV-1 uninfected at their last study visit was implemented.
Statistical Analysis
Randomization was 1:1 between vaccine and placebo, stratified by site and gender based on computer-generated random numbers and provided to site pharmacists by a central statistical and data monitoring center (SDMC). Analyses were of the modified-intent-to-treat cohort (MITT), consisting of all participants HIV-1 uninfected at enrollment. Unless otherwise specified, analyses included the total follow-up period of 3.5 years per HIV-1 uninfected participant, including blinded and unblinded follow-up periods. A formal comparison of rates of infection pre- and post-unblinding was not possible due to the small number of infections pre-unblinding.
Kaplan-Meier cumulative incidence plots of time to HIV-1 infection by treatment, overall and by subgroups were provided, with log-rank p-values. Cox proportional hazards models were used to estimate the HR for HIV-1 infection due to vaccination (vaccine:placebo), overall and within subgroups. Time to HIV-1 infection for infected participants was defined as the time from first study injection to the midpoint between the last plasma HIV-1 RNA negative and first RNA positive test; for uninfected participants the censoring time was defined as the time from first study injection to the last study HIV test. Differences in HRs between subgroups were assessed with Wald tests for the vaccine by subgroup interaction. To assess whether HRs varied over time, Grambsch and Therneau23 tests were used. Differences between vaccinees and placebos in time to dropout and for HIV-1 infected participants’ time to CD4 decay were assessed by log-rank tests. Predictors of drop-out were assessed in univariate and multivariate Cox models. Differences in viral load set-point between HIV-1 infected vaccine and placebo recipients were assessed with Wilcoxon rank sum tests. We defined set-point as the geometric mean of HIV-1 plasma viral-load measurements obtained between 2–3 months after diagnosis. All p-values were 2-sided.
Results
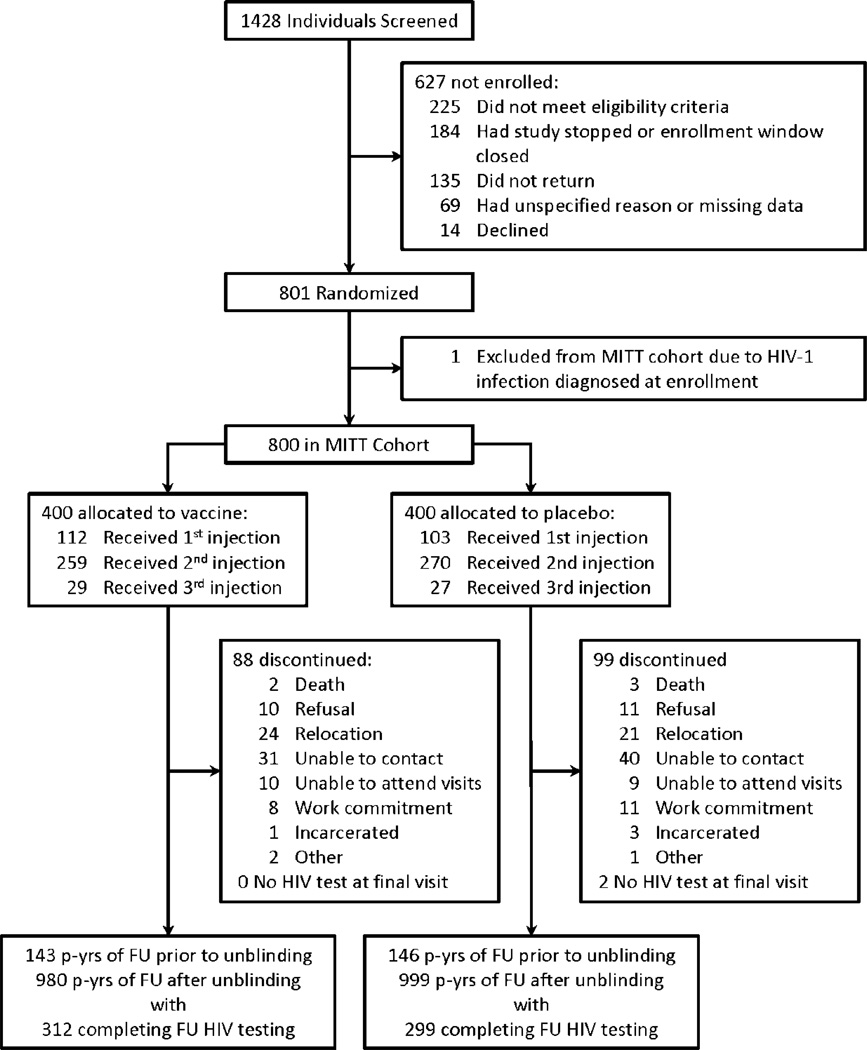
801 (26.7%) of the planned 3000 participants were enrolled before vaccinations were suspended and participants informed of their treatment allocation. One woman who received placebo was diagnosed as HIV-1 infected at enrollment and was therefore excluded from the MITT cohort. Men comprised 55% (n=441) of the MITT cohort, with 129 previously circumcised at the time of enrollment and an additional 139 circumcised while on study (supplemental tables 1 and 2). Among those randomized to receive the vaccine product, 112 (28%) received one vaccination, 259 (65%) received two and 29 (7%) received three vaccinations (figure 1). Since participants were unblinded early in the study, only 289 (13%) of the observed 2268 person years of follow-up occurred while the study was blinded (figure 1).
