Summary
T-helper (Th) cells play critical roles within the mammalian immune system, and the differentiation of naive CD4+ T cells into distinct T-helper subsets is critical for normal immunoregulation and host defense. These carefully regulated differentiation processes are controlled by networks of cytokines, transcription factors, and epigenetic modifications, resulting in the generation of multiple CD4+ T-cell subsets, including Th1, Th2, Th9, Th17, Tfh, and Treg cells. In this review, we discuss the roles of transcription factors in determining the specific type of differentiation and in particular the role of interleukin-2 (IL-2) in promoting or inhibiting Th differentiation. In addition to discussing master regulators and subset-specific transcription factors for distinct T-helper cell populations, we focus on signal transducer and activator of transcription (STAT) proteins and on the cooperative action of interferon regulatory factor 4 (IRF4) with activator protein 1 (AP-1) family proteins and STAT3, in the assembly of complexes that broadly influence T-cell differentiation.
Keywords: transcription factors, cell differentiation, T cells, Th1/Th2/Th17 cells, cytokines, gene regulation
Introduction
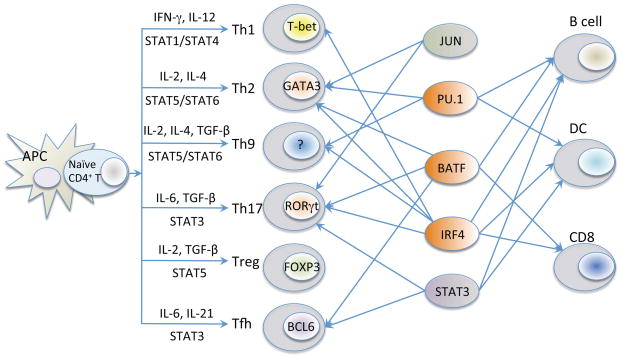
CD4+ T cells critically control the normal mammalian immune response, serving to orchestrate adaptive immune responses and maintain host defense. Following the activation of naive CD4+ T cells by antigen-presenting cells (APCs), signaling via the T-cell receptor (TCR) in the presence of specific cytokines promotes the differentiation of these cells into distinct populations of T-helper (Th) cells with different biological functions (1–4). More than 20 years ago, Mossman and Coffman (5, 6) presented a model of CD4+ T-cell differentiation in which proliferating helper T cells could develop into two major types of effector T cells denoted as Th1 and Th2 cells. Th1 cells are important in host defense against intracellular pathogens, predominantly producing interferon-γ (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor-α (TNFα)(3), whereas Th2 cells mediate host immune responses against multicellular helminthes, mainly producing IL-4, IL-5, IL-10, and IL-13 (1). Subsequent to the original Th1/Th2 paradigm, additional populations of Th cells were identified, including Th9, Th17, induced regulatory T (iTreg) cells, and T-follicular helper (Tfh) cells (Fig. 1).
Fig. 1. Transcription factors involved in the differentiation of CD4+ T cells.
In addition to ‘master regulators’ in the T-helper cells lineages (T-bet in Th1 cells, GATA-3 in Th2 cells, RORγt in Th17, FOXP3 in Treg cells, and BCL6 in Tfh cells), shown are the key STAT proteins that are required for differentiation of these populations of cells as well as other transcription factors known to contribute. For example, BATF-JUN and IRF4 bind to AP-1-IRF4 composite element motifs and form ternary DNA binding complex to regulate IL-21 responsive genes in a range of CD4+ T cells including Th17 cells (discussed later, related to Fig. 4). The PU.1/IRF4 complex is important in B cells and DC and these factors are each known to contribute to the development of Th9 cells.
To better comprehend the complex mechanisms underlying CD4+ T-cell differentiation, it is necessary to understand transcription factor binding profiles during the differentiation process. Enormous advances in DNA high-throughput technologies, such as chromatin immunoprecipitation coupled with sequencing (ChIP-Seq) (7), now make it possible to efficiently characterize transcription factor binding occupancy, the transcriptome, and epigenetic modifications during CD4+ T-cell differentiation. By examining lineage specific transcription factors, master regulators and cytokine expression, our ability to comprehensively investigate complex transcriptional programs has been dramatically enhanced. These studies have revealed many cross-regulatory mechanisms that determine final lineage commitment. In this review, we discuss the roles of transcription factors in T-cell differentiation, including STAT proteins, master regulatory factors, and the BATF/JUN/IRF4 complex. We focus on biochemical complexes and gene/protein networks of interest to both immunological and computational readers.
Transcriptional networks in T-cell differentiation
During CD4+ T-cell differentiation, there are distinct transcription factors and cytokines that act to determine the type of T-helper cells that are produced (Fig. 1). Classically, differentiation of each type of cell was believed to be dependent on a single master regulator, but subsequent studies indicated that the interplay of multiple co-expressed lineage specific transcription factors, rather than a single dominant determinant, is responsible for driving the differentiation of CD4+ T cells both in vivo and in vitro (2, 8–10). Over the past few years, the transcription factors determining the differentiation and actions of these T-cell populations have been intensively studied.
Th1 differentiation
As noted above, Th1 cells dominantly produce IFN-γ to provide host defense against intracellular pathogens, including viruses, and the differentiation of these cells is dependent on stimulation with IL-12 in the presence of TCR stimulation (3). The T box transcription factor T-bet is considered to be a master regulator for Th1 cell differentiation, promoting the expression of IFN-γ, while suppressing Th2 differentiation (11, 12). During Th1 differentiation, two important transcription factors, STAT1 and STAT4, are activated by IFN-γ and IL-12, respectively, resulting in the induction of the Tbx21 gene, which encodes the T-box protein T-bet. T-bet in turn drives Th1 differentiation, thus providing an example of a positive feedback loop. In contrast to its promotion of Th1 differentiation, T-bet antagonizes Th2 and Th17 differentiation by inhibiting the function of GATA-3 and RORγt, respectively (13, 14). IL-2 has broad actions in regulating T cell differentiation (15). It plays an important role in the initial steps leading to Th1 commitment by inducing the expression of the IL-12Rβ2 chain, which is a component of the IL-12 receptor, thereby enhancing responsiveness to IL-12 (16). IL-2 also upregulates expression of Tbx21, thereby stabilizing Th1 lineage commitment (16). Interestingly, T-bet can form complexes with other transcription factors that further enhance Th1 gene expression. For example, BCL6 can form repressive complexes with T-bet to promote the development of the Th1 cell fate by negatively regulating expression of Socs1, Socs3, and Tcf7 (17). In addition, runt-related transcription factor 3 (RUNX3) can cooperate with T-bet to induce Ifng expression while silencing Il4 expression in Th1 cells (18–20). HLX, a homeobox protein, is induced by and genetically interacts with T-bet to promote IFN-γ production in Th1 cells (21); however, whether the two factors physically interact remains to be determined. Moreover, T-bet interacts with RUNX1, thereby blocking the association of RUNX1 with RORγt and inhibiting Th17 differentiation (14). Interestingly, in contrast to CD4+ T cells, in CD8+ T cells, a different TBX family member, Eomesodermin (Eomes), is the major regulator of IFN-γ production (22).
Th2 differentiation
Th2 cells are involved in allergic reactions and host defense to helminthes (1). Th2 differentiation is induced by TCR stimulation in the presence of IL-4, and at least in vitro, Th2 differentiation is enhanced by blocking the actions of IL-12 and IFN-γ. STAT6, which is potently induced by IL-4, drives Th2 differentiation and also contributes to the maintenance of the Th2 phenotype (23, 24). However, IL-2-induced STAT5 signaling plays a critical role in Th2 differentiation (25–27). IL-2 acts at the initiation phase of Th2 differentiation by mediating TCR-induced IL-4Rα expression (27). STAT5A and STAT5B binding to the Il4ra locus are evident within 8 h of Th2 differentiation in vitro (27). By inducing IL-4Rα expression, IL-2 increases IL-4 responsiveness, resulting in an IL-2-to-IL-4 signaling cascade (15, 27). In addition, IL-2 promotes STAT5A and STAT5B binding at multiple sites within the Il4/Il13 Th2 cytokine gene locus, including at well-characterized hypersensitive sites as well as to the locus control region B and C elements in the Rad50 gene, thereby augmenting the production of Th2 cytokines (27). STAT6 and/or STAT5 can also induce expression of the Th2 master regulator, GATA-3, which then drives transcription of the hallmark Th2 cytokine, IL-4, while inhibiting transcription of the hallmark Th1 cytokine, IFN-γ, both by suppressing STAT4 expression and inhibiting RUNX3-mediated Ifng expression. Other transcription factors are also involved in Th2 differentiation. For example, GATA-3 induces expression of c-MAF, which stimulates IL-4 and promotes Th2 differentiation, and JUNB cooperates with c-MAF to augment Il4 expression (28). Interestingly, the transcription factor DEC2 is expressed in Th2 cells and enhances Gata3 expression by binding to its promoter (29). Interferon regulatory factor 4 (IRF4) modulates Il4 gene expression by cooperating with NFATc2 (30). Growth factor independent 1 (GFI-1) is an IL-4-induced STAT6-dependent transcription factor that promotes Th2 cell expansion by enhancing the proliferation of GATA-3high cells (31), while simultaneously suppressing the differentiation of other helper T cells (32, 33). Moreover, chromodomain helicase DNA-binding protein 4 (CHD4) can form a complex with GATA-3 in Th2 cells, which activates Th2 cytokine transcription and represses production of IFN-γ (34). As is evident, Th2 differentiation involves the interaction of multiple transcription factors and signaling pathways that collectively re-enforce this phenotype.