Figure 1. Trial profile.
Abbreviations: MITT – modified intent-to-treat; p-yrs – person years; FU – follow-up
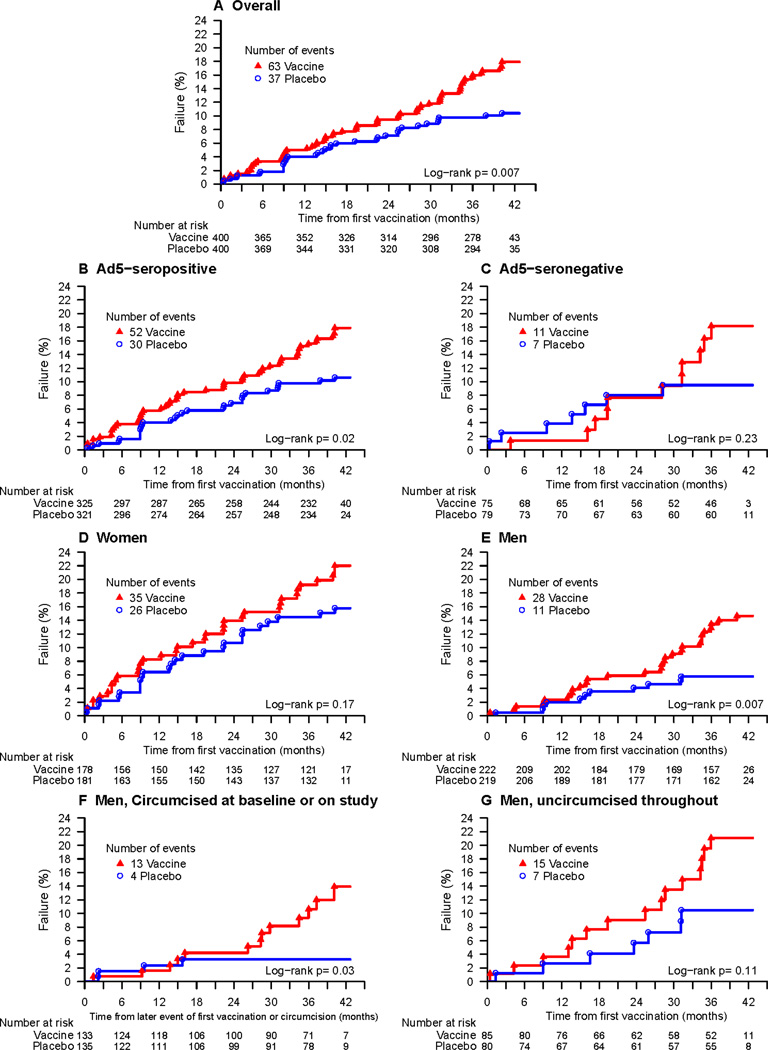
The annualized dropout rate was 7.7% for vaccinees (95% CI 6.2%–9.5%) and 8.8% for placebo (95% CI 7.1%–10.7%)(p=0.40, supplemental figure 1). During the follow-up period, there were 100 HIV-1 infections (table 1). Significantly more HIV-1 infections occurred amongst vaccinees (n=63) as compared to placebo (n=37); the annualized HIV-1 incidence rate was 5.61% (95% CI 4.31%–7.18%) for vaccinees and 3.23% (95% CI 2.28%–4.45%) for placebo recipients (p=0.007, figure 2A). Fourteen of the infections (8 vaccine; 6 placebo) were observed during the 289 person-years of blinded follow-up.
Table 1.
Total number of incident HIV-1 infections by baseline adenovirus serotype 5 (Ad5) serostatus, gender, and circumcision status
| Vaccine | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Category | No. at risk |
No. HIV-1 infections |
Annualized HIV-1 incidence rate |
(95% CI) | No. at risk |
No. HIV-1 infections |
Annualized HIV-1 incidence rate |
(95% CI) |
| Overall | 400 | 63 | 5.61% | (4.31%, 7.18%) | 400 | 37 | 3.23% | (2.28%, 4.45%) |
| Ad5-seronegative | 75 | 11 | 5.43% | (2.71%, 9.72%) | 79 | 7 | 3.05% | (1.23%, 6.28%) |
| Ad5-seropositive | 325 | 52 | 5.65% | (4.22%, 7.41%) | 321 | 30 | 3.28% | (2.21%, 4.68%) |
| Women | 178 | 35 | 7.21% | (5.02%, 10.03%) | 181 | 26 | 5.06% | (3.31%, 7.41%) |
| Men | 222 | 28 | 4.39% | (2.92%, 6.35%) | 219 | 11 | 1.74% | (0.87%, 3.12%) |
| Circumcised mena | 133 | 13 | 3.76% | (2.00%, 6.43%) | 135 | 4 | 1.14% | (0.31%, 2.93%) |
| Uncircumciseda | 157 | 15 | 5.14% | (2.87%, 8.47%) | 147 | 7 | 2.50% | (1.01%, 5.15%) |
| men | ||||||||
Abbreviations: CI, confidence interval; HIV-1, human immunodeficiency virus 1; Ad5, adenovirus serotype 5.
For men who were circumcised on study, their person-years of follow-up from enrollment to end of study is split between the two circumcision subgroups. Their person-years from enrollment to circumcision were counted as uncircumcised time and person-years from circumcision to end of follow-up or HIV infection was counted as circumcised time. Eight men who were uncircumcised at enrollment and did not have a follow-up circumcision assessment were excluded.
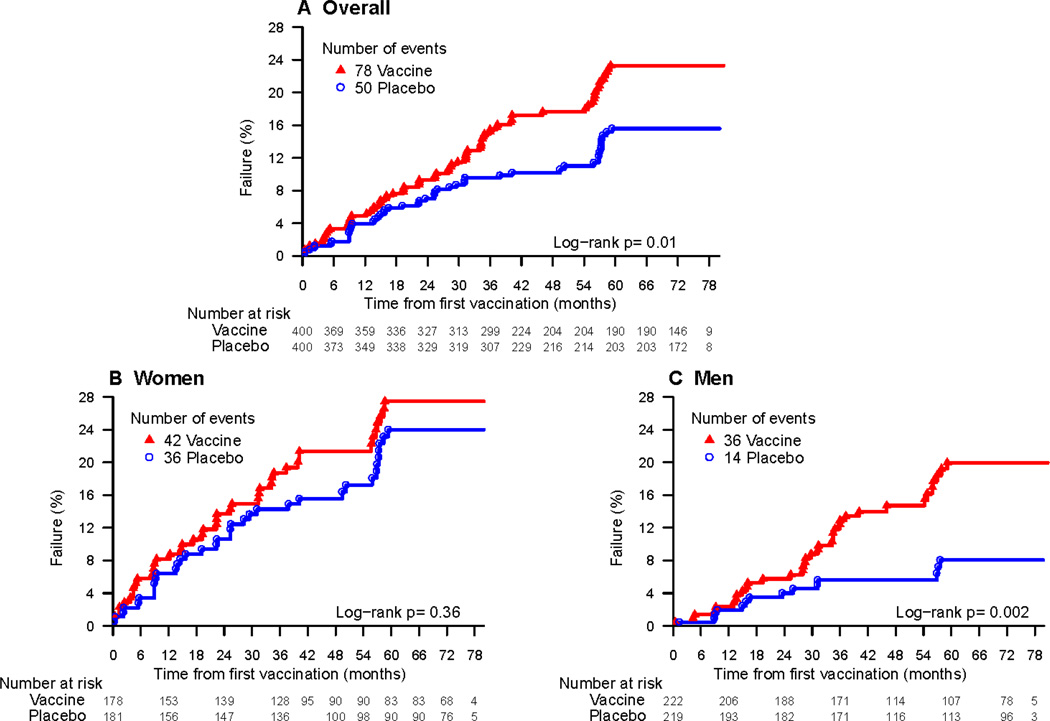
Figure 2.
a. Cumulative HIV-1 incidence curves for vaccine and placebo groups for main Phambili study (excluding sub-study)
b. Cumulative HIV-1 incidence curves for vaccine and placebo groups including additional follow-up from follow-up study
The HR adjusted for baseline HSV-2 status was 1.70 (95% CI: 1.13–2.55, p=0.01, table 2). The overall annualized incidence rate was much higher in women (6.10%) than in men (3.07%). The vaccine:placebo HR was > 1 for both men and women (table 2), with a more pronounced effect in men (interaction p=0.19, table 2, figure 2D, 2E). There was no evidence that the HR varied by baseline Ad5 status, age, or in men, by time-dependent circumcision status (interaction p-values ≥ 0 .38, table 2, supplemental table 2). The HR did not significantly differ among the five study sites (p=0.51, Table 2), and the estimated HR was highest in Soweto (p=0.02), the site with the highest enrollment.