Th9 differentiation
Th9 cells are a subset of helper T cells that produce IL-9 (35–37), which has actions on multiple lineages but is best associated with allergic and inflammatory diseases. Th9 differentiation is induced in vitro by TCR stimulation in the presence of IL-4 and transforming growth factor-β (TGF-β). Several studies have implicated transcription factors PU.1 and IRF4 as vital for Th9 differentiation. PU.1 is an ETS family transcription factor that appears to promote Th9 development by repressing the Th2 program (38), whereas IRF4 can directly bind to and transactivate the Il9 promoter and may additionally contribute to the development and function of Th9 cells (39). Previously, IL-2 was reported to be critical for normal IL-9 production (40), and recently the IL-2-JAK-STAT5 signaling pathway was shown to be vital for Th9 differentiation, with critical STAT5 binding sites at the Il9 promoter (41). Interestingly, expression of transcription factor BCL6, which is induced by IL-21, inversely correlates with Th9 differentiation, and IL-21-induced BCL6 acts as a negative regulatory factor of Th9 differentiation (41). BCL6 binds in close proximity to many binding sites for STAT5 and STAT6, including at the Il9 promoter (41), suggesting a possible binding competition for these factors at the Il9 promoter, given the opposing apparent actions of BCL6 vs. STAT5/STAT6 in Th9 differentiation. Given that Th9 cells are differentiated in the presence of TGF-β and IL-4 and that IL-4 is also the key driver of Th2 differentiation, several transcription factors that are critical for Th2 differentiation also play important roles in Th9 differentiation, including STAT6, GATA-3, and IRF4 (35, 36, 42). IL-4-mediated STAT6 activation promotes Th9 development, based on its repressing the expression of FOXP3 and T-bet (42), which can inhibit IL-9 production. STAT6 also promotes expression of IRF4, which as noted above promotes Th9 differentiation (42). PARP-14 functions as a transcriptional switch and regulates STAT6-dependent activation by increasing the binding efficiency of STAT6 to cognate promoter elements (43) and promotes production of IL-9 (42). TGF-β-activated SMAD2/3 (SMAD transcription factor 2 and 3) can physically interact with IRF4 and cooperatively transactivate the Il9 promoter (44). Finally, an AP-1 family member, BATF, is enriched in Th9 cells and is required for the expression of IL-9 (37).
Th17 differentiation
Th17 cells represent a population of cells that produce the hallmark cytokine IL-17A, as well as IL-17F and IL-22, contributing to the development of inflammatory immune responses including psoriasis and inflammatory bowel disease (45). Th17 differentiation is induced in vitro by stimulation by TCR stimulation, IL-6, and TGF-β, with IL-21 and IL-23 serving important accessory roles. These cytokines activate STAT3 and induce expression of IL-21R and IL-23R, thereby promoting a cytokine signaling cascade (46–48), analogous to the IL-2 to IL-12 cascade for Th1 differentiation and the IL-2 to IL-4 cascade for Th2 differentiation (16, 27), as noted above. During Th17 differentiation, STAT3 activation augments the expression of RORγt, the master regulator for Th17 cells (49). IL-17 production is diminished in RORγt-deficient mice, but RORγt expression alone is insufficient to drive Th17 differentiation, and STAT3 activation is critical for the normal expression of IL-17 and IL-21 (50, 51). Additional transcription factors, including BATF, IRF4, IκBζ, and E2A, act as positive regulators of the expression of RORγt and IL-17 (52–55). BATF, which is discussed below in greater detail, is a FOS-like AP-1 family transcription factor that is required for the differentiation of Th17 cells, with Batf-deficient mice exhibiting defective Th17 differentiation while maintaining normal Th1 and Th2 differentiation (52). The inducible costimulator (ICOS) induces c-MAF, which induces IL-21 production, and in turn IL-21 drives the expansion of Th17 cells (56). Transcription factor E-proteins E2A and HEB act as transcription factors that positively regulate RORγt and IL-17 expression (55). ChIP-Seq studies (57, 58) of a range of transcription factors in Th17 cells indicate the co-localization at the Il17 and Il21 promoters of STAT3, RORγt, BATF, IRF4, c-MAF, JUN/JUNB/JUND, FOSL2 (a FOS family member), as well as other additional factors, suggesting the formation of large protein complexes in which transcription factors either cooperatively or competitively bind to DNA to regulate gene expression (Fig. 2).
Fig. 2. Genome browser graph of Il17a/Il17f locus in Th17 cells, showing a high degree of co-localization among a number of transcription factors including BATF, IRF4, JUN family protein, RORγt, STAT3, c-Maf (MAF), insulator CTCF, co-activator p300 and histone mark H3K4me3.
These data are assembled from Li et al. and Ciofani et al. (57, 58).
In contrast to these inducers of Th17 differentiation, a number of transcription factors, including TCF-1, GFI-1, IRF8 (interferon regulatory factor 8), and T-bet can inhibit Th17 cell differentiation. For example, TCF-1 directly binds to and represses the Il17 gene and can also repress the expression of IL-7R on Th17 cells, thereby constraining the survival of inflammatory Th17 cells (59). Downregulation of GFI-1, which is driven by TGF-β, is important for Th17 differentiation (32, 60). IRF8 also plays a critical role in silencing Th17 cell differentiation (61), acting at least in part through its physical interaction with RORγt. Interestingly, T-bet can negatively regulate Th17 lineage commitment by suppressing IRF4 (62), which is required for optimal Th17 development (53, 63). As noted above, T-bet can also interact with RUNX1, preventing its association with RORγt (14); thus, there appear to be at least two molecular mechanisms by which T-bet can inhibit Th17 differentiation.
Whereas IL-2 promotes Th1, Th2, and Th9 differentiation, it potently inhibits Th17 differentiation (64), although IL-2 can amplify the response of committed Th17 cells (65). A number of mechanisms have been suggested for the inhibitory effect of IL-2 on Th17 differentiation. First, a possible competition of binding at the Il17a gene between IL-2-induced STAT5 and IL-12 induced STAT3 was suggested (64). Second, IL-2 represses expression of Il6ra and Il6st, the genes encoding IL-6Rα and gp130 (16), which are the components of the functional IL-6 receptor. Third, IL-2 induces T-bet, which as indicated above, has two mechanisms by which it represses Th17 differentiation (14, 16)
Treg differentiation
Treg cells serve to limit immune responses and thereby prevent autoimmune and other deleterious immune reactions (45, 66, 67). In naive T cells, TGF-β induces FOXP3 (forkhead/winged helix transcription factor 3), which is a master regulator for the development and function of iTreg and nTreg cells (68–70). IL-2-activated STAT5 plays a critical role in maintaining Treg differentiation by directly binding to and inducing expression of the Foxp3 gene, with FOXP3 in turn regulating the expression of Treg-specific genes (71). TGF-β decreases methylation of a key CpG island in the Foxp3 gene, resulting in increased FOXP3 expression (72). FOXP3 can bind to and block the actions of RORγt, leading to diminished Th17 cell development (73). RUNX1 promotes Treg induction via its association with FOXP3, with a RUNX1/FOXP3 complex maintaining Foxp3 expression (74–77). In the absence of FOXP3, the interaction of RUNX1 with RORγt is favored, with enhanced Th17 differentiation and IL-17 production (75).
Transcription factors downstream of PI3-kinase/AKT activation, particularly FOXO family members FOXO1 and FOXO3, are indispensable for Treg cell function (78). NF-κB family member c-Rel binds to a conserved non-coding sequence, CNS3, in the Foxp3 promoter, thereby augmenting expression of the gene (79, 80). NFAT and SMAD3 are key transcription factors that induce Foxp3 gene expression and control the differentiation of CD4+CD25+ Treg cells, and both factors are essential for histone acetylation in the Foxp3 enhancer region and the induction of Foxp3 expression (81). Transcription factor BACH2 acts as a broad regulator of immune activation, helping to maintain Treg cells while repressing genes associated with the differentiation of other effector populations of CD4+ T cells (82). In the absence of BACH2, Treg cells had diminished expression of FOXP3 and were depleted in number (83). Nr4a nuclear receptors, which are encoded by immediate-early genes induced by TCR stimulation in thymocytes, also have been shown to be essential for the development of Treg cells, with early death in Nr4a-deficient mice due to severe autoimmunity. Interestingly, Nr4a nuclear receptors activate the Foxp3 promoter (84). A range of studies have indicated that a range of other transcription factors, including GATA-3, ETS-1, EOS, Helios and SATB1 are also important in Treg cells. GATA-3 controls Foxp3 expression by directly binding to and promoting the activity of cis-regulatory elements of the Foxp3 gene (85). Transcription factor ETS-1 can interact with the FOXP3 intronic enhancer and is required for the development of natural Treg cells (86). EOS, an Ikaros family zinc-finger transcription factor, directly interacts with FOXP3 and mediates FOXP3-dependent gene silencing in Tregs (87). Another recent study shows downregulation of EOS, rather than loss of FOXP3, underlies reprogramming of Treg cells in response to inflammation (88). Helios, encoded by Ikzf2, is expressed in a subset of Foxp3+ Treg cells and expression of Helios is restricted to the thymic-derived Treg cell population (89). Interestingly, repression of SATB1, which functions as genome organizer to regulate chromatin structure, is crucial for Treg development and function (90).
Tfh Differentiation
T-follicular helper (Tfh) cells provide help to B cells, promoting affinity maturation, germinal center induction, and the generation of plasma cells and memory B cells (91). BCL6 serves as master regulator for the differentiation of Tfh cells. Mice in which Bcl6 is conditionally deleted in T cells have defective Tfh development and lesser effects on other effector T cell lineages (92, 93). IL-6 and IL-21 activate STAT3 and drive Bcl6 expression and Tfh cell differentiation via STAT3 binding to the Bcl6 promoter (94). BLIMP1 is a transcriptional repressor encoded by the Prdm1 gene. BLIMP1 can inhibit Bcl6 expression and thus Tfh differentiation, thereby diminishing germinal center B cell responses (95). Several studies have indicated that STAT1, STAT4, and STAT5 are also involved in Tfh differentiation. IL-6-mediated STAT1 was reported to be required for Bcl6 induction and early Tfh differentiation in vivo (96) and IFN-γ-induced STAT1 directly binds to and regulates the Bcl6 gene (97). STAT5 acts as a negative regulator of Tfh differentiation by suppressing the expression of Tfh-related genes including Bcl6, Batf, Maf and Il21 (98, 99). BATF is an AP-1 family member that directly regulates expression of two transcription factors, BCL6 and c-MAF, which are required for the development of Tfh cells (100). Notably, both BCL6 and c-MAF are required for restoring Tfh cell activity to Batf−/− T cells (101). Recently, achaete-scute homologue 2 (ASCL2), a basic helix–loop–helix transcription factor, was shown to initiate Tfh cell development and promote T-cell migration to the follicles by inducing C-X-C chemokine receptor type 5 (CXCR5), while suppressing the expression of signature genes related to Th1, Th2, and Th17 differentiation (102).