Table 2.
HIV-1 infection hazard ratios (vaccine:placebo), adjusted for baseline HSV-2
| Cohort | Est. (95% CI); P-value | Interaction P-value |
Time-varying HR P-value |
|
|---|---|---|---|---|
| Overall | 1.70 (1.13, 2.55); 0.01 | - | 0.11 | |
| Ad5 seropositive | 1.70 (1.08, 2.66); 0.02 | 0.98 | 0.51 | |
| Ad5 seronegative | 1.70 (0.66, 4.40); 0.27 | 0.01 | ||
| Women | 1.42 (0.85, 2.36); 0.18 | 0.19 | 0.65 | |
| Men | 2.46 (1.22, 4.93); 0.01 | 0.08 | ||
| Men circumcised at baseline or on study | 3.58 (1.16, 11.01); 0.03 | 0.38 | 0.02 | |
| Men uncircumcised throughout study | 1.83 (0.74, 4.54); 0.19 | 0.29 | ||
| Age ≤ 25 years | 1.72 (1.04, 2.84); 0.03 | 0.96 | 0.41 | |
| Age > 25 years | 1.66 (0.83, 3.32); 0.15 | 0.09 | ||
| Soweto | 2.40 (1.19, 4.86); 0.02 | 0.51 | 0.15 | |
| Cape Town | 1.86 (0.84, 4.14); 0.13 | 0.51 | ||
| KOSH | 0.87 (0.33, 2.25); 0.77 | 0.93 | ||
| eThekwini | 1.91 (0.62, 5.94); 0.26 | 0.44 | ||
| Medunsa | 1.05 (0.21, 5.24); 0.95 | 0.50 | ||
While the overall HR did not significantly vary over time (p=0.11), the differences in HIV-1 acquisition rates became evident as infections accumulated during the long follow-up period (figure 2). Among participants who became HIV-1 infected, vaccination had no significant effect on viral load set-point or time to CD4 decay to less than 350. Descriptively, viral load set-point appeared to be slightly lower in the vaccine than placebo group overall and within gender subgroups (supplemental figure 2).
To determine possible explanations for the observed increased rate of HIV-1 infection among vaccinees, we considered the effect of number of vaccinations received and extensively analyzed the dropout and risk behavior data as well as the relationship between on study circumcision and HIV-1 acquisition. We found no evidence that the increase in infection rate amongst vaccinees depended on the number of vaccinations received (supplemental figure 3). The annualized dropout rate for men was higher than for women (9.7% vs. 6.4%), although neither subgroup showed a significant difference in dropout between vaccine and placebo (supplemental figure 1). Of the 100 documented HIV-1 infections, 37 occurred in Soweto and 27 in Cape Town. The annualized dropout rate in the Soweto site was only 4.6%, with no difference between the vaccine and placebo groups (p=0.49). The Cape Town site experienced higher rates of drop-out with more men in the placebo group dropping out compared to the vaccine group although this was not significant (supplemental table3, supplemental figure 1G).
To further assess if differential dropout could account for the increased HR among men, we looked for risk behaviors that were associated with HIV-1 infection and if more of these behaviors were significant predictors of dropout among the placebo men than among vaccine men. Among all men, we found significant associations between HIV-1 infection and certain self-reported risk factors during the trial (multiple partners; casual/anonymous partner; unprotected vaginal/anal sex; apart regularly from main partner), but these behaviors were not associated with dropout among the male vaccine or placebo recipients. Adjustment of the HR estimate for men for behavioral risk in addition to baseline HSV-2 status had little effect on the HR estimate: HR=2.30 after adjustment for behavioral risk (95% CI: 1.14–4.62, p=0.02) versus HR=2.46 (table 2).
To evaluate if differential loss to follow-up was a major factor influencing the higher rates of infection among vaccinees, we implemented a follow-up study in June 2013 to bring back all 695 HIV-1 uninfected participants. To date, of the 422 participants seen, 28 additional HIV-1 infections have been detected (table 3). Of the 189 participants who dropped out, 58 have been located and enrolled into the sub-study. The HIV-1 incidence amongst drop-outs was 5.29% (95% CI: 1.94%–11.51%; n=5 infected men/1 infected woman) in vaccinees vs. 0.96% (95% CI: 0.02%–5.34%; n=1 infected woman) among placebos. Including the additional follow-up time, the adjusted HR is 1.57(95% CI: 1.10–2.23) compared to1.70 (95% CI: 1.13–2.55) for the Phambili study alone.
Table 3.
New HIV-1 infections detected among participants enrolled in the follow-up study with available HIV test resultsa
| Total | Vaccine | Placebo | |
|---|---|---|---|
| All | 28/421 (6.7%) | 15/205 (7.3%) | 13/216 (6.0%) |
| Women | 17/190 (8.9%) | 7/90 (7.8%) | 10/100 (10.0%) |
| Men | 11/231 (4.8%) | 8/115 (7.0%) | 3/116 (2.6%) |
Among the 422 participants enrolled in the follow-up study, one woman who received vaccine is pending HIV testing results.
Discussion
While not significant in our earlier analysis at an average of 22.5 months of follow-up, our long-term follow-up analysis shows a significantly higher rate of HIV-1 infection among vaccinees than placebos. This finding was not related to the number of vaccinations received nor explained by any covariate imbalances between the vaccinated and placebo recipients. The increased vaccine:placebo HR is consistent across all sub-groups (p-values 0.01–0.27), albeit weaker in women than men.
Our data support the Step findings that the MRK Ad5 gag/pol/nef HIV-1 vaccine induced enhancement of HIV-1acquisition among male vaccinees with no significant change in the vaccine: placebo HR over time.22 Step men were primarily MSM, received three vaccinations and were exposed to sub-type B HIV-1. Step demonstrated significant changes in the HR for two sub-groups over time. Among uncircumcised, Ad5 seropositive men, the initially high HR in the first 18 months decreased (interaction test p=0∙04) and the initial low HR among circumcised, Ad5 seronegative men increased after 18 months (p=0∙04). Phambili participants were predominantly heterosexual, exposed to sub-type C HIV-1 and received one or two vaccinations. The increased risk of HIV-1 acquisition due to vaccine was significant in the Ad5 seropositive subgroup (p=0.02) and increased with time in both the Ad5 seronegative subgroup (p=0.01) and in circumcised men (p=0.02).
Given that most of the follow up occurred post-unblinding, we have looked extensively for confounders such as treatment differences in risk behavior to explain our results. Although we did not find any differences in behavior at baseline or during study follow-up between the treatment groups, our staff-administered risk assessments may have led to inaccurate or under-reporting of risk or recall bias. In the future, use of novel platforms to capture sexual practices contemporaneously, or ACASI use at the clinic may improve the reliability of reported sexual practices. After unblinding, under-reporting may have occurred more frequently among vaccinees as they received from staff a stronger message regarding risk based on the Step results. We have previously reported that between 8–11 months after unblinding, more participants in the vaccine group felt they were more likely to get HIV/AIDS than most people, and attributed this to receiving the vaccine.24
To address concerns of ascertainment bias, we initiated a follow-up study to perform HIV testing for all HIV-1 uninfected participants to assess whether higher risk among placebo participants who dropped out could provide an explanation for our results. Based on data accrued to date, we still find a disproportionate amount of HIV-1 infections amongst male vaccinees, particularly those who dropped out of the primary study, consistent with an interpretation of vaccine induced enhancement (table 3). Interestingly, the evidence for enhancement in women lessened based on the additional data.
Our findings differ from that seen in HVTN 505, a study that used DNA priming before a single Ad5 boost.25 The vaccine regimen used in HVTN 505 contained HIV-1 env antigen inserts that induced binding antibodies to HIV-1 envelope. The HVTN 505 study, performed in circumcised Ad5 seronegative MSM, did not demonstrate efficacy nor was there evidence of vaccine-induced enhancement. This suggests that any potential enhancement could have been overcome by anti HIV-1 envelope specific responses, implying that our findings may not be applicable to all Ad5 HIV-1 vaccines.
While unidentified, confounding behavioral factors are potential explanations for our data; the differences between men and women and the consistency between the Step and Phambili studies point to a potential biological explanation for our observation. A recent meta-analysis combining the Step and Phambili data has shown an estimated HR of 1.41 (95% CI: 1.11–1.78, p = 0.005)26 between vaccinees and placebos, that is constant over time. Sub-clinical genital inflammation is a well-recognized risk factor for HIV-1 acquisition,27, 28 as has been demonstrated with HSV-2 infection. We do not have a plausible biological mechanism for this increased susceptibility seen, and postulate that vaccination increased the number of target cells in the genital mucosa. This has been postulated as a mechanism in HSV-2 infection, which has been shown to lead to increased rates of HIV-1 acquisition.29 As with HSV-2, it is possible that similar interactions could exist for potent vectors such as Ad5. Ad5 vector immunization, as well as naturally occurring adenovirus infection have been shown to lead to an expansion of activated Ad5-specific T-cells thereby increasing the number of HIV-1 target cells30, 31, 32. Persistence of adenovirus antigen in the gut from natural infection may further facilitate migration and expansion of Ad5-specific T-cells in the intestinal mucosa with trafficking of target cells to the penile surface. As we did not use an empty viral vector as a control, we cannot determine whether the increased risk of acquisition could be attributed either to an Ad5-vector specific response or to the HIV-1 antigens (gag/pol/nef) used in the vaccine. A study conducted in NHPs utilizing an Ad5-based SIV gag/pol/nef vaccine demonstrated enhancement after a penile challenge, whereas, NHPs immunized with the empty Ad5 vector were not susceptible to low-dose SIV challenge, implying that the SIV-specific immune responses were responsible for the enhanced susceptibility33.