Cross-regulation, diversity, and plasticity in T-cell differentiation
Although a number of transcription factors have been considered to be master regulators of T-helper differentiation, it has become clear that the determination of distinct T-cell subsets is not governed solely by these factors and that additional co-expressed factors are involved as well. These additional factors can sometimes act to suppress other T-cell subsets and balance the commitment towards the ‘favored’ subsets. The CD4+ T-cell phenotype displayed by cells expressing one of these master regulators can be influenced by the cellular context of either cytokine signals or interacting cell signals that influence or refine the activity of the master regulators (103, 104). For example, T-bet is critical for promoting Th1 differentiation, but it also plays a critical role in the inhibition of Th2 differentiation by suppressing GATA-3 expression (105). T-bet induces expression of RUNX3, which can then interact with and attenuate GATA-3, thereby augmenting Th1 differentiation while inhibiting Th2 differentiation (106). As noted above, T-bet also represses Th17 differentiation by interacting with RUNX1 and by decreasing expression of IRF4. GATA-3 promotes Th2 differentiation but also suppresses STAT4, which is critical for Th1 differentiation (107). GATA-3 can also interact with RUNX3 and thereby inhibit RUNX3-dependent IFN-γ production (108). GFI-1 also promotes Th2 expansion (31, 33) but conversely suppresses Th1, Th17 and Treg differentiation (32, 33). Thus, master regulators can induce other factors that serve to re-enforce their own program and/or inhibit other differentiation programs.
Master regulators can be co-expressed. For example, cells co-expressing IL-17 and IFN-γ can be detected in vivo, and remarkably these cells co-express RORγt and Tbet at a single cell level (109). Although BCL6 promotes expression of the classical Tfh program, subsets of Tfh cells within germinal centers can also express IL-4 or IFN-γ, suggesting that there is flexibility in the extent to which other populations of cells are eliminated. Another example of co-expression of master regulators is provided by the ‘Th2+1’ phenotype, in which infection with Th1 cell-promoting lymphocytic choriomeningitis virus (LCMV) can reprogram fully differentiated virus-specific Th2 cells into GATA-3+T-bet+ cells that can produce both IL-4 and IFN-γ (110, 111). Furthermore, IL-12-mediated STAT4 activation can induce expression of Il21 and Bcl6 to generate cells with features of both Tfh and Th1 cells (112), indicating another complexity and underscoring the idea that these populations of Th cells are perhaps best not viewed as rigid lineages.
FOXP3, which is the master regulator of Treg cells (45, 66), can co-express with a range of transcription factors including T-bet, GATA-3, STAT3, and BCL6 to suppress Th1, Th2, Th17, and Tfh cell responses, respectively (85, 104, 113–116). When the composition of FOXP3 complexes in Treg cells was analyzed by mass spectrometry, approximately 100 transcription-related proteins, including lineage-specific transcription factors, were identified (117). The combined activities of co-expressed transcription factors in Treg differentiation thus may result in a range of actions that are influenced or determined by interactions between Th lineage-specific factors and FOXP3. As we discussed above, the classical paradigm of the master regulator in the differentiation of specialized CD4+ T-cell lineages may not truly determine a stable developmental cell fate. Instead, CD4+ T-cell differentiation is flexible, with the potential to respond to the changing environmental conditions and fine-tune the immune functions by altering the expression of critical specific transcription factors.
A BATF/JUN/IRF4 complex in T-cell differentiation
Over the past several years, a distinctive cooperative relationship between IRF4 and other transcription factors in T cells has emerged. The first such functional relationship to be recognized was with STAT3 (118), and then cooperation with AP-1 complexes including BATF and JUN family proteins was subsequently recognized as critical in transcriptional regulation in immune cells, especially in T cells (57, 58, 119, 120). The first indication of global association between IRF4 and STAT3 in T cells emerged from studies of the Prdm1 gene in which a bipartite IL-21 response element that required STAT3 and IRF4 was identified (118). The Prdm1 gene encodes BLIMP1, a zinc finger protein that represses the expression of PAX5 and BCL6 and acts as a master regulator to drive terminal B cell differentiation into plasma cells. Consistent with its regulation of BLIMP1 expression, IRF4 promotes plasma cell maturation and immunoglobulin class switch, but it is also has critical actions for T cells (see below). IRF4 and STAT3 are highly expressed in immune cells and play critical roles in numerous cellular processes and regulate the immune response and lymphoid development.
Interferon regulatory factor 4
Interferon regulatory factors (IRFs) were first characterized and identified as a group of transcription factors induced by type I interferons (IFN-α/β) (121, 122). There are a total of nine mammalian IRF family members, denoted IRF1 to IRF9 (121, 122), which collectively play pivotal roles in the regulation of many biological processes in the immune system (123). Each IRF contains a well-conserved N-terminal DNA binding domain that forms a helix-turn-helix domain and recognizes DNA sequence corresponding to the IFN-stimulated response element (ISRE; 5′-GAAAnnGAAA-3′) (121, 124–126). The crystal structure analysis of the DNA binding domain revealed that 5′-GAAA-3′ is the core motif recognized by IRF family proteins (125).
Among IRF family proteins, the expression of IRF4 (historically also known as Pip/MUM1) and its closely related family member IRF8 (also known as ICSBP1) are restricted to the immune system and are induced in T cells by TCR stimulation. IRF4 is also expressed in other cell populations, including B cells and dendritic cells. IRF4 has a transactivation domain (127)(Fig. 3), and functions as a positive regulator, playing critical roles in mediating development of lymphoid and dendritic cells (128–131). Alone, IRF4 only weakly binds DNA due to the presence of a carboxy-terminal auto-inhibitory domain. However, cooperative binding with the ETS family factor, PU.1, or the closely related ETS family protein, SPIB, can relieve this auto-inhibition and increase binding affinity, allowing IRF4 to recognize composite ETS–IRF consensus motif elements, or EICEs, 5′-GGAAnnGAAA-3′) (125, 127), and regulate a range of genes, including those encoding the κ and λ immunoglobulin light chain genes. Similarly, IRF8 can also interact with PU.1/SPIB because of its structural similarity with IRF4 (125, 132).
Fig. 3. Domain structure of transcription factors that form the BATF-JUN-IRF4 complex.
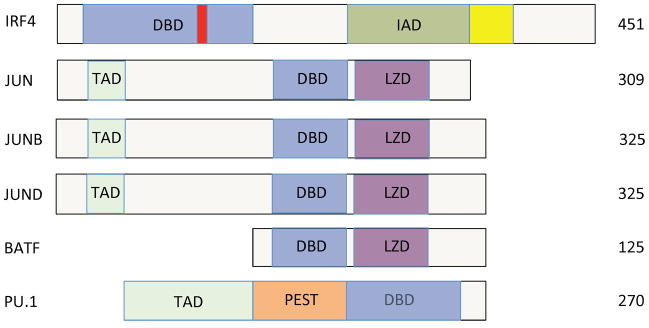
IRF4 is composed of a DNA-binding domain (DBD; blue), an IRF-association domain (IAD; green), a repression domain (yellow) and a nuclear-localization signal (red). JUN family proteins contain transcription-activating domain (TAD), DNA binding domains and leucine-zipper domain (LZD). The structure of BATF is similar to JUN, JUNB, and JUND, but it lacks a transactivation domain. PU.1 has a PEST domain in addition to transactivation and DNA binding domains.
IRF4 plays pivotal roles in both T and B cell biology (133, 134). IRF4 is known to be important for the maturation of B cells (131), plasma cells (135) and T cells (133). We previously demonstrated that IRF4 regulates IL-21-induced Prdm1 expression in B and T cells and that IRF4 globally cooperates with STAT3 as a complex to regulate IL-21-mediated gene expression (118). Notably, STAT3 binding in CD4+ T cells is diminished in Irf4−/− mice, suggesting that the expression of IRF4 is required for STAT3 binding in vivo in CD4+ T cells. IRF4 contributes to the development of multiple T-helper cell subsets (123), with defective Th1 (136), Th2 (30, 137, 138), Th9 (39, 44), Th17 (53, 58, 63), Treg (79), and Tfh (118) differentiation in its absence. IRF4 appears to be dependent on TCR signaling strength and can function to regulate the maintenance of CD8+ T-cell effector differentiation (139, 140). Among dendritic cells (DCs), IRF4 is essential for the development of CD4+CD8α−CD11bhigh DCs (141, 142), and recent studies indicate that it is the migratory function rather than overall development that is dependent on IRF4 (143–145). Notably, Irf4−/− mice did not show apparent phenotypes outside of lymphoid and myeloid lineages (146), underscoring the restriction of important non-redundant actions of IRF4 to the immune system.