The persistence of our effect may be attributed to antigen-experienced CD4 T-cells resident in the long-lasting resting subset of CD4 T-cells that have traditionally been described as “naive”34,35 The increased rate of infection seen in the Phambili vaccine group was weakest amongst women. Given the high background prevalence of genital inflammation seen in women in SA and its association with HIV-1 acquisition, any additional vaccine-related subclinical inflammation may be masked by these already high rates of genital tract inflammation.36 Given the gender differences seen in this study, future vaccine trials should be stratified by sex as was recommended when we published our initial findings37
Given the lack of efficacy of all tested Ad5-vectored HIV-1 vaccines19,20,26, and our observations of vaccine induced enhancement, we do recommend caution with the use of this vaccine platform in high HIV-1 prevalence areas. Further investigations with this vaccine delivery platform in HIV-1 vaccine trials should inform participants of these findings and include judicious monitoring for potential increased risk of HIV acquisition.. In assessing Ad5-vectored vaccines for other diseases, we recommend monitoring participants for HIV-1 acquisition, particularly when trials are conducted in high HIV-1 prevalence areas.
Supplementary Material
Acknowledgments
Our study was funded by grants from the National Institute of Allergy and Infectious Diseases to the HIV Vaccine Trials Network (5U01 AI068614, 5U01 AI068618, 5U01 AI068635, 5U01 AI069453, 5U01 AI069519, 5U01 AI069469) as well as Merck and Co Inc. The South African AIDS Vaccine initiative (SAAVI) of the Medical Research Council provided support to the clinical trial sites. We thank the Phambili Study volunteers and the staff and community members at each of the Phambili Study sites, the staff at the HVTN Administrative Core, SCHARP Statistical Center, and Central Laboratory; the staff at Merck, and Elizabeth Adams at the Division of AIDS in the National Institute of Allergy and Infectious Diseases for their facilitation of the study design, and conduct. The opinions expressed in this Article are those of the authors and do not represent the official views of the US National Institute of Allergy and Infectious Diseases.
Role of the funding source
The study was reviewed by the Division of Acquired Immunodeficiency Syndrome of the US National Institute of Allergy and Infectious Diseases, and the report was reviewed by the sponsors. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010 Sep;5(5):386–390. doi: 10.1097/COH.0b013e32833cfe4c. Review. PubMed PMID: 20978378; PubMed Central PMCID: PMC2967414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheets RL, Stein J, Bailer RT, et al. Biodistribution and toxicological safety of adenovirus type 5 and type 35 vectored vaccines against human immunodeficiency virus-1 (HIV-1), Ebola, or Marburg are similar despite differing adenovirus serotype vector, manufacturer's construct, or gene inserts. J Immunotoxicol. 2008 Jul;5(3):315–335. doi: 10.1080/15376510802312464. PubMed PMID: 18830892; PubMed Central PMCID: PMC2777703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Ewald BA, Lynch DM, et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol. 2008 May;82(10):4844–4852. doi: 10.1128/JVI.02616-07. Epub 2008 Mar 12. PubMed PMID: 18337575; PubMed Central PMCID: PMC2346755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barratt-Boyes SM, Soloff AC, Gao W, et al. Broad cellular immunity with robust memory responses to simian immunodeficiency virus following serial vaccination with adenovirus 5- and 35-based vectors. J Gen Virol. 2006 Jan;87(Pt 1):139–149. doi: 10.1099/vir.0.81445-0. PubMed PMID: 16361426. [DOI] [PubMed] [Google Scholar]
- 5.Santra S, Seaman MS, Xu L, et al. Replication-defective adenovirus serotype 5 vectors elicitdurable cellular and humoral immune responses in nonhuman primates. J Virol. 2005 May;79(10):6516–6522. doi: 10.1128/JVI.79.10.6516-6522.2005. PubMed PMID: 15858035; PubMed Central PMCID: PMC1091731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casimiro DR, Tang A, Chen L, et al. Vaccine-induced immunity in baboons by using DNA and replication-incompetent adenovirus type 5 vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003 Jul;77(13):7663–7668. doi: 10.1128/JVI.77.13.7663-7668.2003. PubMed PMID: 12805466; PubMed Central PMCID: PMC164828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casimiro DR, Chen L, Fu TM, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003 Jun;77(11):6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. PubMed PMID: 12743287; PubMed Central PMCID: PMC154996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Someya K, Xin KQ, Ami Y, et al. Chimeric adenovirus type 5/35 vector encoding SIV gag and HIV env genes affords protective immunity against the simian/human immunodeficiency virus in monkeys. Virology. 2007 Oct 25;367(2):390–397. doi: 10.1016/j.virol.2007.06.012. Epub 2007 Jul 12. PubMed PMID: 17628628. [DOI] [PubMed] [Google Scholar]