AP-1 family members, BATF and JUN
BATF family proteins BATF, BATF2, and BATF3 (147, 148) are AP-1 family transcription factors, along with JUN, FOS, MAF (musculoaponeurotic fibrosarcoma) and ATF (activating transcription factor) family proteins (149). Each of these proteins is a basic leucine-zipper (bZIP) protein (Fig. 3), with the leucine-zipper motif mediating dimerization and the basic domain mediating DNA binding activity (150). In T cells, BATF is highly expressed in pre-activated CD4+ T cells, as well as in Th1, Th2, Th9, Th17, and Treg cells (57, 119). BATF is essential for normal class-switch recombination in B cells and controls Th17 cell differentiation by regulating expression of IL17 and other Th17-specific target genes, including Il22, Rorc, Rora and Ahr. Batf−/− mice are resistant to the induction of EAE, and T cells from these animals have defective expression of the master transcriptional regulator RORγt and of IL-21 (52, 58, 101, 151), both of which are crucial for Th17 differentiation (46–48, 152). The role for BATF in Th2 differentiation remains somewhat unclear, as in one study, it was reported to be required for normal Th2 development, although some Th2 differentiation occurred in its absence (153), whereas in another study, expression of IL-4, IL-10, and CTLA4 were not significantly decreased in Batf−/− mice, whereas, Th2 differentiation was reduced dramatically in Batf−/−Batf3−/− double knockout mice. BATF is essential for the development of Tfh cells (101). In Th1 cells, Ifng expression is not altered in either Batf−/− or Batf3−/− single or Batf−/−Batf3−/− double knockout mice, indicating that IFN-γ and T-bet are not regulated by these BATF family proteins (119). In B cells, BATF can directly regulate the expression of AID (activation-induced cytidine deaminase) and germline transcripts (101, 153).
Like FOS family proteins, BATF, BATF2, and BATF3 can heterodimerize with JUN family proteins. Initially, BATF family proteins were considered as inhibitors of AP-1-dependent transcription, as they lack transactivation domains (Fig. 3) and presumably can function as dominant-negative regulators to block AP-1-mediated gene expression and transcription activity, at least in some cells (154–157). However, BATF and BATF3, respectively, can have positive transcriptional effects on Th cell differentiation and CD8α+ lymphoid-resident dendritic cells (52, 158). BATF and JUN family proteins can form heterodimers and exhibit similar DNA binding profiles as FOS/JUN dimers, preferentially recognizing palindromic AP-1 or TRE (12-O-tetradecanolyphorbol-13-acetate-response element) motifs, with consensus TGA[C/G]TCA DNA binding motifs being preferred to CRE (cyclic AMP-response element) consensus TGACGTCA motifs (148, 155). In addition to JUN, BATF can also heterodimerize with the other two JUN family proteins, JUNB and JUND. Each of these complexes can interact with AP-1 consensus DNA sites, but it remains unclear how the functions of different combinations of BATF-JUN family heterodimers may differ. BATF cannot form homodimers nor dimerize with FOS (155).
Transcriptional regulation via BATF/JUN/IRF4 complexes
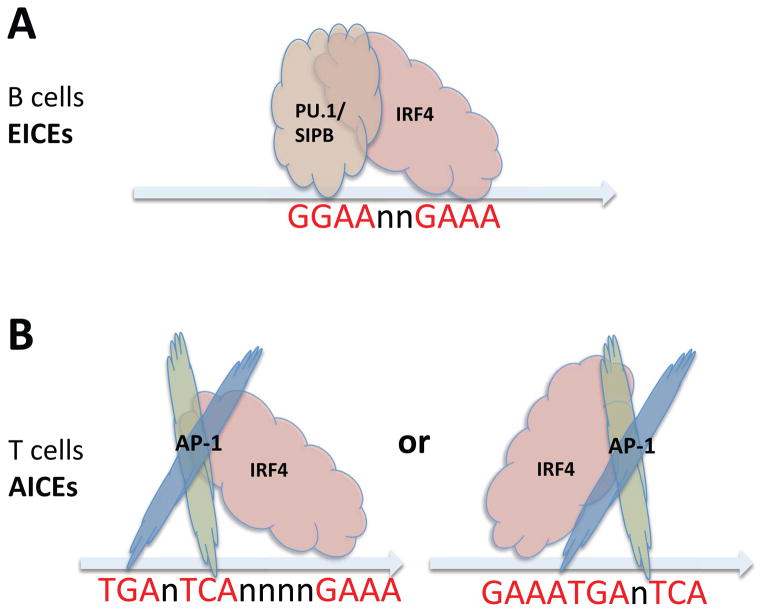
As mentioned above, in B cells, IRF4 can form ternary complex with ETS family factors PU.1 or SPIB (Fig. 4A), relieving auto-inhibition and augmenting IRF4 binding affinity to EICEs (146, 159). In B cells, where PU.1 and SPIB are highly expressed, ChIP-Seq analysis of IRF4 binding from previously published B-cell libraries (118) confirmed that EICEs are the dominant IRF4/PU.1 DNA binding motifs (57). In contrast, in T cells where PU.1 and SPIB are poorly expressed, it was unclear how the C-terminal autoinhibitory effect could be relieved to allow IRF4 binding. Indeed, ChIP-Seq analysis from TCR pre-activated T cells (either unstimulated or stimulated with IL-21), Th2 and Th17 cells (57, 58, 119, 120) revealed that EICEs were not enriched at IRF4 binding sites. Instead, de novo motif discovery from IRF4 binding sites in T cells strikingly revealed that 5′-TGAnTCA-3′ AP-1 motifs were significantly enriched (57, 58, 120). Interestingly, AP-1 like motifs were found at IRF4 ChIP-Seq peaks in B cells as well, although to a much lower extent than EICEs (57). IRF8, which is structurally similar to IRF4 and also can interact with PU.1 (159), instead preferentially bound to tandem canonical IRF core motifs separated by two base pairs (5′-GAAAnnGAAA-3′) in both un-stimulated and IL-21 stimulated cells rather than binding to AICEs (57). Further analysis of how IRF4 interacts with AP-1-like motifs revealed the presence of IRF4 core motifs (5′-GAAA-3′) either adjacent to or separated by four intervening nucleotides (Fig. 4B) from AP-1 motifs (57), indicating binding cooperativity for AP-1 and IRF4. These AP-1–IRF4 composite elements were denoted as AICEs (AP-1-IRF4 composite elements) to correspond to the EICE nomenclature used for classical IRF4 elements binding PU.1/IRF4 complexes in B cells (57, 120). Thus, there are two distinctive IRF4 binding complexes, with PU.1 in B cells and with BATF-JUN in T cells (see Fig. 4).
Fig. 4. Schematic cartoons of the interaction between IRF4 and PU.1/SPIB, and between IRF4 and AP-1 (BATF-JUN) factors.
(A) In B cells, ETS transcription factors PU.1 or SPIB interact with IRF4 to form ternary complexes with ETS–IRF consensus elements (EICEs, 5′-GGAAnnGAAA-3′) on DNA. (B) In contrast, in T cells, where PU.1 is less expressed, AP-1 family heterodimers (mainly BATF-JUN) form complex with IRF4 and recognize AP-1-IRF4 composite elements (AICEs; 5′-TGAnTCAnnnnGAAA-3′, or 5′-GAAATGAnTCA-3′). These AICE complexes either can form with 4 nucleotides or 0 nucleotides between the AP-1 and ETS motifs. These AICEs can also form in B cells.
A motif analysis of BATF-binding sites in T cells, like the analysis for IRF4 binding sites, identified AICEs as well, and BATF binding sites co-localized with those identified for IRF4 and JUN family proteins. BATF binding to a AP-1 consensus element lacking the accessory IRF4 motif does not require interaction with IRF4 (57, 58, 120); however, IRF4 binding to AICEs is substantially dependent on BATF, analogous to the requirement of PU.1/SPIB for the binding of IRF4 to EICEs in B cells. Importantly, BATF binding was markedly diminished in Irf4−/− T cells and conversely IRF4 binding was markedly diminished in Batf−/− T cells, underscoring a functional cooperation between these two factors (57).
Many immunologically important genes that are potently regulated by IL-21 are dependent on AP-1-IRF4 complexes, including Prdm1, Il17a, Il21, Il10, Ikzf2 and Ctla4, and electrophoretic mobility shift assays (EMSAs) revealed direct cooperative binding interactions of AP-1-IRF4. For example, when Th17 nuclear extracts were used with a probe corresponding to a conserved noncoding sequence, CNS9, derived from the Il10 gene, both IRF4 and AP-1 motifs were required for normal binding activity (57). Although BATF2 and BATF3 also interact with JUN family proteins, it is not yet clear whether BATF2-JUN or BATF3-JUN heterodimers are functional binding partners of IRF4 or IRF8. It will be interesting to study how the different BATF family proteins differ in the complexes they form and the degree of overlap in genes that they regulate.
In Th17 cells, BATF and IRF4 appear to function as ‘pioneer’ factors that govern the initial chromatin accessibility for subsequent binding of other transcription factors that are activated following cytokine stimulation (58). For example, together with STAT3, BATF and IRF4 then recruit lineage-specific transcription factor RORγt to regulate a set of Th17-relevant genes. The BATF-IRF4 complex also plays a central regulatory role in IL-6-mediated signaling, particularly in the absence of T-bet (160).
The binding of IRF4 can be complex. For example, in activated B cells, IRF4 can bind to DNA by three different mechanisms—in the context of complexes binding to EICEs and AICEs as noted above, as well via as classical ISREs. Although the binding affinity of these modes varies, these complexes might play distinctive roles in B cell differentiation and development (161). In CD8+ T cells, it has been shown that IRF4 and BATF function as major regulators of antiviral cytotoxic T-cell immunity and are required for sustained CD8+ T-cell effector following infection with lymphocytic choriomeningitis virus (LCMV) (162). The BATF-JUN-IRF4 complex also can function as a “checkpoint” in the differentiation of effector CD8+ T cells via direct binding to and activation of the expression of lineage-specific genes including Tbx21, Prdm1 and Runx3, while simultaneously repressing genes that encode effector molecules such as IFN-γ and granzyme B (163).