- 9.Casimiro DR, Cox K, Tang A, et al. Efficacy of multivalent adenovirus-based vaccine against simian immunodeficiency virus challenge. J Virol. 2010 Mar;84(6):2996–3003. doi: 10.1128/JVI.00969-09. Epub 2009 Dec 30. PubMed PMID: 20042509; PubMed Central PMCID: PMC2826028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zak DE, Andersen-Nissen E, Peterson ER, et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexistingAd5 immunity. Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):E3503–E3512. doi: 10.1073/pnas.1208972109. Epub 2012 Nov 14. PubMed PMID: 23151505; PubMed Central PMCID: PMC3528489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiperl L, Morgan C, Moodie Z, et al. Safety and immunogenicity of a replication-defective adenovirus type 5 HIV vaccine in Ad5-seronegativepersons: a randomized clinical trial (HVTN 054) PLoS One. 2010 Oct 27;5(10):e13579. doi: 10.1371/journal.pone.0013579. PubMed PMID: 21048953; PubMed Central PMCID: PMC2965084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaoko W, Karita E, Kayitenkore K, et al. Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One. 2010 Sep 21;5(9):e12873. doi: 10.1371/journal.pone.0012873. PubMed PMID: 20877623; PubMed Central PMCID: PMC2943475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson O, Dicandilo F, Kublin J, et al. Safety and Immunogenicity of the MRKAd5 gag HIV Type 1 Vaccine in a Worldwide Phase 1 Study of Healthy Adults. AIDS Res Hum Retroviruses. 2010 Nov 23; doi: 10.1089/aid.2010.0151. [Epub ahead of print] PubMed PMID: 20854108; PubMed Central PMCID: PMC3422055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kibuuka H, Kimutai R, Maboko L, et al. A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV Uninfected East Africans(RV 172) J Infect Dis. 2010 Feb 15;201(4):600–607. doi: 10.1086/650299. PubMed PMID: 20078213; PubMed Central PMCID: PMC2811694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Churchyard GJ, Morgan C, Adams E, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204) PLoS One. 2011;6(8):e21225. doi: 10.1371/journal.pone.0021225. Epub 2011 Aug 3.PubMed PMID: 21857901; PubMed Central PMCID: PMC3152265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harro C, Sun X, Stek JE, et al. Safety and immunogenicity of the Merck adenovirus serotype 5 (MRKAd5) and MRKAd6 human immunodeficiency virus type 1 trigene vaccines alone and in combination in healthy adults. Clin Vaccine Immunol. 2009 Sep;16(9):1285–1292. doi: 10.1128/CVI.00144-09. Epub 2009 Jul 15. PubMed PMID: 19605598; PubMed Central PMCID: PMC2745015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koup RA, Lamoreaux L, Zarkowsky D, et al. Replication-defective adenovirus vectors with multiple deletions do not induce measurable vector-specific T cells in human trials. J Virol. 2009 Jun;83(12):6318–6322. doi: 10.1128/JVI.00384-09. Epub 2009 Apr 1.PubMed PMID: 19339347; PubMed Central PMCID: PMC2687372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harro CD, Robertson MN, Lally MA, et al. Safety and immunogenicity of adenovirus-vectored near-consensus HIV type 1 clade B gag vaccines in healthy adults. AIDS Res Hum Retroviruses. 