As noted above, we previously used genome-wide ChIP-Seq analysis to show that IL-21-mediated activation of STAT3 resulted in the binding of this factor to thousands of loci in pre-activated CD4+ T cells and that the majority (over ~70%) of STAT3 binding regions co-localized with IRF4, indicating broad cooperative gene regulation by these two factors (118). Importantly, although no difference in STAT3 phosphorylation was observed in Irf4+/+ and Irf4−/− cells, STAT3 binding was markedly diminished to absent in Irf4−/− T cells, suggesting that IRF4 is essential for normal STAT3 binding activity (118). No direct co-precipitation of IRF4 and STAT3 was reproducibly observed, but it is conceivable that there is a direct or indirect physical association of these two factors, or alternatively it is possible that IRF4 affects the chromatin structure in a fashion that influences STAT3 binding. More recently, it was recognized that in IL-21-stimulated T cells or in Th17 cells, there is co-localization not only of IRF4 and STAT3 but also of BATF and JUN family proteins, generally clustered in very close proximity (within 100 bp) (57, 58, 120), including at the Prdm1 IL-21 response element previously studied (118), as well as in the Il21 promoter and Il17a/Il17f region (57). Notably, STAT3 binds to GAS (γ-interferon activated site) or GAS-like motifs instead of AP-1 motifs, but its co-localization with BATF and IRF4 can potentially be explained by the known physical interaction between the coiled-coil domain of STAT3 and a large COOH-terminal segment of JUN (164–167). However, the mechanism by which STAT3 is recruited to AP-1/IRF4 complex to regulate gene expression is not yet established.
Noncoding RNAs in T-cell differentiation
As noted above, the differentiation of distinctive CD4+ T-cell populations are complex processes involving various cytokines, receptors and transcription factors to determine the ultimate outcome. In addition, a tremendous amount of information has been emerging regarding the functional and dynamic roles of non-coding RNAs, including microRNAs (miRNA) and long non-coding RNAs (lncRNAs) that contribute to the regulation of CD4+ T cells differentiation programs. These RNAs represent additional layers of complexity. Although 20,000 protein-coding genes have been annotated by GENCODE reference database (168, 169), these cover only ~3% of the genome (170) and the remainder largely consists of non-protein coding sequences (171), some of which correspond to microRNAs and lncRNAs.
MicroRNAs
MicroRNAs are endogenous small (~18–23 nucleotide long) non-coding RNAs that function in the transcriptional and post-transcriptional regulation of protein-coding genes (172). Each microRNA can target hundreds of genes or pseudogenes by binding to microRNA response elements, which are generally 6–8 nucleotides long regions within the 3′-UTR (untranslated region) of target mRNAs. MicroRNAs have been shown to influence many important processes, including development, survival, proliferation, differentiation, and the function of immune cells. Growing evidence suggests that microRNAs can critically modulate the development, maintenance, and differentiation of T-helper cells (10, 173, 174). The expression of various cytokines, growth factors, and transcription factors reportedly can be regulated by microRNAs (175).
For CD4+ T cells, microRNAs can control lineage-specific functions at different stages of differentiation (176) (Table 1). MiR-29 is a potent inhibitor of Th1 differentiation, suppressing IFN-γ production to control innate and adaptive immune responses to intracellular bacterial infection by directly targeting 3′ UTR of IFN-γ (177). Moreover, miR-29 also can directly repress expression of T-bet and Eomes, two transcription factors known to induce IFN-γ production that promote Th1 differentiation (178). Several other microRNAs also critically regulate Th1 differentiation. MiR-146a can inhibit Th1 differentiation by directly targeting and inhibiting expression of Stat1, Traf6 and Irak1 (179, 180). MiR-155 enhances Th1 cell-dependent tissue inflammation (181) and inhibits expression of Socs1 and Ship1, which are negative regulators of cytokine signaling (182, 183). Meanwhile, miR155 is upregulated by FOXP3 in Treg cells and it is required to maintain Treg cell proliferative activity by targeting SOCS1 (183). The miR-17~92 cluster (184, 185) can promote Th1 responses while preventing inducible Treg differentiation. MiR-21 (186) plays inhibitory roles in Th1 cells, and loss of miR-21 significantly enhanced the Th1-associated delayed type hypersensitivity cutaneous responses.
Table 1.
MicroRNAs that influence the differentiation of T helper cells
| miRNAs | Targets | Functions | Cells | References |
|---|---|---|---|---|
| miR-29 | Tbet and Eomes 3′UTR of IFNγ | Suppresses IFNγ and inhibits Th1 differentiation | Th1 | 177, 178 |
| miR-146a | Stat1, Traf6 and Irak1 | Directly targets Stat1 and inhibits Th1 differentiation | Th1 | 179, 180 |
| miR-155 | SOCS1, SHIP1 and PU.1 | Enhances Th1 tissue inflammation; promotes development of Th2 and Th17; maintains Treg cell activity | Th1, Th2, Th17, Treg | 181–183, 187 |
| miR-17~92 | IL-12p35 | Promotes Th1 responses; promotes Tfh differentiation; prevents iTreg differentiation | Th1, iTreg, Tfh | 184,185, 193 |
| miR-21 | IL-12, GATA3 | Inhibits IL-12 and limits Th1 differentiation; promotes Th2 differentiation | Th1, Th2 | 186, 188 |
| miR-126 | PU.1 | Indirectly inhibits PU.1, which then increases GATA3 and promotes Th2 differentiation | Th2 | 189 |
| miR-145 | ? | Promotes IL-5 and IL-13 production by Th2 cells | Th2 | 190 |
| miR-10a | BCL6, Ncor2 | Targets BCL-6 and limits the Th17 differentiation | Th17 | 191 |
| miR-326 | ETS-1 | Directly targets ETS-1 and promotes Th17 differentiation | Th17 | 192 |
Th2 differentiation is also influenced by multiple microRNAs. MiR-155 contributes to the regulation of allergic airway inflammation by promoting Th2 responses through its inhibition of PU.1 expression (187). MiR-21, which is repressed by BCL6 but activated by STAT3, can promote Th2 cell differentiation by its induction of GATA-3 and inhibition of Sprouty1 (188). MiR-126 and miR-145 are also shown to be important for Th2 differentiation and effector functions (189, 190). Blocking miR-126 resulted in increased PU.1, diminished GATA-3, and thus diminished Th2 differentiation (189). MiR-145 is also a positive regulator of Th2 differentiation. Inhibition of miR-145 resulted in suppressed Th2 cytokine production and airway hyperresponsiveness (190). In Th17 cells, TGF-β-induced miR-10a can target BCL-6 and functionally attenuate the differentiation of Th17 cells, thus to fine-tune the plasticity and fate of helper T cells (191). MiR-326 promotes Th17 differentiation by directly targeting ETS-1, a negative regulator of Th17 differentiation, and plays a critical role for the pathogenesis of multiple sclerosis and mice with experimental autoimmune encephalomyelitis (EAE) (192). MiR-155 promotes the development of Th17 cells, promoting production by dendritic cells of key cytokines such as IL-6, IL-12 and IL-23 that promote Th17 cell formation (181). In Tfh cells, the miR-17~92 cluster functions to critically regulate Tfh cells differentiation and function (193) and control the migration of CD4+ T cells into B cell follicles, by suppressing expression of the phosphatase PHLPP2 (194). MiR-10a (which can limit Th17 differentiation, as discussed above), can also limit the conversion of iTreg cells into Tfh cells by inhibiting expression of Bcl6 and its co-repressor Ncor2 (191).
Long non-coding RNAs
Long non-coding RNAs (lncRNAs), including long intergenic non-coding RNAs (lincRNAs), are defined as non-protein coding transcripts with lengths ranging from 200 to thousands of nucleotides (195). lncRNAs typically overlap with protein-coding genes or other non-coding RNAs, are often polyadenylated, and lack open reading frames (ORFs) (196, 197). High-throughput transcriptome profiling analysis such as RNA-Seq indicates that there are tens of thousands of mammalian lncRNAs. It has been suggested that the majority of lncRNAs may not have functions (198), and only a very small proportion have proven biological roles according to lncRNAs database (199).
Nevertheless, some lncRNAs are known to regulate cellular processes, including the differentiation and activation of lymphocytes. For example, lincRNA TMEVPG1, which is located near the Ifng gene, is dependent on STAT4 and T-bet. TMEVPG1 expression contributes, although by itself it is not sufficient, to drive the Th1 program and Ifng expression (200). Another lincRNA called LincR-Ccr2-5′AS, along with GATA-3, plays essential roles for the migration of Th2 cells (201). Knockdown of this lincRNA showed decreased expression of neighboring chemokine receptor–encoding genes such as Ccr2 and Ccr3, and compromised the migration of TH2 cells to the lung tissues, indicating a critical function for this lincRNA in Th2 cell differentiation.
Concluding remarks
CD4+ T-cell differentiation into multiple specialized populations of cells comprises multiple layers of regulation, including the induction of a range of cytokines, expression of cytokine receptors, the expression of master regulators and cell-type specific transcription factors, as well as epigenetic control, and the contribution of non-coding RNAs. Here, we discussed T-helper differentiation networks in Th1, Th2, Th9, Th17, Treg, and Tfh cells and also discussed the roles of the BATF/JUN/IRF4 protein complex in the development and differentiation of helper T cells. STAT3 co-localizes with these other factors in a genome-wide fashion and has previously been shown to be capable of interacting with JUN. It remains to be determined whether other cell-type specifying STAT proteins can also interact with this complex. In addition to transcription factors, non-coding RNAs such as shorter ncRNA (miRNA) or longer ncRNAs (lncRNAs, lincRNAs) may affect the expression of neighboring protein-encoding genes to reinforce or attenuate gene regulation by transcription factors, thereby adding another layer of complexity to the regulatory network of transcription factors underlying T-cell development and differentiation.
With the help with next generation sequencing, it has become possible to globally examine the genome and the transcriptome and to integrate these multiple datasets together. Combining multiple complex data sets will help to more completely define the molecular mechanisms underlying CD4+ T-cell differentiation.
Acknowledgments
We thank Dr. Jian-Xin Lin, NHLBI, for critical comments. Supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, NIH, Bethesda, MD.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annual review of immunology. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanno Y, Vahedi G, Hirahara K, Singleton K, O’Shea JJ. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annual review of immunology. 2012;30:707–31. doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annual review of immunology. 2003;21:713–58. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 4.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annual review of immunology. 2006;24:607–56. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Journal of immunology. 1986 Apr 1;136(7):2348–57. [PubMed] [Google Scholar]
- 6.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 7.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007 May 18;129(4):823–37. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009 May;30(5):646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 9.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010 Feb 26;327(5969):1098–102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayamada S, Takahashi H, Kanno Y, O’Shea JJ. Helper T cell diversity and plasticity. Current opinion in immunology. 2012 Jun;24(3):297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000 Mar 17;100(6):655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 12.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002 Jan 11;295(5553):338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 13.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005 Jan 21;307(5708):430–3. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 14.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nature immunology. 2011 Jan;12(1):96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013 Jan 24;38(1):13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nature immunology. 2011 Jun;12(6):551–9. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oestreich KJ, Huang AC, Weinmann AS. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. The Journal of experimental medicine. 2011 May 9;208(5):1001–13. doi: 10.1084/jem.20102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nature immunology. 2007 Feb;8(2):145–53. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 19.Naoe Y, Setoguchi R, Akiyama K, Muroi S, Kuroda M, Hatam F, et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. The Journal of experimental medicine. 2007 Aug 6;204(8):1749–55. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. The Journal of experimental medicine. 2007 Aug 6;204(8):1945–57. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullen AC, Hutchins AS, High FA, Lee HW, Sykes KJ, Chodosh LA, et al. Hlx is induced by and genetically interacts with T-bet to promote heritable T(H)1 gene induction. Nature immunology. 2002 Jul;3(7):652–8. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- 22.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003 Nov 7;302(5647):1041–3. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 23.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996 Apr 18;380(6575):630–3. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996 Mar;4(3):313–9. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 25.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, et al. Interleukin 2 plays a central role in Th2 differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2004 Mar 16;101(11):3880–5. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003 Nov;19(5):739–48. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 27.Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY, et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nature immunology. 2008 Nov;9(11):1288–96. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. The EMBO journal. 1999 Jan 15;18(2):420–32. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XO, Angkasekwinai P, Zhu J, Peng J, Liu Z, Nurieva R, et al. Requirement for the basic helix-loop-helix transcription factor Dec2 in initial TH2 lineage commitment. Nature immunology. 2009 Dec;10(12):1260–6. doi: 10.1038/ni.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. The Journal of experimental medicine. 2002 Apr 15;195(8):1003–12. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Guo L, Min B, Watson CJ, Hu-Li J, Young HA, et al. Growth factor independent-1 induced by IL-4 regulates Th2 cell proliferation. Immunity. 2002 May;16(5):733–44. doi: 10.1016/s1074-7613(02)00317-5. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Davidson TS, Wei G, Jankovic D, Cui K, Schones DE, et al. Down-regulation of Gfi-1 expression by TGF-beta is important for differentiation of Th17 and CD103+ inducible regulatory T cells. The Journal of experimental medicine. 2009 Feb 16;206(2):329–41. doi: 10.1084/jem.20081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Jankovic D, Grinberg A, Guo L, Paul WE. Gfi-1 plays an important role in IL-2-mediated Th2 cell expansion. Proceedings of the National Academy of Sciences of the United States of America. 2006 Nov 28;103(48):18214–9. doi: 10.1073/pnas.0608981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosokawa H, Tanaka T, Suzuki Y, Iwamura C, Ohkubo S, Endoh K, et al. Functionally distinct Gata3/Chd4 complexes coordinately establish T helper 2 (Th2) cell identity. Proceedings of the National Academy of Sciences of the United States of America. 2013 Mar 19;110(12):4691–6. doi: 10.1073/pnas.1220865110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nature immunology. 2008 Dec;9(12):1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature immunology. 2008 Dec;9(12):1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 37.Jabeen R, Goswami R, Awe O, Kulkarni A, Nguyen ET, Attenasio A, et al. Th9 cell development requires a BATF-regulated transcriptional network. The Journal of clinical investigation. 2013 Nov 1;123(11):4641–53. doi: 10.1172/JCI69489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nature immunology. 2010 Jun;11(6):527–34. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010 Aug 27;33(2):192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. Journal of immunology. 1994 Nov 1;153(9):3989–96. [PubMed] [Google Scholar]
- 41.Liao W, Spolski R, Li P, Du N, West EE, Ren M, et al. Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proceedings of the National Academy of Sciences of the United States of America. 2014 Mar 4;111(9):3508–13. doi: 10.1073/pnas.1301138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goswami R, Jabeen R, Yagi R, Pham D, Zhu J, Goenka S, et al. STAT6-dependent regulation of Th9 development. Journal of immunology. 2012 Feb 1;188(3):968–75. doi: 10.4049/jimmunol.1102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehrotra P, Riley JP, Patel R, Li F, Voss L, Goenka S. PARP-14 functions as a transcriptional switch for Stat6-dependent gene activation. The Journal of biological chemistry. 2011 Jan 21;286(3):1767–76. doi: 10.1074/jbc.M110.157768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamiya T, Ichiyama K, Kotani H, Fukaya T, Sekiya T, Shichita T, et al. Smad2/3 and IRF4 play a cooperative role in IL-9-producing T cell induction. Journal of immunology. 2013 Sep 1;191(5):2360–71. doi: 10.4049/jimmunol.1301276. [DOI] [PubMed] [Google Scholar]
- 45.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010 Mar 19;140(6):845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007 Jul 26;448(7152):484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007 Jul 26;448(7152):480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 48.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature immunology. 2007 Sep;8(9):967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 49.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006 Sep 22;126(6):1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 50.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. The Journal of biological chemistry. 2007 Mar 30;282(13):9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 51.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. Journal of immunology. 2007 Apr 15;178(8):4901–7. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 52.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009 Jul 16;460(7253):405–9. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nature immunology. 2007 Sep;8(9):958–66. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 54.Okamoto K, Iwai Y, Oh-Hora M, Yamamoto M, Morio T, Aoki K, et al. IkappaBzeta regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature. 2010 Apr 29;464(7293):1381–5. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- 55.Zhang F, Fuss IJ, Yang Z, Strober W. Transcription of RORgammat in developing Th17 cells is regulated by E-proteins. Mucosal immunology. 2013 Sep 25; doi: 10.1038/mi.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009 Feb;10(2):167–75. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM, et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature. 2012 Oct 25;490(7421):543–6. doi: 10.1038/nature11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, et al. A validated regulatory network for Th17 cell specification. Cell. 2012 Oct 12;151(2):289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu Q, Sharma A, Ghosh A, Sen JM. T cell factor-1 negatively regulates expression of IL-17 family of cytokines and protects mice from experimental autoimmune encephalomyelitis. Journal of immunology. 2011 Apr 1;186(7):3946–52. doi: 10.4049/jimmunol.1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012 Mar 23;36(3):362–73. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 61.Ouyang X, Zhang R, Yang J, Li Q, Qin L, Zhu C, et al. Transcription factor IRF8 directs a silencing programme for TH17 cell differentiation. Nature communications. 2011;2:314. doi: 10.1038/ncomms1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gokmen MR, Dong R, Kanhere A, Powell N, Perucha E, Jackson I, et al. Genome-wide regulatory analysis reveals that T-bet controls Th17 lineage differentiation through direct suppression of IRF4. Journal of immunology. 2013 Dec 15;191(12):5925–32. doi: 10.4049/jimmunol.1202254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huber M, Brustle A, Reinhard K, Guralnik A, Walter G, Mahiny A, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2008 Dec 30;105(52):20846–51. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007 Mar;26(3):371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 65.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature medicine. 2007 Jun;13(6):711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 66.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008 May 30;133(5):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009 May;30(5):636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 68.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003 Apr;4(4):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 69.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003 Feb 14;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 70.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nature immunology. 2007 Mar;8(3):277–84. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 71.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. Journal of immunology. 2007 Jan 1;178(1):280–90. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 72.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. The Journal of experimental medicine. 2007 Jul 9;204(7):1543–51. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008 May 8;453(7192):236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007 Apr 5;446(7136):685–9. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 75.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nature immunology. 2008 Nov;9(11):1297–306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kitoh A, Ono M, Naoe Y, Ohkura N, Yamaguchi T, Yaguchi H, et al. Indispensable role of the Runx1-Cbfbeta transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity. 2009 Oct 16;31(4):609–20. doi: 10.1016/j.immuni.