2009 Jan;25(1):103–114. doi: 10.1089/aid.2008.0212. PubMed PMID: 19108693; PubMed Central PMCID: PMC3256563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008 Nov 29;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. Epub 2008 Nov 13.PubMed PMID: 19012954; PubMed Central PMCID: PMC2721012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray GE, Allen M, Moodie Z, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011 Jul;11(7):507–515. doi: 10.1016/S1473-3099(11)70098-6. Epub 2011 May 11. Erratumin: Lancet Infect Dis. 2011 Jul; 11(7):495. PubMed PMID: 21570355; PubMed Central PMCID: PMC3417349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase II b test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr Opin HIV AIDS. 2010 Sep;5(5):357–361. doi: 10.1097/COH.0b013e32833d2d2b. Review. PubMed PMID: 20978374; PubMed Central PMCID: PMC2995949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duerr A, Huang Y, Buchbinder S, et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J Infect Dis. 2012 Jul15;206(2):258–266. doi: 10.1093/infdis/jis342. Epub 2012 May 4. PubMed PMID: 22561365; PubMed Central PMCID: PMC3490694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 24.Gray GE, Metch B, Churchyard G, et al. Does participation in an HIV vaccine efficacy trial affect risk behaviour in South Africa? Vaccine. 2013 Apr 12;31(16):2089–2096. doi: 10.1016/j.vaccine.2013.01.031. Epub 2013 Jan 29. PubMed PMID: 23370155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer SM, Sobieszczyk ME, Janes H, et al. for the HVTN 505 Study Team. Efficacy Trial of a DNA/rAd5 HIV-1 Preventive Vaccine. N Engl J Med. 2013 Oct 7; doi: 10.1056/NEJMoa1310566. [Epub ahead of print] PubMed PMID: 24099601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert P. Meta-Analysis of Ad 5-vector HIV Vaccine trials to Assess the Vaccine Effect on HIV acquisition. Pl04.06 AIDS Vaccine Barcelona. 2013 [Google Scholar]
- 27.Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993 Jan;7(1):95–102. doi: 10.1097/00002030-199301000-00015. PubMed PMID: 8442924. [DOI] [PubMed] [Google Scholar]
- 28.Mayer KH, Venkatesh KK. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. Am J Reprod Immunol. 2011 Mar;65(3):308–316. doi: 10.1111/j.1600-0897.2010.00942.x. Epub 2011 Jan 9. Review. PubMed PMID: 21214660; PubMed Central PMCID: PMC3077541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Raddad LJ, Magaret AS, Celum C, Wald A, Longini IM, Jr, Self SG, Corey L. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One. 2008 May 21;3(5):e2230. doi: 10.1371/journal.pone.0002230. PubMed PMID: 18493617; Plumed Central PMCID: PMC2377333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calcedo R, Vandenberghe LH, Roy S, Somanathan S, Wang L, Wilson JM. Host immune responses to chronic adenovirus infections in human and nonhuman primates. J Virol. 2009 Mar;83(6):2623–2631. doi: 10.1128/JVI.02160-08. Epub 2008 Dec 30.PubMed PMID: 19116257; PubMed Central PMCID: PMC2648267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganguly S, Manicassamy S, Blackwell J, Pulendran B, Amara RR. Adenovirus type 5 induces vitamin A-metabolizing enzymes in dendritic cells and enhances priming of gut-homing CD8 T cells. Mucosal Immunol. 2011 Sep;4(5):528–538. doi: 10.1038/mi.2011.1. Epub 2011 Feb 2. PubMed PMID: 21289616; PubMed Central PMCID: PMC3097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benlahrech A, Harris J, Meiser A, et al. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):19940–19945. doi: 10.1073/pnas.0907898106. Epub 2009 Nov 16. Plumed PMID: 19918060; PubMed Central PMCID: PMC2785271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qureshi H, Ma ZM, Huang Y, Hodge G, et al. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase II b step trial of a similar HIV-1 vaccine. J Virol. 2012 Feb;86(4):2239–2250. doi: 10.1128/JVI.06175-11. Epub 2011 Dec 7. PubMed PMID: 22156519; PubMed Central PMCID: PMC3302390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell EB, Westermann J. CD4 memory T cells on trial: immunological memory without a memory T cell. Trends Immunol. 2008 Sep;29(9):405–411. doi: 10.1016/j.it.2008.06.002. Epub 2008 Jul 31. PubMed PMID: 18674966. [DOI] [PubMed] [Google Scholar]
- 35.Vrisekoop N, Mandl JN, Germain RN. Life and death as a T lymphocyte: from immune protection to HIV pathogenesis. J Biol. 2009;8(10):91. doi: 10.1186/jbiol198. Epub. Review. PubMed PMID: 19951397; PubMed Central PMCID:PMC2790836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen CR, Moscicki AB, Scott ME, et al. Increased levels of immune activation in the genital tract of healthy young women from sub-Saharan Africa. AIDS. 2010 Aug 24;24(13):2069–2074. doi: 10.1097/QAD.0b013e32833c323b. PubMed PMID: 20588163; PubMed Central PMCID: PMC2914808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhesi Z, Stebbing J, Dhesi Z, Stebbing J. The HVTN 503/Phambili study: efficacy is always the issue. Lancet Infect Dis. 2011 Jul;11(7):490–491. doi: 10.1016/S1473-3099(11)70103-7. Epub 2011 May 11. PubMed PMID: 21570356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.