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Rudra D, Egawa T, Chong MM, Treuting P, Littman DR, Rudensky AY. Runx-CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nature immunology. 2009 Nov;10(11):1170–7. doi: 10.1038/ni.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch’en IL, Stockmann C, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010 Dec 14;33(6):890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009 Mar 19;458(7236):351–6. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009 Dec 18;31(6):932–40. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nature immunology. 2008 Feb;9(2):194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 82.Roychoudhuri R, Hirahara K, Mousavi K, Clever D, Klebanoff CA, Bonelli M, et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature. 2013 Jun 27;498(7455):506–10. doi: 10.1038/nature12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim EH, Gasper DJ, Lee SH, Plisch EH, Svaren J, Suresh M. Bach2 regulates homeostasis of Foxp3+ regulatory T cells and protects against fatal lung disease in mice. Journal of immunology. 2014 Feb 1;192(3):985–95. doi: 10.4049/jimmunol.1302378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sekiya T, Kashiwagi I, Yoshida R, Fukaya T, Morita R, Kimura A, et al. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nature immunology. 2013 Mar;14(3):230–7. doi: 10.1038/ni.2520. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011 Sep 23;35(3):337–48. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mouly E, Chemin K, Nguyen HV, Chopin M, Mesnard L, Leite-de-Moraes M, et al. The Ets-1 transcription factor controls the development and function of natural regulatory T cells. The Journal of experimental medicine. 2010 Sep 27;207(10):2113–25. doi: 10.1084/jem.20092153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009 Aug 28;325(5944):1142–6. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, et al. An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor eos. Immunity. 2013 May 23;38(5):998–1012. doi: 10.1016/j.immuni.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. Journal of immunology. 2010 Apr 1;184(7):3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beyer M, Thabet Y, Muller RU, Sadlon T, Classen S, Lahl K, et al. Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation. Nature immunology. 2011 Sep;12(9):898–907. doi: 10.1038/ni.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 92.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009 Aug 21;325(5943):1001–5. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009 Sep 18;31(3):457–68. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nature immunology. 2012 Apr;13(4):405–11. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009 Aug 21;325(5943):1006–10. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. Journal of immunology. 2013 Apr 1;190(7):3049–53. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakayamada S, Poholek AC, Lu KT, Takahashi H, Kato M, Iwata S, et al. Type I IFN induces binding of STAT1 to Bcl6: divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. Journal of immunology. 2014 Mar 1;192(5):2156–66. doi: 10.4049/jimmunol.1300675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. The Journal of experimental medicine. 2012 Feb 13;209(2):243–50. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, et al. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. The Journal of biological chemistry. 2012 Mar 30;287(14):11234–9. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. Journal of immunology. 2012 Apr 15;188(8):3734–44. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nature immunology. 2011 Jun;12(6):536–43. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014 Mar 27;507(7493):513–8. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nature immunology. 2010 Aug;11(8):674–80. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oestreich KJ, Weinmann AS. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. Nature reviews Immunology. 2012 Nov;12(11):799–804. doi: 10.1038/nri3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, O’Shea JJ, et al. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. The Journal of experimental medicine. 2006 Mar 20;203(3):755–66. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kohu K, Ohmori H, Wong WF, Onda D, Wakoh T, Kon S, et al. The Runx3 transcription factor augments Th1 and down-modulates Th2 phenotypes by interacting with and attenuating GATA3. Journal of immunology. 2009 Dec 15;183(12):7817–24. doi: 10.4049/jimmunol.0802527. [DOI] [PubMed] [Google Scholar]
- 107.Usui T, Nishikomori R, Kitani A, Strober W. GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rbeta2 chain or T-bet. Immunity. 2003 Mar;18(3):415–28. doi: 10.1016/s1074-7613(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 108.Yagi R, Junttila IS, Wei G, Urban JF, Jr, Zhao K, Paul WE, et al. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity. 2010 Apr 23;32(4):507–17. doi: 10.1016/j.immuni.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, Kamradt T, et al. IFN-gamma and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. European journal of immunology. 2010 Nov;40(11):3017–27. doi: 10.1002/eji.201040539. [DOI] [PubMed] [Google Scholar]
- 110.Hegazy AN, Peine M, Helmstetter C, Panse I, Frohlich A, Bergthaler A, et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 2010 Jan 29;32(1):116–28. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 111.Zhu J, Paul WE. CD4+ T cell plasticity-Th2 cells join the crowd. Immunity. 2010 Jan 29;32(1):11–3. doi: 10.1016/j.immuni.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2011 Dec 23;35(6):919–31. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009 Nov 13;326(5955):986–91. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature medicine. 2011 Aug;17(8):983–8. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nature immunology. 2009 Jun;10(6):595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nature medicine. 2011 Aug;17(8):975–82. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, Samstein RM, et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nature immunology. 2012 Oct;13(10):1010–9. doi: 10.1038/ni.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009 Dec 18;31(6):941–52. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tussiwand R, Lee WL, Murphy TL, Mashayekhi M, Wumesh KC, Albring JC, et al. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature. 2012 Oct 25;490(7421):502–7. doi: 10.1038/nature11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Glasmacher E, Agrawal S, Chang AB, Murphy TL, Zeng W, Vander Lugt B, et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science. 2012 Nov 16;338(6109):975–80. doi: 10.1126/science.1228309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annual review of immunology. 2008;26:535–84. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 122.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annual review of immunology. 2001;19:623–55. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 123.Lohoff M, Mak TW. Roles of interferon-regulatory factors in T-helper-cell differentiation. Nature reviews Immunology. 2005 Feb;5(2):125–35. doi: 10.1038/nri1552. [DOI] [PubMed] [Google Scholar]
- 124.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 125.Escalante CR, Yie J, Thanos D, Aggarwal AK. Structure of IRF-1 with bound DNA reveals determinants of interferon regulation. Nature. 1998 Jan 1;391(6662):103–6. doi: 10.1038/34224. [DOI] [PubMed] [Google Scholar]
- 126.Fujii Y, Shimizu T, Kusumoto M, Kyogoku Y, Taniguchi T, Hakoshima T. Crystal structure of an IRF-DNA complex reveals novel DNA recognition and cooperative binding to a tandem repeat of core sequences. The EMBO journal. 1999 Sep 15;18(18):5028–41. doi: 10.1093/emboj/18.18.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brass AL, Kehrli E, Eisenbeis CF, Storb U, Singh H. Pip, a lymphoid-restricted IRF, contains a regulatory domain that is important for autoinhibition and ternary complex formation with the Ets factor PU.1. Genes & development. 1996 Sep 15;10(18):2335–47. doi: 10.1101/gad.10.18.2335. [DOI] [PubMed] [Google Scholar]
- 128.De Silva NS, Simonetti G, Heise N, Klein U. The diverse roles of IRF4 in late germinal center B-cell differentiation. Immunological reviews. 2012 May;247(1):73–92. doi: 10.1111/j.1600-065X.2012.01113.x. [DOI] [PubMed] [Google Scholar]
- 129.Lu R. Interferon regulatory factor 4 and 8 in B-cell development. Trends in immunology. 2008 Oct;29(10):487–92. doi: 10.1016/j.it.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ozato K, Tailor P, Kubota T. The interferon regulatory factor family in host defense: mechanism of action. The Journal of biological chemistry. 2007 Jul 13;282(28):20065–9. doi: 10.1074/jbc.R700003200. [DOI] [PubMed] [Google Scholar]
- 131.Pernis AB. The role of IRF-4 in B and T cell activation and differentiation. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2002 Jan;22(1):111–20. doi: 10.1089/107999002753452728. [DOI] [PubMed] [Google Scholar]
- 132.Schmidt M, Bies J, Tamura T, Ozato K, Wolff L. The interferon regulatory factor ICSBP/IRF-8 in combination with PU.1 up-regulates expression of tumor suppressor p15(Ink4b) in murine myeloid cells. Blood. 2004 Jun 1;103(11):4142–9. doi: 10.1182/blood-2003-01-0285. [DOI] [PubMed] [Google Scholar]
- 133.Mittrucker HW, Matsuyama T, Grossman A, Kundig TM, Potter J, Shahinian A, et al. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science. 1997 Jan 24;275(5299):540–3. doi: 10.1126/science.275.5299.540. [DOI] [PubMed] [Google Scholar]
- 134.Xu WD, Pan HF, Ye DQ, Xu Y. Targeting IRF4 in autoimmune diseases. Autoimmunity reviews. 2012 Oct;11(12):918–24. doi: 10.1016/j.autrev.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 135.Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nature immunology. 2006 Jul;7(7):773–82. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 136.Lohoff M, Mittrucker HW, Prechtl S, Bischof S, Sommer F, Kock S, et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proceedings of the National Academy of Sciences of the United States of America. 2002 Sep 3;99(18):11808–12. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu CM, Jang SY, Fanzo JC, Pernis AB. Modulation of T cell cytokine production by interferon regulatory factor-4. The Journal of biological chemistry. 2002 Dec 20;277(51):49238–46. doi: 10.1074/jbc.M205895200. [DOI] [PubMed] [Google Scholar]
- 138.Tominaga N, Ohkusu-Tsukada K, Udono H, Abe R, Matsuyama T, Yui K. Development of Th1 and not Th2 immune responses in mice lacking IFN-regulatory factor-4. International immunology. 2003 Jan;15(1):1–10. doi: 10.1093/intimm/dxg001. [DOI] [PubMed] [Google Scholar]
- 139.Man K, Miasari M, Shi W, Xin A, Henstridge DC, Preston S, et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nature immunology. 2013 Nov;14(11):1155–65. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- 140.Yao S, Buzo BF, Pham D, Jiang L, Taparowsky EJ, Kaplan MH, et al. Interferon regulatory factor 4 sustains CD8(+) T cell expansion and effector differentiation. Immunity. 2013 Nov 14;39(5):833–45. doi: 10.1016/j.immuni.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O’Shea JJ, et al. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. Journal of immunology. 2005 Mar 1;174(5):2573–81. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 142.Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, et al. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha- dendritic cell development. Proceedings of the National Academy of Sciences of the United States of America. 2004 Jun 15;101(24):8981–6. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bajana S, Roach K, Turner S, Paul J, Kovats S. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. Journal of immunology. 2012 Oct 1;189(7):3368–77. doi: 10.4049/jimmunol.1102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Murphy KM. Transcriptional control of dendritic cell development. Advances in immunology. 2013;120:239–67. doi: 10.1016/B978-0-12-417028-5.00009-0. [DOI] [PubMed] [Google Scholar]
- 145.Akbari M, Honma K, Kimura D, Miyakoda M, Kimura K, Matsuyama T, et al. IRF4 in dendritic cells inhibits IL-12 production and controls Th1 immune responses against Leishmania major. Journal of immunology. 2014 Mar 1;192(5):2271–9. doi: 10.4049/jimmunol.1301914. [DOI] [PubMed] [Google Scholar]
- 146.Shaffer AL, Emre NC, Romesser PB, Staudt LM. IRF4: Immunity Malignancy! Therapy? Clinical cancer research: an official journal of the American Association for Cancer Research. 2009 May 1;15(9):2954–61. doi: 10.1158/1078-0432.CCR-08-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dorsey MJ, Tae HJ, Sollenberger KG, Mascarenhas NT, Johansen LM, Taparowsky EJ. B-ATF: a novel human bZIP protein that associates with members of the AP-1 transcription factor family. Oncogene. 1995 Dec 7;11(11):2255–65. [PubMed] [Google Scholar]
- 148.Murphy TL, Tussiwand R, Murphy KM. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nature reviews Immunology. 2013 Jul;13(7):499–509. doi: 10.1038/nri3470. [DOI] [PubMed] [Google Scholar]
- 149.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunological reviews. 2005 Dec;208:126–40. doi: 10.1111/j.0105-2896.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 150.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nature reviews Cancer. 2003 Nov;3(11):859–68. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 151.Martinez GJ, Dong C. BATF: bringing (in) another Th17-regulating factor. Journal of molecular cell biology. 2009 Dec;1(2):66–8. doi: 10.1093/jmcb/mjp016. [DOI] [PubMed] [Google Scholar]
- 152.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annual review of immunology. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 153.Betz BC, Jordan-Williams KL, Wang C, Kang SG, Liao J, Logan MR, et al. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. The Journal of experimental medicine. 2010 May 10;207(5):933–42. doi: 10.1084/jem.20091548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aronheim A, Zandi E, Hennemann H, Elledge SJ, Karin M. Isolation of an AP-1 repressor by a novel method for detecting protein-protein interactions. Molecular and cellular biology. 1997 Jun;17(6):3094–102. doi: 10.1128/mcb.17.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Echlin DR, Tae HJ, Mitin N, Taparowsky EJ. B-ATF functions as a negative regulator of AP-1 mediated transcription and blocks cellular transformation by Ras and Fos. Oncogene. 2000 Mar 30;19(14):1752–63. doi: 10.1038/sj.onc.1203491. [DOI] [PubMed] [Google Scholar]
- 156.Iacobelli M, Wachsman W, McGuire KL. Repression of IL-2 promoter activity by the novel basic leucine zipper p21SNFT protein. Journal of immunology. 2000 Jul 15;165(2):860–8. doi: 10.4049/jimmunol.165.2.860. [DOI] [PubMed] [Google Scholar]
- 157.Su ZZ, Lee SG, Emdad L, Lebdeva IV, Gupta P, Valerie K, et al. Cloning and characterization of SARI (suppressor of AP-1, regulated by IFN) Proceedings of the National Academy of Sciences of the United States of America. 2008 Dec 30;105(52):20906–11. doi: 10.1073/pnas.0807975106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008 Nov 14;322(5904):1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Escalante CR, Brass AL, Pongubala JM, Shatova E, Shen L, Singh H, et al. Crystal structure of PU.1/IRF-4/DNA ternary complex. Molecular cell. 2002 Nov;10(5):1097–105. doi: 10.1016/s1097-2765(02)00703-7. [DOI] [PubMed] [Google Scholar]
- 160.Koch S, Mousset S, Graser A, Reppert S, Ubel C, Reinhardt C, et al. IL-6 activated integrated BATF/IRF4 functions in lymphocytes are T-bet-independent and reversed by subcutaneous immunotherapy. Scientific reports. 2013;3:1754. doi: 10.1038/srep01754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ochiai K, Maienschein-Cline M, Simonetti G, Chen J, Rosenthal R, Brink R, et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity. 2013 May 23;38(5):918–29. doi: 10.1016/j.immuni.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grusdat M, McIlwain DR, Xu HC, Pozdeev VI, Knievel J, Crome SQ, et al. IRF4 and BATF are critical for CD8 T-cell function following infection with LCMV. Cell death and differentiation. 2014 Feb 14; doi: 10.1038/cdd.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kurachi M, Barnitz RA, Yosef N, Odorizzi PM, Diiorio MA, Lemieux ME, et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8(+) T cells. Nature immunology. 2014 Apr;15(4):373–83. doi: 10.1038/ni.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ginsberg M, Czeko E, Muller P, Ren Z, Chen X, Darnell JE., Jr Amino acid residues required for physical and cooperative transcriptional interaction of STAT3 and AP-1 proteins c-Jun and c-Fos. Molecular and cellular biology. 2007 Sep;27(18):6300–8. doi: 10.1128/MCB.00613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ivanov VN, Bhoumik A, Krasilnikov M, Raz R, Owen-Schaub LB, Levy D, et al. Cooperation between STAT3 and c-jun suppresses Fas transcription. Molecular cell. 2001 Mar;7(3):517–28. doi: 10.1016/s1097-2765(01)00199-x. [DOI] [PubMed] [Google Scholar]
- 166.Schaefer TS, Sanders LK, Nathans D. Cooperative transcriptional activity of Jun and Stat3 beta, a short form of Stat3. Proceedings of the National Academy of Sciences of the United States of America. 1995 Sep 26;92(20):9097–101. doi: 10.1073/pnas.92.20.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang X, Wrzeszczynska MH, Horvath CM, Darnell JE., Jr Interacting regions in Stat3 and c-Jun that participate in cooperative transcriptional activation. Molecular and cellular biology. 1999 Oct;19(10):7138–46. doi: 10.1128/mcb.19.10.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome research. 2012 Sep;22(9):1760–74. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Howald C, Tanzer A, Chrast J, Kokocinski F, Derrien T, Walters N, et al. Combining RT-PCR-seq and RNA-seq to catalog all genic elements encoded in the human genome. Genome research. 2012 Sep;22(9):1698–710. doi: 10.1101/gr.134478.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012 Sep 6;489(7414):101–8. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Consortium EP, Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012 Sep 6;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nature reviews Genetics. 2007 Feb;8(2):93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 173.Ceribelli A, Satoh M, Chan EK. MicroRNAs and autoimmunity. Current opinion in immunology. 2012 Dec;24(6):686–91. doi: 10.1016/j.coi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iborra M, Bernuzzi F, Invernizzi P, Danese S. MicroRNAs in autoimmunity and inflammatory bowel disease: crucial regulators in immune response. Autoimmunity reviews. 2012 Mar;11(5):305–14. doi: 10.1016/j.autrev.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 175.Belver L, Papavasiliou FN, Ramiro AR. MicroRNA control of lymphocyte differentiation and function. Current opinion in immunology. 2011 Jun;23(3):368–73. doi: 10.1016/j.coi.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. The Journal of experimental medicine. 2005 Jul 18;202(2):261–9. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nature immunology. 2011 Sep;12(9):861–9. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 178.Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CD, et al. MicroRNA-29 regulates T-box transcription factors and interferon-gamma production in helper T cells. Immunity. 2011 Aug 26;35(2):169–81. doi: 10.1016/j.immuni.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang L, Boldin MP, Yu Y, Liu CS, Ea CK, Ramakrishnan P, et al. miR-146a controls the resolution of T cell responses in mice. The Journal of experimental medicine. 2012 Aug 27;209(9):1655–70. doi: 10.1084/jem.20112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010 Sep 17;142(6):914–29. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010 Oct 29;33(4):607–19. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huffaker TB, Hu R, Runtsch MC, Bake E, Chen X, Zhao J, et al. Epistasis between microRNAs 155 and 146a during T cell-mediated antitumor immunity. Cell reports. 2012 Dec 27;2(6):1697–709. doi: 10.1016/j.celrep.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009 Jan 16;30(1):80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jiang S, Li C, Olive V, Lykken E, Feng F, Sevilla J, et al. Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood. 2011 Nov 17;118(20):5487–97. doi: 10.1182/blood-2011-05-355644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nature immunology. 2008 Apr;9(4):405–14. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lu TX, Hartner J, Lim EJ, Fabry V, Mingler MK, Cole ET, et al. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. Journal of immunology. 2011 Sep 15;187(6):3362–73. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Malmhall C, Alawieh S, Lu Y, Sjostrand M, Bossios A, Eldh M, et al. MicroRNA-155 is essential for T2-mediated allergen-induced eosinophilic inflammation in the lung. The Journal of allergy and clinical immunology. 2013 Dec 24; doi: 10.1016/j.jaci.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 188.Sawant DV, Wu H, Kaplan MH, Dent AL. The Bcl6 target gene microRNA-21 promotes Th2 differentiation by a T cell intrinsic pathway. Molecular immunology. 2013 Jul;54(3–4):435–42. doi: 10.1016/j.molimm.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proceedings of the National Academy of Sciences of the United States of America. 2009 Nov 3;106(44):18704–9. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Collison A, Mattes J, Plank M, Foster PS. Inhibition of house dust mite-induced allergic airways disease by antagonism of microRNA-145 is comparable to glucocorticoid treatment. The Journal of allergy and clinical immunology. 2011 Jul;128(1):160–7. e4. doi: 10.1016/j.jaci.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 191.Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciume G, Muljo SA, et al. TGF-beta and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nature immunology. 2012 Jun;13(6):587–95. doi: 10.1038/ni.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nature immunology. 2009 Dec;10(12):1252–9. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 193.Baumjohann D, Kageyama R, Clingan JM, Morar MM, Patel S, de Kouchkovsky D, et al. The microRNA cluster miR-17 approximately 92 promotes TFH cell differentiation and represses subset-inappropriate gene expression. Nature immunology. 2013 Aug;14(8):840–8. doi: 10.1038/ni.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kang SG, Liu WH, Lu P, Jin HY, Lim HW, Shepherd J, et al. MicroRNAs of the miR-17 approximately 92 family are critical regulators of T(FH) differentiation. Nature immunology. 2013 Aug;14(8):849–57. doi: 10.1038/ni.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007 Jun 8;316(5830):1484–8. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 196.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013 Mar 14;152(6):1298–307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome research. 2012 Sep;22(9):1775–89. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dinger ME, Pang KC, Mercer TR, Crowe ML, Grimmond SM, Mattick JS. NRED: a database of long noncoding RNA expression. Nucleic acids research. 2009 Jan;37(Database issue):D122–6. doi: 10.1093/nar/gkn617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Amaral PP, Clark MB, Gascoigne DK, Dinger ME, Mattick JS. lncRNAdb: a reference database for long noncoding RNAs. Nucleic acids research. 2011 Jan;39(Database issue):D146–51. doi: 10.1093/nar/gkq1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Collier SP, Collins PL, Williams CL, Boothby MR, Aune TM. Cutting edge: influence of Tmevpg1, a long intergenic noncoding RNA, on the expression of Ifng by Th1 cells. Journal of immunology. 2012 Sep 1;189(5):2084–8. doi: 10.4049/jimmunol.1200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hu G, Tang Q, Sharma S, Yu F, Escobar TM, Muljo SA, et al. Expression and regulation of intergenic long noncoding RNAs during T cell development and differentiation. Nature immunology. 2013 Nov;14(11):1190–8. doi: 10.1038/ni.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]